Abstract
The influence of intensified and reduced training on nocturnal growth hormone (GH) secretion and elimination dynamics was studied in young (1.5 yr) Standardbred geldings to detect potential markers indicative for early overtraining. Ten horses trained on a treadmill for 32 wk in age-, breed-, and gender-matched fixed pairs. Training was divided into four phases (4, 18, 6, and 4 wk, respectively): 1) habituation to high-speed treadmill trotting, 2) normal training, in which speed and duration of training sessions were gradually increased, 3) in this phase, the horses were divided into 2 groups: control (C) and intensified trained (IT) group. In IT, training intensity, duration, and frequency were further increased, whereas in control these remained unaltered, and 4) reduced training (RT). At the end of phases 2, 3, and 4, blood was sampled overnight every 5 min for 8 h for assessment of GH secretory dynamics using pulse detection, deconvolution analysis, and approximate entropy (ApEn). Intensified training induced overtraining (performance decreased by 19% compared with C), which was associated with an increase in concentration peaks number (3.6 vs. 2.0, respectively), a smaller peak secretion pattern with a prolonged half-life (15.2 vs. 7.3 min, respectively), and an increased ApEn (0.89 vs. 0.49, respectively). RT did not lead to full recovery for the overtrained horses. The increased irregularity of nocturnal GH pulsatility pattern is indicative of a loss of coordinated control of GH regulation. Longer phases of somatostatin withdrawal are hypothesized to be the underlying mechanism for the observed changes in GH pulsatility pattern.
Keywords: endocrinology, sports physiology, growth hormone-insulin-like growth factor I axis, hormone pulsatility analysis
overtraining is often suggested to be the result of an accumulation of stressors that exceed an athlete's finite resistance capacity (16, 30). A neuroendocrine disorder at the hypothalamic-pituitary level has been suggested as the underlying cause for overtraining. Considering the overtraining syndrome, it is important to distinguish overtraining from overreaching. Overreaching occurs as a result of intensified training and is often considered normal outcome for elite athletes because of the relatively short time needed for recovery (2–3 wk). Overtraining is defined as an accumulation of training and/or nontraining stress resulting in long-term decrement in performance capacity with or without related physiological and psychological signs and symptoms of overtraining in which restoration of performance capacity may take several weeks or months (16). So far, no reliable marker for the early detection of overtraining has been described. Studies are needed to determine what the best indicator of this state is and the underlying mechanisms that cause fatigue and performance decrements.
Some fatigue states like in obsessive compulsive disorder and major depression as well as the state of overreaching/overtraining are associated with changes in growth hormone (GH) pulsatility (2, 7, 27, 28). GH is thought to play an important role in the anabolic phase after a stressful event and is important for recovery and adaptation to restore homeostatic balance. It is widely known that acute stress like exercise stimulates GH secretion, but the response of the GH axis to chronic stress is still largely unknown.
To study influences of chronic stress on the regulation of the GH axis, measurements should be done during the recovery phase favorably at night. Because, in humans, GH is secreted in a pulsatile fashion with the majority of pulses secreted at night during slow wave sleep; during the day, GH levels are, in general, very low and sometimes undetectable (18, 49). The physiological significance of pulsatile delivery of GH lies in the fact that the episodic pattern of release is thought to be integral in the elicitation of optimal agonist function at the cellular level (36). Because of the pulsatile release and the importance of this pulsatility for the target cells, frequent sampling is necessary for characterizing the effects of changes in GH pulsatility.
One of the reasons for the lack of information on GH pulsatility might be the difficulty to study episodic hormonal secretion, because changes in GH secretory events cannot be directly inferred from GH time series. Plasma GH concentration reflects the result of three simultaneous processes: basal secretion, pulsatile secretion, and clearance. Older pulse detection programs are not able to distinguish between these three processes. However, pulse detection algorithms based on deconvolution analysis are able to provide information about the secretion as well as the elimination kinetics of each pulse at the same moment and can reveal underlying mechanisms. Pulsatility is modulated by feedforward and feedback signals with the neuroendocrine axis, thereby conferring physiological regulation that sometimes results in rather irregular hormone concentrations over time (55). Clear pulse identification and interpretation of the consequences of altered pulsatile hormone secretion can be very difficult in such irregular GH time series. Approximate entropy (ApEn) quantifies the regularity or orderliness of time series without the need to identify discrete pulses (39, 55). Thus, although the pulsatility algorithms analyze individual pulses, ApEn analyzes patterns in the complete data series representing more complex and integrated feedforward and feedback signals. Therefore, ApEn will detect changes in underlying episodic behavior not visible and or reflected in the mean variance of hormone concentrations or in peak occurrences or amplitudes.
The pituitary gland synthesizes different GH isoforms (9, 12). GH at 22 kDa is considered the classic GH and is the most abundant isoform in the circulation of humans. GH at 20 kDa represents ∼10% of the total circulating GH. Fragmentation of intact GH forms (22 and 20 kDa) has been demonstrated (9). However, both the presence and significance of circulating GH fragments require further research and experimental confirmation. Polyclonal or monoclonal antibodies are routinely employed in GH measurement. Considering the molecular heterogeneity of GH and the difference in bioactivity between the GH variants, it is important to take into account the method of measurement and the used antibodies when evaluating studies (9). The available assays for detection of GH concentrations use different monoclonal and polyclonal antibodies directed at specific epitopes on the GH molecule. Because the variant molecular forms of GH may or may not have these intact epitopes spatially accessible to these antibodies, the results obtained for GH concentrations with different assays from the same sample can vary (36). Therefore, the outcome of a study should be related to the possibilities of the selected assay.
It has been shown that overtraining can be experimentally induced in Standardbreds (5, 14, 17, 45, 46, 47), which gives the opportunity to study the pathophysiology of early overtraining and search for potential diagnostic markers. The earliest sign of overtraining in these horses was the inability to complete the intensive training associated with increased irritability and reluctance to exercise (2, 3). The symptoms of overtraining in horses resembles the symptoms in overtrained humans, which might indicate that the horse is a good model to study overtraining syndrome.
In the current study, a protocol for inducing early overtraining was used in horses to study the influence of chronic stress on the GH-IGF-I axis to find a possible marker for early overtraining. It was hypothesized that nocturnal GH secretion would alter in the lightly overtrained horses. More in particular, we hypothesized that, in the overtrained/overreached situation, decrements in mean GH peak amplitude and peak area under the curve (AUC) will occur, whereas the secretion pattern of GH becomes more irregular. In contrast to earlier studies (2, 7, 27, 28), the current study separates the different processes determining plasma GH concentration using deconvolution analysis and ApEn. The deconvolution analysis was performed with data obtained by a polyclonal enzyme-linked immunosorbent assay (ELISA) horse GH assay. Although it is well recognized that this type of assay cannot differentiate between the biological active and inactive epitopes of GH, we hope that this study, which is the first in its kind, instigates future studies with more specific GH assays.
