Abstract
The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) introduced guidelines on the use of contrast-enhanced ultrasound (CEUS) in 2004. This EFSUMB-document focused mainly on liver applications. However, new applications extending beyond the liver were developed thereafter. Increased interest in recent years in CEUS technique and in the application of CEUS in novel fields like endoscopic ultrasound (EUS) has revolutionized indications and applications. As a result, the EFSUMB initiated a new update of the guidelines in 2011 to include this additional knowledge. Some of the contrast-enhanced EUS (CE-EUS) indications are established, whereas others are preliminary; these latter indications are categorized as emergent CEUS applications since the available evidence is insufficient for general recommendation. This article focuses on the use of CE-EUS in various clinical settings. The reader will get an overview of current indications and possible applications of CE-EUS. This involves the introduction of different contrast studies including color Doppler techniques (known as contrast-enhanced high mechanical index endosonography or CEHMI-EUS) as well as more modern high-resolution contrast-enhanced techniques (known as contrast-enhanced low mechanical index endosonography or CELMI EUS).
Keywords: ultrasound, endoscopic ultrasound, contrast-enhanced, differential diagnosis
INTRODUCTION
Contrast-Enhanced endoscopic ultrasound (CE-EUS) is a newly established method which combines the advantage of high-resolution ultrasound (US) of internal organs with the administration of ultrasound contrast agents (UCAs).1 The technique has been described using two different technical subtypes with similar results: contrast-enhanced endoscopic Doppler ultrasound with high-mechanical index (MI) (CEHMI-EUS) which does not require specific software2,3,4,5,6 and contrast-enhanced low-MI endoscopic ultrasound (CELMI-EUS) using the contrast-specific mode,1,7 the technique for the latter being the same as for the transabdominal approach. In CEHMI-EUS the Doppler frequency should be as high as possible, depending on the distance to the tumor. The flow velocity range (pulse repetition frequency, PRF) should be between 4 and 6 cm/s and the gain should be as high as possible without inducing artefacts.8,9
The differential diagnosis between ductal adenocarcinoma and mass forming focal pancreatitis can be improved by analyzing the Doppler spectrum since in adenocarcinomas only arterioles can be detected. Venules are not very often detectable due to the typical vascularization pattern and also probably tumor pressure due to the desmofibrotic reaction. Conversely, in focal pancreatitis both arterioles and venules are detectable by spectral Doppler analysis.10,11 The method can be improved even further by estimation of the resistance index (RI) of the arterioles. The cut-off RI for suspected malignant lesions is >0.70 versus <0.70 for non-malignant, inflammatory lesions.10
The differential diagnosis of solid and cystic pancreatic lesions is similar to that described for the transabdominal route.12,13,14,15,16 Both focal and diffuse autoimmune pancreatitis typically show hyperenhancement.17,18,19 The recommended uses and indications are mainly for the pancreas and additionally for the discrimination of mass-forming chronic pancreatitis from ductal adenocarcinoma in patients with chronic pancreatitis.20,21
GENERAL ADVICE
The best performance in CE-EUS is typically achieved by investigators who are highly skilled in B-mode ultrasound. An adequate ultrasound machine (equipped with low-MI contrast imaging mode) and optimal machine settings are needed. The particular skills needed to perform CE-EUS examinations are relatively easy to learn compared to the time and effort needed to optimize conventional examination skills. The recommended dose of SonoVue® is 4.8 mL under most circumstances. This can be decreased to 2.4 mL for examination of the pancreas depending on the sensitivity of the equipment used and the type of transducer and the organ under investigation. Extravascular applications need just a few drops of SonoVue® in 10 to 100 mL physiologic saline solution depending on the pathology examined.20,21
