1. Introduction
Numerous studies have shown that emotional reactivity is one of the core aspects of temperament that influence children's adaptive behavior, including the ability to regulate behavior and attention in the presence of emotional demands (Cicchetti & Cohen, 2006; Cole, Dennis, Martin, & Hall, 2008; Gottman, Katz, & Hooven, 1997). Temperament theory has focused on two over-arching dimensions of temperamental reactivity in early childhood: surgency and negative affectivity (Rothbart, Ahadi, Hershey, & Fisher, 2001). Negative affectivity (NA) is a reactive dimension of temperament reflecting the degree to which a child displays discomfort, fear, anger/frustration, sadness, and reduced soothability (Rothbart & Bates, 2006; Thomas & Chess, 1977).
NA has been associated with a broad array of emotional pathologies, in particular those that are related to disruptions in how emotional information is attended to and processed, such as anxiety and depression (Compas, Connor-Smith, & Jaser, 2004; Dougherty, Klein, Durbin, Hayden, & Olino, 2010; Klein, Durbin, Shankman, & Santiago, 2002; Laurent, Joiner, & Catanzaro, 2011; Mikolajewski, Allan, Hart, Lonigan, & Taylor, 2012; Morgan, Shaw, & Olino, 2012). For example, anxiety is characterized by an attention threat bias, or disrupted attention towards threat-relevant stimuli (e.g., angry faces). This is evidenced at multiple stages of attention, depending on when and how attention is measured (Amir, Foa, & Coles, 1998), including exaggerated vigilance for threat, greater difficulty disengaging attention from threat, and attentional avoidance of threat (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Mogg & Bradley, 1998; Williams, Mathews, & MacLeod, 1996).
Nakagawa and Sukigara (2012) examined the relation between temperamental NA in 12-month-old infants and individual differences in attentional disengagement from threatening stimuli (e.g., fearful faces). They found that infants displaying greater NA had difficulty disengaging their attention from fearful faces compared to happy or neutral faces, and showed overall increased negative affect across the 12-36 month period. This suggests a developmental trajectory whereby children displaying greater NA, who also show greater attentional capture and interference by threat-relevant stimuli, may be at elevated risk for developing affective psychopathologies (Derryberry & Rothbart, 1997; Pérez-Edgar et al., 2011). Thus, a critical research goal is to increase the understanding of how NA influences disrupted attention towards emotional stimuli.
1.1 Negative Affectivity and EEG Asymmetry
One method that can be useful in investigating how temperamental characteristics, like NA, confer risk for later affective disruptions is EEG asymmetry. EEG asymmetry (relative difference in activity between left and right hemispheres) has traditionally been thought to reflect trait-like differences in emotional reactivity. Individual differences in temperament correlate with distinct patterns of asymmetric EEG activity in anterior (frontal and anterior-temporal) and posterior hemispheres of the brain (Davidson, Ekman, Saron, Senulis, & Friesen, 1990; Heller, Etienne & Miller, 1995; Heller & Nitschke, 1998). Activity in the left anterior regions of the brain reflects trait-like tendencies to experience and express approach-related emotions such as happiness and anger, whereas activity in the right anterior regions of the brain reflects withdrawal-related emotions such as fear (Davidson, 1992; Davidson et al., 1990; Davidson & Fox; 1982, 1989; Fox & Davidson, 1987; Gable & Harmon-Jones, 2008; Miskovic & Schmidt, 2010).
Anterior EEG asymmetry has been successfully used to predict the developmental trajectory of children with temperamental characteristics such as behavioral inhibition (Fox, Schmidt, Calkins, Rubin, & Coplan, 1996; Henderson, Fox, & Rubin, 2001). For example, in a study by Fox, Henderson, Rubin, Calkins, and Schmidt (2001), children identified as behaviorally inhibited in infancy and who remained inhibited and wary towards novel situations as toddlers displayed greater right anterior asymmetry, while those exhibiting less inhibition and wariness exhibited a shift in asymmetry from greater right to greater left anterior asymmetry.
Although anterior EEG asymmetry has been linked to parental report measures of temperament in infancy (e.g., LoBue, Coan, Thrasher, & DeLoache, 2011), and to the continuity of temperamental characteristics (e.g., Fox et al., 2001), few studies have examined whether EEG asymmetry helps explain the association between NA and cognitive biases that might put children at risk for affective psychopathology. Given that NA is associated with withdrawal type-behaviors and traits, and anterior EEG asymmetries (characterized as greater right-than-left activity) are also associated with withdrawal-type behaviors and traits, it is of interest to examine whether anterior EEG asymmetry will moderate or mediate responses between NA and executive attention (Coan & Allen, 2004). In the present study, we pursued this goal by testing the hypothesis, through both mediation and moderation analyses, that greater right anterior asymmetry will either partially or fully explain which children showing high NA will also show greater attentional capture by threat (i.e., measured as more interference on an attention task in the context of threat-relevant angry faces versus neutral or happy faces).
Less is understood about temperament and posterior EEG asymmetry. Greater right posterior asymmetry is thought to reflect more general emotional arousal (Bruder et al., 1997; Heller, 1993; Heller & Nitschke, 1998; Levin, Heller, Mohanty, Herrington, & Miller, 2007; Sobotka, Davidson & Senulis, 1992). For example, Heller, Nitschke, and Miller (1998) found that posterior regions of the brain are not only specialized for cognitive processes as was previously assumed, but for affective behaviors as well. Heller and colleagues posit that arousal, independent of high or low valence, is dependent on functions of the right posterior brain regions. Given that arousal is a key feature of anxiety, a posterior asymmetry (greater right activity than left) has been theorized to be related to the arousal facet of anxiety disorders (Bruder et al., 1997; Nitschke, Heller, Palmieri, & Miller, 1999). Greater activation in the right posterior brain regions has also been linked to performance on attention tasks. Specifically, greater activity in the right hemisphere has been associated with faster reaction times on attention tasks as well as the maintenance of attention in monitoring the occurrence of sensory stimuli (Heller, 1993). However, EEG studies on left posterior asymmetries have failed to show consistent associations with emotion regulation, emotion processing, or attention. Furthermore, studies examining adults evidencing depression and/or anxiety have documented individual differences in patterns of posterior EEG asymmetry. Non-anxious/depressed patients showed greater left posterior asymmetry (due to decreased right posterior activation) whereas anxious-depressed patients showed greater right posterior asymmetry (due to increased right posterior activation; Bruder et al., 1997). These findings are consistent with the model that, in adults, greater right posterior asymmetry is indicative of anxious arousal (Heller et al., 1995).
