Abstract
Objective:
The objective of this study is to compare the efficacy of central (single) vs bilateral (2-injections) endoscopic ultrasound (EUS)-celiac plexus neurolysis (CPN) for palliation of patients with pain related to pancreatic cancer.
Materials and Methods:
Patients with unresectable pancreatic cancer were included. Central EUS CPN was used in the first group and bilateral EUS CPN in the second. The measurement of pain was made with a visual analog pain scale (VAPS) applied before and after the procedure. Follow-up was made at weeks 2 and 4 after the procedure. The use of morphine before and after EUS CPN was evaluated. Complications related to the procedure were recorded.
Results:
A total of 53 patients underwent EUS CPN, 21 (39.6%) with the central technique and 32 (60.4%) with bilateral injection; 29 were women (54.7%) and the median age was 59 (30-85) years. The tumor was located in the head of the pancreas in 24 (45.3%) patients, the neck in 14 (26.4%), the body in 26 (49.1%) and in the tail of the pancreas in 8 (15.1%). Nearly, 14 (26.4%) patients had more than one pancreatic segment involved. There was no difference in the median (range) percent pain reduction from baseline-4 weeks later was 50% (0-100) vs 60% (0-100), for central and bilateral techniques, respectively; P = 0.18. In total, 60.4% of patients had a reduction of 50% punctuation in the VAPS. No major complications were detected.
Conclusions:
EUS CPN is useful for the management of pain in patients with unresectable pancreatic cancer, but there is no significant difference between central vs bilateral techniques.
Keywords: Celiac plexus, neurolysis, endoscopic ultrasound
INTRODUCTION
The management of abdominal pain in patients with inoperable pancreatic is significantly associated with an impaired quality-of-life.1 There are different modalities of treatment for this problem: Non-steroidal anti-inflammatory drugs (NSAIDs), narcotics, fluoroscopy and computed tomography (CT)-guided celiac plexus neurolysis (CPN) and surgery.2 Narcotics are the most commonly used treatment; however, some problems related to their chronic use are difficult to tolerate, e.g., dependency, constipation, delirium, nausea and vomiting.2
The percutaneous injection of absolute alcohol into the celiac plexus under either fluoroscopic/CT guidance are both effective techniques, but previous reports show that endoscopic ultrasound-guided CPN (EUS CPN) is safer, effective and less expensive than the percutaneous route.3,4,5 Different studies with modifications to the traditional technique have reported good results, but scarce information is available at this moment.6,7,8 The aim of the present study was to compare the efficacy of central vs bilateral CPN for the palliation of patients with pain related to inoperable pancreatic cancer.
MATERIALS AND METHODS
Data obtained prospectively were analyzed in a retrospective manner. Electronic and paper records of consecutive patients with pain caused by pancreatic cancer who were referred to a tertiary center and underwent EUS CPN from May 2007 to July 2011 were evaluated. Central EUS CPN was used in the first group of the study and bilateral EUS CPN in the second group. All patients had unresectable pancreatic cancer confirmed by CT, magnetic resonance imaging and/or EUS; EUS criteria and fine-needle aspiration were used when a tissue diagnosis was not available before EUS. They underwent EUS CPN during the same EUS procedure for diagnosis.
