Abstract
Objective:
Esophageal tumors arising in the muscularis propria are difficult to be resected endoscopically using standard electro-surgical techniques, even the endoscopic submucosal dissection (ESD) technique appeared recently. Our purpose is to investigate the efficacy of endoscopic ultrasound (EUS)-assisted tunnel-type ESD for resection of these tumors.
Methods:
A total of 17 patients were included in this study. A standard endoscope was used. The submucosal tunnel was created with the triangle knife according to the standard ESD technique, about 5 cm proximal to the lesion. EUS was performed within the tunnel to detect the tumor, and then the tumor was separated both from the submucosal and the muscle layers. After the tumor was removed, several clips were used to close the mucosal defect. EUS was performed to evaluate the healing quality 1 week after the procedure.
Result:
In all the cases, the tumors were completely resected. Mean tumor size was 24.2 mm (12-50 mm) in diameter. The histo-logical diagnoses were leiomyoma (16/17) and gastrointestinal stromal tumor (GIST, 1/17). Subcutaneous emphysema was found in 2 patients after the procedure, but disappeared by the third day. No patients sustained perforation or developed significant hem-orrhage, and there were no other immediate severe complications after the procedure. The healing quality was satisfying in 16/17 patients evaluated by EUS 1 week after the procedure. No recurrence has been found during follow-up (mean 7 months, range 3-13 months).
Conclusion:
EUS-assisted tunnel-type ESD is effective and safe in treatment of esophageal tumors arising in the muscularis pro-pria.
Keywords: endoscopic submucosal dissection, tunnel-type, endoscopic ultrasound, submucosal tumor, leiomyoma, gastrointestinal stromal tumor
INTRODUCTION
Tumors arising form the muscularis propria are mainly refer to the leiomyoma, which is the most frequent mesenchymal tumors of esophagus. Gastrointestinal stromal tumor (GIST) has also been documented, but very rare. The traditional therapy for these tumors is surgery, which is traumatic. Endoscopic submucosal dissection (ESD) is a minimally invasive technique by endoscopy, and it is an important technique for resection of lesions in the gastrointestinal tract, including early cancers and submucosal tumors (SMTs) in the muscle layer.1,2 When a tumor originates from the muscularis propria layer of the esophagus, it can be difficult to achieve complete resection by ESD because of a high risk of perforation.3 The tunnel-type ESD (T-type ESD) procedure presented in this study is a modified technique for treatment of tumors in the muscle layer of the gastrointestinal tract. However, tumors may also be hard to identify by endoscope in the tunnel sometimes. In this study, we applied endoscopic ultrasound (EUS) to assist the dissection procedure and evaluate the healing quality of submucosal tunneling. The aim of this current pilot study, which has been approved by The Institutional Review Board of China Medical University, is to assess the feasibility of EUS-assisted T-type ESD for the removal of SMTs arising from the muscularis propria.
PATIENTS AND METHODS
Patients
In this study, 17 patients underwent T-type ESD for 17 lesions between October 2009 and December 2011. The patient characteristics are outlined in Table 1. The inclusion criteria were as follows. First, muscular layer tumors were identified by EUS. Second, the tumor size should be larger than 10 mm in diameter. If the tumor size is less than 10 mm in diameter, band ligation or band ligation-assisted ESD technique is recommended. Third, patient candidates for this study should be older than 18 years. Forth, patients did not take aspirin or other nonsteroidal anti-inflammatory drugs for at least 1 week prior to the procedure. At last, routine blood test, prothrombin time and partial thromboplastin time should be normal. Patients with tumors near the proximal end of the esophagus were not candidates for T-type ESD, because there would be not enough space for the tunnel. The patients who were not willing to accept the general anesthesia should also be excluded form this study. Written informed consents were obtained from all the patients.
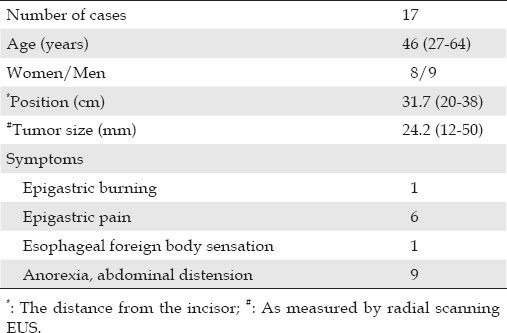
Table 1.
