Abstract
Background
Rheumatoid arthritis (RA) patient preferences may account for some of the variability in treatment amongst different racial groups. How and why treatment preferences differ by race is not well understood. We sought to determine whether Black and White RA patients differ in how they evaluate the specific risks and benefits related to medications.
Methods
136 RA patients completed a conjoint analysis interactive computer survey to determine how they valued the specific risks and benefits related to treatment characteristics. We calculated the importance that respondents assigned to each characteristic and the ratio of the importance that patients attached to overall benefit versus overall risk. Subjects having a risk ratio of less than one were classified as being risk averse.
Results
The mean age of the study sample was 55 years (range 22–84), 49% were Black and 51% were White. Black subjects assigned the greatest importance to the risk of cancer, whereas White subjects were most concerned with the likelihood of remission and halting radiographic progression. Fifty-two percent of Black subjects were found to be risk averse compared to 12% of the White subjects (p<0.0001). Race remained strongly associated with risk aversion [adjusted odds ratio (95% CI) = 8.4 (3.1 – 23.1)] after adjusting for relevant covariates.
Conclusions
Black patients attach greater importance to the risks of toxicity and less importance to the likelihood of benefit than their White counterparts. Effective risk communication and improved understanding of expected benefits may help decrease unwanted variability in healthcare.
Racial disparities in the delivery of health services have been well-documented in patients with arthritis as well as other disorders (1–5). These disparities occur even amongst insured populations with access to care (6, 7). Differences in patient preferences may account for some of the variability in healthcare utilization amongst different racial groups (2, 3).
Some data suggest that Black patients with rheumatoid arthritis (RA) are less likely to receive aggressive care compared to White patients (8, 9) and that this difference might be explained in part by patients’ preferences (10). How and why treatment preferences differ by race, however, is not well understood. RA treatment decisions are frequently complex and require multiple trade-offs involving relief of symptoms, long-term reduction in disability, common reversible adverse events, and rare serious complications. In this study, we sought to determine whether Black and White RA patients differ in how they make trade-offs between the specific risks and benefits related to treatment.
To quantify how patients evaluate specific treatment risks and benefits, we used conjoint analysis. Conjoint analysis is a well-established method that has been used to elicit patients’ preferences for diverse domains in healthcare including treatment options, cancer screening, and healthcare delivery (11–14). We chose to use this approach, because data generated from a conjoint study can be used to quantify the importance that respondents attach to specific risks and benefits, thereby enabling one to identify the impact of each treatment characteristic on patients’ preferences. Using conjoint analysis, it is therefore possible to determine if a patient’s preference is driven primarily by concerns related to the risk of toxicity or the expectations of benefit.
Patients and Methods
Participants
We recruited RA patients from Washington Hospital Center, Washington, DC and Virginia Commonwealth University, Richmond, VA. Patients were recruited and interviewed after their appointments in the outpatient rheumatology clinics. Inclusion criteria were: RA diagnosed by, and currently under the care of a rheumatologist, a positive serum test for at least one of the RA-associated autoantibodies (rheumatoid factor or anti-cyclic citrullinated peptide), self-identified as Black or White, and able to read and write English. One hundred fifty consecutive patients willing to hear about the study agreed to participate. Of these, nine refused to complete the questionnaire (because it was too long or too difficult) and five could not complete the computer survey due to time constraints, resulting in a total of 136 subjects.
Data Collection
We examined how subjects made trade-offs between specific treatment characteristics using Adaptive Conjoint Analysis (ACA, SSI Web version 6.4, Sawtooth Software ®). We included characteristics related to commonly used DMARDs: benefits (chance of remission, symptom improvement, and radiographic progression), route of administration, and risks (injection reaction, nausea, lung or liver injury, tuberculosis, neurological disease, and theoretical risk of cancer). All characteristics were defined by a range of estimates based on the best available data from the literature (Table 1) (15–18) and a list of standardized descriptions were provided (Appendix A). We chose to omit the names of medications from the conjoint questionnaire to ensure that preferences were based on values for specific risks and benefits and not biased by recognition of brand names.
Table 1.