MATERIALS AND METHODS
Animals.
Twelve Standardbred geldings were used in this study. They were trained in two groups of six for logistical reasons. Per year, six horses were trained in three couples of two horses. Each test horse had its own age-matched control, and these formed a couple (pair) during the whole session. These individual couples underwent identical daily routines. Horses were aged 20 ± (SD) 2 mo and had a body weight of 368 ± 45 (SD) kg at the beginning of the experiment. The horses had no known history of health and exercise problems and had not previously been involved in any kind of organized exercise or training regimen. The horses were owned by the Faculty of Veterinary Medicine of the University of Utrecht. The horses were individually housed in box stalls, and their diet consisted of grass silage supplemented with concentrated feed and vitamin supplements and met nutrient requirements for maintenance and performance [58 MJ net energy (range 54–66)]. Salt blocks and water were available ad libitum. The experiments were approved by the Committee for Animal Welfare at the Faculty of Veterinary Medicine, Utrecht University.
Experimental setup.
Before the start of the experiment, the horses were acquainted with the Equine Exercise Laboratory and were acclimatized to running on the high-speed treadmill (Mustang 2000; Kagra, Graber, Fahrwangen, Switserland). The training period consisted of a total of 32 wk divided into four phases. A schematic representation of these phases is provided in Fig. 1. At the end of phases 1, 2, 3, and 4, the horses performed a standardized exercise test in the morning between 0730 and 1230 to monitor performance improvement. GH profiles were collected on the same day from 2200–0600 at the end of phases 2, 3, and 4.
Fig. 1.
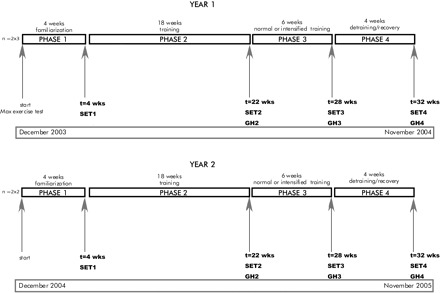
Schematic view of the experimental set up. GH, growth hormone; SET, standardized exercise test.
Training.
All training sessions and exercise tests were performed on a high-speed treadmill.
Each training session was preceded by a 30-min warm-up walk in a horse walker followed by an 8-min warm-up at the treadmill, which consisted of 4 min of walking at a speed of 1.6 m/s and 4 min of slow trotting at a speed of 3.0–4.0 m/s (no incline). Each training session ended with a cooldown that consisted of a 5-min walk at the treadmill followed by a 30-min walk in the horse walker. On the resting days, the horses walked for 60 min in a horse walker.
To standardize training to the individual exercise capacity of the horses, training and exercise intensity was adapted to the maximal individual heart frequency. However, an incremental exercise test was difficult to perform for the young, relatively untrained horses. In a previous study with 2- to 3-yr-old Standardbred stallions, an average maximal heart frequency (HFmax) of 240 beats/min was obtained (5). This HFmax was used in this study as an estimated maximum (HFest-max) to guide training intensity (speed and inclination) on the treadmill. Training intensity was adjusted on a weekly basis to the measured peak HFs (Polar S610; Polar Electro, Kempele, Finland) during training sessions. The study was divided into four phases after accommodation to exercise. The training program was constructed as follows.
Phase 1 (habituation phase, 4 wk).
Week 1 of phase 1 consisted of 3 times/wk exercise at 30% HFest-max for 20–30 min/session, week 2 consisted of 4 times/wk exercise at 30% HFest-max for 25–45 min/session, week 3 consisted of 4 times/wk exercise at 40% HFest-max for 30–45 min/session, and week 4 consisted of 4 times/wk exercise at 50% HFest-max for 35–45 min/session.
Phase 2 (normal training phase, 18 wk).
Training in phase 2 consisted of mixed endurance training (ET) and high-intensity interval training (HIT). Days of ET were alternated with HIT. An ET session consisted of continuous trotting for 20–24 min at 60% HFest-max or trotting for 16–18 min at 75% HFest-max. Each HIT session contained either three 3-min bouts or four 2-min bouts of exercise at 80–85% HFest-max interspersed by 3- or 2-min recovery bouts at 60% HFest-max. The horses exercised 4 days/wk throughout this training period. We considered that, in this phase, training could be performed without excessive effort by the horses; therefore, we called this the normal training phase.
Phase 3 (intensified training phase, 6 wk).
In phase 3, one horse of each couple was randomly selected and subjected to an intensified training program, whereas the other horse continued training at the volume, intensity, and frequency it received in phase 2. The intensified training regimen consisted of alternating days of HIT and ET as described in phase 2 for 6 days/wk during the first 3 wk. For the last 3 wk, horses were trained 7 days a week, HIT four times and ET three times. Exercise intensity during ET was gradually increased to 24–35 min at 60–75% HFest-max. High-intensity exercise gradually increased to five 3-min bouts at 80–85% HFest-max interspersed with 2-min periods at 60% HFest-max or six 2-min bouts at 80–85% HFest-max interspersed with 1-min periods at 60% HFest-max. We anticipated that this training schedule was heavy for the animals.
Phase 4 (reduced training phase, 4 wk).
In phase 4, all horses performed ET for 20 min at 60% HFest-max for 3 days and 70% HFest-max for 1 day a week.
Standard exercise test.
Four standardized exercise tests (SETs 1–4) were performed on the final day of each phase to monitor performance. Before the SET started, horses walked for 30 min in the horse walker followed by a warm-up on the treadmill that consisted of 4 min walk (1.6 m/s) and 4 min of trotting at 4.5 m/s. Next, after 1 min of additional walking at 1.5 m/s, horses trotted for 20 min at ∼80% HFest-max. Finally, horses were allowed to cool down for 5 min at 1.5 m/s. Heart frequency was monitored constantly with a Polar S610 heart rate meter and continuous electrocardiographic monitoring (Cardio Perfect Stress 4.0; Cardio Perfect, Atlanta, GA). In phases 3 and 4, the speed and inclination were not increased further. This made a comparison between the tests possible. Venous blood was drawn from the jugular vein before the test [time (t) = 0 min], after the warming up (t = 9 min), every 5 min during the SET (t = 14, 19, 24, and 29 min), and after the cooling down (t = 34 min) for measuring whole blood lactate concentrations. Samples were kept on ice until analysis (ABL-605 Radiometer; Copenhagen, Westlake, OH).
GH profiles.