The main elements and advantages of CE-EUS include real-time imaging of microvascularity and microperfusion, real-time intervention guidance, on-site performance ability and impressively good detail resolution. This is why CE-EUS has been so successful in recent years and has gained importance in daily routine examination. CE-EUS has numerous advantages over computed tomography (CT) and magnetic resonance imaging (MRI). It can be performed immediately without any previous laboratory testing for renal function, and it is appropriate for repeated follow-up examinations due to the lack of ionising radiation. In addition, the physical size of UCAs such as SonoVue® (equivalent to or smaller than red blood cells) means that they act as pure blood pool agents, with no extravasation into the interstitial fluid. This leads to prolonged enhancement of the vascular system which can be advantageous compared to CT and MRI contrast enhancement.20
PANCREAS
Ultrasound was always a good diagnostic tool for diagnosing pancreatic diseases. However, in the era before the introduction of ultrasound contrast enhancers certain conditions of the pancreas could not be diagnosed properly. The introduction of contrast enhancers could provide additional information about the vascularisation of the organ. This increased the value of the method especially for diagnosing necrotic pancreatic areas or vascularisation patterns of pancreatic neoplastic diseases that could be demonstrated in many studies22,23 including a recently published multicenter trial with more than 1000 patients.24 CE-EUS was able to combine the advantage of high-resolution ultrasound with real-time contrast enhancing visualization of pancreatic lesions. The combination led to the discovery of a unique vascularisation behaviour of inflammatory lesions compared to malignant lesions and was therefore able to discriminate both conditions. Furthermore cystic wall vascularisation could be visualized with the highest resolution possibly in current clinical medicine and is able to help endoscopists to introduce new clinical pathways.20
PANCREATIC PATHOLOGIES
Solid pancreatic lesions
The introduction of contrast agents has strongly enhanced the value of ultrasound, increasing the accuracy of the first-line examination for the characterization of pancreatic tumors. Ductal adenocarcinoma, the most common primary malignancy of the pancreas, is typically (about 90%) hypoenhancing in all phases because of the low mean vascular density (Fig. 1).22,23,25,26,27,28,29,30 This effect was already known from CT and MRI studies. The advantage of ultrasound analysis of the vascularization however is the real-time investigation of the pancreas. This enables the investigator to be independent from blood circulation times and therefore increases the value of the method.20 In clinical practice there is still the problem of discrimination of chronic pancreatitis to pancreatic carcinoma due to the similar vascularization behavior. CE-EUS, however, provided the possibility to analyze the macrovessels (arteriols and venoules) of the pancreas for neovascularization patterns. The main differentiation between the neovascularization of adenocarcinomas of the pancreas and chronic pancreatitis is the analysis of arterial and venous macrovessels. Contrast-Enhanced Doppler method reveals an irregular vessel system of the carcinoma without displaying venous vessels in adenocarcinomas in contrast to a netlike homogenous vessel system with both arterial and venous vessels in chronic pancreatitis. Using these criteria a discrimination sensitivity and specificity of over 90% can be achieved.10,20
Figure 1.
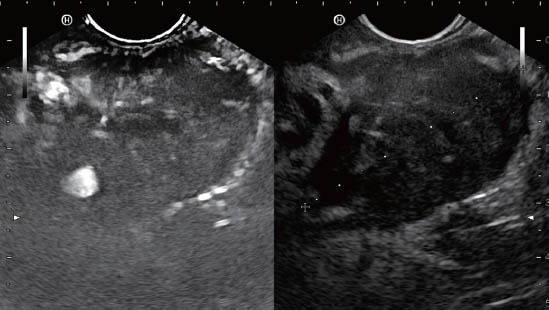
Typically hypoenhancing ductal adenocarcinoma of the pancreas.
Neuroendocrine tumors (Fig. 2) and serous microcystic neoplasia present typically as hyperenhancing masses in the arterial phase, owing to their abundant arterial vascularisation.22,23,31,32 Larger cystic tumors, however, can have the same central necrotic areas, which could let the lesion appear inhomogenous and make the correct diagnosis more difficult.33 Pancreatic intraepithelial neoplasias (PanIN) have been recognized as precursor lesions of ductal adenocarcinoma. PanIN-3 is a polypoid lesion in a papillar or micropapillar structure with signs of necrosis. Whereas PanIN 1-2 lesions are invisible at EUS, and PanIN-3 lesions can be visualized by pancreatic duct irregularities, which can be confirmed histologically by biopsy or surgery.34 If PanIN-3 lesions can be visualized by EUS, contrast studies show typically a hyperenhancing lesion.
Figure 2.
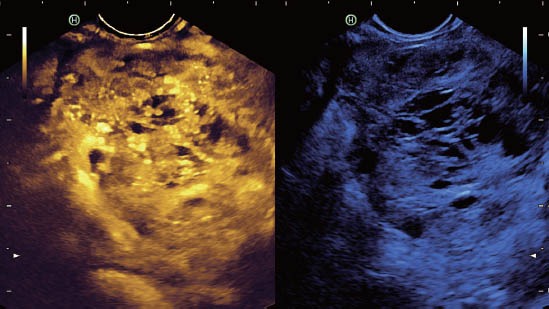
Typically hyperenhancing neuroendocrine tumor of the pancreas.