Patient characteristics
Equipment
EUS examinations were performed with a radial scanning echoendoscope (Pentax EG3670URK) before the procedure and at the follow-up intervals. Miniprobe echoendoscopy (Fujinon SP701) was performed in the tunnel to confirm the tumors. A standard endoscope (EG 2770K, Pentax, Tokyo, Japan), hook knife (Olympus, Tokyo, Japan), and insulation-tipped diathermic knife-2 (IT-2, Olympus, Tokyo, Japan) were used to dissect the submucosal layer and to separate the tumors from the muscle layer. A transparent hood was attached at the tip of the endoscope, allowing an easy and safe dissection with good visualization. Positive pressure ventilation and carbon dioxide (CO2) insufflation were used during the procedure. Hemoclips (standard size 8 mm open, Olympus Medical system, Olympus Optical Co., Ltd, Tokyo, Japan) were used for closure of the mucosa. The agent for the submucosa was glycerol and fructose injection with 0.007% epinephrine.
Procedure
The patients were all under general anesthesia with nasotracheal intubation. CO2 insufflation was controlled through the endoscope. Steps from the procedure are shown in Fig. 1. The site chosen for mucosal entry was 3-5 cm proximal to the lesion. A submucosal injection with 10 mL glycerol and fructose with 0.3% methyl-blue and epinephrine was performed at this site. A satisfied submucosal fluid rushing (SFC) was created. Then, a longitudinal mucosal incision of approximately 2 cm was made with the hook knife, providing the mucosal entry to the submucosal space.
Figure 1.
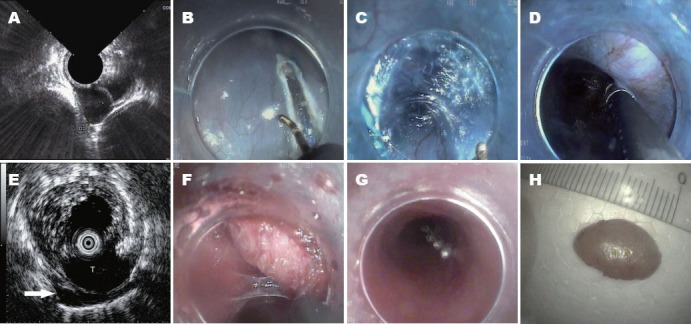
The tunnel type endoscopic submucosal dissection procedure and EUS in tunnel. A: Muscle layer tumor viewed on EUS; B: Longitudinal mucosal incision; C: Submucosal tunnel creation; D: Endoscopic view of tumor in tunnel, difficult to distinguish from the muscular layer or aortas; E: Tumor(T) identified by EUS in tunnel. A small amount of irrigated saline leaked through the muscular layer of esophagus to the mediastinal space (arrow); F: Tumor dissection in the tunnel; G: Mucosal entrance closing; H: Tumor ex vivo. EUS: endoscopic ultrasound. T: tumor.
The submucosal tunnel was created with the IT-2 knife and triangle knife according to the standard ESD technique. The endoscope was advanced with the knife cutting the submucosal fibers. Repeated submucosal injections were administered to aid the dissection and guide the endoscope to the lesion.
EUS scanning in the tunnel was carried out by an echoendoscope or a miniprobe with saline irrigation (video 1). This procedure helped to confirm the tumors, especially for those presented extraluminal extension.
Once the tunnel and the endoscope reached the tumor, the triangle knife was used to separate it both from the submucosa and the muscle layers. After the tumor was removed, several clips were used to close the mucosal defect. The clips were released from the distal end of the opening, and they were placed one by one until the defect was closed tightly. A tissue adhesive was sprayed onto the surface of the wound in order to reinforce the closure. Post-procedure antibiotic drugs were also routinely used for 2 days in this study.
All the tumors resected were sent for histopathological and immunohistochemical analysis. EUS was repeated 1 week after the procedure, and the patients were subjected to additional follow-up examinations at 1, 6, and 12 months.