Characteristics included in the ACA questionnaire
| Characteristics | Estimates |
|---|---|
| Remission | 45 out of 100 patients go into remission 25 out of 100 patients go into remission 15 out of 100 patients go into remission |
| Improvement | 70 out of 100 patients feel much better, but occasionally have some joint pain or swelling 50 out of 100 patients feel much better, but occasionally have some joint pain or swelling 40 out of 100 patients feel much better, but occasionally have some joint pain or swelling |
| Radiographic progression | No further bone damage seen on X-rays in 80 out of 100 patients No further bone damage seen on X-rays in 50 out of 100 patients No further bone damage seen on X-rays in 30 out of 100 patients |
| Route | Pill you take once a week Injection you give yourself once every 1–2 weeks Intravenous infusion you get every 6–8 weeks |
| Injection reaction | No injection reactions 30 in 100 patients get a rash or local burning at the site of injection 3 in 100 patients will get a reaction during the infusion (headache, nausea, fever) |
| Reversible adverse events | No increased risk of nausea, dizziness or unusual tiredness 10 in 100 people will have nausea, dizziness or unusual tiredness |
| Risk of lung injury | No increased risk of lung or liver injury Rare risk of lung injury (2 in 100 patients) or liver injury (about 1 in 1000 patients) |
| Risk of tuberculosis | No increased risk of tuberculosis Extremely rare risk of tuberculosis (about 1 in 10,000 patients) |
| Extremely rare adverse events | No increased risk of neurologic disease or heart failure. Extremely rare risk of neurologic disease or heart failure (about 1 in 10,000 patients) |
| Risk of cancer | No increased risk of cancer Possible increased risk of cancer (about 1 in 1000 patients) |
The specifics related to ACA have been previously described (11, 13, 14). Briefly, ACA is an interactive computer program that determines how respondents value specific product characteristics by asking them to answer a series of rating and paired-comparison tasks (examples are provided in Appendix B). Each task includes a subset of the characteristics under consideration. For details describing how ACA constructs specific questions please see http://www.sawtoothsoftware.com/download/techpap/acatech.pdf.
ACA uses each respondent’s answers to calculate values for each estimate of each medication characteristic with least squares regression analysis (19). We calculated the importance that respondents assigned to each characteristic by dividing the range of values for each characteristic by the sum of ranges, and multiplying by 100. The relative importances are proportions and sum to 100 (19). We then created a variable representing the ratio of the importance that patients attach to overall benefit (average of values for all benefits) versus overall risk (average of values for all risks). Subjects having a risk ratio of less than one, i.e. attaching greater importance to the risk of toxicity than to the likelihood benefit, were classified as being risk averse.
Participants also completed a questionnaire to ascertain gender, race, maximum level of education obtained, marital status, employment status, annual household income, insurance, functional status [Health Assessment Questionnaire (HAQ) (20)], and use of disease modifying drugs for RA. For the purpose of analyses, income was dichotomized at $40,000. Patients who refused to report their income and were on Medicaid were classified as having an income < $40,000; those who refused and had private insurance were classified as having an income of at least $40,000. Other refusals were classified as missing. All data (ACA and patient characteristics) were collected in face-to-face interviews with the help of a research assistant who used a standardized script to describe the medications characteristics.
Statistical Analyses
Patient characteristics were entered into SAS computer files (SAS Software, version 8.0, SAS Institute, Inc., Cary, North Carolina). Preference data derived from ACA were imported into SAS and merged with the patient characteristics data set.
We examined the association between race and the relative importance assigned to each medication characteristic using the Mann-Whitney U test. The relation of risk aversion to each covariate was assessed using the chi-square or Mann-Whitney U test for categorical and continuous variables respectively. We subsequently used logistic regression to ascertain associations between respondent characteristics (found to be significant at p< 0.05 in bivariate analyses) and risk aversion as defined above. Sensitivity analysis using cut-offs of p < 0.2 or p < 0.1 did not affect the results.
Results
Subject Characteristics
The mean age of the study sample was 55 years (range 22–84), 83% percent were women, 49% were Black and 51% were White. Fifty-seven subjects were recruited from the Washington Hospital Center and 79 subjects were recruited from Virginia Commonwealth University. Participant characteristics by race are described in Table 2. White subjects reported having a higher household income and were more likely to be married, college educated and employed compared to Black subjects.
Table 2.
Patient characteristics by race
| Characteristic | Black (Number=67) | White (Number=69) |
|---|---|---|
| Mean age (range) | 55 (22–84) | 55 (22–77) |
| Women (%) | 61 (91) | 52 (75) |
| Married (%) | 12 (19) | 42 (61) |
| At least some college education (%) | 33 (49) | 44 (64) |
| Currently employed (%) | 16 (24) | 30 (43) |
| Income at least $40,000 (%) | 14 (23) | 34 (52) |
| Insured (%) | 64 (96) | 60 (88) |
| Median number of disease modifying drugs used (range) | 2 (0–5) | 2 (0–5) |
| Mean HAQ* (range) | 1.4 (0–2.6) | 0.91 (0–2.4) |
| Median duration of RA (in years, range) | 7 (0.2–32) | 8 (0.1–49) |
Possible score can range from 0 to 3.
Relative Importances
The relative importances that subjects assigned to each characteristic are presented in Table 3. The distribution of the relative importances suggests that subjects assigned some importance to almost all characteristics. However, Black subjects were most influenced by the risk of cancer (relative importance of 13%), whereas White subjects were most influenced by the likelihood of remission and radiographic progression (relative importance of 15%). Black subjects were more concerned about the risks of toxicity, whereas White subjects assigned greater importance to the likelihood of benefit (Figure 1).
Table 3.
