Abstract
The primary goal of this study was to develop descriptive image interpretation criteria and a classification of endoscopic ultrasound-confocal microscopy (EUS-CM) findings in pancreatic masses and lymph nodes through a review of prospectively obtained EUS-CM videos from proven malignant and benign cases, and to propose diagnostic criteria for predicting malignancy. The material used was a 19-G EUS-needle in which the stylet was replaced by the confocal mini-probe. The mini-probe pre-loaded in the EUS-needle was guided endosonographically in the target then the mini-probe was pushed under EUS guidance into the lesion. Eleven patients mean age 62.3 years underwent EUS for the staging of a pancreatic mass (3 cystic and 4 solid) or for the diagnosis of celiac and/or mediastinal LN (n = 4). Benign intraductal papillary mucinous neoplasm (IPMN) was characterized by the aspect of finger-like projections which correspond to the villous changes of intestinal IPMN type. In pancreatic adenocarcinomas, EUS-CM found vascular leakage with irregular vessels with leakage of fluorescein into the tumor, large dark clumps which correspond to humps of malignant cells. Inflammatory lymph nodes were characterized by the presence of diffuse small cells into a homogeneous stroma with a normal vascularization. At the opposite, EUS-CM showed in malignant lymph node glandular structures with dark cells, large dark clumps and an important neo-vascularization with huge leakage of fluorescein.
INTRODUCTION
Confocal endomicroscopy is an emergent technique and allows real optical biopsies in the gastrointestinal tract. The aim of this study was to evaluate a new intratumoral confocal miniprobe under endoscopic ultrasound (EUS) guidance in patients with solid or cystic pancreatic tumors and celiac or mediastinal lymph nodes. The primary goal of this study was to develop descriptive image interpretation criteria and a classification of EUS-confocal microscopy (CM) findings in pancreatic masses and lymph nodes through a review of prospectively obtained EUS-CM videos from proven malignant and benign cases, and to propose diagnostic criteria for predicting malignancy. Secondary aims include assessing procedure-related adverse events, technical feasibility of EUS-CM and developing an atlas of EUS-CM images in pancreatic masses and lymph nodes. Informed consent was obtained for all patients included in this study.
PATIENTS AND METHODS
From December 2011 to March 2012, 11 patients (7 males & 4 females), mean age 62.3 years (range: 22-76 years), underwent an EUS for the staging of pancreatic masses (3 cystic and 4 solid) or for the diagnosis of celiac and/or mediastinal lymph nodes (n = 4). The results of the EUS-CM were compared with the histologic findings of EUS-FNA in 6 patients and with the histologic examination of the resected specimen in 5 patients (2 cystic and 3 solid tumors of the pancreas). The EUS-CM images were reviewed and interpreted by an experienced pathologist specialized in digestive oncology.
The material used was a 19-G EUS-needle (Cook-endoscopy) in which the stylet was replaced by the confocal miniprobe (Cellvizio Technology, Mauna-Kea company-France). The miniprobe pre-loaded in the EUS-needle was guided endosonographically in the target, and then pushed under EUS guidance into the lesion (Fig. 1). The intratumoral CM examination started after injection of 2.5-mL fluorescein intravenously. No complication occurred during this study. The final diagnosis was 1 serous cystadenoma, 1 benign intraductal papillary mucinous neoplasm (IPMN), 1 malignant IPMN, 1 pancreatic sarcoma, 3 adenocarcinomas of the pancreas, 7 malignant lymph nodes (2 celiac and 5 in the mediastinum) [from gastric cancer (n = 2), colon cancer (n = 2), cardia carcinoma (n = 2), and lung cancer (n = 1), respectively] and 2 inflammatory lymph nodes.
Figure 1.