The standard for complete healing of the tunnel should include: complete closure of the mucosal entrance; submucosal space for the tunnel disappeared, which means the five layers of the esophagus can clearly be identified. It was not suppose to find much exudation around the resection bed of the tumors and the tunnel.
RESULTS
All of the tumors in the 17 patients were completely resected. The results are outlined in Tab. 2. The mean tumor size measured by EUS was 24.2mm (range 12-50 mm) in diameter. The histological diagnoses were leiomyoma (16/17) and GIST (1/17). The mean operative time was 97.6 min (range 60-150 min), and the average time for defect closing was 9.6 minutes (range 7-16 min).
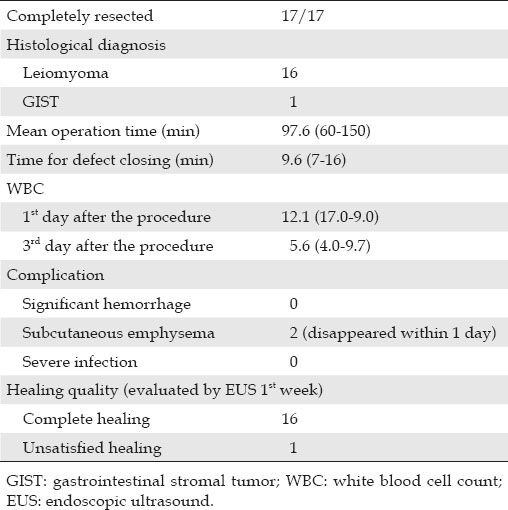
Table 2.
Outcomes for patients treated by tunnel type ESD
No patients sustained perforation or developed significant hemorrhage, and there were no other immediate severe complications after the procedure. White blood cell counts measured on the first day after the procedure were increased, but returned to the normal level by the third day. No patients developed fever during the hospital stay. Subcutaneous emphysema was found in 2 patients after the procedure, but disappeared by the third day.
Histopathological evaluations of 16/17 tumors confirmed the diagnosis of leiomyoma. Immunohistochemical stains were negative for c-Kit (CD117), CD34, and DOG-1 markers, but positive for smooth muscle actin (SMA) and Desmin markers. Histopathological evaluations of 1/17 tumors confirmed the diagnosis of GIST. Immunohistochemical stains for c-Kit (CD 117), CD34, Desmin and DOG-1 markers were positive, but for S100 were negative.
All patients underwent the first follow-up EUS at 1 week after the procedure (Fig. 2). In most cases, each layer of the esophagus was clearly displayed, and the ultrasound images demonstrated complete healing of the tunnel. The upper two layers of the mucosal entry sites were seen as hypoechoic areas that were covered by a hyperechoic structure. The resection bed formed a small space filled with hypoechoic granulation tissue. The only change noted on the endoscopic view was found at the site of the mucosal entry, and a great amount of tissue adhesive remained on the surface. One patient was noticed with unsatisfactory healing of the tunnel by EUS scanning. In the endoscopic view, we found the entrance of the tunnel was completely open (Fig. 3). The surface mucosa from where the tumor was resected became defected. The EUS scanning revealed that the tunnel had not been closed; the mucosa and the submucosa were stilled separated. The patient did not have any uncomfortable feelings during the whole week after the operation. When EUS was performed 2 months later during follow-up, we found the tunnel was completely healed. For the rest of the patients, follow-up endoscopic examinations at 1 month showed only a small scar at the mucosal entry site. No clips remained.
Figure 2.
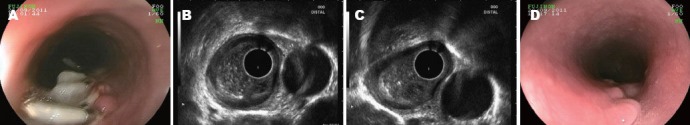
EUS following up. A: Endoscopic view of mucosal entry; B: EUS image showing healing of the tunnel; C: Hypoechoic structure of the site of resection bed indicate the seroma; D: Following up after 1 month-small scar. EUS: endoscopic ultrasound.
Figure 3.