Thus, one of the aims of the present study was to investigate the extent to which posterior EEG asymmetries relate to NA and emotional interference on attention. Given rapid neural development occurring during the early school years (Casey, Giedd, & Thomas, 2000; Casey, Tottenham, Liston, & Durston, 2005), it remains unclear whether individual differences in posterior and anterior asymmetries will be associated with similar or distinct patterns of attention to emotion. Such developmental shifts have been documented in a previous fMRI study, in which researchers examined developmental differences in attentional control among 8-to-12 year old children and adults on a combined flanker task and go/no-go paradigm (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002). Children who had greater attention interference showed greater activation in posterior regions of the right hemisphere while adults who had greater attention interference had greater activation in anterior regions of the right hemisphere. Thus, we tested the exploratory hypothesis that, like children with greater right anterior asymmetries, children with greater right posterior EEG asymmetries, if indicative of individual differences in anxious arousal, will be more likely to show attention interference in the presence of threat stimuli. We predicted that this pattern would be enhanced among those children showing high temperamental NA.
Given that the processes through which NA relates to attention to threat are not fully understood, there is a need for studies that combine the examination of EEG asymmetry and NA with that of attention performance in an emotional context. Accordingly, the overall goal of the present study was to merge these literatures and investigate whether NA is related to disrupted attention towards threat, measured as emotional interference on attention during an executive attention task. In addition, we examined whether individual differences in EEG asymmetry influences these associations. School-aged children were recruited in order to examine these processes during a period of rapid neural development – preschool and early elementary school.
We tested the hypothesis that children showing greater NA, combined with greater right anterior or posterior asymmetry, may be more likely to have disrupted patterns of attention towards threat, measured as greater interference on an executive attention task in the context of angry versus neutral or happy faces. As EEG asymmetry might also represent a specific biological mechanism through which NA creates risk for attention disruptions, we tested whether individual differences in asymmetry fully mediate the associations between NA and attention interference.
2. Methods
2.1 Participants
Participants were 31 children between the ages of five and seven (17 girls, M age in years = 5.74, SD =.58). The age distributions for the girls were eight five-year olds and nine six-year olds. The boys included in the study were two five-year olds, ten six-year olds and two seven-year olds. Participants were an ethnically diverse group of children (13 Caucasians, eight African-Americans, four Hispanics, one Asian, and five identified by their mother as “Other” ethnicity). Maternal report on their children's handedness indicated that all participants were right-handed. Mothers reported no diagnosed developmental disorders or attention problems were present, and completed questionnaires assessing child temperament and emotional behavior. Participants spent approximately three hours in the laboratory and, at the end of the study, families were compensated with $100.00 for their time.
2.2 Procedures and Measures
Children were accompanied by their mothers to the laboratory where mothers gave informed consent and children gave verbal assent. Mothers filled out questionnaires assessing their child's temperament and behavior. During the laboratory visit, children completed a computerized task measuring EEG baseline followed by a computerized attention task while continuous EEG data was recorded. Specifically, EEG was measured at baseline while children sat quietly for eight 1-minute periods, counterbalanced in order for eyes closed (C, O, O, C, O, C, C) or eyes open conditions (O, C, C, O, C, O, C, C). Other computer-based tasks (e.g.,directed emotion regulation) and behavioral assessments (e.g., observed emotional behavior, observed parent-child interactions) followed but are not included in the present report.
2.3 EEG Acquisition
Using the BioSemi system (BioSemi; Amsterdam, NL), EEG activity was recorded continuously via 64 Ag/AgCl scalp electrodes embedded in an elasticized nylon cap based on the international 10/20 system. Eye movements were monitored by electro-oculogram (EOG) signals from electrodes placed 1 cm above and below the left eye (to measure vertical eye movements) and 1 cm on the outer edge of each eye (to measure horizontal eye movements). The EEG signal was preamplified at the electrode to improve the signal-to-noise ratio. EEG was recorded at a sampling rate of 512 Hz and amplified with a band pass of .16 – 100 Hz. The voltage from each active electrode was referenced online with respect to a common mode sense active electrode producing a monopolar (nondifferential) channel. All data were re-referenced offline to an average reference and filtered with a high pass frequency of .1 Hz and a low pass frequency of 30 Hz.
2.4 EEG Data Reduction
The raw EEG data was scanned through a computerized artifact scan batch for ocular and movement artifacts using Brain Electrical Source Analysis version 5.1 (BESA; MEGIS Software GmbH, Munich, Germany). Trials with EEG or EOG activity above ± 100 mV were excluded from further analysis.
All artifact-free EEG data measured at baseline were transformed to raw power scores with a Fast Fourier Transformation (FFT) filtered using a Hanning window of 1-s width and 50% overlap to retain as much data as possible. Power (μ2) was computed in the alpha frequency band (8-12 Hz) for anterior (FC5, FC6), anterior-temporal (T8, T7) and posterior (P8, P7) sites. These sites were selected based on the greatest power values displayed for each region, respectively. As power values are expressed in units of voltage squared, the distributions tend to be skewed and the data were log transformed to normalize the distributions. Alpha activity is associated with lower levels of awareness or increased levels of relaxation, therefore decreased alpha activity is considered to be an indication of increased mental activity. In addition, because asymmetrical activity was of interest to this study, a difference score subtracting left power scores from right power scores was calculated for anterior (FC6-FC5), anterior-temporal (T8-T7) and posterior (P8-P7) sites. The difference score provides a unidimensional scale representing relative activity of the right and left hemispheres. Higher or positive scores indicate relatively greater left activity and lower or negative scores indicate relatively greater right activity.