Before the procedure, all patients had laboratory tests including prothrombin time and a full blood count. Patients were placed in left decubitus position and sedated using a combination of midazolam, propofol and fentanyl by the anesthetist. Patients were continually monitored with an automated non-invasive blood pressure device, electrocardiogram and pulse oximetry throughout the procedure. EUS CPN was performed with a linear array echoendoscope GFUCT-140 (Olympus America Inc.; Center Valley, PA) by an experienced Echoendoscopist. The angle formed by the aorta and celiac trunk was identified through the posterior gastric wall. For central injection, under direct EUS visualization, a standard EchoTip 22 gauge (Cook Medical, Inc. Winston-Salem, NC) primed with normal saline solution was placed immediately adjacent and anterior to the aorta at the level of the celiac trunk. After injecting 2 mL of saline solution to clear the needle, an aspiration test was performed; if no blood was obtained, 10 mL of 1% xylocaine was injected. The aspiration test was repeated and if no blood was seen, 10 mL of dehydrated 98% absolute alcohol was injected. The needle was then flushed with 3 mL of saline solution and withdrawn from the patient. In cases with bilateral injection, the same procedure was done, but injections were done at both sides of the celiac trunk with clockwise and counter-clockwise rotation (10 mL of dehydrated 98% absolute alcohol was injected each side and 5 mL of 1% xylocaine each side). In both cases, after the procedure, a Doppler ultrasound of the celiac trunk and aorta was made to evaluate permeability. The average estimated time for the EUS CPN portion of the procedure was 10 min. After the procedure, all patients remained under observation for at least 2 h to rule out any complications. All patients were reevaluated for complications 7 days after the procedure.
The measurement of pain intensity was made with a validated9 visual analog pain scale (VAPS) (0-10) in all patients by a different physician (pain specialist). Measurements 2 and 4 weeks after the procedure were made. The complications related to the EUS CPN were determined to be in agreement with the medical records.
Statistical analysis
Medians, ranges and proportions were used to summarize the demographics and clinical variables because of non-normal distribution. To assess differences between groups at basal time, data were tested with Mann-Whitney U test and χ2 test according to the variable evaluated. EUS CPN pain scores paired before and after (15 days and 30 days) were compared with the Friedman test. The difference in percent pain reduction from baseline-2 weeks and baseline-4 weeks between groups was tested by using a Chi-squared test. A two-tailed P < 0.05 was considered to be significant. All analyses were performed by using SPSS version 20.
RESULTS
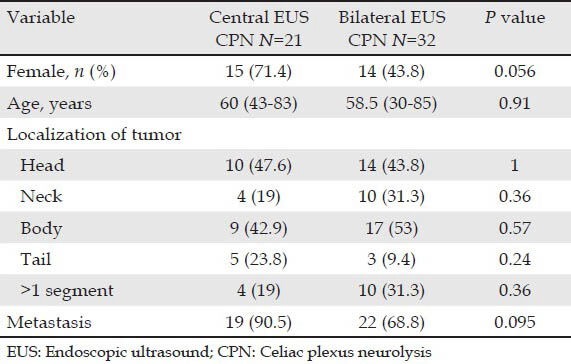
The 53 patients underwent EUS CPN, 21 (39.6%) with central technique and 32 (60.4%) with bilateral injection. Twenty-four men (45.3%) and twenty-nine women (54.7%) were included (median age was 59 years; range 30-85 years). The tumor was located in the head of the pancreas in 24 (45.3%) patients, the neck in 14 (26.4%), the body in 26 (49.1%) and in the tail of the pancreas in 8 (15.1%). Fourteen (26.4%) patients had more than one pancreatic segment involved. The characteristics of the patients classified by EUS CPN technique (central vs. bilateral) are shown in (Tab. 1). No differences between groups were detected. All, but three patients (neuroendocrine tumors) had adenocarcinoma. All patients had major arterial (mesenteric, celiac trunk) or venous invasion (portal vein) based on EUS and/or CT findings, 41 (77.4%) patients had metastases and 12 (22.6%) had duodenum infiltration by the tumor.
Table 1.
Baseline characteristics with patients classified by groups
Overall, pain scores decreased significantly from a median of 9 (range 5-10) before EUS CPN to a median of 3 (range 0-10) at 2 weeks after EUS CPN. The differences persisted at 4 weeks after EUS CPN (median 3; range 0-10) (P < 0.001). 2 weeks after the procedure, compared with baseline, 32 (60.4%) patients had a reduction of 50% punctuation in the VAPS. At 4 weeks, 28 (52.8%) patients continued with this improvement. In (Tab. 2), data with patients classified by technique (central vs. bilateral) are shown. There was no difference in the median (range) percent pain reduction from baseline-2 weeks later with central vs bilateral technique (60% [30-100] vs. 60% [0-80]; P = 0.76 (Fig. 1A)). Median (range) percent pain reduction from baseline-4 weeks later was 50% (0-100) vs 60% (0-100) for central and bilateral techniques, respectively (P = 0.18) (Fig. 1B).
