Abstract
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is a technique which allows the study of cells obtained through aspiration in different locations near the gastrointestinal tract. EUS-FNA is used to acquire tissue from mucosal/submucosal tumors, as well as peri-intestinal structures including lymph nodes, pancreas, adrenal gland, gallbladder, bile duct, liver, kidney, lung, etc. The pancreas and lymph nodes are still the most common organs targeted in EUS-FNA. The overall accuracy of EUS is superior to computed tomography scan and magnetic resonance imaging for detecting pancreatic lesions. In most cases it is possible to avoid unnecessary surgical interventions in advanced pancreatic cancer, and EUS is considered the preferred method for loco-regional staging of pancreatic cancer. FNA improved the sensitivity and specificity compared to EUS imaging alone in detection of malignant lymph nodes. The negative predictive value of EUS-FNA is relatively low. The presence of a cytopathologist during EUS-FNA improves the diagnostic yield, decreasing unsatisfactory samples or need for additional passes, and consequently the procedural time. The size of the needle is another factor that could modify the diagnostic accuracy of EUS-FNA. Even though the EUS-FNA technique started in early nineteen's, there are many remarkable progresses culminating nowadays with the discovery and performance of needle-based confocal laser endomicroscopy. Last, but not least, identification and quantification of potential molecular markers for pancreatic cancer on cellular samples obtained by EUS-FNA could be a promising approach for the diagnosis of solid pancreatic masses.
Keywords: endoscopic ultrasound, fine needle aspiration, confocal laser endomicroscopy
INTRODUCTION
Endoscopic ultrasound fine-needle aspiration (EUS-FNA) is a technique which allows the study of human cells obtained through aspiration in different locations near the gastrointestinal tract. The procedure is usually performed after detection of a suspected mass through other imaging methods, including endoscopy, ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI), etc.1 Thus, FNA is a minimally invasive method for cytological sampling of peripheral and deep-seated mass lesions.2
The attachment of ultrasound probes to endoscopes in the early 1980s (by DiMagno)3 allowed an improved visualization of the gastrointestinal wall and abdominal organs4. Nevertheless, a need for tissue diagnosis was evident from the early work on EUS.5 EUS-FNA has started in 1991 for pancreatic cancer6 and at present is performed on a routine basis at many endoscopic centers, being evident that this procedure has a major impact on the therapeutic management of patients, by obtaining a definite tissue diagnosis from lesions outlined by EUS7. The ability to obtain cytologic material under direct visualization adds a new dimension to the diagnostic usefulness of this technique because it offers an opportunity for prompt and accurate diagnosis.4
EUS-FNA is used to acquire tissue from mucosal/ submucosal tumors, as well as peri-intestinal structures including lymph nodes, pancreas, adrenal gland, gallbladder, bile duct, liver, kidney, lung, etc.8 Based on the published literature, the pancreas and lymph nodes (intra-thoracic and intra-abdominal) are the most common organs targeted in EUS-FNA.4
Even though the EUS-FNA technique started twenty years ago, there are remarkable progresses culminating nowadays with the application of through the needle-based confocal laser endomicroscopy (CLE) methods that allow real-time optical biopsies.
FROM THE PAST...
It is obvious that EUS-FNA is not at all limited to gastroenterology, as the gastrointestinal tract traverses through anatomical regions related to other medical specialties such as pulmonology, thoracic surgery, internal medicine, oncology, urology, gynecology and endocrinology.7,9 In the early 1990s, a special biopsy equipment was developed by Peter Vilmann and Søren Hancke in collaboration with Medi-GlobeGmbH, Tübingen, Germany, and this gave the final breakthrough for the EUS-guided biopsy method.6,7,9 All needle assemblies that are commercially available today have basically similar characteristics and designs (Fig. 1A, 1B and 1C).
Figure 1.
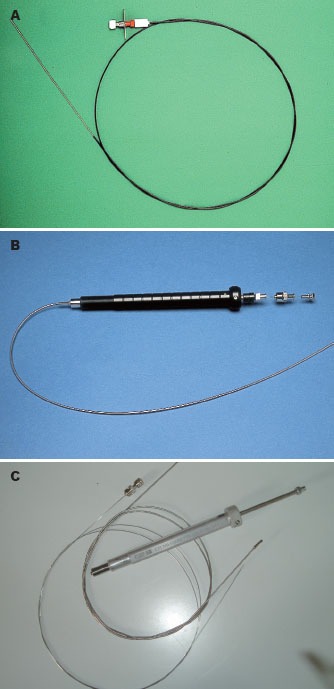
Initial designs of the endoscopic ultrasound-guided fine needle aspiration needle (Vilmann-Hancke needle).
Nevertheless, FNA was developed many centuries ago. The first report on the use of needles for therapeutic purposes can be found in the most influential book of Arab Medieval Medicine, who described for the first time therapeutic punctures of the thyroid gland, using instruments resembling modern aspiration needles. But the last century showed remarkable progress in terms of FNA, the use of surgical needles for the diagnosis and treatment of various diseases being widely used.1 In 1912, a German haematologist, Hans Hirschfeld (1873-1944), reported on FNA biopsy for diagnosis of cutaneous lymphomas and other tumors with the use of needle aspiration biopsy and histological process of the acquired cellular material.10 Leonard Stanley Dudgeon was the first who scientifically established the technique of needle biopsy,11 while Hayes Martin and Edward Ellis12,13 applied needle biopsies on a wide range of samples and clinical cases. But, the first report of the use of fine (22-G) needle should be attributed to Ernst Manheim (a German pathologist), in 1931.14
Diagnostic role of EUS-FNA
The first EUS-FNA for cytologic diagnosis of a pancreatic lesion was performed by Peter Vilmann in 1991 (Fig. 2) and published in 1992.6 His thesis which is considered a landmark study was published as a book on EUS using curved linear array transducer with description and development of the biopsy needle and the EUS-FNA procedure (Fig. 3)9, EUS-FNA from upper and lower gastrointestinal tract was further described in 1992 in separate studies by Wiersema et al.15 and Vilmann et al.2,6
Figure 2.