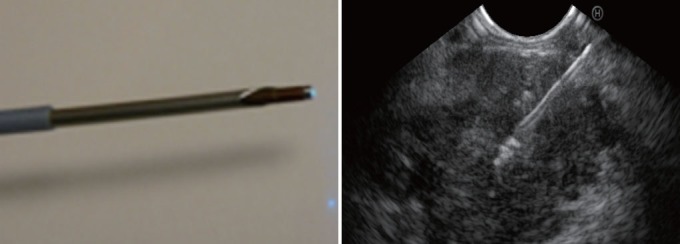
Probe of confocal passed in a 19-G needle inserted in a pancreatic mass.
RESULTS AND DESCRIPTIONS OF THE EUS-CM IMAGES
Cystic pancreatic lesion
EUS-CM showed ultra-thin straight bright grey bands in the serous cystadenoma and an aspect of dark clumps with a neo-vascularization and large vessels (> 20 μm of diameter) in the malignant IPMN. Benign IPMN was characterized by the aspect of finger-like projections which correspond to the villous changes of intestinal IPMN type (Fig. 2). Benign IPMN was also characterized by the presence of regular vessels (Fig. 3).
Figure 2.
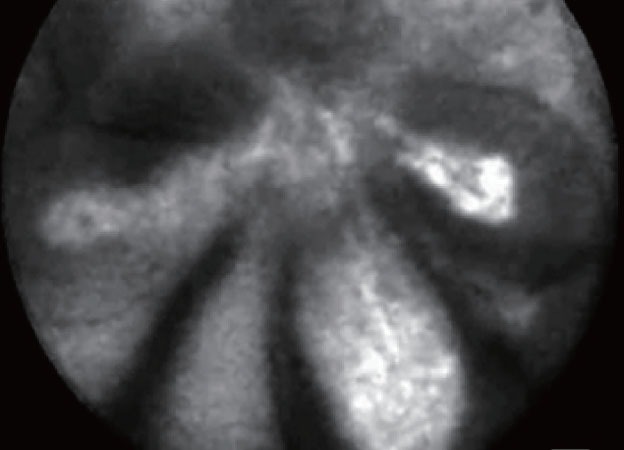
Finger like projections: benign intraductal papillary mucinous neoplasm intestinal type.
Figure 3.
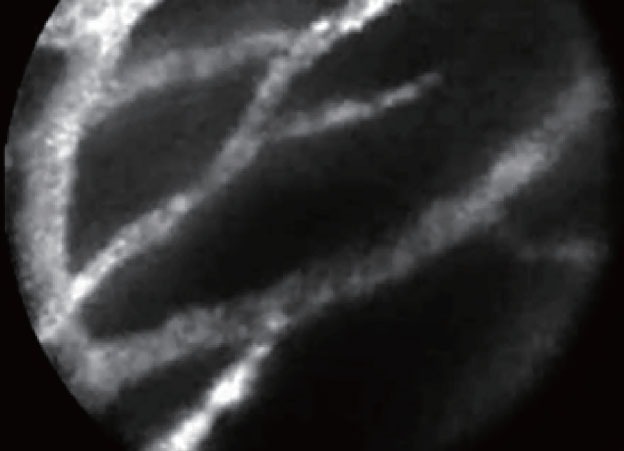
Normal vessels in the wall of benign intraductal papillary mucinous neoplasm.
Solid pancreatic mass
In pancreatic adenocarcinomas, EUS-CM found vascular leakage with irregular vessels with leakage of fluorescein into the tumor (Fig. 4), large dark clumbs which correspond to humps of malignant cells (Fig. 5). Regarding the pancreatic sarcoma, EUS-CM showed no dark clumps but an aggregation of small round cells, fibrous grey and thin bands and irregular neo-vessels. Normal pancreatic tissue appears as “coffee bean” aspects with regular vessels (Fig. 6)
Figure 4.