To study pulsatile GH secretion, blood was drawn via an indwelling catheter in the jugular vein at 5-min intervals for 8 h from 2200 until 0600 at the end of phases 2, 3, and 4. During the experiment, the horses were kept in their own stables, allowing them to follow their normal nightly activities such as sleeping. The last feeding of concentrates was at 1800 and of grass silage at 1930. Blood samples for GH were drawn into lithium heparin tubes on ice and centrifuged (Rotina 48R; Hettich Zentrifugen, Tuttlingen, Germany) at 4°C for 10 min at 2,778 g. The resulting plasma was stored at −20°C until assayed for hormone concentrations.
In addition, serum was sampled for the determination of basal IGF-I concentrations at the end of each phase on the same day as the GH profiles were collected. Serum was stored at −20°C until assayed for hormone concentration.
Hormonal assay.
GH concentrations were quantitated by an automated polyclonal ELISA designed for measurement of equine GH (DSL-10–75100 Equine GH Coated Well ELISA kit; Diagnostic Systems Laboratories, Webster, TX). The assay protocol described by the manufacturer was followed. The assay was validated with respect to specificity, parallelism, and repeatibility. Aliquots of equine heparinized plasma with GH concentrations of 50 ng/ml were diluted 1:2, 1:4, 1:8, 1:16, 1:32, and 1:64 with zero assay standard. In plasma diluted with zero standard, parallelism was observed with 97.9% of expected GH concentrations measured on the average. Sensitivity of the assay, defined as 10% suppression of the calibration curve (n = 8 zero-concentration standards), was 0.16 ng/ml for GH in plasma. When equine GH standards were used with concentrations of 0, 1.5, and 10 ng/ml, 101, 106, and 98.4% of exogenous hormone, respectively, was measured in the assay. Assay repeatability was assessed by calculation of intra- and interassay coefficients of variation (CV) for two pools of equine plasma with mean GH concentrations of 1.5 and 10 ng/ml. Respective intra-assay CV were 9.59 and 9.91% (n = 10 and 15 determinations, respectively). Respective interassay CV were 9.48 and 8.71% (n = determinations in 12 assays).
In addition, uncertainties (SD) of each GH concentration were estimated empirically considering that variances were associated with assay response and standard curve evaluations. Subsequently, confidence limits for the standard curve parameters were calculated. Briefly, 10 standard solutions (range 1,5–50 μg/l) were obtained from the manufacturer. These control solutions were assayed 10 times. From the resultant absorbance values, a precision profile (CV against concentration) over the dynamic range of the assay was calculated according to Ekins (11) and Hunter (21). With the resulting mathematical function, CV values for all measured GH data points were calculated.
Total serum IGF-I concentrations were measured in a heterologous radioimmunoassay as described previously by Nap et al. (35), after extraction with acid ethanol. The efficiency of extraction was 85–90%. Curves obtained with serial dilutions of equine plasma spiked with IGF-I were parallel to the standard curve, and the intra-assay and interassay CV were 3.2 and 15.6%, respectively (8, 31).
Pulse analysis algorithms.
GH concentration data series were analyzed with a pulse detection program not based on deconvolution analysis called Cluster 8 (UVA Pulse Analysis Software, Charlottesville, VA; see Ref. 52). This program provides parameters like the overall mean GH concentration (μg/l), 8-h integrated AUC, and characteristics of GH concentration peaks, such as the number of concentration peaks in 12 h, the mean interval between peaks, the mean peak width (i.e., duration), the mean peak height (i.e., amplitude), and the mean area under the peaks. For the 5-min interval GH curves, the Cluster program was configured in a 3 × 3 fashion, defining significant peaks with three samples and significant nadirs with three samples and a t-statistic of 3.0 for the upstroke and downstroke as advised by Johnson (25).
A pulse analysis algorithm based on deconvolution analysis called Autodecon (UVA Pulse Analysis Software) was used to separate the three continuous processes determining the GH concentration at one time. It calculates from the GH concentration data series a secretion curve (Fig. 3) and provides parameters on basal secretion (basal secretion velocity), pulsatile secretion (the number of secretory bursts per 8 h), half-duration of burst (duration at half-maximal amplitude), mean interval between secretory bursts, mean secretory burst mass, mean secretory burst amplitude (i.e., maximal secretory rate), and elimination (GH half-life) (53).
Additional calculations included the 8-h basal GH secretion (basal secretion rate × 480 min), 8-h pulsatile GH secretion (mean burst mass × number of secretory bursts), 8-h total GH secretion (i.e., the sum of basal and pulsatile secretion), and the ratio of pulsatile to total GH secretion.
Initially the PULSE2 program (UVA Pulse Analysis Software) was used to estimate the number and position of secretory bursts that might comprise the final GH concentration profile. The file generated by PULSE2 was then used in the deconvolution analysis program, where iterative nonlinear least squares parameter estimations at 95% statistical confidence intervals were used to quantify all aforementioned parameters of secretion and elimination. Values below the minimal detectable concentration of the assay were reported by the laboratory and used for the analysis with an uncertainty based on the precision profile as advised by Johnson (24).
Peaks are defined as the GH peaks in the GH concentration data series. Bursts are defined as the GH peaks in the calculated secretion curve.
ApEn (UVA Pulse Analysis Software, Charlottesville, Virginia) statistic was used as an estimate of the regularity of the GH release process in each condition. ApEn is a single value calculated for a hormone time series, where a higher ApEn denotes greater process irregularity or greater disorderliness of hormone release (39, 40). The ApEn analysis was applied with parameters of m = 1 (i.e., run length), r = 0.20 (i.e., tolerance window), and 1,000 Monte Carlo simulations/series.
Statistics.
All data are expressed as means ± SD. Statistical analysis was performed using a linear mixed-effects model with a one-step autoregressive process (SPSS version 14.0 for Windows; SPSS, Chicago, IL). Random factor used was horse; fixed factors used were couple, group, phase, and group × phase. There were no significant differences found between couples for the calculated parameters; therefore, a significant year effect could be excluded. A P value <0.05 was considered significant.
RESULTS
Because of injury, two horses [1 control horse and 1 horse from the intensified training group (IT) from the same pair] were not able to complete the training. Analysis is therefore based on the remaining 10 horses.
The SETs were performed throughout the experiment to monitor performance. During SET 1, horses trotted on a speed of 6.5–7.0 m/s with no incline, during SET 2, 3, and 4, the horses trotted on a speed of 7.5–8.5 m/s with a treadmill inclination of 1–4%. SET 2, 3, and 4 were performed on the same incline and speed to make valid comparisons possible.