Cystic pancreatic lesions
CE-EUS improves the ultrasonographic differential diagnosis between pseudocysts and mucious cystic neoplasia [mucinous cystadenoma, intraductal papillary mucinous neoplasms (IPMNs)] of the pancreas by accurately revealing microvascularization of intralesional septa and parietal nodules.12,16 Pseudocysts typically contain non-vascularized material (debris), with the exception of transversing (large) vessels, which are frequently found in the early stages. Pseudocysts do not show any signal on CE-EUS and remain completely non-enhancing in all phases, even when they are heterogeneous on US. IPMNs are divided into main-duct and side branch-duct types with different prognosis depending on the age of patients and comorbidity. CE-EUS is helpful in differentiating perfused (nodules) from non-perfused (clots) regions (Fig. 3, 4). Serous oligo-and macrocystic cystadenomas are benign cystic lesions, typically with a lobulated appearance with thin walls and centrally orientated arteries. CE-EUS enables the investigators to discriminate pseudocysts from tumorous pancreatic lesions because of the vascularisation behaviour described above. However, the discrimination of different benign cystic lesions or even of benign from malignant cystic lesions is not so easy. If no typical cystic lesions (per example microcystic serous cyst adenoma can already be safely diagnosed by the anatomic structure) have to be discriminated, every enhancing cystic lesion can be further discriminated by using endoscopic fine needle aspiration (FNA). Beside the cytology and the estimation of the CEA level, the most important result is the analysis of the fluid. Mucinous cysts have a higher potential of malignancy and should be surgical treated if the patient's condition allows.
Figure 3.
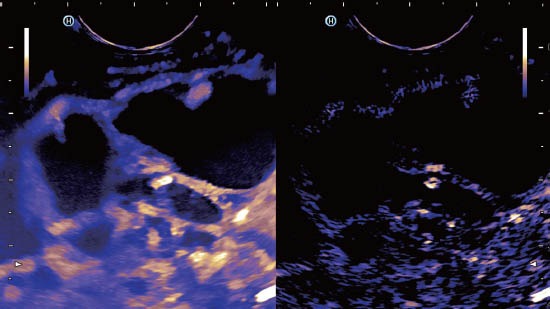
Macrocystic cystadenoma with contrast enhancing septae.
Figure 4.
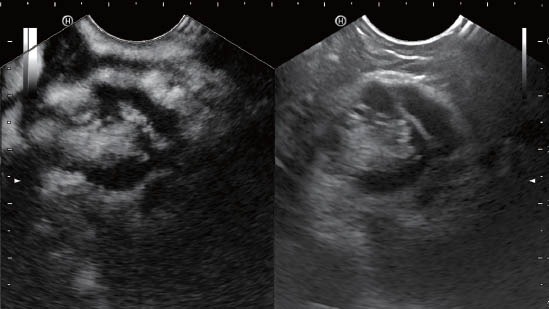
Intraductal papillary mucinous neoplasm of the pancreas with perfused intraductular nodules.
PANCREATITIS
In acute pancreatitis, CE-EUS may help to identify and delineate necrotic areas, which do not enhance at the very early stage.35 The lack of nephrotoxicity of CE-EUS contrast agents is of particular importance in patients with pancreatitis because of the fact that most severely ill patients develop renal failure. In those cases, CT contrast enhancers are contraindicated. Focal mass-forming pancreatitis25 and autoimmune pancreatitis17 have similar or stronger enhancement to or than the normal pancreatic parenchyma which is of importance when it comes to a differential diagnosis for ductal adenocarcinoma. The homogenous increased contrast enhancing behaviour of the pancreas can be a sign of diffuse autoimmune pancreatitis and might led the investigator into the right direction (Fig. 5).
Figure 5.
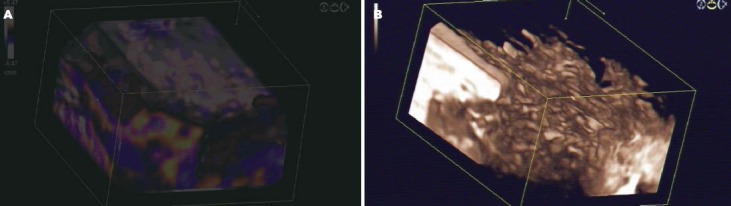
3D-reconstruction of CEHMI-EUS and CELMI-EUS of the pancreatic corpus of a patient with autoimmune pancreatitis. A: The Colour Doppler signals of the arterioles and venoules of the pancreas. B: The contrast enhancing effect in the smaller vessels in the same area of the pancreas using low mechanical index technique with 3D reconstruction. CELMI-EUS: contrast-enhanced low-MI endoscopic ultrasound; CEHMI-EUS: contrast-enhanced endoscopic Doppler ultrasound with high-mechanical index.
