Abstract
Cholangiocarcinoma is a malignancy that arises from biliary epithelium and is associated with a poor prognosis. Accurate preopera-tive diagnosis and staging of cholangiocarcinoma continues to remain difficult. Endoscopic retrograde cholangiopancreatography (ERCP) is the most commonly performed procedure for cholangiocarcinoma and can provide a tissue diagnosis through brush cytology of the bile duct. However, the sensitivity of biliary brush cytology to diagnose cholangiocarcinoma may be as low as 30%. Endoscopic ultrasound (EUS) is a diagnostic modality which may overcome the limitations of other imaging and biopsy techniques in this setting. EUS can complement the role of ERCP and provide a tissue diagnosis through fine needle aspiration (FNA) and staging through ultrasound imaging. There is currently a paucity of data about the exact role of EUS for the diagnosis of cholan-giocarcinoma in patients with indeterminate extrahepatic biliary strictures. Although multiple studies have shown that EUS is more accurate than ERCP and radiologic imaging for identifying a biliary mass and diagnosing cholangiocarcinoma, the sensitivities are variable. More importantly, the incidence of false negative results is not negligible, though the specificity is close to 100%. There is also controversy regarding the role of EUS-FNA, since even though this may increase diagnosis, it can also lead to tumor seeding.
Keywords: cholangiocarcinoma, endosonography, fine needle aspiration
INTRODUCTION
Cholangiocarcinoma is a neoplasm derived from the bile duct epithelium of the intrahepatic and extrahepatic biliary tree. Although this type of malignancy is relatively rare, its prognosis without medical or surgical intervention is very poor with a 3-year mortality rate of greater than 95%.1 The poor prognosis in part can be attributed to the advanced stage of the disease at time of diagnosis.
However, even the more advanced stages of cholangio-carcinoma have been shown to have improved outcomes with surgical intervention. Tumor resection has been associated with a 5-year overall survival of 21%-63%, while liver transplantation for hilar cholangiocarcinoma had an even better survival of 54%-82% at 5 years.2,3,4,5,6,7,8,9 Surgical interventions carry with them a relatively high rate of complications. Studies looking at various forms of operative management identified morbidity rate of 30%-70%.10,11,12 It is therefore imperative to select the optimal surgical candidates, in whom the benefits of intervention would outweigh the risks.
Currently, endoscopic retrograde cholangio-pancreatography (ERCP) is the modality of choice for evaluating biliary strictures for malignancy. However, even the combination of biopsy and brush cytology done via ERCP frequently fails to diagnose a biliary tract malignancy. Although the specificity is close to 100%, the sensitivity of brush cytology and biopsy is only 48%-55%.13,14
Given the relatively low sensitivity and diagnostic accuracy of ERCP-based techniques to diagnose cholangiocarcinoma, new diagnostic modalities have emerged, in particular endoscopic ultrasound (EUS). This review will discuss the utility of EUS in differentiating cholangiocarcinoma from benign biliary strictures. It will also address the role of EUS-FNA in identifying nodal disease and how this information can be used for better selecting patients who would be good surgical candidates. The limitations and risks associated with this technique are also highlighted.
ROLE OF ENDOSCOPIC ULTRASOUND IN DIAGNOSING CHOLANGIOCARCINOMA
Although ERCP remains the first line modality for evaluating biliary strictures for malignancy, in recent years, EUS has emerged as a potential alternative. In EUS, the echoendoscope is advanced into the duodenum, where it can be manipulated to visualize the biliary tract both in the cross-sectional and longitudinal views. If a mass is present, it usually appears hypoechoic or, less frequently, heterogenous (Fig. 1). Moreover, EUS allows for the identification of hilar, celiac axis, and para-aortic lymph nodes, which can facilitate the staging of cholangiocarcinoma (Fig. 2). Once a target of interest is identified, fine needle aspiration (FNA) can be performed to obtain cytology (Fig. 3).
Figure 1.