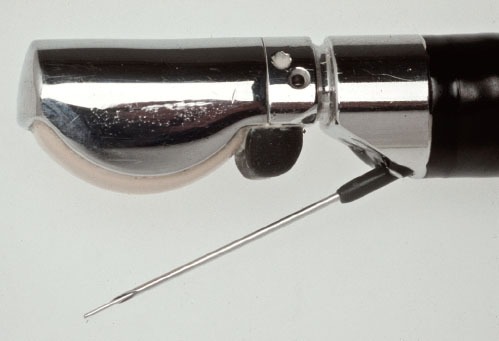
Endoscopic ultrasound (EUS)-guided fine needle aspiration consisting of a linear EUS scope with the FNA needle exiting the biopsy channel in the plane of the ultrasound image.
Figure 3.
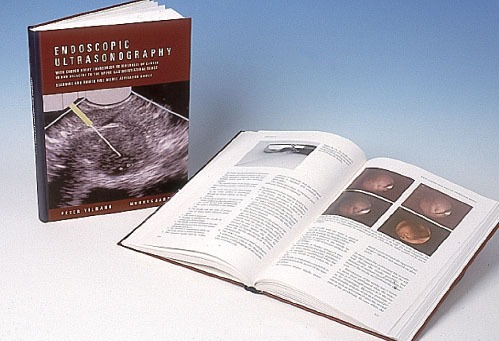
Initial thesis was considered a landmark study and published as a book on endoscopic ultrasound (EUS) using curved linear array transducer with description and development of the biopsy needle and the EUS-guided fine needle aspiration procedure.
Figure 4.
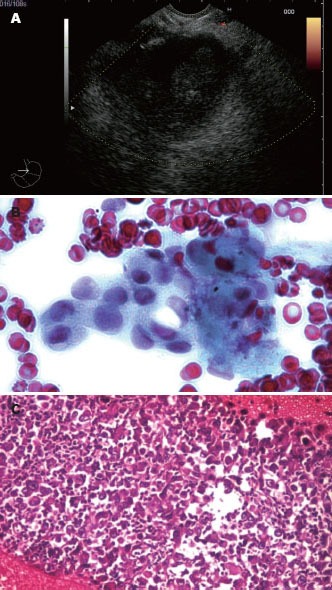
Endoscopic ultrasound-guided fine needle aspiration performed with a 22-G needle on the side of a hypoechoic pancreatic tumor mass, with both cytology and microhistology performed after at least 3 passes. Cytology showed a clump of malignant cells, while microhistology confirmed the diagnosis of adenocarcinoma.
Figure 5.
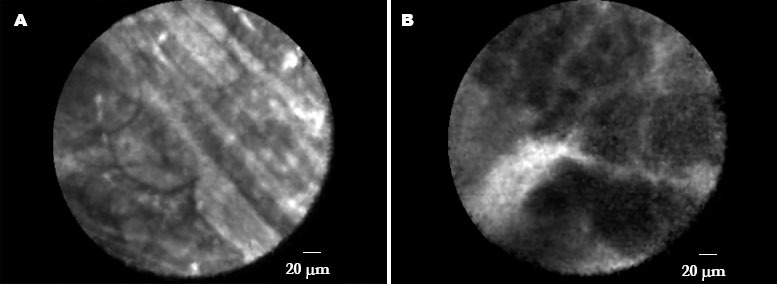
Needle based confocal laser endomicroscopy (nCLE) image of pancreatic tissue using fluorescein as a systemic contrast agent, presenting grey lobular structures of tissue with thin white lines crossing representing fibrosis in a patient with chronic pancreatitis. Another nCLE image of pancreatic tissue, demonstrating dark lobular structures and large vessels obtained in a patient with pancreatic ductal carcinoma.
The regional anatomy of the pancreas is complex, making procurement of cytologic samples difficult without exploratory laparotomy. Traditionally, transcutaneous ultrasound, CT or EUS-FNA has been used for imaging guidance of the needle to obtain biopsies of the pancreas.16 Since its development in the 1980s, EUS has been found utility in the investigation and staging of suspected pancreatic cancer. As compared to other imaging modalities (CT, MRI), EUS has the advantages of a better detection rate of pancreatic lesions under 3 cm in size and the ability to collect samples for cytopathologic analysis, being an integral part of the diagnosis and staging of pancreatic tumors.17 Thus, the overall accuracy of EUS is superior to CT scan and MRI for detecting pancreatic lesions.4,18,19,20,21 Actually, the main role of EUS-FNA in pancreatic lesions is to obtain the tissue diagnosis in suspected pancreatic masses. Thus, in most cases it is possible to avoid unnecessary surgical interventions in advanced pancreatic cancer, and EUS is considered the preferred method for loco-regional staging of pancreatic cancer. A medical literature review to evaluate the role of EUS-FNA for diagnosis of solid pancreatic masses showed a 78%-95% sensitivity, 75%-100% specificity, and 78%-95% accuracy,17,22,23,24,25,26,27,28,29,30,31,32,33 confirming that EUS-FNA is an effective and safe method to obtain a cytological diagnosis of pancreatic tumors.30
Many studies have noted the importance of performing EUS-FNA for mediastinal and intra-abdominal lymph nodes. Most of these studies have evaluated the use of EUS-FNA in the staging of malignant neoplasms of the lung, gastrointestinal tract, and pancreas4 but many other such as sarcoidosis34, lymphomas35 and tuberculosis36 have been diagnosed by this method. Lymph node staging and the detection of metastatic lesions are essential issues of pancreatic cancer staging. EUS-FNA allows for sampling of suspicious-appearing lymph nodes and liver lesions. EUS-FNA has been shown to increase the accuracy of lymph node staging and thereby reduce the number of unnecessary surgical interventions.16
A meta-analysis from 76 studies (9310 patients) on mediastinal lymph nodes, demonstrated that FNA improved the sensitivity (from 84.7% to 88%) and specificity (from 88% to 96.4%) compared to EUS imaging alone.37 Thus, EUS-FNA should be the diagnostic test of choice for assessment of mediastinal lymph nodes.38,39 A recent study also demonstrated an excellent specificity (100%) and a high accuracy (90%) for EUS-FNA of mediastinal lymph nodes in patients with known or suspected lung cancer, indicating that the procedure has a high clinical impact.40
Some meta-analyses and multicenter studies evaluated the accuracy of EUS-FNA in diagnosing other organ lesions like: mucinous pancreatic cystic neoplasms, solid pseudopapillary tumors, autoimmune pancreatitis, metastatic lesions of the pancreas, bile duct strictures and gallbladder masses, esophageal and gastric cancer, gastrointestinal stromal tumors (GISTs), non-small cell lung cancer staging, etc.41,42,43,44,45,46,47,48,49 For diagnosing mucinous pancreatic cystic lesions, EUS-FNA has a pooled sensitivity of 63% and specificity of 88%.42,50,51,52 Over a 6-year period, a retrospective, multicenter study demonstrated the usefulness of EUS-FNA in diagnosing a rare but important cause of focal pancreatic lesions (metastatic lesions of the pancreas).44,53,54,55,56 Another meta-analysis (including 284 patients) has shown that EUS-FNA has a pooled sensitivity of 84% and a pooled specificity of 100% in the evaluation of bile duct strictures and gallbladder masses.45 A meta-analysis has also demonstrated that EUS-FNA substantially improves the sensitivity (from 84.7% to 96.7%) and specificity (from 84.6% to 95.5%) compared to EUS imaging alone in evaluating N stage disease of esophageal cancer.46,57,58,59,60,61 Last, but not least, a recent study (over a 4-year period), has shown that the EUS-FNA performance characteristics for diagnosing GISTs yielded a sensitivity of 82%, a specificity of 100%, and an overall accuracy of 86%.48,61,62,63 Thus, EUS-FNA is an extremely valuable tool for diagnosing and characterizing submucosal lesions of the upper gastrointestinal tract.49