During the intensified training phase, the IT horses changed their gait on the treadmill to canter instead of trotting. Regarding the IT group, horses maintained trotting at high speeds during SET 3 for 15.8 ± 2.1 min only compared with 19.2 ± 1.6 min (P = 0.036) in control horses. IT horses started cantering frequently at the beginning of SET 3 despite humane encouragement to trot or even stopped. As a consequence, the mean duration of trotting during SET 3 was decreased significantly by 19% in the IT group compared with control horses.
Complete lactate datasets were only present for the first 15 min of the SET because of the decrease in SET duration for the IT group after the intensified training phase (Table 1). A right shift of the lactate curve after the intensified training phase compared with the training phase was observed for the IT group with a significant difference in mean blood lactate concentration at t = 14 min (P = 0.034), t = 19 min (P = 0.043), and t = 24 min (P = 0.011) (see Table 1). Also, a significantly decreased mean maximum blood lactate concentration was measured at the end of the SET for the IT horses (4.3 ± 2.2 vs. 8.3 ± 1.5 mmol/l, respectively). At the end of reduced training, the maximum lactate levels were significantly lower for the IT group (5.1 ± 2.1 mmol/l) compared with the control group (9.1 ± 4.8 mmol/l) (data not shown). Maximum lactate levels were obtained at the end of the SETs for each individual horse.
Table 1.
Whole blood lactate concentrations before (t = 0), after warming up (t = 9), and during the first 10 min (t = 14, 19, and 24) of the SET for the C and IT groups
| Time, min | SET 2 |
SET 3 |
SET 4 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| C | IT | C | IT | C | IT | ||||
| 0 | 0.6±0.1 | 0.6±0.1 | 0.6±0.3 | 0.8±0.3 | 0.8±0.3 | 0.7±0.2 | |||
| 9 | 0.5±0.1 | 0.4±0.1 | 0.4±0.1 | 0.4±0.1 | 0.5±0.2 | 0.4±0.1 | |||
| 14 | 3.1±1.0 | 3.5±1.1* | 2.4±1.0 | 1.9±1.3† | 3.6±1.3 | 2.4±1.1 | |||
| 19 | 4.6±3.2 | 4.8±1.5* | 3.1±1.3 | 3.0±1.4† | 4.4±1.9 | 3.5±1.3 | |||
| 24 | 7.0±4.1 | 6.2±1.1* | 4.9±2.2 | 3.2±1.6† | 5.9±2.5 | 4.2±2.0 | |||
Values are means ± SE. Units are mmol/l. SET, standardized exercise test; C, control; IT, intensified trained; t, time. Within a row, values with different superscripts differ significantly (P < 0.05).
In Fig. 2, mean GH data series are shown for the control and the IT group after the training and the overtraining phase, showing an obvious increase in the baseline for the IT group compared with the control group after a period of intensified training (Fig. 2B). Figure 3 shows the GH concentration curve and the calculated GH secretion curve for an intensively trained and a control horse after the intensified training phase. Figure 4 shows the individual GH data series for the IT group and the control group after the intensified training phase. The mean results of the pulse detection and the deconvolution analysis/ApEn are shown in Tables 2 and 3, respectively.
Fig. 2.
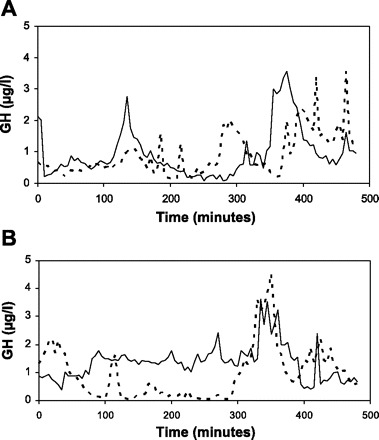
Mean nocturnal GH profiles for intensified trained (solid line) horses and control horses (broken line) after the training period (A) and intensified training period (continued normal training for the control horses) (B).
Fig. 3.
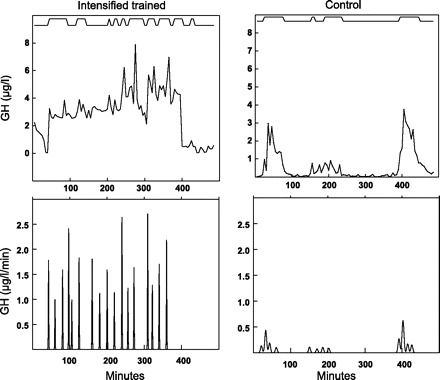
Cluster and deconvolution analyses of GH for the 8-h overnight sampling period. Top: hormone concentrations from cluster analysis for both control and intensified trained horse after intensified training phase. Bottom: corresponding secretory bursts from deconvolution analysis.
Fig. 4.
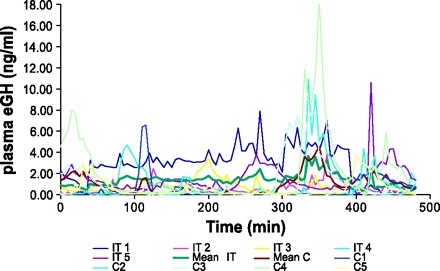
Individual nocturnal GH profiles for the intensified trained (IT) and the control (C) group as well as the mean nocturnal GH profile for the IT and C group after the intensified training phase.
Table 2.
Results of peak detection analysis of nocturnal GH time series for C and IT Standardbreds after a period of training, intensified training, and reduced training
| Parameter | Training |
Intensified Training |
Reduced Training |
||||||
|---|---|---|---|---|---|---|---|---|---|
| C | IT | C | IT | C | IT | ||||
| 8-h Mean GH, μg/l | 0.91±0.47 | 0.96±0.34 | 0.95±0.71 | 1.36±0.91 | 0.97±0.67 | 1.16±1.05 | |||
| Total GH AUC, μg/l | 441±229 | 464±165 | 463±346 | 659±439 | 472±325 | 561±510 | |||
| No. of GH concentration peaks | 2.6±0.6 | 2.4±1.1* | 2.0±0.0* | 3.6±1.1† | 2.0±0.7 | 1.8±0.4* | |||
| Mean interval between peaks, min | 126±83 | 186±116 | 206±152 | 124±73* | 189±78 | 236±73† | |||
| Mean duration of peaks, min | 66±29 | 85±26 | 58±23 | 72±20* | 80±38 | 109±35† | |||
| Mean amplitude of peaks, μg/l | 4.3±4.3 | 5.2±3.6 | 5.2±4.7 | 4.6±2.4 | 5.2±2.3 | 8.2±7.1 | |||
| Mean AUC of peaks, μg/l | 81.9±92.8 | 168.8±119.2 | 137.0±125.3 | 100.1±57.0 | 147.1±119.5 | 236.6±232.5 | |||
| Mean nadir GH concentration, μg/l | 0.26±0.35 | 0.25±0.31 | 0.09±0.11 | 0.74±0.92 | 0.36±0.34 | 0.04±0.00 | |||
Values are means ± SE. GH, growth hormone; AUC, area under the curve. Within a row, values with different superscripts differ significantly (P < 0.05).