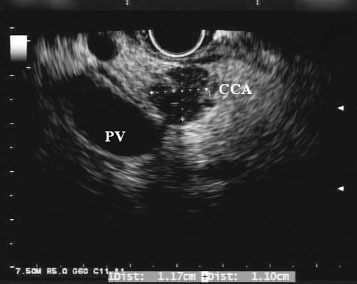
Endoscopic ultrasound view of a hypoechoic biliary lesion 1.2 cm in diameter (cholangiocarcinoma). There is no invasion of the portal vein (PV).
Figure 2.
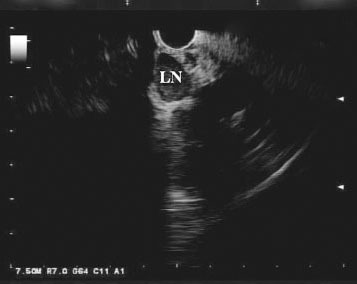
Endoscopic ultrasound view of a hypoechoic homogenous malignant appearing lymph node (LN) at the hilum of the liver.
Figure 3.
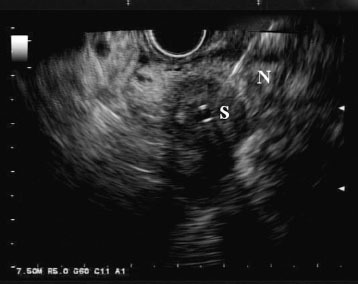
Endoscopic ultrasound view of a hypoechoic bile duct lesion undergoing aspiration via a 22-G needle (N). There is a plastic biliary stent (S) traversing the lesion.
EUS appears to play an important role in evaluating biliary strictures and diagnosing cholangiocarcinoma. Mohamadnejad et al. conducted an observational study that compared EUS to ERCP and radiologic imaging in the diagnosis and preoperative evaluation of extrahepatic biliary tumors. The study found that detection of a biliary cancer was superior by EUS (94%) when compared with computed tomography (CT, 30%) or magnetic resonance imaging/ magnetic resonance cholangiopancreatography (MRI/MRCP, 42%); this difference was found to be of a statistical significance. In this study, the overall sensitivity of EUS-FNA to diagnose cholangiocarcinoma was found to be 73%, with a sensitivity of 81% for distal tumors and 59% for proximal lesions. In the same population, ERCP with brush cytology had only a 25% and 28% sensitivity in proximal and distal tumors, respectively.15 The difference in EUS-FNA sensitivities between distal and proximal biliary tumors can be attributed to the degradation of EUS quality with distance resulting in less accurate targets for needle aspiration. Multiple other studies have shown similar results, documenting an EUS-FNA sensitivity of 60%-89% for detecting cholangiocarcinoma among patient cohorts with negative ERCP brush cytology and showing better diagnostic accuracy than alternative imaging modalities (Tab. 1).8,16,17,18 However, not all the literature is in agreement about the superiority of EUS over ERCP for assessment of malignant biliary strictures. Rosch et al. found that in the assessment of biliary strictures, EUS was more sensitive in the diagnosis of pancreatic tumors, but ERCP was a better diagnostic modality for cholangiocarcinoma.19 Therefore, the optimal diagnostic approach may be to combine both EUS and ERCP for the diagnosis of extrahepatic cholangiocarcinoma.
Table 1.