How to decrease the rate of false negative?
The specificity of EUS can be increased close to 100% with FNA with an accuracy of 95%. However, the negative predictive value of EUS-FNA is relatively low, especially when the diagnosis of pancreatic lesions is suspected. Data from the medical literature for diagnosis of solid pancreatic masses using EUS-FNA showed a 46%-80% negative predictive value.17,22,23,24,25,26,27,28,31 For lymph nodes, the negative predictive value of EUS-FNA is higher, approximately 80% (78% - 85%).16,32,33,34,35,36,37,38,39,40 Hence, negative results do not completely exclude malignancy. Therefore, the need for routine EUS-FNA of potentially resectable pancreatic mass lesions noted on other imaging modalities is still controversial.64,65
For effective assessment of the EUS-FNA samples, a cytopathologist or an advanced trainee in cytopathology should be on site to ensure that the samples taken are adequate. The presence of a cytopathologist during EUS-FNA, improves the diagnostic yield, decreasing unsatisfactory samples, reducing the need for additional passes, and consequently the procedural time.4,8 In many centers, EUS-FNA samples are obtained by the endoscopist and samples are sent in a fixative to the cytopathology laboratory for sample preparation. In other institutions, technologists and trainees in cytopathology go to the endoscopy suite and provide on-site assessment of sample adequacy. In a few centers, cytopathologists go to the endoscopy suite and provide an on-site, prompt interpretation.4 So, onsite cytopathological assessment is accepted as a quality control measure for EUS and is the standard of care at most academic EUS centers. In a recent study of 182 patients with pancreatic masses who underwent EUS-FNA, the presence of an on-site cytopathologist was associated with a significantly lower number of inadequate samples and a higher diagnostic sensitivity.66,67,68,69
But another emerging concept is telecytopathology which may be a valid substitute for onsite cytopathologic evaluation. The slides are initially prepared and prescreened by a cytotechnologist or pathology resident and then analyzed by an offsite cytopathologist using real-time remotely operated system.64 A retrospective study demonstrated the potential use of telecytopathology as a valid substitute for onsite evaluation of pancreatic carcinoma by EUS-FNA.70
A retrospective review showed that the cytotechnologists and cytopathologists are comparably accurate in on-site evaluations of adequacy of EUS-guided pancreatic FNA. The adequacy and accuracy of the on-site evaluation increases with institutional experience, and this increase is not solely attributable to cytologist factors.71,72
The goal of performing FNA is to obtain a positive diagnosis in the quickest possible time with the least number of passes. The number of passes to be made depends on the presence or absence of on-site cytopathologist for assessment of specimen adequacy, for establishment of on-site diagnosis, and to guide the need for further sampling. In the absence of an on-site cytopathologist, adequate number of passes should be performed to avoid the need for repeat procedures.8 Studies have shown that with solid pancreatic mass lesions, the optimal number of EUS-FNA needle passes ranged from 2 to 6.73,74 Moreover, using suction increases the diagnostic yield of a higher number of slides without increasing bloodiness.75
The size of the needle is another factor that could modify the diagnostic accuracy of EUS-FNA. 19-G needles seem to be able to provide a higher amount of tissue material as compared to 22-G needles, without supplemental complications. Anyway, EUS-FNA with a 19-G aspiration needle may be a valuable method for the diagnosis of pancreatic/peri-pancreatic masses when the cytopathologist is not available in endoscopy room.76 There was no difference in accuracy, number of passes or complications between 25-G and 22-G needles. Anyway, targeting of lesions in the distal duodenum may be easier by using the 25-G needle due to less friction and easier penetration.77 Another recent study demonstrated that with an adequate tissue sample, broad application of cytometry and immunohistochemistry increased the diagnostic yield of EUS-FNA.78 Recently, EUS-FNA biopsy can be performed with a new 19-G histology needle, being feasible for histopathology diagnosis of intraintestinal and extraintestinal mass lesions, offering the possibility of obtaining a core sample for histological evaluation in the majority of cases, with an overall diagnostic accuracy of over 85%.79
However, EUS-FNA is technically challenging and requires special training, being a difficult technique to master, with a prolonged learning curve. The American Society for Gastrointestinal Endoscopy (ASGE) recommends that for “comprehensive competence in all aspects of EUS, a minimum of 150 supervised cases, of which 75 should be pancreaticobiliary, and 50 EUS-FNA” should be performed. The current ASGE guideline of 25 supervised EUS-FNA procedures for the diagnosis of pancreatic adenocarcinoma is reasonable.80,81,82,83,84