2.5 Attention Task
Children completed a child version of the Attention Network Test (ANT; Dennis, Malone, & Chen, 2009; Rueda et al., 2004) in the experimental booth. This task is a combination of a cued reaction time (Posner, 1980) and a flanker task (Eriksen & Eriksen, 1974) designed to assess three attention networks: alerting, orienting, and executive attention. Cues are presented prior to the target flanker task, in which participants indicate the direction of a central stimulus that is accompanied by either congruent or incongruent flanking stimuli. Since the executive attention system is primarily implicated when a task involves conflicting response options such as the flanker task (Eriksen & Eriksen, 1974), we focused our analyses on the executive attention system only, measured as the degree of conflict interference during the ANT (detailed further below).
Participants were seated approximately at a distance of 65 cm from a personal IBM computer with a 14-inch monitor (Windows XP) in a dimly lit room. The ANT was presented using E-Prime software (Psychological Software Tools, Pittsburgh, PA). Based on previous studies (Birk, Dennis, Shin, & Urry, 2011; Dennis & Chen, 2009; Dennis, Malone, & Chen, 2009), the task was modified by briefly presenting faces (angry, happy, or neutral faces) for 200ms prior to each trial of the task in order to create an emotional context and thus measure the degree to which distinct types of emotional faces influenced executive attention performance. Participants were presented with three experimental blocks, with each block presenting either angry, happy, or neutral faces prior to each trial of the task. Order of blocks was counterbalanced across participant. Each block consisted of 64 trials.
The original ANT designed for adults used arrows to represent the central stimuli and flankers (Fan, McCandliss, Sommer, Raz, & Posner, 2002). To make the task more relevant and interesting to young children, the central stimuli and flankers were changed to fish (Rueda et al., 2004).
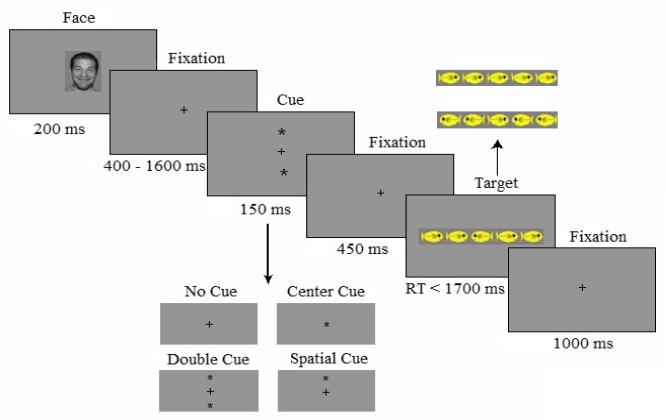
Each trial of the ANT consisted of a series of events (see Figure 1). First the emotional face stimuli (angry, happy, or neutral) were presented for 200ms followed by a fixation cross, which was variably presented on the screen from 400-1600ms. Subsequently, one of the four cueing conditions (no cue, center cue, double cue, or spatial cue) was presented for 150ms. This was again followed by the fixation cross that was presented for 450ms. After the fixation period, the central target along with the flankers was displayed up to 1700ms. The final event consisted of a fixation period which was variably presented for 1000ms. Each trial lasted for approximately 5,100ms.
Figure 1.
Experimental procedure for the modified ANT (see also Dennis, Malone, & Chen, 2009).
The target measure, executive conflict interference, was calculated as the mean reaction time (RT) of congruent flankers subtracted from the mean RT of incongruent flankers (summed for all cueing conditions). RTs ± 2 standard deviations from the mean were replaced with sample means to account for high variability in RTs on the ANT. Higher scores indicate greater conflict interference – thereby signifying decreased or worse executive attention performance.
2.6 Emotional face stimuli
The emotional face stimuli presented at the beginning of each ANT trial were taken from a battery of faces developed by the Research Network on Early Experience and Brain Development (Tottenham et al., 2009). This battery consisted of 646 facial expression (anger, fear, sad, neutral, disgust, and happy) modeled by male and female actors of various ethnicities. This study included 48 black-and-white photographs of angry, happy, and neutral adult faces, equally divided between the three emotion types. Actors were equally divided between males and females, and males and females were equally divided among Caucasian, African American, Latino, and Asian ethnicities. Faces were presented randomly, for a total of four times each, for each block of trials (64 per block).
2.7 Temperament Questionnaire
Mothers completed the Children's Behavior Questionnaire (CBQ; Rothbart, Ahadi, Hershey & Fisher, 2001) to assess child characteristics on various dimensions of temperament, rating each dimension on a 1-7 Likert scale. The CBQ is a 195-item questionnaire designed to measure 15 dimensions of temperament in children aged three to seven years. The CBQ scale included in this study was NA (α = .83). NA measures negative affective arousal and includes 5 subscales: anger, fear, falling reactivity/soothability, distress, and sadness. Sample items for NA include “has temper tantrums when he/she doesn't get what s/he wants” and “gets quite frustrated when prevented from something s/he wants to do”.
3. Results
3.1 Descriptive Statistics
Descriptive statistics for all study variables are shown in Table 1. Simple Pearson zero-order correlations between EEG asymmetry, negative affectivity and executive attention scores are shown in Table 2. Asymmetry scores for anterior, anterior temporal and posterior regions were all positively associated, whereas conflict interference scores across the three emotion conditions were not significantly associated with each other. Furthermore, negative affectivity was not correlated with EEG asymmetry and conflict interference scores.
Table 1.