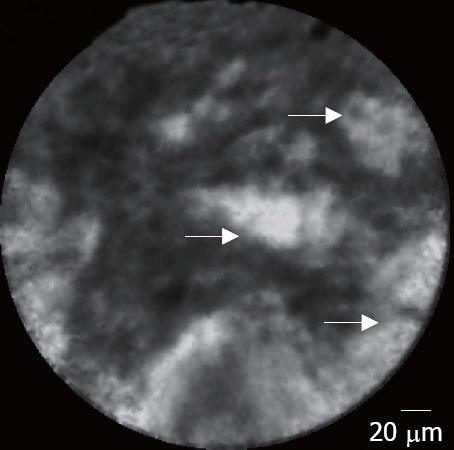
Pancreatic adenocarcinoma: leakage of fluorescein (arrows).
Figure 5.
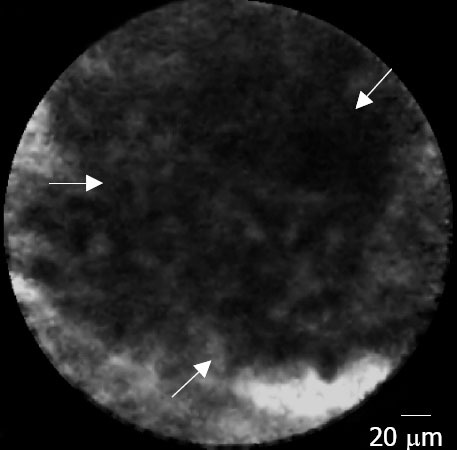
Pancreatic adenocarcinoma: large black clumb (arrows).
Figure 6.
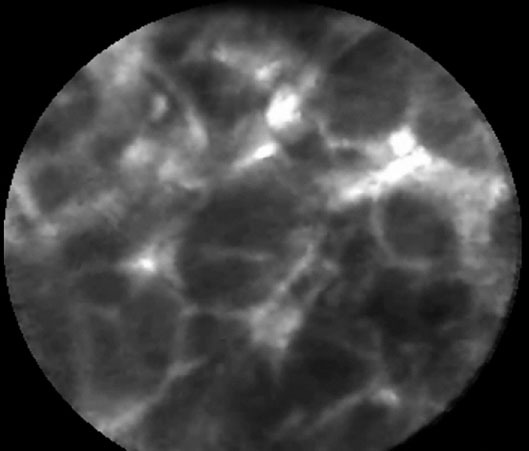
Normal pancreatic tissue: coffee bean aspect.
Lymph nodes
Inflammatory lymph nodes were characterized by the presence of diffuse small cells into a homogeneous stroma with a normal vascularization (Fig. 7). At the opposite, EUS-CM showed in malignant lymph node glandular structures with dark cells, large dark clumbs and an important neovascularization with huge leakage of fluorescein (Fig. 8).
Figure 7.
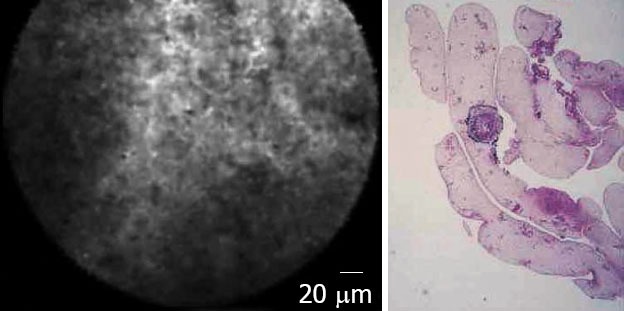
Inflammatory lymph node: confocal microscopy aspect and histology.
Figure 8.
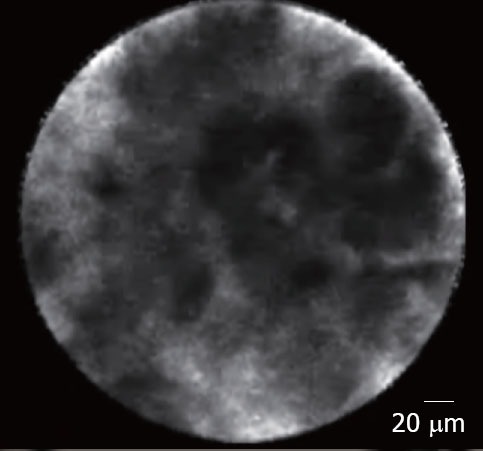
Malignant lymph node: metastasis from a cardia cancer.