TO THE FUTURE
CLE is a novel endoscopic method that was first introduced in 2004 into the endoscopist's toolbox.85 Whereas techniques such as chromoendoscopy and conventional magnification endoscopy try to predict histology from mucosal patterns, CLE actually allows intravital microscopy of the human gastrointestinal mucosa during ongoing endoscopy, enabling real-time optical biopsy.86
CLE is based on tissue illumination with a low-power laser with subsequent detection of the fluorescence light reflected from the tissue.87,88 Confocal imaging can be based on tissue reflectance or tissue fluorescence.89,90 CLE based on tissue fluorescence uses local and/or intravenous contrast agents and generates high-quality images comparable with traditional histological examination. The contrast agents can be applied systemically (fluorescein, tetracycline) or topically (acriflavine, cresyl violet) by using a spraying catheter.90 Development of organ- and tissue-specific contrast agents will further expand the indications for confocal endomicroscopy, which can potentially be used to assess extraluminal (e.g., biliary, pancreatic, intraperitoneal) pathology.
Since the first description of use of an endoscope-based CLE (eCLE) system in humans in 2004, the technology of eCLE has been studied for several indications. These include use to identify Barrett esophagus, esophageal and gastric cancer, gastric intestinal metaplasia, celiac disease, colorectal polyps, ulcerative colitis surveillance, graft-versus-host disease, biliary tract strictures, pancreatic cysts, and use in association with endoscopic mucosal resection.91,92,93,94,95,96 Recent technology allows a confocal miniprobe (pCLE) to be passed through the biopsy channel of the endoscope, while the optical biopsy images are usually co-registered with the histopathology results performed at the conclusion of pCLE imaging. Based on the same CLE system, microprobe-based CLE has been combined with EUS-guided puncture of pancreatic cystic lesions, a procedure termed needle-based CLE (nCLE), in a proof-of-principle approach. Although follow-up trials are to be awaited, nCLE may be proved valuable in the evaluation of such cystic lesions97, but also focal masses98.
Molecular markers
Pancreatic cancer is characterized by a variety of molecular alterations99, and therefore, identification and quantification of potential molecular markers for pancreatic cancer on cellular samples obtained by EUS-FNA could be a promising approach for the diagnosis of solid pancreatic masses.100 EUS-FNA can provide material for molecular biology analysis101; moreover EUS-FNA allows the extraction of sufficient quantities of RNA to perform quantitative realtime polymerase chain reaction (PCR) analysis99, which is currently the most accurate method for detecting differential gene expression. A recent study demonstrated that molecular studies on EUS-FNA material (using low density array on EUS-FNA biopsies from solid pancreatic masses) are promising investigational techniques for the identification and quantification of markers in pancreatic adenocarcinoma patients diagnosed with non-resectable tumors.102
Accurate diagnosis of malignant and benign pancreatic masses can be challenging, potentially delaying treatment for cancer and subjecting patients with benign disease to unnecessary surgery. EUS-FNA of pancreatic masses remains inconclusive in a subset of patients and several other innovative approaches have been proposed. Broad panel microsatellite loss and k-ras point mutation analysis can be reliably performed on EUS-FNA samples from pancreatic masses and improves the diagnostic accuracy. Furthermore, it accurately differentiates between malignant and benign pancreatic masses.103 K-ras mutation analysis may be helpful in patients with suspected pancreatic ductal adenocarcinoma (PDAC) yet inconclusive EUS-FNA findings, a study from this year demonstrating that k-ras mutations were extremely rare in pancreatic inflammation and other pancreatic tumors.104,105 Autoimmune pancreatitis may mimic pancreatic cancer, and thus the detection of DNA mutations in EUS-FNA material may improve discrimination between autoimmune pancreatitis and pancreatic cancer. A k-ras mutation in EUS/FNA material from a pancreatic mass is associated with malignancy and may help to discriminate from benign conditions such as autoimmune pancreatitis.106 Mucins (MUC) are aberrantly expressed in various malignancies,107 while alterations in mucin glycosylation and expression have been described in cancer. MUC7 could serve as a potential biological marker to identify malignant lesions, especially pancreatic adenocarcinoma.101 Another approach is to use mismatch excision repair (MMR), a DNA repair system that eliminates mismatched base pairs and it plays an important role in the maintaining of genomic integrity. The use of MMR genes for the differentiation between pseudotumoral chronic pancreatitis and pancreatic cancer using a minimally invasive sampling technique could be a promising technique.99
In conclusion, large-scale gene analysis has been widely proposed as a powerful method for diagnosis of malignancy, but also to predict prognosis, invasion and metastasis through the identification of biomarkers. Future studies will clarify the role of molecular markers-based techniques associated with EUS-FNA.