Table 2.
VAPS basal and at follow-up and complications in included patients classified by technique (central vs bilateral)
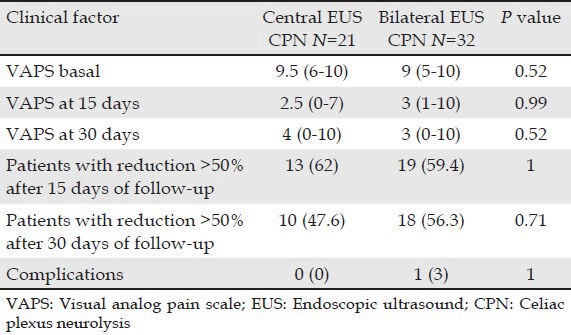
Figure 1.
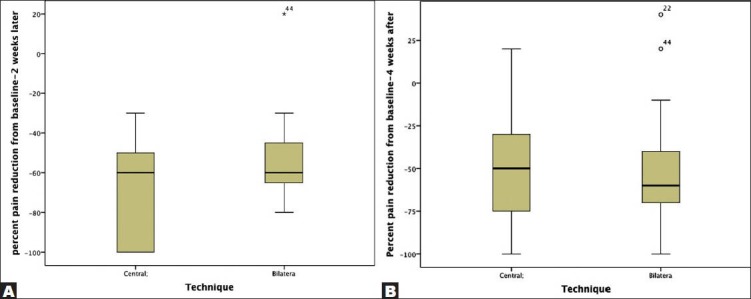
Percent pain reduction from baseline-2 weeks later (A) and baseline-4 weeks (B) after classification by central vs bilateral technique
All patients were treated with narcotics before EUS CPN. After the procedure, 10 patients went without any medical treatment and another 14 patients only received NSAID. No differences between groups were seen. Morphine usage was not significantly different over time in 24 (45.2%) patients; however their score on the VAPS had reduced.
Complications
There were no major complications. No patient was hospitalized after an out-patient procedure. Among the patients that were already hospitalized, none had prolongation of their hospital stay because of the procedure. One (3%) patient undergoing the bilateral technique of EUS CPN had transient abdominal pain after the procedure.
DISCUSSION
In this study, results are consistent with previous reports about the usefulness of EUS CPN in the management of abdominal pain in patients with unresectable pancreatic cancer. There was no significant difference for abdominal pain management between central vs bilateral techniques.
In a previous report from our group, EUS CPN reduced pain scores in 81% of the patients and scores in the VAPS decreased by at least 50% from baseline within 2 and 4 weeks after the procedure.10 Current data are similar, but some of the included patients had a different technique.