Table 3.
Results of nocturnal GH secretory parameters analyzed by deconvolution analysis for C and IT Standardbreds after a period of training, intensified training, and reduced training
| Parameter | Training |
Intensified Training |
Reduced Training |
||||||
|---|---|---|---|---|---|---|---|---|---|
| C | IT | C | IT | C | IT | ||||
| Basal secretion, μg/l | 0.014±0.017 | 0.014±0.025 | 0.008±0.005 | 0.010±0.008 | 0.008±0.006 | 0.009±0.009 | |||
| Half-life, min | 9.6±4.2 | 14.3±2.3‡ | 7.3±4.4* | 15.2±1.2†‡ | 10.8±6.2 | 9.1±3.7§ | |||
| Half-duration burst, min | 3.1±0.6† | 2.8±0.4 | 5.7±3.9 | 4.6±4.9 | 7.9±4.4* | 3.3±1.6† | |||
| No. of bursts/8 h | 8.6±2.9 | 10.6±0.9 | 9.4±2.7* | 10.0±5.4 | 6.2±1.8† | 9.2±4.1 | |||
| Mean amplitude burst, μg·l−1·min−1 | 0.86±0.35 | 0.63±0.22 | 0.96±0.30 | 0.86±0.72 | 0.72±0.67 | 1.12±0.43 | |||
| Mean mass burst, μg/l | 2.8±1.1 | 1.9±0.8 | 6.3±5.8 | 2.5±1.2 | 4.5±2.8 | 4.3±3.5 | |||
| Mean interval between bursts, min | 49.4±15.1 | 39.1±7.6 | 51.6±15.1 | 49.1±25.2 | 70.4±28.9* | 38.9±19.9† | |||
| 8-h Total secretion (μg/l) = AUC | 441±229 | 464±165 | 462±346 | 659±439 | 472±325 | 561±510 | |||
| 8-h basal secretion, μg/l | 6.5±7.9 | 6.9±11.8 | 3.6±2.4 | 4.6±3.9 | 3.7±2.8 | 4.2±4.2 | |||
| 8-h pulsatile secretion, μg/l | 434.7±228.2 | 456.7±154.0 | 458.8±346.6 | 654.4±439.9 | 467.9±326.4 | 557.0±506.0 | |||
| Ratio pulsatile/total secretion, % | 98.4±1.7 | 98.9±1.6 | 98.7±1.2 | 99.1±0.8 | 98.8±0.9 | 99.3±0.2 | |||
| Approximate entropy | 0.63±0.26 | 0.56±0.24 | 0.49±0.1* | 0.82±0.29† | 0.6±0.22 | 0.54±0.42* | |||
| IGF-I, ng/ml | 164±69 | 157±42 | 189±74 | 162±17 | 145±57 | 179±33 | |||
Values are means ± SE. AUC, area unser the curve. Within a row, values with different superscripts differ significantly (P < 0.05).
GH pulsatility parameters were not significantly different between the two groups after a period of training (phase 2). Intensified training increased the number of concentration peaks, prolonged GH half-life, and increased the ApEn value for the IT group compared with the control group (P = 0.004, 0.011, and 0.039, respectively). Reduced training significantly decreased peak half-duration (P = 0.031) and the interval between secretion peaks (P = 0.020) for the IT group compared with the control group.
Intensified training significantly increased the number of concentration peaks (P = 0.042) for the IT group compared with phase 2. Reduced training significantly decreased ApEn values (P = 0.023), the number of concentration peaks (P = 0.000), and half-life (P = 0.045), whereas interval between the concentration peaks (P = 0.036) and the duration of the concentration peaks (P = 0.042) significantly increased for the IT group compared with the intensified training phase.
For the control group, reduced training significantly decreased the number of secretion bursts (P = 0.043) compared with phase 3, in which the animals continued training as in the last couple of weeks of phase 2.
Basal IGF-I concentrations did not differ significantly between phases or groups, as shown in Table 3.
DISCUSSION
In this study, we were able to quantitate the influences of prolonged exercise stress on nocturnal GH pulsatility modalities and regularity. We showed, for the first time, an increase in irregularity (ApEn) combined with an increase in GH pulse frequency and half-life in intensively trained horses, indicating that the GH-IGF-I axis shows pathophysiological adaptation to increased exercise stress.
SET.
The intensified trained group in the current study showed an 19% reduction on the average in treadmill trotting-time-to fatigue compared with the control horses. The drop in performance seen in the current study is similar with the reduction on the average in treadmill run-time-to fatigue by 14% as reported by others in horses (14). Extending the trot can have profound energetic requirements that could limit performance compared with cantering (58). Given the fact that Standardbred horses are bred for trotting and taking into account the lower energetic costs of cantering, IT group clearly showed a loss of performance, indicative for overreaching or maybe even overtraining (29, 32).
In addition, the intensified training phase induced a right shift of the lactate curve and diminished maximum lactate levels at SET 3 for the intensified trained horses. A right shift of the blood lactate curve is often associated with an improvement in performance in humans, but both optimal training and overtraining can induce a right shift of the lactate curve, (4, 23). A decrease in submaximal blood lactate can result from a decreased production, or an increased utilization by muscle and other organs (4). ET improves lactate utilization in humans (4, 10), whereas overtraining seems to decrease muscle lactate production capacity in humans (4, 23). Several mechanisms have been proposed to explain this decrease. It is suggested that, not the amount of substrate in the muscle, but the capacity to mobilize the substrate available is the critical factor. Mobilization of substrate is influenced by several hormones, among them the stress hormones epinephrine/norepinephrine, corticosteroids, and GH (1, 2, 3). The combination of a loss of performance and a right shift in the lactate curve might indicate that overreaching/overtraining has caused this right shift.
GH pulsatility.
An important finding was the increase in ApEn after the intensified training phase for the IT group. ApEn quantities the orderliness of the subordinate (= nonpulsatile) secretory GH patterns. A larger GH ApEn value denotes greater irregularity and has been reported during puberty and in patients with acromegaly and Cushing's disease (41, 48). Decreased regularity could reflect impaired coordinate control of GH secretion by GH-releasing hormone (GHRH), somatostatin, GH, and/or IGF-I.