CONCLUSIONS
EUS-FNA is used to acquire tissue from different tissue structures located in the vicinity of the gastrointestinal tract. The pancreas and lymph nodes are still the most common organs targeted in EUS-FNA. This minimally invasive procedure has an excellent sensitivity and specificity for the diagnosis of solid pancreatic masses and lymph nodes. Nevertheless, the negative predictive value of EUS-FNA is relatively low, especially for the diagnosis of pancreatic neoplasms. There are several issues to be taken into consideration to improve this: the presence of a cytopathologist during the procedure or the alternative of telecytopathology, application of immunohistochemistry, performance of various sampling techniques, including fine needle aspiration biopsy (FNB), the prolonged learning curve, etc. Even though the EUS-FNA technique started in early nineteen's, there are many remarkable progresses culminating nowadays with the discovery and performance of nCLE. Last, but not least, identification and quantification of potential molecular markers for pancreatic cancer on cellular samples obtained by EUS-FNA could be a promising approach for the diagnosis of solid pancreatic masses
ACKNOWLEDGMENTS
Establishement of nCLE in Copenhagen was possible based on the contribution of A.P. Møller and Chastine McKinney Møllers Foundation, The Foundation of Arvid Nilsson, Foundation Juchum, The Toyota Foundation Denmark, Lundbeck Foundation and The Foundation of Aase and Ejnar Danielsen. The activity of several authors (CMI, SI, AS) was supported by the research grant “Minimal invasive assessment of angiogenesis in pancreatic cancer based on imaging methods and molecular techniques (Angio-PAC)”, Ideas programme, 164/2011, NRC - UEFISCDI, project number PN-II-ID-PCE-2011- 3-0589. Also, a great thanks to Pia Helene Klausen and Daniela Burtea, they have been of great help organizing the work in both centers.
REFERENCES
- 1.Diamantis A, Magiorkinis E, Koutselini H. Fine-needle aspiration (FNA) biopsy: historical aspects. Folia Histochem Cytobiol. 2009;47:191–7. doi: 10.2478/v10042-009-0027-x. [DOI] [PubMed] [Google Scholar]
- 2.Logroño R, Waxman I. Interactive role of the cytopathologist in EUS-FNA. Gastrointest Endosc. 2001;54:485–90. doi: 10.1067/mge.2001.118445. [DOI] [PubMed] [Google Scholar]
- 3.DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet. 1980;1:629–31. doi: 10.1016/s0140-6736(80)91122-8. [DOI] [PubMed] [Google Scholar]
- 4.Jhala NC, Jhala DN, Chhieng DC, et al. Endoscopic Ultrasound–Guided Fine-Needle Aspiration: A Cytopathologist's Perspective. Am J Clin Pathol. 2003;120:351–67. doi: 10.1309/MFRF-J0XY-JLN8-NVDP. [DOI] [PubMed] [Google Scholar]
- 5.Caletti GC, Brocchi E, Ferrari A, et al. Guillotine needle biopsy as a supplement to endosonography in the diagnosis of gastric submucosal tumors. Endoscopy. 1991;23:251–4. doi: 10.1055/s-2007-1010679. [DOI] [PubMed] [Google Scholar]
- 6.Vilmann P, Hancke S, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 7.Vilmann P, Saftoiu A. Endoscopic ultrasound-guided fine needle aspiration biopsy: Equipment and technique. J Gastroenterol Hepatol. 2006;21:1646–55. doi: 10.1111/j.1440-1746.2006.04475.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramesh J, Varadarajulu S. How can we get the best results with endoscopic ultrasound-guided fine needle aspiration? Clin Endosc. 2012;45:132–7. doi: 10.5946/ce.2012.45.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilmann P. Copenhagen: Munksgaard; 1998. Endoscopic ultrasonography with curved array transducer in diagnosis of cancer in and adjacent to the upper gastrointestinal tract. Scanning and guided fine needle aspiration biopsy [Dissertation] [Google Scholar]
- 10.Hirschfeld H. Über isolierte aleukämische Lymphadenose der Haut. Z Krebsforsch. 1912;11:397–407. [Google Scholar]
- 11.Dudgeon LS, Patrick SV. A new method for the rapid microscopical diagnosis of tumors: with an account of 200 cases so examined. Br J Surg. 1927;15:250–61. [Google Scholar]
- 12.Martin H, Ellis E. Biopsy by needle puncture and aspiration. Ann Surg. 1930;92:169–81. doi: 10.1097/00000658-193008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin H, Ellis E. Aspiration biopsy. Surg Gynecol Obstet. 1934;59:578–89. [Google Scholar]
- 14.Mannheim E. Die Bedeutung der Tumorpunktion für die Tumordiagnose. Z Krebsforsch. 1931;34:572–93. [Google Scholar]
- 15.Wiersema MJ, Hawes RH, Tao LC, et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992;38:35–9. doi: 10.1016/s0016-5107(92)70327-7. [DOI] [PubMed] [Google Scholar]
- 16.Noh KW, Wallace MB. Endoscopic Ultrasound-Guided Fine-Needle Aspiration in the Diagnosis and Staging of Pancreatic Adenocarcinoma. Med Gen Med. 2005;7:15. [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Volmar KE, Vollmer RT, Jowell PS, et al. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–61. doi: 10.1016/s0016-5107(05)00364-0. [DOI] [PubMed] [Google Scholar]
- 19.Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc. 1999;50:786–91. doi: 10.1016/s0016-5107(99)70159-8. [DOI] [PubMed] [Google Scholar]
- 20.Will U, Mueller A, Topalidis T, et al. Value of endoscopic ultrasonography-guided fine needle aspiration (FNA) in the diagnosis of neoplastic tumor(-like) pancreatic lesions in daily clinical practice. Ultraschall Med. 2010;31:169–74. doi: 10.1055/s-0028-1109491. [DOI] [PubMed] [Google Scholar]
- 21.Horwhat JD, Paulson EK, McGrath K, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966–75. doi: 10.1016/j.gie.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Gress F, Gottlieb K, Sherman S, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459–64. doi: 10.7326/0003-4819-134-6-200103200-00010. [DOI] [PubMed] [Google Scholar]