Recommended Uses and Indications According to the EFSUMB Guidelines36
Focal pancreatic lesions found at US can be studied with CEUS in order to improve: (1) Characterization of ductal adenocarcinoma (level of recommendation: A; 1b) since ductal adenocarcinoma is typically hypoenhancing; (2) Differential diagnosis between pseudocysts and cystic tumors (level of recommendation: A; 1b) by analyzing microperfusion of (parietal) vascularized nodules and septa; (3) Differentiation of vascular (solid) from avascular (liquid/necrotic) components of a lesion (level of recommendation: A; 1b) which is of importance for the early detection of necrosis in acute pancreatitis and for the differential diagnosis of pseudocysts and neoplasia in chronic pancreatitis; (4) Defining the dimensions and margins of a lesion, including its relationship with adjacent vessels (level of recommendation: B; 2b) which is of importance for operability; and (5) Diagnosis in cases that are indeterminate on CT (vascularisation of solid pancreatic lesions; differential diagnosis between pseudocysts and pancreatic cystic tumors, especially mucinous cystic tumor) (level of recommendation: C; 5) which is mainly true for contrast-enhanced endoscopic ultrasound.
BILIARY DISEASE
The vascular phases of the gallbladder wall after US contrast administration are different from those of the liver because the blood supply is provided entirely by the cystic artery and not by portal vein branches. CE-EUS is helpful in differentiating non-enhancing sludge from enhancing neoplasia. Gallbladder carcinomas are typically hyperenhancing in the arterial phase and hypoenhancing in the venous phase. In acute cholecystitis, detection or exclusion of abscess formation in the surrounding liver parenchyma can be performed with CE-EUS. Interruption of the gallbladder wall suggests perforation, which can be confirmed by the lack of enhancement of the perforated wall. The role of CE-EUS in patients with Klatskin tumors is underestimated in our view but convincing data have not been published yet.
Promising findings have been observed in extrahepatic cholangiocarcinoma, e.g., determining the depth of wall infiltration and surrounding tissue mainly by using CE-EUS but lesions can also be detected by transabdominal US.37,38,39,40
GASTROINTESTINAL TRACT
Gastrointestinal stromal tumors (GIST) show typical arterial hyperenhancement and often central necrosis. Central necrosis is not often displayed in other subepithelial lesions (Fig. 6-8). Leiomyoma (Fig. 7) shows less arterial enhancement and lipoma often only little enhancement (Fig. 8). Subepithelial lesions so far are not part of the EFSUMB guidelines.36,41,42,43 Oncological principles and locoregional complications have to be regarded.44,45,46,47,48,49
Figure 6.
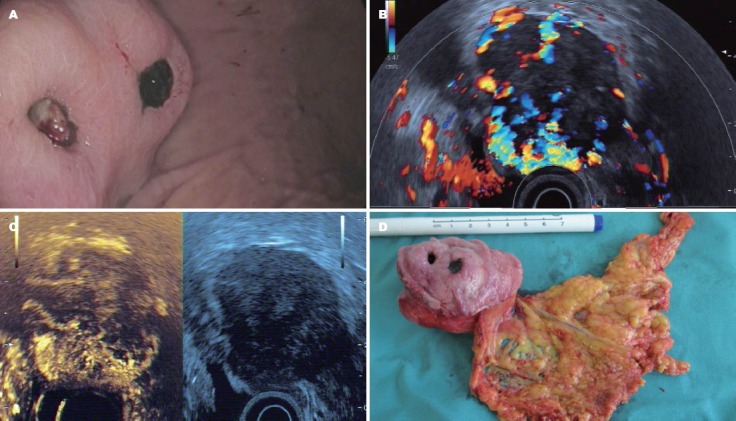
Gastrointestinal stromal tumor. A: Typical endoscopic features; B: CEHMI-EUS image of the same tumor, and please notice the rich vessel system; C: CELMI-EUS image, and the necrotic area was displayed in the upper area of the tumor; D: The surgical specimen of the tumor. CELMI-EUS: contrast-enhanced low-MI endoscopic ultrasound; CEHMI-EUS: contrast-enhanced endoscopic Doppler ultrasound with high-mechanical index.
Figure 8.
CELMI-EUS of a hypoenhancing lipoma. CELMI-EUS: contrast-enhanced low-MI endoscopic ultrasound.
Figure 7.
CELMI-EUS of a hypoenhancing leiomyoma. CELMI-EUS: contrast-enhanced low-MI endoscopic ultrasound.
SPLEEN
CE-EUS has been used in few patients with splenic abnormalities, and not been established so far in the routine use. CE-EUS is especially useful for characterizing ectopic splenic tissue (accessory spleens and post-splenectomy splenosis). Accessory spleens usually exhibit a typical location and appearance with a central or eccentric but symmetric vascular tree.