The main difference found with the peak analysis program (Cluster8) is an increase in GH peak frequency for the IT group at the end of the intensified training phase (Table 2). This increase is accompanied by a significant increase in mean peak duration and interval after the reduced training phase, reflecting the decrease after the overtraining phase. Summarized, the GH pulsatility pattern changed to more frequent, smaller concentration peaks for the intensified trained horses. There are no reports on the influence of overtraining on nocturnal GH pulsatility, but a few studies report on the influence of training on nocturnal GH pulsatility in humans (3, 57). Weltman et al. (57) reported an amplification of the pulsatile release of GH in women undergoing 1 yr of aerobic training above the lactate threshold. Training increased GH peak amplitude, nadir GH concentration, GH peak area, and 24-h GH concentration. These data might suggest that amplitude modulation of GH pulses induced by moderate training precedes alterations in pulse number induced by intensified training/early overtraining. Studies in humans reporting on the influence of fasting on nocturnal GH pulsatility found similar results. Partial fasting resulted in increases in amplitude (37), and pure fasting in increases in GH pulse frequency (6, 19).
The increased frequency in concentration peaks after intensified training (Table 2) was not accompanied by an increase in secretion bursts (Table 3) in the current study. This is in contrast with our expectations, because large peaks of plasma GH concentrations often reflect the occurrence of a succession of secretory pulses. Hartman et al. (18) found that only 4% of GH secretion occurred as isolated single bursts in humans, and that these single secretory episodes had very low amplitudes. This is also the case in our horses, because the deconvolution analysis revealed the existence of ∼80% more pulses than detected in the plasma profiles by pulse detection with the Cluster program in both groups. An explanation for the discrepancy found during the intensified training phase for the IT group might be the fact that the deconvolution program had more difficulty in fitting the curves of the IT group because of the increase in disorderliness as shown by the increase in ApEn. This could have led to an underestimation of the GH pulse frequency. In addition, Fig. 3 shows that the interpeak (basal) serum GH concentrations rose simultaneously with increasing GH secretory pulse frequency, probably because the decay of the serum GH concentration from previous peaks was not complete before the next high-amplitude pulse occurred. This has already been described for exceptionally irregular GH patterns associated with acromegaly in humans (51). When identification of the individual secretory pulses fails, secretory pulses are combined, which leads to longer half-lives.
The underestimation of secretory pulses probably caused the prolonged GH half-life after phase 3 for the IT horses. However, other less likely explanations could be 1) a decrease in metabolic clearance rate as described in patients with chronic liver disease because of decreased liver production of IGF-I and hence an impaired negative feedback (34) and in renal disease because of impaired glomerular clearance of GH (43); and 2) an increased concentration of binding proteins, and consequently an increase in the amount of bound GH (54).
Four weeks of reduced training were integrated in the current experiment to evaluate the duration of the effects induced by intensified training. The control group did not show any differences in GH pulsatility resulting from the prolongation of the normal training (phase 2 compared with phase 3). However, reduced training significantly decreased the number of bursts (Table 3) back to training levels, indicating that the (prolongation of the) training might have had some influence on GH pulsatility. Based on these data, the above-mentioned hypothesis on adaptation of GH pulsatility to training stress could be modified into the following. Moderate training seems to influence amplitude of GH pulses, prolonged moderate training influences number of GH pulses, and prolonged heavy training or early overtraining seems to induce a loss of regularity of GH pulsatility.
The nocturnal GH profile of the IT group was changing in the same direction as the control group during reduced training; however, secretion pulses were still smaller and more frequent, indicating that complete recovery was not yet reached.
The combination of changes in GH secretion dynamics drove us to hypothesize about which pathophysiological mechanism could cause these alterations. Based on the model that GHRH predominantly increases GH pulse amplitude whereas somatostatin primarily controls GH pulse frequency (19), these results suggest that intensified training in young Standardbred horses primarily increases somatostatin withdrawal intervals so that GH burst frequency is increased, whereas less-demanding training predominantly influences GHRH by increasing GH pulse amplitude. This corresponds readily to the decrease in serial regularity of GH release implicit in the higher ApEn values in the intensified trained horses, suggesting a relative loss of coordinated somatostatin and GHRH release, which normally would give rise to organized pulses of GH release (56). It also correlates to the reported finding that stress hormones like endogenous opiates and catecholamines stimulate GH secretion by inhibiting somatostatin release in hamsters (3). It is hypothesized that the intensified training might have induced increased levels of stress hormones like catecholamines with a result being decreased levels of somatostatin allowing high-frequency GH pulsatility.
What could be the beneficial effects of this adaptation of GH pulsatility for the overtrained horses? Pulsatile delivery of intravenous GH has been shown to be more effective than continuous delivery for upregulation of GH-modulated tissue (3, 22). GH pulses tend to promote optimal linear and muscular growth, whereas continuous GH stimulation modulates carbohydrate and lipid metabolism via induction of hepatic IGF-I synthesis and expression of GH receptor as well as low-density lipoprotein receptor (50). This might explain the adaptations seen after training in which increases in pulse amplitude mimic a more continuous GH stimulation important for adaptations in metabolism, whereas overtraining asks for more rigorous and effective adaptations like increases in pulsatile delivery to keep homeostatic balance.
Several reports describe the influence of acute exercise on nocturnal GH secretory dynamics in humans by pulse detection analysis (26, 27, 36). In general, exercise of sufficient duration and intensity at the end of the day influences the temporal release of overnight GH release not at all, or it shifts GH secretion toward the second half of the night, possibly caused by an increase in the stress hormone cortisol during early sleep induced by intensive exercise at the end of the day. Increased cortisol concentrations impair the GH secretion during early hours of sleep and diminish the anabolic response (27, 36). One study used deconvolution analysis to analyze GH secretory dynamics after acute heavy resistance exercise at the end of the day. They reported increased secretory burst frequency and decreased mean interval between bursts, mean burst mass, and mean burst amplitude and thereby increased ApEn (44), which are similar results as we found in the intensively trained horses. It is possible that acute stress and chronic stress might elicit the same alteration in GH pulsatility. However, further research is needed to confirm this hypothesis.
Perspectives and Significance
To the best of our knowledge, we have demonstrated for the first time that intensified training for 6 wk alters nocturnal GH pulsatility in young Standardbred horses compared with a control group by a diminished coordinated GH secretion. The signs and symptoms occurring at the end of the intensified training phase and the persistence of some of the signs and symptoms during the reduced training might indicate that the IT group was not fully recovered at the end of reduced training. This might lead to the conclusion that an early stage of overtraining was induced during this experiment. It is hypothesized that the change in nocturnal GH pulsatility pattern is of benefit for the intensively trained horse by promoting a reestablishment of homeostatic balance, which improves recovery of and adaptation to the increased training load/stress. Longer periods of somatostatin withdrawal are expected to be the underlying mechanism for the observed changes in GH pulsatility pattern. Diminished GH secretion is described for athletes suffering overtraining in a more advanced stage (2). The mental and physical stress implied by this approach seemed to be enough to induce symptoms associated with early overtraining. Under these circumstances, the horse could serve as a model for humans for studying overtraining or possibly other stress-related pathophysiological mechanisms, since patterns of inducing overtraining seemed to be similar to humans.