- 23.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–91. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 24.Ylagan LR, Edmundowicz S, Kasal K, et al. Endoscopic ultrasound guided fine-needle aspiration cytology of pancreatic carcinoma: a 3-year experience and review of the literature. Cancer. 2002;96:362–9. doi: 10.1002/cncr.10759. [DOI] [PubMed] [Google Scholar]
- 25.Bhutani MS, Hawes RH, Baron PL, et al. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854–8. doi: 10.1055/s-2007-1004321. [DOI] [PubMed] [Google Scholar]
- 26.Wilson JL, Kalade A, Prasad S, et al. Diagnosis of solid pancreatic masses by endoscopic ultrasound-guided fine-needle aspiration. Int Med J. 2009;39:32–7. doi: 10.1111/j.1445-5994.2008.01633.x. [DOI] [PubMed] [Google Scholar]
- 27.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Defrias DV, Alasadi R, et al. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA): experience of an academic centre in the USA. Cytopathology. 2010;21:35–43. doi: 10.1111/j.1365-2303.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Yang R, Lu Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: a systematic review. J Cancer Res Clin Oncol. 2012;138:1433–41. doi: 10.1007/s00432-012-1268-1. [DOI] [PubMed] [Google Scholar]
- 30.Baghbanian M, Shabazkhani B, Ghofrani H, et al. Efficacy of endoscopic ultrasound guided fine needle aspiration in patients with solid pancreatic neoplasms. Saudi J Gastroenterol. 2012;18:358–63. doi: 10.4103/1319-3767.103426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshinaga S, Suzuki H, Oda I, et al. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011;23(S1):29–33. doi: 10.1111/j.1443-1661.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 32.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1996;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 33.Fisher L, Segarajasingam DS, Stewart C, et al. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: performance and outcomes. J Gastroenterol Hepatol. 2009;24:90–6. doi: 10.1111/j.1440-1746.2008.05569.x. [DOI] [PubMed] [Google Scholar]
- 34.von Bartheld MB, Veselic-Charvat M, Rabe KF, et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of sarcoidosis. Endoscopy. 2010;42:213–7. doi: 10.1055/s-0029-1243890. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro A, Pereira D, Escalon MP, et al. EUS-guided biopsy for the diagnosis and classification of lymphoma. Gastrointest Endosc. 2010;71:851–5. doi: 10.1016/j.gie.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 36.Puri R, Vilmann P, Sud R, et al. Endoscopic ultrasound-guided fine needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy. 2010;42:462–7. doi: 10.1055/s-0029-1244133. [DOI] [PubMed] [Google Scholar]
- 37.Puli SR, Batapati Krishna Reddy J, Bechtold ML, et al. Endoscopic ultrasound: it's accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028–37. doi: 10.3748/wjg.14.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–195. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen BH, Vilmann P, Folke K, et al. Endoscopic ultrasonography and real-time guided fine-needle aspiration biopsy of solid lesions of the mediastinum suspected of malignancy. Chest. 1996;110:539–44. doi: 10.1378/chest.110.2.539. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan R, Bhutani MS, Thosani N, et al. Clinical impact of EUS-FNA of mediastinal lymph nodes in patients with known or suspected lung cancer or mediastinal lymph nodes of unknown etiology. J Gastrointestin Liver Dis. 2012;21:145–52. [PubMed] [Google Scholar]
- 41.Jani N, Dewitt J, Eloubeidi M, et al. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: a multicenter experience. Endoscopy. 2008;40:200–3. doi: 10.1055/s-2007-995364. [DOI] [PubMed] [Google Scholar]
- 42.Thosani N, Thosani S, Qiao W, et al. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci. 2010;55:2756–66. doi: 10.1007/s10620-010-1361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanno A, Ishida K, Hamada S, et al. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76:594–602. doi: 10.1016/j.gie.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 44.DeWitt J, Jowell P, Leblanc J, et al. EUS-guided FNA of pancreatic metastases: a multicenter experience. Gastrointest Endosc. 2005;61:689–96. doi: 10.1016/s0016-5107(05)00287-7. [DOI] [PubMed] [Google Scholar]
- 45.Wu LM, Jiang XX, Gu HY, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy in the evaluation of bile duct strictures and gallbladder masses: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:113–20. doi: 10.1097/MEG.0b013e3283426313. [DOI] [PubMed] [Google Scholar]
- 46.Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479–90. doi: 10.3748/wjg.14.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest. 2007;131:539–48. doi: 10.1378/chest.06-1437. [DOI] [PubMed] [Google Scholar]
- 48.Watson RR, Binmoeller KF, Hamerski CM, et al. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci. 2011;56:1757–62. doi: 10.1007/s10620-011-1646-6. [DOI] [PubMed] [Google Scholar]
- 49.Turhan N, Aydog G, Ozin Y, et al. Endoscopic ultrasonography-guided fine-needle aspiration for diagnosing upper gastrointestinal submucosal lesions: a prospective study of 50 cases. Diagn Cytopathol. 2011;39:808–17. doi: 10.1002/dc.21464. [DOI] [PubMed] [Google Scholar]
- 50.Hernandez LV, Mishra G, Forsmark C, et al. Role of endoscopic ultrasound (EUS) and EUS-guided fine needle aspiration in the diagnosis and treatment of cystic lesions of the pancreas. Pancreas. 2002;25:222–28. doi: 10.1097/00006676-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Frossard JL, Amouyal P, Amouyal G, et al. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol. 2003;98:1516–24. doi: 10.1111/j.1572-0241.2003.07530.x. [DOI] [PubMed] [Google Scholar]
