Abstract
Objective:
Dysphagia as a presenting manifestation of tuberculosis is rare and there is paucity of data on the clinical, endoscopic and endosonographic features of these patients. We present our data related to the features over last four years.
Methods:
We analyzed retrospectively the clinical, endoscopic, radiological, endosonographic and cytological findings in 14 patients (male: 10; mean age: 37.7 ± 10.4 years) with dysphagia due to tuberculosis presenting to us over last 4 years.
Results:
Nine patients (64.3%) had Grade 1 dysphagia, 4 (28.6%) patients had Grade 2 and 1 patient (7.1%) had Grade 3. Mid esophagus was the commonest site of involvement. Endoscopic findings were extrinsic bulge (50%), linear ulcers (28.6%) and pol-ypoidal ulcerated lesion (7.1%). Endoscopic biopsies were inconclusive. Endoscopic ultrasound (EUS) demonstrated mediastinal lymph nodes being responsible for endoscopic bulge and their infiltration into esophageal wall leading on to ulcers. EUS-guided fine needle aspiration from these nodes established diagnosis in all patients.
Conclusion:
Dysphagia in tuberculosis is most commonly caused by compression by the surrounding mediastinal lymph nodes. EUS is a useful investigation for assessment of these patients.
Keywords: endoscopic ultrasound, mediastinum, tuberculosis, computed tomography, dysphagia, fine needle aspiration
INTRODUCTION
Gastrointestinal (GI) tuberculosis can involve any site in the GI tract with the terminal ileum, cecum and the peritoneum being the usual sites.1 Dysphagia as a presenting manifestation of tuberculosis is rare and occurs either due to primary involvement of the esophagus by tuberculosis or secondary to direct extension from adjacent structures like mediastinal or hilar lymph nodes, pulmonary sites, larynx or pharynx or spine.2,3,4,5,6,7,8,9,10 Primary esophageal tuberculosis is very rare because of various protective mechanisms like presence of stratified squamous epithelium covered with mucus as well as saliva and rapid transit of luminal contents through the esophagus.2,3,4,5,6,7,8,9,10 Hence, most of the cases of esophageal tuberculosis are because of secondary involvement from the surrounding infected structures. The clinical and endoscopic findings are non-specific and can mimic esophageal cancer. Endoscopic ultrasound (EUS) can also help in diagnosis of esophageal tuberculosis by demonstrating the invasion of the esophagus by surrounding infected structures.2,7 EUS-guided fine needle aspiration (EUS-FNA) has been shown to be an useful modality for diagnosis of mediastinal tuberculosis.11 In this article, we present the clinical, endoscopic, radiological and endosonographic features of patients of tuberculosis presenting with dysphagia seen by us over last four years.
PATIENTS AND METHODS
A retrospective analysis of the collected data based on the patients with tuberculosis presenting to us with dysphagia in the last 4 years was done. The clinical data retrieved from patient records included the presenting symptoms, findings on clinical examination, human immunodeficiency virus (HIV) status, findings of endoscopy, EUS examination, computed tomography (CT), EUS-FNA cytology, and histopathology of the endoscopic biopsy specimens. The dysphagia was graded on a scale of 0 to 4: 0, normal diet possible; 1, unable to swallow certain solids; 2, able to swallow only semisolid soft diet; 3, able to swallow liquids only; and 4, unable to swallow even liquids in adequate amounts.12 An informed consent was obtained from all the patients prior to endoscopic procedures. A diagnosis of tuberculosis was made if the cytological examination revealed acid-fast bacilli or caseating necrosis with granulomas or culture showing growth of Mycobacterium tuberculosis. The diagnosis of tuberculosis was further confirmed by documenting response to antitubercular therapy in these patients.
All the patients were given four-drug antitubercular therapy (Isoniazid 5 mg/kg per day, Rifampicin 10 mg/kg per day, Ethambutol 15 mg/kg per day and Pyrazinamide 25 mg/kg per day) along with 10 mg/day of pyridoxine for the first two months and then Isoniazid and Rifampicin for further 7 months. Any adverse effects, if observed, from the antitubercular therapy were also retrieved.