Acknowledgments
We thank Maarten Boswinkel, Cornélie Westermann, Machteld van Dierendonck, Peggy Laan, Petty Vos, Kim Hardeveld, Sophie Wolfert, Ilvy van den Broek, Annemiek Maaskant, Maarten van den Burg, Marleen Jager, Galina Peeters, Saskia Terpstra, and Esmee Smiet for collecting the blood samples; Marjorie Pollack for technical assistance; Andries Klaarenbeek and Henk van Voorst for training of the horses; and Jan van den Broek for statistical analysis.
REFERENCES
- 1.Ahlborg G. Mechanisms for glycogenolysis in nonexercising human muscle during and after exercise. Am J Physiol Endocrinol Metab 248: E540–E545, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Barron JL, Noakes TD, Levy W, Smith C, Millar RP. Hypothalamic dysfunction in overtrained athletes. J Endocrinol Metab 60: 803–806, 1985 [DOI] [PubMed] [Google Scholar]
- 3.Borer KT. Exercise induced facilitation of pulsatile Growth hormone (GH) secretion and somatic growth. In: Hormones and Sport, edited by Laron Z and Rogol AD. New York, NY: Serona Symposia Publications/Raven Press, 1989, vol. 55, p. 21–40. [Google Scholar]
- 4.Bosquet L, Léger L, Legros P. Blood lactate response to overtraining in male endurance athletes. Eur J Appl Physiol 84: 107–114, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bruin G. Limits of Training and Testing in Horses (PhD dissertation). Maastricht, The Netherlands: Maastricht University, 1996
- 6.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111: 1409–1421, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleare JA. The neuroendocrinology of chronic fatigue syndrome. Endocr Rev 24: 236–252, 2003 [DOI] [PubMed] [Google Scholar]
- 8.De Graaf-Roelfsema E, Tharasanit T, Van Dam K, Keizer HA, Van Breda E, Wijnberg ID, Stout TAE, Van der Kolk JH. Effects of short-term administration of recombinant equine growth hormone or hydrocortisone or long-term administration of recombinant equine growth hormone to horses on tissue sensitivity to insulin. Am J Vet Res 66: 1907–1913, 2005 [DOI] [PubMed] [Google Scholar]
- 9.De Palo EF, De Filippis V, Gatti R, Spinella P. Growth hormone isoforms and segments/fragments: Molecular structure and laboratory measurement. Clin Chimica Acta 364: 67–76, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Donovan CM, Brooks GA. Endurance training affects lactate clearance, not lactate production. Am J Physiol Endocrinol Metab 244: E83–E92, 1983 [DOI] [PubMed] [Google Scholar]
- 11.Ekins RP. The “Precision profile”: its use in RIA; assessment and design. Ligand Quat 4: 33–44, 1981 [Google Scholar]
- 12.Farrington M, Hymer WC. Growth hormone aggregates in the rat adenohypophysis. Endocrinology 126: 1630–1638, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg AL. Mechanisms of growth and atrophy of skeletal muscle. Muscl Biol 1: 89–118, 1972 [PubMed] [Google Scholar]
- 14.Golland LC, Evans DL, Stone GM, Tyler-McGowan CM, Hodgson DR, Rose RJ. Plasma cortisol and β-endorphin concentrations in trained and over-trained Standardbred racehorses. Pflügers Arch 439: 11–17, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Grossman EJ, Grindeland RE, Roy RR, Talmadge RJ, Evans J, Edgerton VR. Growth hormone, IGF-1, and exercise effects on non-weight-bearing fast muscles of hypophysectomized rats. J Appl Physiol 83: 1522–1530, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Halson SL, Jeukendrup AE. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med 34: 967–981, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Hamlin MJ, Shearman JP, Hopkins WG. Changes in physiological parameters in overtrained Standardbred racehorses. Equine Vet J 34: 383–388, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Hartman ML, Faria ACS, Vance ML, Johnson ML, Thorner MO, Veldhuis JD. Temporal structure of in vivo growth hormone secretory events in humans. Am J Physiol Endocrinol Metab 260: E101–E110, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Hartman ML, Veldhuis JD, Johnson ML, Lee MM, Alberti KGMM, Samojlik E, Thorner MO. Augmented growth hormone (GH) secretory burst frequency and amplitude mediate enhanced GH secretion during a two-day fast in normal men. J Clin Endocrinol Metab 74: 757–765, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Howrie DL. Growth hormone for the treatment of growth failure in children. Clin Pharm 6: 283–291, 1987 [PubMed] [Google Scholar]
- 21.Hunter WM. Recent Advances in radioimmunoassay and related procedures. In: Radioimmunoassay and Related Procedures in Medicine, Vienna, Austria: IAEA, 1982, p. 3–18.
- 22.Isgaard J, Carlsson L, Isaksson OGP, Jansson JO. Pulsatile intravenous growth hormone (GH) infusion to hypophysectomized rats increases insulin-like growth factor 1 messenger RNA in skeletal tissues more effectively than continuous GH infusion. Endocrinology 123: 2605–2610, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Jeukendrup AE, Hesselink MK, Snyder AC, Kuipers H, Keizer HA. Physiological changes in male competitive cyclists after two weeks of intensified training. Int J Sports Med 13: 534–41, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Johnson ML. Pulse_XP User's Guide, version 1.0. 2004a, http://mljohnson.pharm.virginia.edu/pulseXP, chapt. 1, p. 18
- 25.Johnson ML. Pulse_XP User's Guide, version 1.0. 2004, http://mljohnson.pharm.virginia.edu/pulseXP, chapt. 2, cluster 8, p. 4
- 26.Kanaley JA, Weltman JY, Veldhuis JD, Rogol AD, Hartman ML, Weltman A. Human growth hormone response to repeated bouts of aerobic exercise, J Appl Phys 83: 1756–1761, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Kern W, Perras B, Wodick R, Fehm HL, Born J. Hormonal secretion during nighttime sleep indicating stress of daytime exercise. J Appl Phys 79: 1461–1468, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Kluge M, Schüssler P, Weikel J, Dresler M, Zuber V, Querfurt F, Yassouridis A, Steiger A. Altered nocturnal growth hormone (GH) secretion in obsessive compulsive disorder. Psychoneuroendocrinology 31: 1098–1104, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kreider R, Fry AC, O'Toole ML. Overtraining in sport: terms, definitions, and prevalence. In: Overtraining in Sport, edited by Kreider R, Fry AC, and O′Toole ML. Champaign, IL: Human Kinetics, 1998, vii-ix.