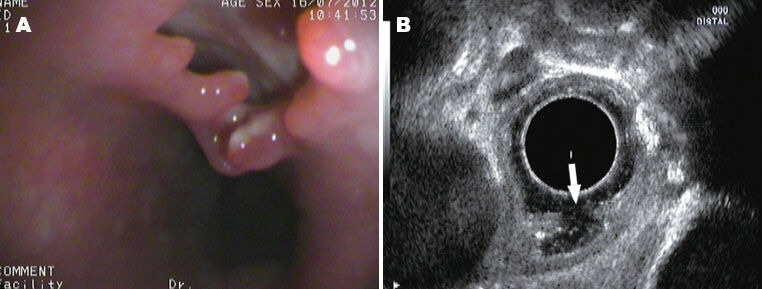
Unsatisfied healing of tunnel detected by EUS. A: The surface mucosa from where the tumor was resected became defected; B: The EUS scanning revealed that the tunnel had not been closed; the mucosa and the muscularis propria were stilled separated, but the muscularis propria was intact endosonographically. EUS: endoscopic ultrasound.
All the patients have been followed up post-operatively at regular intervals with standard endoscopy and EUS. No recurrence has been found during follow-up (mean 7 months, range 3-13 months).
DISCUSSION
ESD is considered a minimally invasive technique that offers the possibility of complete resection of neoplasms by endoscopy.1,2,4 When a tumor originates from the muscularis propria layer of the esophagus, it is difficult to achieve complete resection by ESD because of a high risk of perforation. Band ligation is a simple technique for resection of small tumors (less than 1 cm in diameter) from muscularis propria, but it has the limitation, i.e., without pathological examination.5,6
Very recently, preliminary studies about T-type ESD for treatment of SMTs of the esophagus have been published.7,8 However, none of these studies applied EUS guidance during the tunneling process or the following-up period.
For esophagus, EUS is the only tool to distinguish the SMTs from the physiological protrusions like trachea, left atrium, aorta and vertebra. Aorta is the most dangerous one in this technique. EUS is also necessary in tumor identification within the tunnel. In this study, we observed that the endoscopic view of tumors within the tunnel was also hard to distinguish from the physiological protrusions and the normal muscular layer. Tumor dissection should begin after the EUS scanning. This procedure may prevent the additional injury of the aorta by mistake.
Before this study, no researches reported whether liquid would leak through the muscular layer during the tunnel creation or the tumor dissection procedure. In our study, although the muscular layer was kept intact during the tunnel creation process in the submucosal space, the saline irrigated for EUS leaked outside the esophagus. This proved that mucosa is the key barrier of the esophagus from water and gas. This observation also confirmed that mucosal layer closure is sufficient for restoring the intactness of the esophagus. In this study, the WBC rose right after the procedure in all the patients, but returned to normal the next day. The small amount of leaked saline was absorbed quickly and did not cause the severe infection of mediastinum. But large amount of liquid flush into the tunnel should be avoided.
Another concern of this technique is whether the tunnel creation and tumor resection will cause a structure change of the esophageal wall. Actually, it is still unknown whether the tunnel or the resection bed of tumors will form dead space after the entrance closed. EUS studies 1 week after the procedure showed a complete healing of the tunnel in most cases of our study. The five echo-layers of the esophagus were all clearly identified by EUS 1 week after the procedure. However in 1 patient, the unhealed tunnel, the open mucosal entrance and the broken mucosa of the resection bed were revealed during the follow-up EUS examinations 1 week later. The causes remained unclear, and early falling-off of the clips may be one. The tumor site was reduced to a small submucosal space filled with granulation tissue which was hypoechnoic. Follow-up endoscopic examinations at 1 month showed only a small scar at the mucosal entry site with no surgical clips remaining.
In our pathological results, most of the cases are leiomyomas which were definitely a benign tumor without any symptoms. However, GIST was confirmed in 1 patient, which was seldom published. This result further proved the clinical significance of this therapy.