Most focal splenic lesions are incidentally discovered by US. In this setting and when they are single or scanty, especially if they are hyperechoic on B-mode US, they are benign in the large majority of cases. Analysis of comorbidity is often conclusive for the determination of focal splenic lesions including patients with lymphoma. If small splenic lesions with uncertain origin are discovered and the diagnosis might change the clinical pathway, biopsy is recommended. Sometimes those lesions are hard to visualize by percutaneous US but easy to visualize by EUS. In those cases, contrast-enhanced studies are possible and endoscopic FNA is safely performed.
ADRENAL GLANDS
CEUS and CE-EUS, both cannot reliably differentiate between benign (endocrine tumors and adenomas) and malignant adrenal masses50 despite some controversial discussion of the subject.51 Adrenal cysts, lipoma and myelolipoma demonstrate a characteristic B-mode US morphology. When it comes to a differential diagnosis in patients with arterial hypertension, it is important to know that adenomas in Conn's syndrome are often smaller than 20 mm in diameter and pheochromocytomas are often larger than 40 mm and that they display typical B-mode US characteristics at the time of final diagnosis. CEUS may reveal the characteristic hypervascularity of pheochromocytoma, which also have typically necrotic regions that do not enhance.50 Adrenal carcinomas are often large at the time of diagnosis and incidental in patients without underlying malignant diseases. Adrenal lesions are rarely malignant if <40 mm in diameter. Therefore, CEUS is rarely indicated if the clinical background of the patient is known and typical B-mode features of the most common tumors are evident. Incidentaloma show typically homogenous contrast enhancement. The most challenging task is the newly detected incidentaloma in patients with underlying malignant diseases, e.g. bronchial carcinoma. Typically, benign lesions occur in about 50% of the patients while malignant lesions and some rare diseases, e.g., lymphangioma occur in the remaining 50%.52 EUS of the left adrenal gland can be easily performed but might be difficult on the right side.
CEUS typically shows an hypoenhancing effect in non-small cell lung carcinomas. In case of the left adrenal gland, endoscopic FNA cytology can be easily performed to prove the metastatic origin.
LYMPH NODES
CEUS has been shown to increase the accuracy of the analysis of the vascular pattern (angioarchitecture) in patients with neoplastic infiltration of lymph nodes,53 although controlled prospective data are lacking. Demonstration of malignant neovascularization, seen as vessels penetrating the node's capsule away from the hilum, has been used in most reports as the characteristic feature of lymph node metastasis. Demonstration of vascular anatomy can be facilitated by adding CEUS to the conventional US examination. Lymphoma must be considered as a specific entity because there is evidence that the vascular pattern of lymphomatous lymph node infiltration resembles that of non-malignant nodes.54,55 There are limitations of CEUS due to the fact that about one third of malignant infiltrations occur in lymph nodes with a size of less than 5 mm at least in gastrointestinal cancers; therefore, exclusion of lymph node infiltration by all imaging methods is impossible.56 So far CE-EUS is not recommended for routine discrimination between benign and malignant lymph nodes.
ENDORECTAL ULTRASOUND, PERINEAL (PERIANAL) ULTRASOUND
Contrast-Enhanced perineal US is an effective, easily available but so far not well-known diagnostic tool. Perineal US is particularly useful if clinical examination and endorectal US cannot be performed (e.g., due to severe pain when introducing the probe). Contrast-Enhanced perineal US requires good knowledge of the anatomy of the pelvic floor and the sphincter apparatus. Patient preparation is not required. The localization of inflammatory and neoplastic lesions should be described in relation to the sphincter apparatus. Fistulas can be further differentiated by US examination into intersphincteric, transsphincteric and extrasphincteric types.
Conventional B-mode perineal US is inadequate for complex fistulas, as its sensitivity for the complex branching structure is poor. However, discrimination of fistulas and abscesses with contrast-enhanced perineal US is possible in most cases, although studies have not been published yet. Additionally, the extent of a fistula can be imaged by instillation of contrast agent into its external ostium.
Contrast-Enhanced ultrasound has been established over the last decade. Initially, a set of guidelines for the use of ultrasound contrast agents was published in 2004 dealing only with liver applications. A second edition of the guidelines in 2008 reflected changes in the available contrast agents and updated the guidelines for the liver, as well as implementing some non-liver applications.57 Time has moved on, and the need for international guidelines on the use of CEUS in the liver58 and non-liver36 has become apparent. A joint WFUMB-EFSUMB initiative has implicated experts from major leading ultrasound societies worldwide. These liver CEUS guidelines are simultaneously published in the official journals of both organizing federations (i.e., Ultrasound in Medicine and Biology for WFUMB and Ultraschall in der Medizin/European Journal of Ultrasound for EFSUMB). These guidelines and recommendations provide general advice on the use of all currently clinically available ultrasound contrast agents (UCA). They are intended to create standard protocols for the use and administration of UCA on an international basis and improve the management of patients worldwide. Similarly, we strongly promote CELMI-EUS for the benefit of our patients worldwide.