- 52.Attasaranya S, Pais S, LeBlanc J, et al. Endoscopic ultrasound-guided fine needle aspiration and cyst fluid analysis for pancreatic cysts. JOP. 2007;8:553–63. [PubMed] [Google Scholar]
- 53.DeWitt JM, Chappo J, Sherman S. EUS-FNA of metastatic malignant melanoma to the pancreas: report of two cases and review. Endoscopy. 2003;35:219–22. doi: 10.1055/s-2003-37258. [DOI] [PubMed] [Google Scholar]
- 54.Béchade D, Palazzo L, Fabre M, et al. EUS-guided FNA of pancreatic metastasis from renal cell carcinoma. Gastrointest Endosc. 2003;58:784–8. doi: 10.1016/s0016-5107(03)02034-0. [DOI] [PubMed] [Google Scholar]
- 55.Roland CF, van Heerden JA. Nonpancreatic primary tumors with metastasis to the pancreas. Surg Gynecol Obstet. 1989;168:345–7. [PubMed] [Google Scholar]
- 56.David O, Green L, Reddy V, et al. Pancreatic masses: a multi-institutional study of 364 fine-needle aspiration biopsies with histopathologic correlation. Diagn Cytopathol. 1998;19:423–7. doi: 10.1002/(sici)1097-0339(199812)19:6<423::aid-dc4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 57.Vilmann P. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of lymph nodes. Gastrointest Endosc. 1996;43:S24–S29. doi: 10.1016/s0016-5107(96)81510-0. [DOI] [PubMed] [Google Scholar]
- 58.Kondo D, Imaizumi M, Abe T, et al. Endoscopic ultrasound examination for mediastinal lymph node metastases of lung cancer. Chest. 1990;98:586–93. doi: 10.1378/chest.98.3.586. [DOI] [PubMed] [Google Scholar]
- 59.Vickers J. Role of endoscopic ultrasound in the preoperative assessment of patients with oesophageal cancer. Ann R Coll Surg Engl. 1998;80:233–9. [PMC free article] [PubMed] [Google Scholar]
- 60.Familiari P, Marchese M, Larghi A, et al. Staging of esophageal carcinoma: endoscopic ultrasonography. Rays. 2005;30:357–62. [PubMed] [Google Scholar]
- 61.Vazquez-Sequeiros E, Norton ID, Clain JE, et al. Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc. 2001;53:751–7. doi: 10.1067/mge.2001.112741. [DOI] [PubMed] [Google Scholar]
- 62.Chatzipantelis P, Salla C, Karoumpalis I, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy in the diagnosis of gastrointestinal stromal tumors of the stomach: A study of 17 cases. J Gastrointest Liver Dis. 2008;17:15–20. [PubMed] [Google Scholar]
- 63.Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–82. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iqbal S, Friedel D, Gupta M, et al. Endoscopic-Ultrasound-Guided Fine-Needle Aspiration and the Role of the Cytopathologist in Solid Pancreatic Lesion Diagnosis. Pathol Res Internat. 2012 doi: 10.1155/2012/317167. ID 317167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallace MB, Woodward T, Raimondo M. Endoscopic ultrasound and fine-needle aspiration for pancreatic cancer. Digest Endosc. 2004;16:S193–S196. [Google Scholar]
- 66.Vignesh S, Hoffe SE, Saif MW. EUS-Guided Pancreatic Diagnosis and Beyond JOP. J Pancreas. 2011;12:86–91. [PubMed] [Google Scholar]
- 67.Chang KJ, Katz KD, Durbin TE, et al. Endoscopic ultrasound–guided fine-needle aspiration. Gastrointest Endosc. 1994;40:694–9. [PubMed] [Google Scholar]
- 68.Jihala D, Eloubeidi M, Chhieng D, et al. Accuracy of preliminary malignant diagnosis on endoscopic ultrasound guided fine needle aspiration: analysis of 120 cases [abstract] Acta Cytol. 2001;45:859. [Google Scholar]
- 69.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of en¬doscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 70.Kim B, Chhieng DC, Crowe DR, et al. Dynamic telecytopathology of on site rapid cytology diagnoses for pancreatic carcinoma. Cytojournal. 2006;3:27. doi: 10.1186/1742-6413-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buxbaum JL, Eloubeidi MA, Lane CJ, et al. Dynamic Telecytology Compares Favorably to Rapid Onsite Evaluation of Endoscopic Ultrasound Fine Needle Aspirates. Dig Dis Sci. 2012;57:3097–7. doi: 10.1007/s10620-012-2275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olson MT, Ali SZ. Cytotechnologist on-site evaluation of pancreas fine needle aspiration adequacy: comparison with cytopathologists and correlation with the final interpretation. Acta Cytol. 2012;56:340–6. doi: 10.1159/000338646. [DOI] [PubMed] [Google Scholar]
- 73.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–81. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 74.Möller K, Papanicolaou IS, Toermer T, et al. EUS-guided FNA of solid pancreatic masses: high yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60–9. doi: 10.1016/j.gie.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Puri R, Vilmann P, Săftoiu A, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 76.Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22-G and 19-G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–45. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 77.Affolter KE, Schmidt RL, Matynia AP, et al. Needle size has only a limited effect on outcomes in eus-guided fine needle aspiration: a systemic reviw and meta-analysis. Dig Dis Sci. 2013;58:1026–34. doi: 10.1007/s10620-012-2439-2. [DOI] [PubMed] [Google Scholar]
- 78.Sodikoff JB, Johnson HL, Lewis MM, et al. Increased diagnostic yield of endoscopic ultrasound-guided fine needle aspirates with flow cytometry and immunohistochemistry. Diagn Cytopathol. 2012 Jul 26; doi: 10.1002/dc.22903. doi: 10.1002/dc.22903. Epub head of print. [DOI] [PubMed] [Google Scholar]
- 79.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 80.Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: a learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–8. doi: 10.1016/s0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 81.Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811–4. doi: 10.1016/s0016-5107(01)70082-x. [DOI] [PubMed] [Google Scholar]