RESULTS
Fourteen patients (male: 10; mean age: 37.7 ± 10.4 years; age range: 26-62 years) with dysphagia due to tuberculosis were studied. None of these patients were seropositive for HIV. Nine patients (64.3%) had Grade 1 dysphagia, 4 (28.6%) patients had Grade 2 and only 1 patient (7.1%) had Grade 3. None of the patients had absolute dysphagia.
Associated Clinical Features
None of the patients had cough, expectoration or hemoptysis and the clinical examination of the chest was normal. Along with dysphagia the other associated symptoms were loss of appetite in 11 (78.6%), loss of weight in 10 (71.4%) and fever in 5 (35.7%) patients. None of these patients had palpable peripheral lymphadenopathy or organomegaly.
Endoscopic Features
Abnormal findings on upper gastrointestinal endoscopy were observed in 12/14 (85.7%) patients. The site of involvement was mid esophagus in all these 12 patients. Endoscopic findings were extrinsic bulge (50%), linear ulcers (28.6%) and polypoidal ulcerated lesion (7.1%). All the patients with esophageal ulcers had also an underlying bulge and these ulcers were located on the summit of this bulge (Fig. 1). The endoscope was negotiable across the esophageal lesion in all these patients. Endoscopic biopsy was performed in patients with ulcers or polypoidal lesion and revealed chronic nonspecific inflammation with no granulomas or acid fast bacilli.
Figure 1.
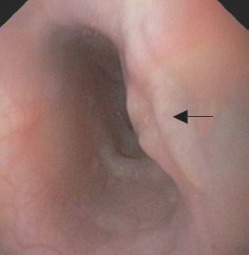
Endoscopy showing an extrinsic bulge with linear ulcer (arrow).
Radiological features
Contrast enhanced computed tomography (CECT) of the chest was done in all the patients and all the 14 patients had enlarged subcarinal lymph nodes ranging in size from 1.8 cm to 4.2 cm (Fig. 2). Other locations of the mediastinal lymph nodes were left paratracheal in 4 (28.6%) and right paratracheal in 1 (7.1%) patients. Concomitant esophageal wall thickening was observed in 5/14 (35.7%) patients. None of these patients had lung lesion.
Figure 2.
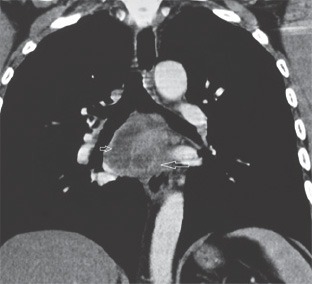
Contrast enhanced computed tomography: large subcarinal lymph nodes (arrows).
Endosonographic features
The mediastinal lymph nodes could be well visualized in all the patients. EUS could demonstrate that the mediastinal lymph nodes were responsible for the bulge visualized on the endoscopy. In patients with esophageal ulcers, EUS could demonstrate that the mediastinal lymph nodes had infiltrated the esophageal wall and at this site there was thickening of the esophageal wall along with loss of stratification (Fig. 3). On EUS, echogenic foci/strands in the lymph nodes were observed in all 14 patients and focal anechoic or hypoechoic areas were seen in 3 (21.4%) patients. Echogenic foci with shadowing suggestive of calcification were observed in only 1 (7.1%) patient (Fig. 4). EUS guided FNA could be done in all the patients with no adverse effects.
Figure 3.
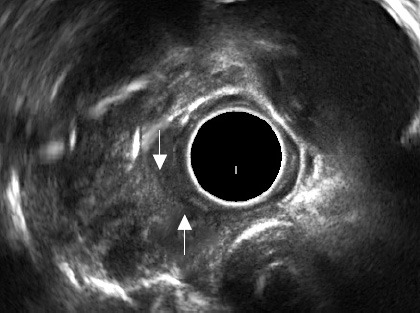
Endoscopic ultrasound: right paratracheal lymph node invading the esophageal wall (arrows).
Figure 4.
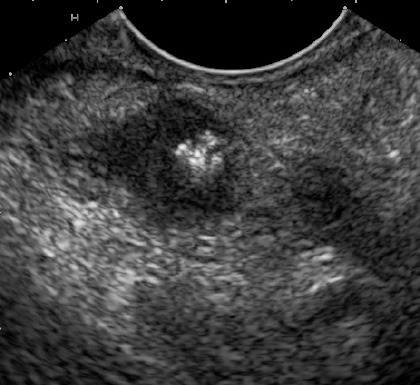
Endoscopic ultrasound: lymph node with calcification.