REFERENCES
- 1.Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: a new technique. Z Gastroenterol. 2005;43:1219–23. doi: 10.1055/s-2005-858662. [DOI] [PubMed] [Google Scholar]
- 2.Becker D, Strobel D, Bernatik T, et al. Echo-enhanced color-and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc. 2001;53:784–9. doi: 10.1067/mge.2001.115007. [DOI] [PubMed] [Google Scholar]
- 3.Bhutani MS, Hoffman BJ, van Velse A, et al. Contrast-enhanced endoscopic ultrasonography with galactose microparticles: SHU508 A (Levovist) Endoscopy. 1997;29:635–9. doi: 10.1055/s-2007-1004270. [DOI] [PubMed] [Google Scholar]
- 4.Giovannini M. Contrast-enhanced endoscopic ultrasound and elastosonoendoscopy. Best Pract Res Clin Gastroenterol. 2009;23:767–79. doi: 10.1016/j.bpg.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Giovannini M. Contrast-enhanced and 3-dimensional endoscopic ultrasonography. Gastroenterol Clin North Am. 2010;39:845–58. doi: 10.1016/j.gtc.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Giovannini M. Endosonography: new developments in 2006. ScientificWorldJournal. 2007;7:341–63. doi: 10.1100/tsw.2007.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitano M, Sakamoto H, Matsui U, et al. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–50. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich CF, Ignee A, Hocke M, et al. Pitfalls and artefacts using contrast enhanced ultrasound. Z Gastroenterol. 2011;49:350–6. doi: 10.1055/s-0029-1245851. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich CF. Liver tumor characterization--comments and illustrations regarding guidelines. Ultraschall Med. 2012;33(Suppl 1):S22–S30. doi: 10.1055/s-0032-1312892. [DOI] [PubMed] [Google Scholar]
- 10.Hocke M, Schulze E, Gottschalk P, et al. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–50. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saftoiu A, Iordache SA, Gheonea DI, et al. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos) Gastrointest Endosc. 2010;72:739–47. doi: 10.1016/j.gie.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 12.D’Onofrio M, Megibow AJ, Faccioli N, et al. Comparison of contrast-enhanced sonography and MRI in displaying anatomic features of cystic pancreatic masses. AJR Am J Roentgenol. 2007;189:1435–42. doi: 10.2214/AJR.07.2032. [DOI] [PubMed] [Google Scholar]
- 13.D’Onofrio M, Malago R, Zamboni G, et al. Contrast-enhanced ultrasonography better identifies pancreatic tumor vascularization than helical CT. Pancreatology. 2005;5:398–402. doi: 10.1159/000086540. [DOI] [PubMed] [Google Scholar]
- 14.Faccioli N, D’Onofrio M, Malago R, et al. Resectable pancreatic adenocarcinoma: depiction of tumoral margins at contrast-enhanced ultrasonography. Pancreas. 2008;37:265–8. doi: 10.1097/MPA.0b013e31816c908b. [DOI] [PubMed] [Google Scholar]
- 15.Numata K, Ozawa Y, Kobayashi N, et al. Contrast-enhanced sonography of pancreatic carcinoma: correlations with pathological findings. J Gastroenterol. 2005;40:631–40. doi: 10.1007/s00535-005-1598-8. [DOI] [PubMed] [Google Scholar]
- 16.Beyer-Enke SA, Hocke M, Ignee A, et al. Contrast enhanced transabdominal ultrasound in the characterisation of pancreatic lesions with cystic appearance. JOP. 2010;11:427–33. [PubMed] [Google Scholar]
- 17.Hocke M, Ignee A, Dietrich CF. Contrast-enhanced endoscopic ultrasound in the diagnosis of autoimmune pancreatitis. Endoscopy. 2011;43:163–5. doi: 10.1055/s-0030-1256022. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich CF, Hirche TO, Ott M, et al. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718–20. doi: 10.1055/s-0029-1214866. [DOI] [PubMed] [Google Scholar]