DISCUSSION
The introduction of EUS represented a major advance in the diagnosis and staging of gastrointestinal malignancies.
In addition to providing imaging of tumors and enhancing TMN staging, EUS also provides guidance for fine needle aspiration (FNA) and biopsies of undiagnosed masses and lymph nodes suspicious for malignant invasion, providing further diagnostic and staging information. However, FNA is technically demanding and multiple punctures of lymph nodes or masses are sometimes required to obtain sufficient tissue for histologic assessment. For further consideration, the fact is that pancreatic masses have a wide differential diagnosis that includes benign and malignant etiologies and FNA of the pancreas is associated with an accuracy around 85% to 90% but a negative predictive value of 35% to 40%.1,2,3 Hence, the ability of EUS-CM is to more accurately evaluate masses and lymph nodes prior to their puncture in an effort to have an optical biopsy and it will be a “minute” diagnosis of malignancy in the future.
The first study on pig models confirmed the feasibility of the technique.4 Ten pigs were examined while they were under general anesthesia. Either EUS-guided organ puncture or natural-orifice transluminal endoscopic surgery (NOTES) procedure was used. The confocal miniprobe was inserted through the 22-G needle, and puncture of various intra- abdominal structures and organs was performed (lymph nodes, diaphragm, ovaries, liver, spleen, and pancreas) after intravenous injection of fluorescein (5-10 mL 1% or 2 mL 10% solution). Real-time sequences were recorded. Biopsy specimens were taken for standard histopathology. It was technically feasible to introduce the needle-based confocal miniprobe into various organs at the time of EUS or NOTES procedures. The device enabled real-time in vivo collection of images at histologic resolutions and of acceptable image quality from several intra-abdominal organs interrogated. The authors concluded that needle-based confocal laser endomicroscopy (nCLE) in the pancreas was technically feasible via a 19-G needle under endosonographic guidance.
Recently, Konda et al.5 published their data on 18 patients presenting for EUS-FNA. Patients were injected with 2.5 mL of 10% fluorescein. The lesion was interrogated with the nCLE probe positioned at the tip of a 19-G FNA needle. Cases included 16 cysts and 2 masses. There were no device malfunctions. Technical challenges were encountered in 6 of 18 attempts to image, reflecting challenges with a postloading technique, the longer ferule tip, and a transduodenal approach. Technical feasibility to perform imaging with nCLE during a pancreatic EUS-FNA procedure was achieved in 17 of 18 cases. Ten cases had good to very good image quality. Two serious adverse events occurred; both were pancreatitis requiring hospitalization.
CONCLUSION
EUS-CM is feasible during an EUS examination; these preliminary results are very encouraging. An international multicenter study focused in cystic and solid pancreatic lesions and lymph nodes (CONTACT study) is ongoing.
REFERENCES
- 1.Giovannini M, Seitz JF, Monges G, et al. Fine needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy. 1995;27:171–77. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 2.Wieserma M, Vilmann P, Giovannini M, et al. Endosonography-guided fine needle aspiration biopsy : diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 4.Becker V, Wallace MB, Fockens P, et al. Needle-based confocal endomicroscopy for in vivo histology of intra-abdominal organs: first results in a porcine model (with videos) Gastrointest Endosc. 2010;71:1260–6. doi: 10.1016/j.gie.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Konda VJ, Aslanian HR, Wallace MB, et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos) Gastrointest Endosc. 2011;74:1049–60. doi: 10.1016/j.gie.2011.07.018. [DOI] [PubMed] [Google Scholar]