Cytological features
EUS-FNA yielded adequate material in all the patients and caseous material was aspirated in 6/14 (42.8%) patients. Epitheloid granulomas were seen in 13/14 (92.9%) patients and of these 9 (64.3%) patients had caseating necrosis. Stain for AFB was positive in 3(21.4%) patients.
Follow-up clinical data
All these patients were treated with four-drug antitubercular therapy and they had disappearance of dysphagia within 10 to 22 days of initiation of the therapy. Repeat endoscopy was done in patients with esophageal ulcers or polypoidal lesions 3 months after initiation of the therapy and there was complete resolution of these lesions. No adverse effects of the therapy were observed in any patient.
DISCUSSION
In patients with tuberculosis, dysphagia can occur because of various mechanisms like compression by the surrounding tubercular peri-esophageal lymph nodes, associated mediastinal fibrosis, ulceration and/or polypoidal lesions in the esophagus, or altered motility.2,3,4,5,6,7,8,9,10 In our study, a majority of the patients (50%) had dysphagia because of extrinsic compression by the mediastinal lymph nodes whereas 36% patients had dysphagia because of esophageal mucosal involvement. In 2 patients the esophagus was normal on endoscopy, but both of them had enlarged subcarinal lymph nodes. The dysphagia in these 2 patients could be because of associated mediastinal fibrosis or dysmotility. There were no associated pulmonary symptoms in these 14 patients but associated constitutional symptoms like loss of appetite and loss of weight were seen in >2/3 of the patients. Fever was not reported by the majority of the patients suggesting that the clinical symptoms closely mimic those of esophageal cancer except these patients being younger and therefore a high index of suspicion for its diagnosis is to be kept especially if a younger patient from tropical countries presents with dysphagia.
The chest CECT can help by identifying lung lesions, mediastinal lymph nodes and complications such as esophago-bronchial fistulae.9 However, none of the radiological findings are specific for tuberculosis and similar radiological findings can be seen in esophageal cancers as well as metastatic mediastinal lymph nodes. Similarly, endoscopic findings are also non-specific. A majority of patients with esophageal tuberculosis have linear ulcerative lesion with or without extrinsic bulge in the mid esophagus as was also in our study.2,3,4,5,6,7,8,9,10 The mid esophagus is the most common site of involvement because of its proximity to the hilar and mediastinal lymph nodes surrounding the bifurcation of the trachea.2,3,4,5,6,7,8,9,10 The endoscopic mucosal biopsy specimens can help in the diagnosis of esophageal tuberculosis, but one of the study has shown that the diagnostic yield of biopsies obtained after a single endoscopic session was 50% and obtaining biopsies from multiple endoscopic sessions increased the diagnostic yield.9 In our study, single session of endoscopic mucosal biopsy was non-diagnostic in all the 5 patients with abnormal esophageal mucosal findings.
EUS has been demonstrated to be an excellent tool for detection for tubercular mediastinal lymph nodes as well as to obtain specimens from these lymph nodes using FNA.11,13 An earlier study had described EUS features of mediastinal tubercular lymph nodes and found that hyperechoic foci in the lymph nodes were seen in 77.2% of patients with tubercular mediastinal lymphadenopathy and in 5% of patients with non-tubercular mediastinal lymphadenopathy (P = 0.00). Patchy anechoic or hypoechoic areas were seen in 40.9% patients with tubercular mediastinal lymphadenopathy whereas none of the patients with non-tubercular mediastinal lymphadenopathy presented these (P = 0.01).11 In the current study also, echogenic foci in the lymph nodes were observed in all the 14 patients and focal anechoic or hypoechoic areas were seen in 3 (21.4%) patients. These hyperechoic foci/strands probably represent patchy fibrosis or speckled calcification in the lymph nodes.11 Moreover, EUS could demonstrate mediastinal lymph nodes causing the bulge visualized on the endoscopy. In patients with esophageal ulcers, EUS could demonstrate that the mediastinal lymph nodes had infiltrated the esophageal wall.