- 19.Hocke M, Dietrich CF. New technology--combined use of 3D contrast enhanced endoscopic ultrasound techniques. Ultraschall Med. 2011;32:317–8. doi: 10.1055/s-0031-1274695. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich CF, Cui XW, Schreiber-Dietrich DG, Ignee A. EFSUMB guidelines 2011: comments and illustrations. Ultraschall Med. 2012;33(Suppl 1):S11–S21. doi: 10.1055/s-0032-1312890. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich CF. Comments and illustrations regarding the guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS)--update 2008. Ultraschall Med. 2008;29(Suppl 4):S188–S202. doi: 10.1055/s-2008-1027799. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich CF, Braden B, Hocke M, et al. Improved characterisation of solitary solid pancreatic tumours using contrast enhanced transabdominal ultrasound. J Cancer Res Clin Oncol. 2008;134:635–43. doi: 10.1007/s00432-007-0326-6. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich CF, Ignee A, Braden B, et al. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–7. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 24.D’Onofrio M, Barbi E, Dietrich CF, et al. Pancreatic multicenter ultrasound study (PAMUS) Eur J Radiol. 2011;81:630–638. doi: 10.1016/j.ejrad.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 25.D’Onofrio M, Zamboni G, Tognolini A, et al. Mass-forming pancreatitis: value of contrast-enhanced ultrasonography. World J Gastroenterol. 2006;12:4181–4. doi: 10.3748/wjg.v12.i26.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kersting S, Konopke R, Kersting F, et al. Quantitative perfusion analysis of transabdominal contrast-enhanced ultrasonography of pancreatic masses and carcinomas. Gastroenterology. 2009;137:1903–11. doi: 10.1053/j.gastro.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 27.Ryschich E, Schmidt J, Loeffler T, et al. Different radiogenic effects on microcirculation in healthy pancreas and in pancreatic carcinoma of the rat. Ann Surg. 2003;237:515–21. doi: 10.1097/01.SLA.0000059984.75871.B5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryschich E, Schmidt E, Maksan SM, et al. Expansion of endothelial surface by an increase of vessel diameter during tumor angiogenesis in experimental and hepatocellular and pancreatic cancer. World J Gastroenterol. 2004;10:3171–4. doi: 10.3748/wjg.v10.i21.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt J, Ryschich E, Daniel V, et al. Vascular structure and microcirculation of experimental pancreatic carcinoma in rats. Eur J Surg. 2000;166:328–35. doi: 10.1080/110241500750009195. [DOI] [PubMed] [Google Scholar]
- 30.Makinen K, Loimas S, Nuutinen P, et al. The growth pattern and microvasculature of pancreatic tumours induced with cultured carcinoma cells. Br J Cancer. 2000;82:900–4. doi: 10.1054/bjoc.1999.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Onofrio M, Mansueto G, Falconi M, et al. Neuroendocrine pancreatic tumor: value of contrast enhanced ultrasonography. Abdom Imaging. 2004;29:246–58. doi: 10.1007/s00261-003-0097-8. [DOI] [PubMed] [Google Scholar]
- 32.Malago R, D’Onofrio M, Zamboni GA. Contrast-enhanced sonography of nonfunctioning pancreatic neuroendocrine tumors. AJR Am J Roentgenol. 2009;192:424–30. doi: 10.2214/AJR.07.4043. [DOI] [PubMed] [Google Scholar]
- 33.Mork H, Ignee A, Schuessler G, et al. Analysis of neuroendocrine tumour metastases in the liver using contrast enhanced ultrasonography. Scand J Gastroenterol. 2007;42:652–62. doi: 10.1080/00365520601021765. [DOI] [PubMed] [Google Scholar]
- 34.Dietrich CF, Hocke M, Gallotti A, et al. Ultrasonography of the Pancreas. 2012;95:112. [Google Scholar]
- 35.Ripolles T, Martinez MJ, Lopez E, et al. Contrast-enhanced ultrasound in the staging of acute pancreatitis. Eur Radiol. 2010;20:2518–23. doi: 10.1007/s00330-010-1824-5. [DOI] [PubMed] [Google Scholar]
- 36.Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich CF, Cui XW, Boozari B, et al. Contrast-enhanced ultrasound (CEUS) in the diagnostic algorithm of hepatocellular and cholangiocellular carcinoma, comments on the AASLD guidelines. Ultraschall Med. 2012;33(Suppl 1):S57–S66. doi: 10.1055/s-0032-1312903. [DOI] [PubMed] [Google Scholar]
- 38.Cui XW, Ignee A, Braden B, et al. Biliary Papillomatosis and New Ultrasound Imaging Modalities. Z Gastroenterol. 2012;50:226–31. doi: 10.1055/s-0031-1281967. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich CF, Cui XW, Barreiros AP, et al. EFSUMB guidelines. 2011: comment on emergent indications and visions. Ultraschall Med. 2012;33(Suppl 1):S39–S47. doi: 10.1055/s-0032-1312895. [DOI] [PubMed] [Google Scholar]