Descriptive Statistics for Asymmetry Scores, NA, and Executive Attention (Conflict Interference)
| M | SD | Range | |
|---|---|---|---|
| Anterior Asymmetry (FC6-FC5) | .12 | .42 | −.76 - 1.12 |
| Anterior-temporal Asymmetry (T8-T7) | .14 | .43 | −.61 - 1.46 |
| Posterior Asymmetry (P8-P7) | .39 | .53 | −.44 - 1.63 |
| Negative Affectivity | 6.72 | 3.34 | 3.53 - 18.49 |
| Conflict Interference Angry | 33.48 | 75.86 | −11.97 - 183.91 |
| Conflict Interference Happy | 17.94 | 129.74 | −226.75 - 385.57 |
| Conflict Interference Neutral | 24.82 | 112.25 | −184.16 - 248.84 |
Note. M = mean. SD = standard deviation.
Table 2.
Correlations between Asymmetry Scores, NA, and Executive Attention (Conflict Interference)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Anterior Asymmetry (FC6-FC5) | - | ||||||
| 2. Anterior-Temporal Asymmetry (T8-T7) | .57** | - | |||||
| 3. Posterior Asymmetry (P8-P7) | .43* | .40* | - | ||||
| 4. Negative Affectivity | −.10 | −.01 | −.06 | - | |||
| 5. Conflict Interference Angry | .11 | −.04 | −.01 | .02 | - | ||
| 6. Conflict Interference Neutral | .10 | −.25 | .19 | −.27 | .21 | - | |
| 7. Conflict Interference Happy | −.22 | .11 | −.33† | .02 | .23 | −.13 | - |
Note.
p < .01.
p < .05.
p < .10
Before conducting the primary analyses, age, and gender were examined in relation to the primary measures of interests: EEG asymmetry and NA. Overall, no age differences were found for EEG asymmetry in anterior (r = .11, p = .58) and posterior (r = .11, p = .58) regions. Age was related to anterior-temporal asymmetry (r = .35, p = .05) with younger children showing greater right asymmetry. Additionally, there were associations with age and NA (r = −.37, p = .05) with younger children displaying greater levels of NA, t(28) = 2.36, p = .03. Furthermore, gender differences emerged in relation to EEG asymmetry with boys (M = −0.04, SD = 0.45) showing greater right anterior asymmetry than girls (M = 0.25, SD = 0.36), t(29) = -2.04, p = .05. No other differences emerged for anterior-temporal [t(29) = .02, p = .98] and posterior [t(29) = .12, p = .90] regions. Similarly, no gender differences were found for NA, t(28) = −.88, p =.39.
3.2 Moderation Analyses
The primary hypothesis was that children showing greater NA combined with greater right asymmetry in either anterior, anterior-temporal, or posterior regions would show reduced attention performance following threat-relevant angry faces. Nine hierarchical linear regressions were conducted to evaluate the association between patterns of EEG asymmetry and attention performance. The moderator variables were EEG asymmetry for anterior (FC6 – FC5), anterior-temporal (T8 – T7), and posterior regions (P8 – P7). The dependent variables were executive attention following angry, happy, and neutral faces. NA scores were entered in the first step followed by EEG asymmetry in the second step of each regression1. The final step of each regression was the interaction between NA and EEG asymmetry scores.
Given recommendations concerning probing interaction effects (Aiken & West, 1991; Finney, Mitchell, Cronkite, & Moos, 1984), if interaction terms’ contributions to R2 approached significance (p = .10), the interactions were followed up with the PROCESS macro for SPSS (Hayes, 2013) by using simple regression equations. The dependent variable on the y-axis (attention performance) was plotted against three levels of the predictor (NA): two standard deviation below the mean (low), 0 (mean value), and two standard deviation above the mean (high). Plotted regression lines represent primarily right (two standard deviations below the mean for moderator variable) or left (two standard deviations above the mean for moderator variable) asymmetry and a simple slopes analysis was conducted to determine whether the slope of each regression lines was significantly different from zero. For all steps of the analyses, predictor variables were centered to reduce problems of lack of invariance of regression coefficients and multicollinearity.
3.2.1 Anterior Asymmetry
No significant effects emerged.
3.2.2 Anterior-Temporal Asymmetry
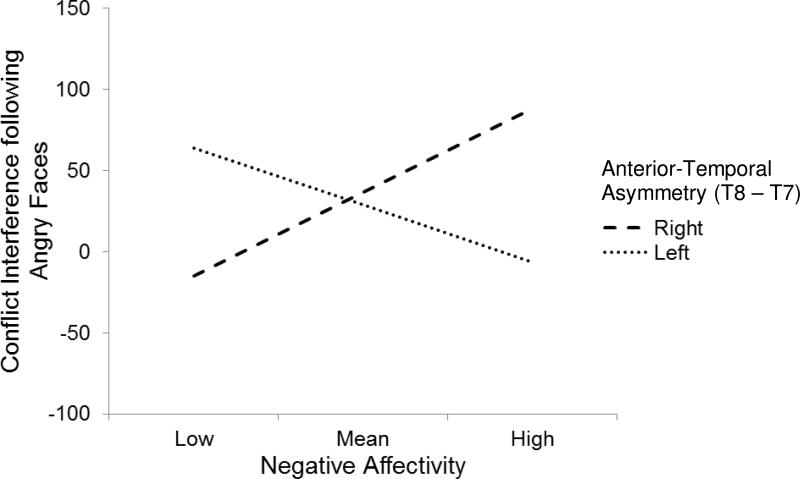
Predictors explained significant variance in conflict interference following angry faces for the regression with EEG asymmetry in anterior-temporal regions [F(3, 26) = 2.99, p = .049, R2 = .26]. As predicted, there was a significant interaction between EEG asymmetry for anterior-temporal electrodes and NA following angry faces [interaction step change statistics: F(1, 26) = 8.84, p = .006, R2 = .25; interaction coefficient: t = −2.97, p = .006]. As seen in Figure 2, as NA increased, conflict interference following angry faces also increased, but only for those children showing greater right anterior-temporal asymmetry (t = 2.41, p = .02). Conversely, as NA increased, conflict interference following angry faces decreased, but only for those children showing greater left anterior-temporal asymmetry (t = −1.97, p = .06).
Figure 2.