Accuracy of EUS-FNA in diagnosing cholangiocarcinoma
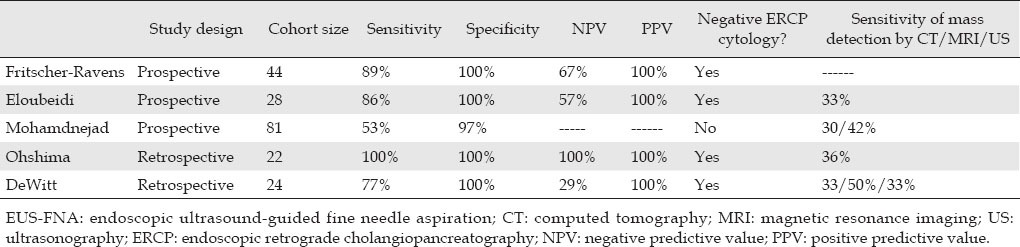
It is also important to note that the negative predictive values of EUS-FNA in most of the studies were relatively low, ranging from 29% to 67%.8,16,17,20 The relatively low negative predictive value of EUS-FNA underscores the fact that false negatives do occur and in the setting of significant clinical evidence concerning for malignancy, more aggressive diagnostic techniques, including exploratory surgery, should be employed. On the other hand, the positive predictive value of EUS-FNA was found to be 100% in almost every study.8,16,17,18 As a result, patients with benign diseases or alternative diagnoses are far less likely to be misdiagnosed as having a biliary malignancy and are therefore spared inappropriate procedures or treatments.8,20 This is an important advantage of EUS-FNA over other diagnostic modalities, given the fact that with alternative modes of diagnosis, including radiologic imaging and ERCP, the rate of unnecessary surgical intervention for benign biliary lesions has been shown to be as high as 15%-25%.21,22
IMPACT OF ENDOSCOPIC ULTRASOUND ON CLINICAL MANAGEMENT OF CHOLANGIOCARCINOMA
When evaluating patients with cholangiocarcinoma, one of the main goals is determining those who are candidates for tumor resection or liver transplantation. In general, vascular infiltration, lymph node invasion and distant metastases are all contraindications to surgical intervention. Determination of tumor resectability usually requires exploratory surgery, but ideally, this determination should be made with a less interventional approach, such as imaging or endoscopy.
Multiple studies have also established that EUS is superior to alternative imaging modalities that included CT, MRI, abdominal ultrasound, and angiography in detecting tumor vascular invasion and determining resectability status in patients with cholangiocarcinoma.8,23 Fritscher-Ravens et al. demonstrated that EUS correctly identified unresectable diseases in 83% of patients who were confirmed to have loco-regional metastases by exploratory surgery.17 Similarly, Mohamadnejad found that EUS was more accurate than CT scan in determining the unresectability status (53% vs. 33% respectively) as confirmed by exploratory surgery.15 In the same study, EUS also correctly diagnosed 97% patients with resectable cholangiocarcinoma. It should be noted that Mohamadnejad's study found that both EUS and CT scan are limited in their ability to predict unresectability of the cancer.
Determination of lymph node involvement is another important criterion for treatment planning in cholangiocarcinoma. In particular, nodal disease needs to be taken into account when selecting candidates for liver transplantation, a surgical option for patients with cholangiocarcinoma who are otherwise deemed unresectable due to anatomic location or local invasion. Studies from various centers have shown worse outcomes among transplanted patients found to have lymph node metastases and this is generally considered a contraindication to liver transplanatation.24,25,26
Previously, patients had undergone laparotomies or laparoscopies with lymphadenectomy in order to assess for nodal diseases. EUS characteristics of malignant lymph nodes include a larger size (>1 cm), round shape, well-defined margins, and a hypoechoic homogenous appearance.27 No single feature independently predicts malignant invasion. When all four of the above features are present in the same lymph node, the accuracy for predicting malignant invasion may be as high as 80%. However, Bhutani et al have shown that all four features of malignant involvement were present in only one-fourth of malignant lymph nodes.28
EUS with FNA of the suspicious lymph nodes may provide a less invasive alternative to obtain a diagnosis for nodal involvement. Gleeson et al. compared the accuracy of EUS in detecting malignant lymph nodes and compared them to CT, MRI and laparotomy in a cohort of 47 patients with cholangiocarcinoma being evaluated for liver transplant.29 In the study, EUS visualized all suspicious lymph nodes, unlike CT and MRI, which failed to identify the presence of nodes in 26% of cases. Of note, although some studies have correlated certain features of nodal morphology and echogenicity being predictive of a node being malignant, this study did not find any such association.27,30 In terms of diagnostic accuracy, cytology from the EUS-FNA detected metastatic disease in the nodes of 8 of 47 individuals, thus sparing 38% of the cohort from a more invasive diagnostic laparotomy. Of the patients who ultimately did undergo surgical staging, EUS-FNA was found to have missed metastatic nodal involvement in 2 patients, demonstrating an overall sensitivity of 80%. Conversely, Mohamadnejad et al. found that EUS-FNA correctly detected lymph node metastases in only 9% of the patients as compared to 18% identified by CT/MRI, though the specificity of EUS remained high at 95%. It should be noted that in Mohamadnejad's study, EUS-FNA of benign appearing lymph nodes was not performed which may have contributed to the very low detection of nodal metastasis.15 It is well documented that there is significant inter-observer variability in predicating malignant lymphadenopathy when using EUS characteristics alone and when EUS-FNA of nodes is not performed; diagnostic accuracy to detect cancer will decrease.