In this study, EUS-FNA from the mediastinal lymph nodes could establish diagnosis in all the 14 patients. Epithelioid granulomas were seen in 92.9% patients and 64.3% patients had caseating necrosis. Stain for AFB was positive in 21.4% patients. Fritscher-Ravens et al.13 have also reported that EUS-FNA has a high diagnostic yield for the diagnosis of mediastinal tubercular lymphadenopathy with sensitivity, specificity, and positive and negative predictive values being 86%, 100%, 100%, and 91%, respectively.
In conclusion, dysphagia in tuberculosis can be caused by diverse mechanisms with most common mechanism being compression by the surrounding mediastinal lymph nodes. EUS is a useful investigation approach for assessment of these patients and EUS-FNA can help in achieving correct diagnosis in these patients.
DISCLOSURE
None of the authors have any conflicts of interest.
REFERENCES
- 1.Werbeloff L, Novis BH, Bank S, et al. The radiology of tuberculosis of the gastro-intestinal tract. Br J Radiol. 1973;46:329–36. doi: 10.1259/0007-1285-46-545-329. [DOI] [PubMed] [Google Scholar]
- 2.Rana SS, Bhasin DK, Sharma V, et al. Dysphagia as the first manifestation of tuberculosis. Endoscopy. 2011;43(Suppl 2 UCTN):E300–1. doi: 10.1055/s-0030-1256456. [DOI] [PubMed] [Google Scholar]
- 3.Welzel TM, Kawan T, Bohle W, et al. An unusual cause of dysphagia: esophageal tuberculosis. J Gastrointestin Liver Dis. 2010;19:321–4. [PubMed] [Google Scholar]
- 4.Rathinam S, Kanagavel M, Tiruvadanan BS, et al. Dysphagia due to tuberculosis. Eur J Cardiothorac Surg. 2006;30:833–6. doi: 10.1016/j.ejcts.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Mokoena T, Shama DM, Ngakane H, et al. Oesophageal tuberculosis: a review of eleven cases. Postgrad Med J. 1992;68:110–5. doi: 10.1136/pgmj.68.796.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peixoto PC, Ministro PS, Sadio AD, et al. Esophageal tuberculosis: an unusual cause of dysphagia. Gastrointest Endosc. 2009;69:1173–6. doi: 10.1016/j.gie.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Aydin A, Tekin F, Ozutemiz O, et al. Value of endoscopic ultrasonography for diagnosis of esophageal tuberculosis: report of two cases. Dig Dis Sci. 2006;51:1673–6. doi: 10.1007/s10620-005-9028-6. [DOI] [PubMed] [Google Scholar]
- 8.Gomes J, Antunes A, Carvalho A, et al. Dysphagia as a manifestation of esophageal tuberculosis: a report of two cases. J Med Case Reports. 2011;8(5):447. doi: 10.1186/1752-1947-5-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Kim SU, Sohn JW, et al. Endoscopic findings and clinical features of esophageal tuberculosis. Scand J Gastroenterol. 2010;45:1269–72. doi: 10.3109/00365521.2010.501524. [DOI] [PubMed] [Google Scholar]
- 10.Jain SK, Jain S, Jain M, et al. Esophageal tuberculosis: is it so rare? Report of 12 cases and review of the literature. Am J Gastroenterol. 2002;97:287–91. doi: 10.1111/j.1572-0241.2002.05456.x. [DOI] [PubMed] [Google Scholar]
- 11.Rana SS, Bhasin DK, Srinivasan R, et al. Endoscopic ultrasound (EUS) features of mediastinal tubercular lymphadenopathy. Hepatogastroenterology. 2011;58:819–23. [PubMed] [Google Scholar]
- 12.Atkinson M, Ferguson R, Ogilvie AC. Management of malignant dysphagia by intubation at endoscopy. J Roy Soc Med. 1979;27:894–7. doi: 10.1177/014107687907201206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritscher-Ravens A, Ghanbari A, Topalidis T, et al. Granulomatous mediastinal adenopathy: can endoscopic ultrasound-guided fine-needle aspiration differentiate between tuberculosis and sarcoidosis? Endoscopy. 2011;43:955–61. doi: 10.1055/s-0031-1271110. [DOI] [PubMed] [Google Scholar]