Negative affectivity X anterior-temporal asymmetry predicting conflict interference following angry faces.
No significant effects emerged for attention performance following happy and neutral faces.
3.2.3 Posterior Asymmetry
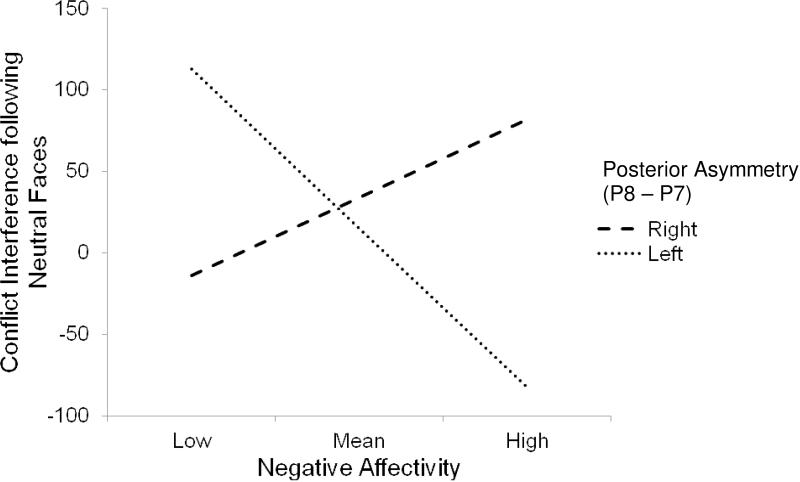
Predictors explained significant variance in conflict interference following neutral faces for the regression with EEG asymmetry in posterior regions at the level of a trend [F(3, 26) = 2.42, p = .09, R2 = .22]. There was a marginally significant interaction between EEG asymmetry for posterior electrodes and NA following neutrals faces [interaction step change statistics: F(1, 26) = 3.63, p = .07, R2 = .11; interaction coefficient: t = −1.91, p = .07]. As seen in Figure 3, as NA increased, conflict interference following neutral faces decreased, but only for those children showing greater left posterior asymmetry (t = −2.40, p = .02). The slope for right posterior asymmetry was not significant (t = 1.07, p = .30).
Figure 3.
Negative affectivity X posterior asymmetry predicting conflict interference following neutral faces
No significant effects emerged for attention performance following angry and happy faces.
3.3 Mediation Analyses
To explore the hypothesis that children's asymmetry mediates the relationship between NA and conflict interference following threat-relevant angry faces we conducted a series of nine hierarchical multiple regressions using the PROCESS macro for SPSS (Hayes, 2013). This procedure tests the product of the coefficient for (α) the effect of the independent variable (NA) to the mediator (asymmetry) with (β) the effect of the mediator to the dependent variable (conflict interference) when the independent variable is taken into account. The 95% confidence interval for the direct path (αβ) was calculated; an overlap with zero indicates no significant mediation effects (MacKinnon, Fairchild, & Fritz, 2007; MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002).
No significant effects emerged.
4. Discussion
The present study examined NA and EEG asymmetry in relation to executive attention performance following emotional stimuli. As predicted, we found that greater NA along with greater right anterior-temporal EEG asymmetry was associated with more attention interference following angry faces. Conversely, as might be expected, greater NA along with greater left anterior-temporal EEG asymmetry was associated with less attention interference following angry faces. A novel finding also emerged in which greater NA along with greater left posterior EEG asymmetry was associated with less attention interference, but only following neutral faces. Findings have the potential to extend the existing literature on the links between NA and disruptions in attention to emotion, and the neurophysiological substrates for these associations. Moreover, findings may shed light on the neural processes through which temperamental NA confers risk for later affective psychopathology.
Before testing study hypotheses, we examined associations among all study variables as well as age and gender effects. Asymmetry scores for anterior, anterior-temporal and posterior regions were all positively associated. This finding counters studies that have found inverse associations between anterior and posterior regions (Davidson, Schaffer, & Saron, 1985). However, these findings have typically been based on measuring EEG asymmetries during a cognitive performance task (Davidson et al., 1985; Davidson & Hugdahl, 1996. Other studies, in contrast, have found that EEG asymmetries observed during a resting baseline may reflect relatively independent cortical processes (Hoptman & Davidson, 1998), which may account for concomitant increases in the activity of anterior, anterior-temporal and posterior regions, rather than expected inverse associations. In a pediatric sample, in particular, as specialization of discrete cortical regions mature through both segregation and integration of cognitive control networks (Fair et al., 2007), the inverse link between anterior and posterior functions may become less variable and more robust.
Correlational analyses also revealed that associations between EEG asymmetry and NA scores did not reach significance. This finding is in line with studies that have not found a relation between EEG asymmetry and dispositional negative and positive affect (Harmon Jones & Allen, 1998), suggesting that these broad constructs may not directly map onto specific patterns of EEG asymmetry. Indeed, as suggested by our moderation analyses, dispositional affective tendencies like NA and EEG asymmetry may represent orthogonal and interacting individual differences that are rapidly maturing and changing during this developmental period. This is interesting to consider in light of age-related differences found in NA – specifically, that younger children displayed more NA than older children. This age shift is likely linked to the ongoing development of cognitive and emotional skills that support affect regulation (Forbes, Fox, Cohn, Galles, & Kovacs, 2006).