The main complications of this procedure are pneumome-diastinum and subcutaneous emphysema, which are confirmed by computed tomography scan. Some patients will have bilateral subcutaneous emphysema over the neck and anterior chest wall on physical examination. All of the patients in this group remained asymptomatic. Because CO2 insufflation was used for the procedure, the subcutaneous emphysema and pneumomediastinum were resolved within a week, without incidence of mediastinitis. The study from Tamiya et al. has concluded that in esophageal ESD, pneumomediastinum detected by chest CT only did not cause significant clinical complications.9 Being rapidly absorbed, CO2 has already be used to lower the risk of complications such as subcutaneous emphysema and pneumomediastinum.10,11,12 But the safety of a longer-time insufflation is still needed to be proven, especially for the elderly patients.13,14
Hemorrhage within the tunnel is another potential complication of ESD tunnel techniques, so definite hemostasis within the tunnel is important. Delayed bleeding in the tunnel after the procedure is difficult to manage. There was no post-procedure hemorrhage observed in the present group of patients.
In conclusion, the results of EUS-assisted T-type ESD for resection of esophageal tumors in the muscularis propria have indicated that the procedure is feasible and safe. All tumors in this small pilot study were resected completely with minimal complications. Regular follow-up examinations by EUS have demonstrated excellent healing without signs of recurrence. Results from larger series with a longer follow-up period and controlled trials are needed to evaluate this technique more thoroughly.
REFERENCES
- 1.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–83. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 2.Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331–6. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 3.Messmann H, Probst A. Management of endoscopic submucosal dissection complications. Endoscopy. 2009;41:712–4. doi: 10.1055/s-0029-1214992. [DOI] [PubMed] [Google Scholar]
- 4.Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications form a large consecutive series. Dig Endosc. 2005;17:54–8. [Google Scholar]
- 5.Sun SY, Ge N, Wang C, et al. Endoscopic band ligation without electrosurgery: a new technique forexcision of small upper-GI leiomuoma. Gastrointest Endosc. 2004;60:218–22. doi: 10.1016/s0016-5107(04)01565-2. [DOI] [PubMed] [Google Scholar]
- 6.Ge N, Sun SY, Sun SW, et al. Hemoclip-reinforced and EUS-assisted band ligation as an effective and safe technique to treat small GISTs in the Gastric Fundus. Am J Gastroenterol. 2011;106:1560–1. doi: 10.1038/ajg.2011.144. [DOI] [PubMed] [Google Scholar]
- 7.Gong W, Xiong Y, Zhi F, et al. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231–5. doi: 10.1055/s-0031-1291720. [DOI] [PubMed] [Google Scholar]
- 8.Inoue H, Ikeda H, Hosoya T, et al. Submucosal endoscopic tumor resection for subepitheial tumors in the esophagus and cardia. Endoscopy. 2012;44:225–30. doi: 10.1055/s-0031-1291659. [DOI] [PubMed] [Google Scholar]
- 9.Tamiya Y, Nakahara K, Kominato K, et al. Pneumomediastinum is a frequent but minor complication during esophageal endoscopic submucosal dissection. Endoscopy. 2010;42:8–14. doi: 10.1055/s-0029-1215215. [DOI] [PubMed] [Google Scholar]
- 10.Saito Y, Uraoka T, Matsuda T, et al. A pilot trial study to assess safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2006;63:231. doi: 10.1016/j.gie.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Minami H, Komatsu T, et al. Prolonged carbon dioxide insufflation under general anesthesia for endoscopic submucosal dissection. Endoscopy. 2010;42:1021–9. doi: 10.1055/s-0030-1255969. [DOI] [PubMed] [Google Scholar]
- 12.Maeda Y, Hirasawa D, Fujita N, et al. A pilot study to assess mediastinal emphysema after esophageal endoscopic submucosal dissection with carbon dioxide insufflation. Endoscopy. 2012;44:71. doi: 10.1055/s-0031-1291664. [DOI] [PubMed] [Google Scholar]
- 13.Halpern P, Raskin Y, Sorkine P, et al. Exposure to extremely high concentrations of carbon dioxide: A clinical description of a mass casualty incident. Ann Emerg Med. 2004;43:196–9. doi: 10.1016/j.annemergmed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Singh K, Singhal A, Saggar VR, et al. Subcutaneous Carbon Dioxide Emphysema Following Endoscopic Extraperitoneal Hernia Repair: Possible Mechanisms. J Laparoendosc Adv Surg Tech. 2004;14:317–20. doi: 10.1089/lap.2004.14.317. [DOI] [PubMed] [Google Scholar]