- 40.Barreiros AP, Piscaglia F, Dietrich CF. Contrast enhanced ultrasound for the diagnosis of hepatocellular carcinoma (HCC): Comments on AASLD guidelines. J Hepatol. 2012;57(4):930–932. doi: 10.1016/j.jhep.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Dietrich CF, Jenssen C. Evidence based endoscopic ultrasound. Z Gastroenterol. 2011;49:599–621. doi: 10.1055/s-0029-1246021. [DOI] [PubMed] [Google Scholar]
- 42.Dietrich CF, Jenssen C, Hocke M, et al. Imaging of gastrointestinal stromal tumours with modern ultrasound techniques - a pictorial essay. Z Gastroenterol. 2012;50:457–67. doi: 10.1055/s-0031-1282076. [DOI] [PubMed] [Google Scholar]
- 43.Dietrich CF. Gastrointestinal ultrasound update 2011. Praxis (Bern 1994) 2012;101:239–50. doi: 10.1024/1661-8157/a000836. [DOI] [PubMed] [Google Scholar]
- 44.Nan G, Siyu S, Shiwei S, et al. Hemoclip-reinforced and EUS-assisted band ligation as an effective and safe technique to treat small GISTs in the gastric fundus. Am J Gastroenterol. 2011;106:1560–1. doi: 10.1038/ajg.2011.144. [DOI] [PubMed] [Google Scholar]
- 45.Siyu S, Sheng W, Guoxin W, et al. Gastric perforations after ligation of GI stromal tumors in the gastric fundus. Gastrointest Endosc. 2010;72:615–6. doi: 10.1016/j.gie.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 46.Bories E, Caillol F, Pesenti C, et al. Frontiers in interventional endoscopy. Bull Cancer. 2007;94:1091–8. doi: 10.1684/bdc.2007.0526. [DOI] [PubMed] [Google Scholar]
- 47.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 48.Landi B, Bouche O, Guimbaud R, et al. Management of gastrointestinal stromal tumours of limited size: proposals from a French panel of physicians. Dig Liver Dis. 2011;43:935–9. doi: 10.1016/j.dld.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Polkowski M, Larghi A, Weynand B. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich CF, Ignee A, Barreiros AP, et al. Contrast-enhanced ultrasound for imaging of adrenal masses. Ultraschall Med. 2010;31:163–8. doi: 10.1055/s-0028-1109357. [DOI] [PubMed] [Google Scholar]
- 51.Friedrich-Rust M, Glasemann T, Polta A. Differentiation between benign and malignant adrenal mass using contrast-enhanced ultrasound. Ultraschall Med. 2011;32:460–71. doi: 10.1055/s-0031-1273408. [DOI] [PubMed] [Google Scholar]
- 52.Trojan J, Schwarz W, Sarrazin C, et al. Role of ultrasonography in the detection of small adrenal masses. Ultraschall Med. 2002;23:96–100. doi: 10.1055/s-2002-25190. [DOI] [PubMed] [Google Scholar]
- 53.Schmid-Wendtner MH, Partscht K, Korting HC, et al. Improved differentiation of benign and malignant lymphadenopathy in patients with cutaneous melanoma by contrast-enhanced color Doppler sonography. Arch Dermatol. 2002;138:491–7. doi: 10.1001/archderm.138.4.491. [DOI] [PubMed] [Google Scholar]
- 54.Nakase K, Yamamoto K, Hiasa A, et al. Contrast-enhanced ultrasound examination of lymph nodes in different types of lymphoma. Cancer Detect Prev. 2006;30:188–91. doi: 10.1016/j.cdp.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Hocke M, Menges M, Topalidis T, et al. Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes. J Cancer Res Clin Oncol. 2008;134:473–80. doi: 10.1007/s00432-007-0309-7. [DOI] [PubMed] [Google Scholar]
- 56.Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Best Pract Res Clin Gastroenterol. 2009;23:743–59. doi: 10.1016/j.bpg.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich CF, D’Onofrio M, Evans D, Filice C, Greiner L, Jäger K, de Jong N, Leen E, Lencioni R, Lindsell D, Martegani A, Meairs S, Nolsøe C, Piscaglia F, Ricci P, Seidel G, Skjoldbye B, Solbiati L, Thorelius L, Tranquart F, Weskott HP, Whittingham T. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) - Update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 58.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver - Update 2012. Ultraschall Med. 2012 epub in advance. [Google Scholar]