Different modalities for EUS CPN have been used with good results.6,7,8,11 In 2008, Levy et al. showed their results regarding EUS-guided direct ganglia neurolysis and block.7 These data were very promising, however to date there are no new consistent reports regarding the efficacy of direct ganglia neurolysis. In a very interesting study by Sakamoto et al.,8 data about broad plexus neurolysis were shown with good results. Regarding the question of which technique is better, one (central) injection at the celiac trunk vs two (bilateral) injections on either side of the celiac trunk, there is still controversial data. To the best of our knowledge, only two prospective studies6,11 designed to evaluate this question have been reported. In the study by Sahai et al., the aim was to compare the short-term safety and efficacy of central and bilateral techniques. The bilateral technique was more effective than central injection (mean percent pain reduction 70.4% [61-80] vs. 45.9% [32.7-57.4]; P = 0.0016).6 In the study of LeBlanc et al., the time until the onset of pain relief, duration of pain relief and complications were evaluated. In this study, 69% of the patients had an improvement in the 1-injection group and 81% in the 2-injection group (P = 0.340).11 Although both of the formerly mentioned studies were prospective, only the study of LeBlanc et al. was randomized; other differences to consider between these studies were: The main outcome was not exactly the same, scales for measuring pain, definitions for “pain relief,” “positive response” and “complete response,” time of follow-up and the amount and concentration of the substances used for EUS CPN (bupivacaine and 98% alcohol). Regardless of these differences, the conclusion seems to be in contrast with the main question of whether there is any difference in efficacy between central single injection vs bilateral injections. Results in this study are consistent with the conclusions of LeBlanc et al.11 When data from this study, regarding the main outcome, were analyzed in VAPS score or in percent pain reduction, the results are consistent: There was no difference between central (single) injection vs bilateral injections. The 2-injection approach has been associated with severe injury to the left adrenal artery;6 because there is a lack of clear advantages with this technique, we believe that is better to avoid it. However, in this study, the complication rate was not different between groups so further randomized studies are necessary.
The limitations of this study include the design, the sample-size and difficulties in measuring pain, which is a variable and subjective experience. In a recent meta-analysis from 2010 published by Kaufman et al.12 only 3 studies (gathering 119 patients in total) of EUS CPN for pancreatic cancer were included and only two of these were prospective. If we add patients with pancreatic cancer included by Sahai et al.6 (n = 72) and LeBlanc et al.11 (n = 50), a total of 241 patients have been published. The present study included 53 patients; only two previous studies6,11 have a bigger sample-size. In this study, pain specialists obtained pain scores rather than endoscopic team members, which eliminate a potential source of bias.
CONCLUSION
EUS CPN is useful for the management of abdominal pain in patients with unresectable pancreatic cancer, but there is no significant difference between central vs bilateral injections. Because of the possibility of severe injury to the vasculature and left adrenal gland, a single injection (central) may be more advisable.
REFERENCES
- 1.Müller-Nordhorn J, Roll S, Böhmig M, et al. Health-related quality of life in patients with pancreatic cancer. Digestion. 2006;74:118–25. doi: 10.1159/000098177. [DOI] [PubMed] [Google Scholar]
- 2.Caraceni A, Portenoy RK. Pain management in patients with pancreatic carcinoma. Cancer. 1996;78:639–53. doi: 10.1002/(SICI)1097-0142(19960801)78:3<639::AID-CNCR45>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 3.Gress F, Schmitt C, Sherman S, et al. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900–5. doi: 10.1111/j.1572-0241.1999.01042.x. [DOI] [PubMed] [Google Scholar]
- 4.Gunaratnam NT, Sarma AV, Norton ID, et al. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–24. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 5.Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656–62. doi: 10.1016/s0016-5107(96)70047-0. [DOI] [PubMed] [Google Scholar]
- 6.Sahai AV, Lemelin V, Lam E, et al. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: A comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326–9. doi: 10.1038/ajg.2008.64. [DOI] [PubMed] [Google Scholar]
- 7.Levy MJ, Topazian MD, Wiersema MJ, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98–103. doi: 10.1111/j.1572-0241.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto H, Kitano M, Kamata K, et al. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle. Am J Gastroenterol. 2010;105:2599–606. doi: 10.1038/ajg.2010.339. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: A comparison of six methods. Pain. 1986;27:117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez-Luna MA, Chavez-Tapia NC, Franco-Guzman AM, et al. Endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. Rev Gastroenterol Mex. 2008;73:63–7. [PubMed] [Google Scholar]
- 11.LeBlanc JK, Al-Haddad M, McHenry L, et al. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: One injection or two? Gastrointest Endosc. 2011;74:1300–7. doi: 10.1016/j.gie.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman M, Singh G, Das S, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–34. doi: 10.1097/MCG.0b013e3181bb854d. [DOI] [PubMed] [Google Scholar]