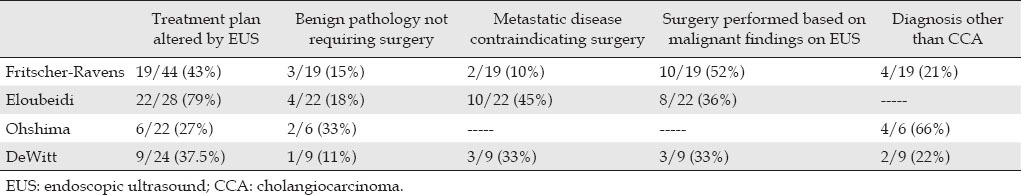
Overall, EUS-FNA appears to have an impact on clinical decision-making for patients with cholangiocarcinoma in as much as 79% of the time (Tab. 2). 8,17,20 By identifying invasive or metastatic disease, EUS spares patients with unresectable tumors more invasive staging procedures and by confirming benign disease, EUS precludes healthy patients from undergoing unnecessary surgical resections. However, the data currently available is fairly limited and inconsistent, with some studies demonstrating an excellent accuracy for EUS, while others showing only marginal results. EUS should definitely play a role in staging cholangiocarcinoma; however, it is unclear whether the sensitivity of the approach is good enough to eliminate more invasive alternatives to confirm the diagnosis and overall staging.
Table 2.
Impact of EUS on clinical management of cholangiocarcinoma
INTRADUCTAL ULTRASONOGRAPHY
Intraductal ultrasonography (IDUS) is an alternative method for evaluating indeterminate biliary strictures. The technique involves performing an ERCP to canulate the biliary tract followed by advancing a high frequency ultrasound probe over a guide wire through the duodenoscope and into the biliary tract. Once this is accomplished, the ultrasound probe can generate high resolution images of the bile duct wall and evaluate for any evidence of malignancy. In a normal biliary system, the ultrasound distinguishes three layers: an inner hyperechoic layer of the bile duct mucosa, middle hypoechoic layer of fibromuscular tissue, and the outer hyperechoic layer of the subserosal adipose tissue. If IDUS demonstrates a hypoechoic mass with irregular borders or a mass showing disruption in the bile wall structure is identified, suspicion for a malignancy is high.31,32
Studies have found that IDUS is more accurate than EUS in identifying malignant biliary strictures. The high frequency of the ultrasound probes allows for a better accuracy in evaluating local tumor invasion and longitudinal extension. Menzel et al found that IDUS exceeded EUS in terms of accuracy (IDUS, 89%; EUS, 75%), sensitivity (IDUS, 91%; EUS, 75%), and T-staging (IDUS, 77%; EUS, 54%) for the diagnosis and staging for cholangiocarcinoma. The accuracy rate for lymph node staging using IDUS (60%) was comparable with that of EUS (62.5%).31 Other trials conversely show IDUS to be inferior to conventional EUS for malignant lymph node detection.33
IDUS is rarely performed now due to several disadvantages. One limitation of the high frequency IDUS probe is a decrease in depth of penetration. As a result, IDUS fails to evaluate tumor extension beyond the hepatoduodenal ligament nor is it able to assess distant metastases or regional lymph node involvement.31,33 Additionally, these probes are fragile and expensive.