- 30.Kuipers H, Keizer HA. Overtraining in elite athletes: review and directions for the future. Sports Med 6: 79–92, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Laron Z, Karp M, Pertzelan A, Kauli R. Insulin, growth, and growth hormone. Isr J Med Sci 8: 440–452, 1972 [PubMed] [Google Scholar]
- 32.Lehmann M, Foster C, Gastmann U, Keizer H, Steinacker JM. Definition, types, symptoms, findings, underlying mechanisms, and frequency of overtraining and overtraining syndrome. In: Overload, Performance Incompetence, and Regeneration in Sport. New York, NY: Kluwer Academic/Plenum Publishers, 1999, p. 1–7.
- 33.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87: 873–907, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Moller S, Becker U. Insulin-like growth factor 1 and growth hormone in chronic liver disease. Dig Dis 10: 239–248, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Nap RC, Mol JA, Hazewinkel HA. Age-related plasma concentrations of growth hormone (GH) and insulin-like growth factor 1 (IGF-1) in Great Dane pups fed different dietary levels of protein. Domest Anim Endocrinol 10: 237–247, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Nindl BC, Hymer WC, Deaver DR, Kraemer WJ. Growth hormone pulsatility profile characteristics following acute heavy resistance exercise. J Appl Phys 91: 163–172, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Nindl BC, Rarick KR, Castellani JW, Tuckow AP, Patton JF, Young J, Montain SJ. Altered secretion of growth hormone and luteinizing hormone after 84 h of sustained physical exertion superimposed on caloric and sleep restriction. J Appl Phys 100: 120–128, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Pertzelan A, Blum I, Grunebaum M, Laron Z. The combined effect of growth hormone and methandrostenolone on the linear growth of patients with multiple pituitary hormone deficiencies. Clin Endocrinol (Oxf) 6: 271–276, 1977 [DOI] [PubMed] [Google Scholar]
- 39.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA 88: 2297–2301, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pincus SM, Keefe DL. Quantification of hormone pulsatility via an approximate entropy algorithm. Am J Physiol Endocrinol Metab 262: E741–E754, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Pincus SM, Veldhuis JD, Rogol AD. Longitudinal changes in growth hormone secretory process irregularity assessed transpubertally in healthy boys. Am J Physiol Endocrinol Metab 279: E417–E424, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Sonntag WE, Hylka VW, Meites J. Growth hormone restores protein synthesis in skeletal muscle of old male rats. J Gerontol 40: 689–694, 1985 [DOI] [PubMed] [Google Scholar]
- 43.Tonshoff B, Blum WF, Mehls O. Derangements of the somatotropic hormone axis in chronic renal failure. Kidney Int Suppl 58: S106–S113, 1997 [PubMed] [Google Scholar]
- 44.Tuckow AP, Rarick KR, Kraemer WJ, Marx JO, Hymer WC, Nindl BC. Nocturnal growth hormone secretory dynamics are altered after resistance exercise: deconvolution analysis of 12 hour immunofunctional and immunoreactive isoforms. Am J Physiol Regul Integr Comp Physiol 291: R1749–R1755, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Tyler CM, Golland LC, Evans DL, Hodgson DR, Rose RJ. Changes in maximum oxygen uptake during prolonged training, overtraining and detraining in horses. J Appl Physiol 81: 2244–2249, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Tyler CM, Golland LC, Evans DL, Hodgson DR, Rose RJ. Skeletal muscle adaptations to prolonged training, overtraining and detraining in horses. Pflügers Arch 436: 391–397, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Tyler-McGowan CM, Golland LC, Evans DL, Hodgson DR, Rose RJ. Haematological and biochemical responses to training and overtraining. Equine Vet J Suppl 30: 621–625, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Van Aken MO, Pereira AM, Frolich M, Romijn JA, Pijl H, Veldhuis JD, Roelfsema F. Growth hormone secretion in primary adrenal Cushing's syndrome is disorderly and inversely correlated with body mass index. Am J Physiol Endocrinol Metab 288: E63–E70, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Vaughan GM, Allen JP, Tullis W, Siler-Khodr TM, de la Pena A, Sackman JW. Overnight plasma profiles of melatonin and certain adenohypophyseal hormones in men. J Clin Endocrinol Metab 47: 566–571, 1978 [DOI] [PubMed] [Google Scholar]
- 50.Veldhuis JD, Anderson SM, Shah N, Bray M, Vick T, Gentili A, Mulligan T, Johnson ML, Weltman A, Evans WS, Iranmanesh A. Neurophysiological regulation and target-tissue impact of the pulsatile mode of growth hormone secretion in the human. Growth Horm IGF Res 11, Suppl A: S25–S37, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Veldhuis JD, Faria A, Vance ML, Evans WS, Thorner MO, Johnson ML. Contemporary tools for the analysis of episodic growth hormone secretion and clearance in vivo. Acta Paediatr Scand Suppl 347: 63–82, 1988 [PubMed] [Google Scholar]
- 52.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol Endocrinol Metab 250: E486–E493, 1986 [DOI] [PubMed] [Google Scholar]
- 53.Veldhuis JD, Johnson ML. Deconvolution analysis of hormone data. Methods Enzymol 210: 539–575, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Veldhuis JD, Johnson ML, Faunt LM, Mercado M, Baumann G. Influence of the high-affinity growth hormone (GH)-binding protein on plasma profiles of free and bound GH and on the apparent half-life of GH. J Clin Invest 91: 629–641, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veldhuis JD, Johnson ML, Veldhuis OL, Straume M, Pincus SM. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol Regul Integr Comp Physiol 281: R1975–R1985, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Veldhuis JD, Liem AY, South S, Weltman A, Weltman J, Clemmons DA, Abbott R, Mulligan T, Johnson ML, Pincus S, Straume M, Iranmanesh A. Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab 80: 3209–3222, 1995 [DOI] [PubMed] [Google Scholar]
- 57.Weltman A, Weltman JY, Schurrer R, Evans WS, Veldhuis JD, Rogol AD. Endurance training amplifies the pulsatile release of growth hormone: effects of training intensity. J Appl Physiol 72: 2188–2196, 1992 [DOI] [PubMed] [Google Scholar]
- 58.Wickler SJ, Hoyt DF, Cogger EA, McGuire R. The cost of transport in an extended trot. Equine Vet J suppl 34: 126–130, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Young VR. Regulation of protein synthesis and skeletal muscle growth. J Anim Sci 38: 1054–1070, 1974 [DOI] [PubMed] [Google Scholar]