- 82.Konge L, Vilmann P, Clementsen P, et al. Reliable and valid assessment of competence in endoscopic ultrasonography and fine-needle aspiration for mediastinal staging of non-small cell lung cancer. Endoscopy. 2012;44:928–33. doi: 10.1055/s-0032-1309892. [DOI] [PubMed] [Google Scholar]
- 83.Barthet M, Gasmi M, Boustiere C, et al. EUS training in a live pig model: does it improve echo endoscope hands-on and trainee competence? Endoscopy. 2007;39:535–9. doi: 10.1055/s-2007-966336. [DOI] [PubMed] [Google Scholar]
- 84.Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33–7. doi: 10.1016/s0016-5107(03)02028-5. [DOI] [PubMed] [Google Scholar]
- 85.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–13. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 86.Goetz M. Confocal laser endomicroscopy: Current indications and future perspectives in gastrointestinal diseases. Endoscopia. 2012;24:67–74. [Google Scholar]
- 87.Wang TD. Confocal microscopy from the bench to the bedside. Gastrointest Endosc. 2005;62:696–7. doi: 10.1016/j.gie.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.American Society For Gastrointestinal Endoscopy Confocal Laser Endomicroscopy. Gastrointest Endosc. 2009;70:197–200. doi: 10.1016/j.gie.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 89.Yoshida S, Tanaka S, Hirata M, et al. Optical biopsy of GI lesions by reflectance-type laser-scanning confocal microscopy. Gastrointest Endosc. 2007;66:144–9. doi: 10.1016/j.gie.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 90.Kiesslich R, Neurath MF. Endomicroscopy is bornddo we still need the pathologist? Gastrointest Endosc. 2007;66:150–3. doi: 10.1016/j.gie.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 91.Giovanni D, De Palma Confocal laser endomicroscopy in the “in vivo ” histological diagnosis of the gastrointestinal tract. World J Gastroenterol. 2009;15:5770–5. doi: 10.3748/wjg.15.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology. 2009;136:1509–13. doi: 10.1053/j.gastro.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 93.Becker V, Vercauteren T, von Weyhern CH, et al. High-resolution miniprobe-based confocal microscopy in combination with video mosaicing (with video) Gastrointest Endosc. 2007;66:1001–7. doi: 10.1016/j.gie.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 94.Gheonea DI, Cârţână T, Ciurea T, et al. Confocal laser endomicroscopy and immunoendoscopy for real-time assessment of vascularization in gastrointestinal malignancies. World J Gastroenterol. 2011;17:21–7. doi: 10.3748/wjg.v17.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rispo A, Castiglione F, Staibano S, et al. Diagnostic accuracy of confocal laser endomicroscopy in diagnosing dysplasia in patients affected by long-standing ulcerative colitis. World J Gastrointest Endosc. 2012;4:414–20. doi: 10.4253/wjge.v4.i9.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hundorfean G, Chiriac MT, Siebler J, et al. Confocal laser endomicroscopy for the diagnosis of diversion colitis. Endoscopy. 2012;44(Suppl 2):E358–9. doi: 10.1055/s-0032-1310019. [DOI] [PubMed] [Google Scholar]
- 97.Konda VJ, Aslanian HR, Wallace MB, et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos) Gastrointest Endosc. 2011;74:1049–60. doi: 10.1016/j.gie.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 98.Saftoiu A, Vilmann P, Bhutani MS. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy: Using the Optical Needle into the Acoustic Haystack. Euro J Ultrasound. 2012;33:607–10. [Google Scholar]
- 99.Gheonea DI, Ciurea ME, Saftoiu A, et al. Quantitative RT-PCR analysis of MMR genes on EUS-guided FNA samples from focal pancreatic lesions. Hepatogastroenterology. 2012;59:916–20. doi: 10.5754/hge11463. [DOI] [PubMed] [Google Scholar]
- 100.Laurell H, Bouisson M, Berthelemy P, et al. Identification of biomarkers of human pancreatic adenocarcinomas by expression profiling and validation with gene expression analysis in endoscopic ultrasound-guided fine needle aspiration samples. World J Gastroenterol. 2006;12:3344–51. doi: 10.3748/wjg.v12.i21.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carrara S, Cangi MG, Arcidiacono PG, Perri F, et al. Mucin expression pattern in pancreatic diseases: findings from EUS-guided fine-needle aspiration biopsies. Am J Gastroenterol. 2011;106:1359–63. doi: 10.1038/ajg.2011.22. [DOI] [PubMed] [Google Scholar]
- 102.Bournet B, Pointreau A, Souque A, et al. Gene expression signature of advanced pancreatic ductal adenocarcinoma using low density array on endoscopic ultrasound-guided fine needle aspiration samples. Pancreatology. 2012;12:27–34. doi: 10.1016/j.pan.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 103.Khalid A, Nodit L, Zahid M, et al. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493–500. doi: 10.1111/j.1572-0241.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 104.Ogura T, Yamao K, Sawaki A, et al. Clinical impact of K-ras mutation analysis in EUS-guided FNA specimens from pancreatic masses. Gastrointest Endosc. 2012;75:769–74. doi: 10.1016/j.gie.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 105.Wang X, Gao J, Ren Y, et al. Detection of KRAS gene mutations in endoscopic ultrasound-guided fine-needle aspiration biopsy for improving pancreatic cancer diagnosis. Am J Gastroenterol. 2011;106:2104–11. doi: 10.1038/ajg.2011.281. [DOI] [PubMed] [Google Scholar]
- 106.Khalid A, Dewitt J, Ohori NP, et al. EUS-FNA mutational analysis in differentiating autoimmune pancreatitis and pancreatic cancer. Pancreatology. 2011;11:482–6. doi: 10.1159/000331505. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Gao J, Li Z, et al. Diagnostic value of mucins (MUC1, MUC2 and MUC5AC) expression profile in endoscopic ultrasound-guided fine-needle aspiration specimens of the pancreas. Int J Cancer. 2007;121:2716–22. doi: 10.1002/ijc.22997. [DOI] [PubMed] [Google Scholar]