RISK OF TUMOR SEEDING WITH EUS-FNA
One of the most concerning complications of FNA is the risk of tumor seeding along the needle tract. Several studies have evaluated this risk among patients undergoing percutaneous needle aspiration for abdominal malignancies and found it to range from 1/1,000-1/33,000.34,35 A meta-analysis conducted by Silva et al. reported an even higher tumor seeding rate of 2.7% among patients who underwent percutaneous needle aspiration during evaluation for hepatocellular carcinoma.36 In turn, Heimbach et al. conducted a retrospective study comparing the frequency of peritoneal metastases between patients who did or did not undergo FNA as part of staging for hilar cholangiocarcinoma.37 The results found that 83% patients with cholangiocarcinoma and underwent an EUS-FNA had peritoneal metastases at the time of operative restaging, as compared to 8% of patients who did not undergo FNA. In light of this data, the study's conclusion was that cholangiocarcinoma patients who are liver transplant candidates should not undergo EUS-FNA as this may increase the risk of metastases due to tumor seeding, and therefore eliminate them as candidates for a potentially curative surgery.
It is important to note that percutaneous needle aspiration may carry a greater risk of tumor seeding when compared to EUS-FNA. Micames et al. conducted a retrospective study that found a significant difference in the incidence of peritoneal carcinomatosis in patients with pancreatic adenocarcinoma after needle aspiration by the two approaches (2.2% in the EUS group vs. 16.3% in the percutaneous group).38
In summary, several case reports have described tumor seeding from EUS-FNA and it is definitely a phenomenon that needs to be taken into account when deciding among diagnostic approaches.39,40,41 Unfortunately, there are only a handful of retrospective and no prospective studies that have looked at tumor seeding or its effect on disease outcomes.
Therefore, no definitive rule exists about when EUS-FNA is appropriate and when it is contraindicated. However, tumor seeding is something that all clinicians should take into consideration when deciding on a diagnostic modality for cholangiocarcinoma.
LIMITATIONS OF ENDOSCOPIC ULTRASOUND
There are several confounding factors that can affect the accuracy of EUS in detecting cholangiocarcinoma. Primary sclerosing cholangitis (PSC), a rare condition in only 2%-9% of study populations, is associated with multiple strictures and benign lymphadenopathy. Thus, accuracy of EUS performed for evaluation of an indeterminate biliary stricture can be impeded for diagnosing a malignancy in the setting of PSC.8,15,16
Another possible confounder for the diagnosis of cholangiocarcinoma is the presence of biliary stents at the time of EUS evaluation. The presence of a stent at the time of the EUS appears to decrease the EUS sensitivity in detecting malignant lesions.8,15,42
This can be explained by acoustic shadowing from the stent which then creates artifact and decreases the EUS probe's ability to accurately evaluate the bile ducts or any surrounding masses. Additionally, the presence of the stent can physically interfere with the FNA, as it limits the portion of the duct that can be used for needle aspiration.
CONCLUSIONS
Cholangiocarcinoma is a rare, difficult to treat neoplasm with poor survival outcomes. In part, its poor prognosis is due to its advanced stage at the time of diagnosis. Improved outcomes can only be achieved with better diagnostic modalities that can diagnose this disease at an earlier stage. In recent years, EUS has emerged as a new tool for evaluating the hepato-biliary tract and obtaining cytology by means of FNA. Although multiple studies have shown that EUS is more accurate than ERCP and radiologic imaging for identifying a biliary mass and diagnosing cholangiocarcinoma, the sensitivities are variable. More importantly, the incidence of false negative results is not negligible, though the specificity is close to 100%. Therefore, EUS-FNA can be considered a reliable tool for confirming malignancy, but negative cytology is not sufficient to fully rule out a diagnosis of cholangiocarcinoma in the presence of clinical suspicion.
EUS also plays a role in determining the presence of metastatic and locally invasive disease, which is essential for treatment planning. Vascular invasion and distant metastases are contraindications to resection, whereas locally advanced disease without lymph node involvement makes patients potential candidates for liver transplantation. EUS-FNA appears to have a very high specificity, but a variable sensitivity in staging cholangiocarcinoma. The benefit of minimizing the need for more invasive diagnostic approaches also has to be weighed against the risk of tumor seeding associated with needle aspiration. In this setting, if a patient is a candidate for resection or liver transplantation, it may be beneficial to bypass EUS-FNA and go straight to surgical staging in order to limit the chance of disseminating the tumor, which would disqualify the patient from potentially curative treatment.