In addition to age differences, gender differences emerged with boys showing relatively greater right anterior asymmetry and girls showing relatively greater left anterior asymmetry. Such patterns of asymmetry may reflect the tendency for males to use more visuospatial processing, which relies heavily on the right hemisphere, and females to use more verbal processing which relies heavily on the left hemisphere (Bryden, McManus, & Bulman-Fleming, 1994; Shankman et al., 2005). However, age and gender did not influence primary study hypotheses, and thus cannot be used to interpret key findings. Counter to study hypotheses, no significant effects emerged for children showing high NA and greater anterior asymmetry. Though studies with adults have typically found an association with NA and anterior asymmetry, it is likely that ongoing development of the frontal cortex during early childhood could lead to variability in how basic cognitive processes such as attention are represented in the brain. Indeed, given the relative immaturity of the prefrontal cortex, young children may utilize more posteriorly-mediated control processes compared to adults (Casey, Giedd, & Thomas, 2000; Segalowitz & Davies, 2004). This variability and posterior-shift of cognitive control in early development is consistent with our finding that children showing high NA and greater right anterior-temporal asymmetry showed more attention interference (worse executive attention) following the presentation of threat-relevant angry faces. Conversely, children showing high NA and greater left anterior-temporal asymmetry showed less attention interference (better executive attention) following the presentation of threat-relevant angry faces, although this finding was at the level of a trend (p = .06). Indeed previous asymmetry research has linked patterns of greater right anterior asymmetry with affective and anxiety disorders in adults (Davidson, Marshall, Tomarken, & Henriques, 2000) and school-aged children (Baving, Laucht, & Schmidt, 2003). In that same vein, research on negative emotionality has documented associations with attention biases – a propensity to be hypersensitive to threatening stimuli – and a risk for the development of anxiety disorders (Caspi, Henry, McGee, Moffitt, & Silva, 1995; Derryberry & Reed, 2002). Taken together, the present findings could reflect the links between attentional biases to threat and NA in children (Dennis & Chen, 2007, 2009; Derryberry & Reed, 1997, 2000), which could ultimately influence developmental trajectories towards affective psychopathology. In the present study, the predicted interference effect by angry faces may indicate that those children with high NA and right anterior-temporal asymmetry had exaggerated attention and reactivity to the threat-relevant angry faces. This in turn would detract from the ability to allocate their attentional resources to the subsequent target attention task (Derryberry & Reed, 1998).
Children with high NA and left anterior-temporal asymmetry performed better following the presentation of threat-relevant angry faces. These findings appear to be consistent with previous research that has linked greater left anterior asymmetry to the processing of social threat and approach-related emotions such as anger (d'Alfonso, van Honk, Hermans, Postma & de Haan, 2000; Fox & Davidson, 1988; van Honk, Hermans, d'Alfonso, Schutter, van Doornen, & de Haan, 2002). Anger is an emotion that has been associated with heightened approach tendencies, which in turn can motivate an individual to respond to threat in a constructive and vigilant manner (Berkowitz, 1993; Harmon-Jones & Allen, 1998) thereby increasing children's performance on the task. This interpretation is bolstered by the timing of the task: emotional stimuli (angry faces) were briefly presented for 200ms prior to each trial of the attention task, suggesting enduring capture of attention by threat.
We also examined whether posterior asymmetry moderated the impact of NA on attention. First, the non-significant main effect of posterior asymmetry showed that greater posterior asymmetry was not associated with attention interference following neutral faces. However, the trend-level interaction between posterior asymmetry and NA further specified that for children showing high NA, greater left posterior asymmetry was associated with decreased attention interference (better executive attention) following neutral faces. This suggests that in the context of high temperamental NA, greater left posterior activation (or decreased right posterior activation) represents a biological individual difference that may reduce emotional interference on attention. It is interesting that this effect of posterior asymmetry emerged only for neutral faces, but is consistent with the suggestion that neutral faces are inherently ambiguous (Thomas et al., 2001) and may be interpreted as either novel or threatening by those with exaggerated NA.
Results should be interpreted in the context of some study limitations. First, the sample used in this study consisted of typically developing children, thus limiting the generalizability of results. A second limitation was the inclusion of emotional stimuli that are not strongly arousing. Future research should also examine more salient and arousing affective stimuli, such as complex emotional pictures. The use of more arousing emotional pictures, or the inclusion of mood inductions in the design, may elicit stronger associations between EEG asymmetry in anterior and posterior regions and emotional interference effects. For example, one study found that using negative emotional film clips prior to recording EEG resulted in greater right posterior activity – indicating increased emotional arousal (Aftanas & Pavlov, 2005) and that EEG asymmetry moderated the impact of mood induction on attention interference effects (Dennis & Solomon, 2010).
Findings from this study highlight the potential utility of using EEG asymmetry in the study of emotion and attention, and extend the existing literature on EEG asymmetry and NA and its relation to biased attention and attention interference effects in children. Attention was targeted in the present study because impaired attention has been considered one of the cardinal features in the DSM-IV for depression and anxiety. Individuals who allocate excessive neural resources to “threatening stimuli” – in the case of individuals with anxiety related disorders – may have difficulty allocating further resources to other tasks that have attentional demands (Ferneyhough, Kim, Phelps, & Carrasco, 2012). Similarly, individuals who allocate excessive neural resources to disengage from emotional stimuli during attention demanding tasks may be at an increased risk for developing affective psychopathologies (e.g., anxiety and depression; Mathews & MacLeod, 2002). Future studies could investigate these individual differences in affective style and its effect on attention using EEG as a measure that is sensitive to the interaction between cognitive, affective, and attentional processes. In addition, results may have implications for understanding how temperament confers risk for adaptive and maladaptive emotional attention in children.
Research Highlights.
EEG asymmetry influenced whether NA confers risk for emotion-attention disruptions
High NA and right anterior-temporal asymmetry predicted more attention interference
High NA and left anterior-temporal asymmetry predicted less attention interference
High NA and left posterior asymmetry predicted less attention interference
Table 3.
Hierarchical Regression Analysis for Variables Predicting Conflict Interference following Angry Faces (N = 30)
| Variable | B | SE B | β |
|---|---|---|---|
| Negative affectivity (NA) | 2.42 | 3.97 | .04 |
| Anterior-Temporal Asymmetry | −11.29 | 36.63 | −.05 |
| Anterior-Temporal Asymmetry x NA | −36.48 | 12.27 | −.50 ** |
| R2 | .26 | ||
| F for change in R2 | 8.84 ** |
Note:
p < .01.
Table 4.