Overall, the available data on the role of EUS to diagnose and stage cholangiocarcinoma is limited by the small size of the cohorts and the lack of randomized, controlled trials. As EUS becomes more frequently utilized in the work up of biliary strictures and the staging of cholangiocarcinoma, a higher volume of data will likely become available to better assess the utility of this modality in diagnosing cholangiocarcinoma and evaluating tumor resectability.
DISCLOSURE
The authors attest that they have no commercial associations (e.g., equity ownership or interest, consultancy, patent and licensing agreement, or institutional and corporate associations) that might be a conflict of interest in relation to the submitted manuscript.
REFERENCES
- 1.Farley DR, Weaver AL, Nagorney DM. “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425–9. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 2.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–8. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–17. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24:201–7. doi: 10.1055/s-2004-828896. [DOI] [PubMed] [Google Scholar]
- 5.Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–7. doi: 10.1097/01.tp.0000253551.43583.d1. [DOI] [PubMed] [Google Scholar]
- 6.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer: A single-center experience. Ann Surg. 1996;224:628–38. doi: 10.1097/00000658-199611000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eloubeidi MA, Chen VK, Jhala NC, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209–13. doi: 10.1016/s1542-3565(04)00005-9. [DOI] [PubMed] [Google Scholar]
- 9.Pandey D, Lee KH, Tan KC. The role of liver transplantation for hilar cholangiocarcinoma. Hepatobil Pancreat Dis Int. 2007;6:248–53. [PubMed] [Google Scholar]
- 10.Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33:1247–54. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 11.Hanazaki K, Kajikawa S, Shimozawa N, et al. Prognostic factors of intrahepatic cholangiocarcinoma after hepatic resection: univariate and multivariate analysis. Hepatogastroenterology. 2002;49:311–6. [PubMed] [Google Scholar]
- 12.Sakamoto Y, Shimada K, Nara S, et al. Surgical management of infrahilar/suprapancreatic cholangiocarcinoma: an analysis of the surgical procedures, surgical margins, and survivals of 77 patients. J Gastrointest Surg. 2010;14:335–43. doi: 10.1007/s11605-009-1072-7. [DOI] [PubMed] [Google Scholar]
- 13.Sachdev A, Duseja A, Bhalla A, et al. Efficacy of endoscopic wire guided biliary brushing in the evaluation of biliary strictures. Trop Gastroenterol. 2003;24:215–7. [PubMed] [Google Scholar]
- 14.Pugliese V, Antonelli G, Vincenti M, et al. Endoductal tissue sampling of biliary strictures through endoscopic retrograde cholangiopan creatography (ERCP) Tumori. 1997;83:698–702. doi: 10.1177/030089169708300314. [DOI] [PubMed] [Google Scholar]
- 15.Mohamadnejad M, DeWitt JM, Sherman S, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71–8. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 16.DeWitt J, Misra VL, LeBlanc JK, et al. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325–33. doi: 10.1016/j.gie.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 17.Fritscher-Ravens A, Broering DC, Knoefel WT, et al. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45–51. doi: 10.1046/j.1572-0241.2003.04006.x. [DOI] [PubMed] [Google Scholar]
- 18.Manu NK, Derek MM, Viney W, et al. Role of EUS/EUS-guided FNA in the management of proximal biliary strictures. Hepatogastroenterology. 2011;58:1862–5. doi: 10.5754/hge10531. [DOI] [PubMed] [Google Scholar]
- 19.Rosch T, Hofrichter K, Frimberger E, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390–6. doi: 10.1016/s0016-5107(04)01732-8. [DOI] [PubMed] [Google Scholar]
- 20.Ohshima Y, Yasuda I, Kawakami H, et al. EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J Gastroenterol. 2011;46:921–8. doi: 10.1007/s00535-011-0404-z. [DOI] [PubMed] [Google Scholar]
- 21.Clayton RA, Clarke DL, Currie EJ, et al. Incidence of benign pathology in patients undergoing hepatic resection for suspected malignancy. Surgeon. 2003;1:32–8. doi: 10.1016/s1479-666x(03)80006-9. [DOI] [PubMed] [Google Scholar]
- 22.Gerhards MF, Vos P, van Gulik TM, et al. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg. 2001;88:48–51. doi: 10.1046/j.1365-2168.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama M, Hagi H, Atomi Y, et al. Diagnosis of portal venous invasion by pancreatobiliary carcinoma: value of endoscopic ultrasonography. Abdom Imaging. 1997;22:434–8. doi: 10.1007/s002619900227. [DOI] [PubMed] [Google Scholar]
- 24.Schüle S, Altendorf-Hofmann A, Uteß F, et al. Liver transplantation for hilar cholangiocarcinoma--a single-centre experience. Langenbecks Arch Surg. 2013;398:71–7. doi: 10.1007/s00423-012-1007-8. [DOI] [PubMed] [Google Scholar]
- 25.Friman S, Foss A, Isoniemi H, et al. Liver transplantation for cholangiocarcinoma: selection is essential for acceptable results. Scand J Gastroenterol. 2011;46:370–5. doi: 10.3109/00365521.2010.533384. [DOI] [PubMed] [Google Scholar]
- 26.Robles R, Figueras J, Turrion VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–71. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faige DO. EUS in patients with benign and malignant lymphadenopathy. Gastrointest Endosc. 2001;53:593–8. doi: 10.1067/mge.2001.114060. [DOI] [PubMed] [Google Scholar]
- 28.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474–9. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 29.Gleeson FC, Rajan E, Levy MJ, et al. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438–43. doi: 10.1016/j.gie.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: a prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol. 2004;99:628–33. doi: 10.1111/j.1572-0241.2004.04064.x. [DOI] [PubMed] [Google Scholar]
- 31.Menzel J, Poremba C, Dietl KH, et al. Preoperative diagnosis of bile duct strictures--comparison of intraductal ultrasonography with conventional endosonography. Scand J Gastroenterol. 2000;35:77–82. doi: 10.1080/003655200750024579. [DOI] [PubMed] [Google Scholar]
- 32.Tamada K, Ueno N, Tomiyama T, et al. Characterization of biliary strictures using intraductal ultrasonography: comparison with percutaneous cholangioscopic biopsy. Gastrointest Endosc. 1998;47:341–9. doi: 10.1016/s0016-5107(98)70216-0. [DOI] [PubMed] [Google Scholar]
- 33.Tamada K, Ido K, Ueno N, et al. Preoperative staging of extrahepatic bile duct cancer with intraductal ultrasonography. Am J Gastroenterol. 1995;90:239–46. [PubMed] [Google Scholar]
- 34.Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Radiology. 1991;178:253–8. doi: 10.1148/radiology.178.1.1984314. [DOI] [PubMed] [Google Scholar]
- 35.Lundstedt C, Stridbeck H, Andersson R, et al. Tumor seeding occurring after fine-needle biopsy of abdominal malignancies. Acta Radiol. 1991;32:518–20. [PubMed] [Google Scholar]
- 36.Silva MA, Hegab B, Hyde C, et al. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–6. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 37.Heimbach JK, Sanchez W, Rosen CB, et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–60. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690–5. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 39.Doi S, Yasuda I, Iwashita T, et al. Needle tract implantation on the esophageal wall after EUS-guided FNA of metastatic mediastinal lymphadenopathy. Gastrointest Endosc. 2008;67:988–90. doi: 10.1016/j.gie.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Paquin SC, Gariepy G, Lepanto L, et al. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610–1. doi: 10.1016/s0016-5107(05)00082-9. [DOI] [PubMed] [Google Scholar]
- 41.Shah JN, Fraker D, Guerry D, et al. Melanoma seeding of an EUS-guided fine needle track. Gastrointest Endosc. 2004;59:923–4. doi: 10.1016/s0016-5107(04)00340-2. [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Salem R, Aslanian H, et al. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069–73. doi: 10.1111/j.1572-0241.2004.30223.x. [DOI] [PubMed] [Google Scholar]