Hierarchical Regression Analysis for Variables Predicting Conflict Interference following Neutral Faces (N = 30)
| Variable | B | SE B | β |
|---|---|---|---|
| Negative affectivity (NA) | −7.59 | 5.56 | −.23 |
| Anterior-Temporal Asymmetry | −17.61 | 46.70 | −.09 |
| Anterior-Temporal Asymmetry x NA | −40.62 | 21.32 | −.43† |
| R2 | .22 | ||
| F for change in R2 | 3.63† |
Note:
* p < .05.
p < .10.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Regressions with age and gender entered in the first step did not differ from the reported results, thus were not included in the analyses.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Aftanas LI, Pavlov SV. Trait anxiety impact on posterior activation asymmetries at rest and during evoked negative emotions: EEG investigation. International Journal of Psychophysiology. 2005;55:85–94. doi: 10.1016/j.ijpsycho.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Amir N, Foa EB, Coles ME. Automatic activation and strategic avoidance of threat-related information in social phobia. Journal of Abnormal Psychology. 1998;107:285–290. doi: 10.1037//0021-843x.107.2.285. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Baving L, Laucht M, Schmidt MH. Frontal EEG correlates of externalizing spectrum behaviors. European Child and Adolescent Psychiatry. 2003;42:36–42. doi: 10.1007/s00787-003-0307-5. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Towards a general theory of anger and emotional aggression: Implications of the cognitive-neoassociationistic perspective for the analysis of anger and other emotions. In: Wyer RS, Snail TK, editors. Perspectives on anger and emotion: Advances in social cognition. Erlbaum; Hillsdale, NJ: 1993. pp. 1–46. [Google Scholar]
- Birk JL, Dennis TA, Shin LM, Urry HL. Threat-related facilitation of executive control efficiency during state anxiety. Emotion. 2011;11:1291–1304. doi: 10.1037/a0026152. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Bryden MP, McManus IC, Bulman-Fleming MB. Evaluating the empirical support for the Geschwind-Behan-Galaburda model of cerebral lateralization. Brain and Cognition. 1994;26:103–167. doi: 10.1006/brcg.1994.1045. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Science. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Caspi A, Henry B, McGee R, Moffitt TE, Silva P. Temperamental origins of child and adolescent behavior problems: From age 3 to age 15. Child Development. 1995;66:55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Cohen DJ. Developmental psychopathology. 2nd ed. Wiley; Hoboken, NJ: 2006. [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cole PM, Dennis TA, Martin SE, Hall SE. Emotion regulation and the early development of psychopathology. In: Vandekerckhove M, von Scheve C, Ismer S, Jung S, Kronast S, editors. Regulating emotions: Culture, social necessity and biological inheritance. Blackwell Publishing; Malden, MA: 2008. pp. 171–188. [Google Scholar]
- Compas BE, Connor-Smith JK, Jaser SS. Temperament, stress reactivity, and coping: Implications for depression in childhood and adolescence. Journal of Clinical Child and Adolescent Psychology. 2004;33:21–31. doi: 10.1207/S15374424JCCP3301_3. [DOI] [PubMed] [Google Scholar]
- d'Alfonso AAL, van Honk J, Hermans E, Postma A, de Haan EHF. Laterality effects in selective attention to threat after repetitive transcranial magnetic stimulation at the prefrontal cortex in female subject. Neuroscience Letters. 2000;280:195–198. doi: 10.1016/s0304-3940(00)00781-3. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron C, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: Emotional expression and brain physiology I. Journal of Personality and Social Psychology. 1990;58:330–341. [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218:1235–1236. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of Abnormal Psychology. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Hugdahl K. Baseline asymmetries in brain electrical activity predict dichotic listening performance. Neuropsychology. 1996;10:241–246. [Google Scholar]
- Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: Regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry. 2000;47:85–95. doi: 10.1016/s0006-3223(99)00222-x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Schaffer CE, Saron C. Effects of lateralized presentations of faces on self-reports of emotion and EEG asymmetry in depressed and nondepressed subjects. Psychophysiology. 1985;22:353–364. doi: 10.1111/j.1469-8986.1985.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Chen C. Emotional face processing and attention performance in three domains: Neurophysiological mechanisms and moderating effects of trait anxiety. International Journal of Psychophysiology. 2007;65:10–19. doi: 10.1016/j.ijpsycho.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Chen C. Trait anxiety and conflict monitoring following threat: An ERP study. Psychophysiology. 2009;46:122–131. doi: 10.1111/j.1469-8986.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Malone MM, Chen C. Emotional face processing and emotion regulation in children: An ERP study. Developmental Neuropsychology. 2009;34:85–102. doi: 10.1080/87565640802564887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Solomon B. Frontal EEG and emotion regulation: Electrocortical activity in response to emotional film clips is associated with reduced mood induction and attention interference effects. Biological Psychology. 2010;85:456–464. doi: 10.1016/j.biopsycho.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Motivational and attentional components of personality. In: Matthews G, editor. Cognitive science perspectives on personality and emotion. Elsevier; Amsterdam: 1997. pp. 443–473. [Google Scholar]
- Derryberry D, Reed MA. Anxiety and attentional focusing: Trait, state and hemispheric influences. Personality and Individual Differences. 1998;25:745–761. [Google Scholar]
- Derryberry D, Reed MA. Attention and Voluntary Self-Control. Self and Identity. 2000;1:105–111. [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Durbin CE, Hayden EP, Olino TM. Temperamental positive and negative emotionality and children's depressive symptoms: A longitudinal prospective study from age three to age ten. Journal of Social and Clinical Psychology. 2010;29:464–490. [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Ferneyhough E, Kim MK, Phelps EA, Carrasco M. Anxiety modulates the effects of emotion and attention on early vision. Cognition and Emotion. 2012:1–11. doi: 10.1080/02699931.2012.689953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney JW, Mitchell RE, Cronkite RC, Moos RH. Methodological issues in estimating main and interactive effects: Examples from coping/ social support and stress field. Journal of Health and Social Behavior. 1984;25:85–98. [PubMed] [Google Scholar]
- Forbes EE, Fox NA, Cohn JF, Galles SF, Kovacs M. Children's affect regulation during a disappointment: Psychophysiological responses in relation to parent history of depression. Biological Psychology. 2006;71:264–277. doi: 10.1016/j.biopsycho.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. EEG asymmetry in 10-month-old infants in response to approach of a stranger and maternal separation. Developmental Psychology. 1987;23:233–240. [Google Scholar]
- Fox NA, Davidson RJ. Patterns of brain electrical activity during facial signs of emotion in ten month old infants. Developmental Psychology. 1988;24:230–236. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1, 1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Calkins SD, Rubin KH, Coplan RJ. The role of frontal activation in the regulation and dysregulation of social behavior during the preschool years. Development and Psychopathology. 1996;8:89–102. [Google Scholar]
- Gable PA, Harmon-Jones E. Approach-motivated positive affect reduces breadth of attention. Psychological Science. 2008;19:476–482. doi: 10.1111/j.1467-9280.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- Gottman JM, Katz LF, Hooven C. Meta-emotion: How families communicate emotionally. Lawrence Erlbaum Associates; Mahwah, NJ: 1997. [Google Scholar]
- Harmon-Jones E, Allen JJB. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74:1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis. The Guilford Press; New York: 2013. [Google Scholar]
- Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;7:476–489. [Google Scholar]
- Heller W, Etienne MA, Miller GA. Patterns of perceptual asymmetry in depression and anxiety: Implications for neuropsychological models of emotion and psychopathology. Journal of Abnormal Psychology. 1995;104:327–333. doi: 10.1037//0021-843x.104.2.327. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Cognition and Emotion. 1998;12:421–447. [Google Scholar]
- Heller W, Nitschke JB, Miller GA. Lateralization in emotion and emotional disorders. Current Directions in Psychological Science. 1998;7:26–32. [Google Scholar]
- Henderson HA, Fox NA, Rubin KH. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Hoptman JM, Davidson RJ. Baseline EEG asymmetries and performance on neuropsychological tasks. Neuropsychologia. 1998;36:1343–1353. doi: 10.1016/s0028-3932(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Klein DN, Durbin CE, Shankman SA, Santiago NJ. Depression and personality. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 1st ed. Guilford Press; New York: 2002. pp. 115–140. [Google Scholar]
- Lamb MR, Robertson LC, Knight R. Attention and interference in the processing of global and local information: Effects of unilateral temporal-parietal junction lesions. Neuropsychologia. 1989;27:471–483. doi: 10.1016/0028-3932(89)90052-3. [DOI] [PubMed] [Google Scholar]
- Laurent J, Joiner TE, Catanzaro SJ. Positive affect, negative affect, and physiological hyperarousal among referred and nonreferred youths. Psychological Assessment. 2011;23:945–957. doi: 10.1037/a0024080. [DOI] [PubMed] [Google Scholar]
- Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research. 2007;31:211–233. [Google Scholar]
- LoBue V, Coan J, Thrasher C, DeLoache JS. Prefrontal asymmetry and parent-rated temperament in infants. PLoS One. 2011;6:e22694. doi: 10.1371/journal.pone.0022694. doi:10.1371/journal.pone.0022694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, Colin MacLeod. Induced processing biases have causal effects on anxiety. Cognition and Emotion. 2002;16:331–354. [Google Scholar]
- Mikolajewski AJ, Allan NP, Hart SA, Lonigan CJ, Taylor J. Negative affect shares genetic and environmental influences with symptoms of childhood internalizing and externalizing disorders. Journal of Abnormal Child Psychology. 2012 doi: 10.1007/s10802-012-9681-0. doi:10.1007/s10802-012-9681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA. Frontal brain electrical asymmetry and cardiac vagal tone predict biased attention to social threat. International Journal of Psychophysiology. 2010;75:332–338. doi: 10.1016/j.ijpsycho.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, Olino TM. Differential susceptibility effects: The interaction of negative emotionality and sibling relationship quality on childhood internalizing problems and social skills. Journal of Abnormal Child Psychology. 2012;40:885–899. doi: 10.1007/s10802-012-9618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa A, Sukigara M. Difficulty in disengaging from threat and temperamental negative affectivity in early life: A longitudinal study of infants aged 12-36 months. Behavioral and Brain Function. 2012;8:1–8. doi: 10.1186/1744-9081-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. [PubMed] [Google Scholar]
- Pérez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, Fox NA. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology. 2011;39:885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey K, Fisher P. Investigation of temperament at three to seven years: The Children's Behavior Questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of Child Psychology: Vol. 3. Social, emotional, and personality development. 6th ed. Wiley; New York, NY: 2006. pp. 99–166. [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, Posner MI. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: An electrophysiological strategy. Brain and Cognition. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Tenke CE, Bruder GE, Durbin CE, Hayden EP, Klein DN. Low positive emotionality in young children: Association with EEG asymmetry. Development and Psychopathology. 2005;17:85–98. doi: 10.1017/s0954579405050054. [DOI] [PubMed] [Google Scholar]
- Sobotka SS, Davidson RJ, Senulis JA. Anterior brain electrical asymmetries in response to reward and punishment. Electroencephalography and Clinical Neurophysiology. 1992;83:236–247. doi: 10.1016/0013-4694(92)90117-z. [DOI] [PubMed] [Google Scholar]
- Thomas A, Chess S. Temperament and development. Brunner/Mazel; Oxford: 1977. [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Hermans EJ, d'Alfonso AAL, Schutter DJLG, van Doornen L, de Haan EHF. A left-prefrontal lateralized, sympathetic mechanism directs attention towards social threat in humans: evidence from repetitive transcranial magnetic stimulation. Neuroscience Letters. 2002;319:99–102. doi: 10.1016/s0304-3940(01)02558-7. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Yamagata S, Kobayashi S. Cerebral asymmetry of the “top-down” allocation of attention to global and local features. Journal of Neuroscience. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-09-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]