Relative importances by race
| Treatment Characteristic | Relative Importances (Median*) | p-value | |
|---|---|---|---|
| Black | White | ||
| Likelihood of remission | 11 | 15.7 | <0.0001 |
| Likelihood of symptoms improving | 9.9 | 14.5 | <0.0001 |
| Likelihood of arresting radiographic progression | 9.8 | 15.4 | <0.0001 |
| Route of administration | 10.2 | 7.8 | 0.02 |
| Risk of injection reaction | 8.6 | 6.3 | 0.03 |
| Risk of nausea | 7.4 | 5.9 | 0.3 |
| Risk of lung injury | 10.5 | 9.3 | 0.3 |
| Risk of TB | 8.6 | 4.4 | <0.0001 |
| Risk of neurologic disease | 8.6 | 6.3 | 0.002 |
| Risk of cancer | 13.6 | 10.3 | 0.008 |
The relative importances are proportions. The relative importances do not sum to 100 because the values presented are medians and not means.
Figure 1.
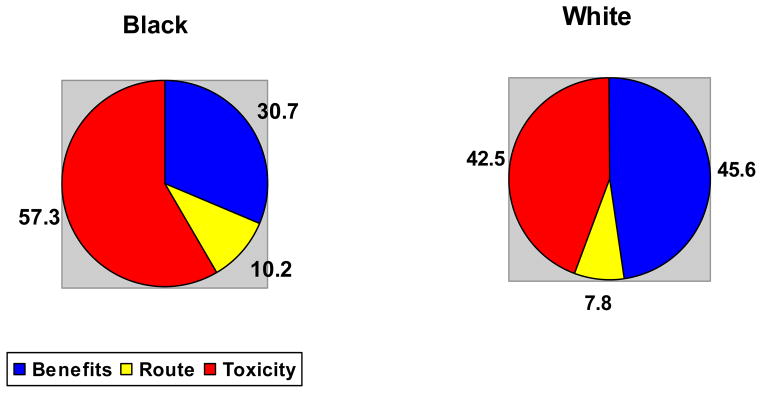
Relative impact of risks versus benefits amongst Black and White subjects.
Risk Aversion
Fifty-two percent of Black subjects were found to be risk averse compared to 12% of the White subjects (p< 0.0001). We found no associations between duration of RA (p= 0.5), disability (p= 0.9) or number of previous anti-rheumatic drugs used (p= 0.9) and risk aversion. Risk aversion was significantly associated with marital status and education as reported in Table 4.
Table 4.
Association between patient characteristics and risk aversion (Bivariate Analyses)
| Characteristic | Risk Averse (%) | P-value |
|---|---|---|
| Race | ||
| Black | 35 (52) | <0.0001 |
| White | 8 (12) | |
| Marital Status | ||
| Married | 11 (20) | 0.02 |
| Not married | 32 (39) | |
| Employment status | ||
| Employed | 11 (24) | 0.2 |
| Not employed | 32 (36) | |
| Education | ||
| At least some college education | 16 (21) | 0.002 |
| No college education | 27 (46) | |
| Income | ||
| At least $40,000 | 10 (21) | 0.05 |
| Less than $40,000 | 29 (38) | |
| Age | ||
| Less than 65 | 37 (35) | 0.2 |
| 65 or older | 6 (21) | |
In a multivariate model adjusting for marital status, education, and income, race remained strongly associated with risk aversion [adjusted odds ratio (95% CI) = 8.4 (3.1 – 23.1)] (Table 5). Adjusting for research site in the multivariate model did not affect the results and the association between risk aversion and race persisted when examined separately in each research location (p <0.01 at both sites).
Table 5.
Association between patient characteristics and risk aversion
| Characteristic | Adjusted Odds Ratio (95% CI) |
|---|---|
| Black | 8.4 (3.1 – 23.1) |
| No college education | 3.5 (1.4 – 8.6) |
| Married | 0.7 (0.2 – 2.01) |
| Income < $40,000 | 1.3 (0.4 – 3.8) |
Given the strong association between education and race, we performed further analyses and found that risk aversion was greater among Black compared to White subjects among subjects with a college education (36% versus 9%, p= 0.004) as well as those without a college education (68% versus 16%, p< 0.0001).
Discussion
In this study, we found significant differences in the ways that Black and White patients evaluate treatment characteristics. Specifically, we found that Black patients attach greater importance to the risks of toxicity (particularly for serious, albeit rare, adverse events) and less importance to the likelihood of benefit than their White counterparts. Use of conjoint analysis enabled us to quantify the importance that patients attach to specific medication characteristics, thus making it possible to gain insight into the reasons underlying differences in patients’ preferences. Because results were based solely on trade-offs between treatment characteristics, the relative importance data were not biased by physicians’ preferences or previously formed opinions based on external sources of information.
To date, studies of differences in treatment by race have been looked upon largely as correctable via changes either in healthcare practitioners or the healthcare system (21). This view stems from the disparities literature which has largely focused on access to care (22–24), insurance (25), quality of care (presumably because of unconscious practitioner bias) (26–29), and social determinants of health (30). Some studies have now shown that physicians’ practice environment may also contribute to health disparities. Primary care physicians caring for poorer, more likely minority, patients may often not have the training, practice or financial resources to deliver highest quality of care (31) including more technically complicated, more expensive, or newer therapies.
Included in the Institute of Medicine’s causal model of health disparities (21) is an acknowledgement that a component of disparities in care received may be related to disparate preferences for care by race. Yet, few studies have tested this notion (32–36). Our study is important because, to the best of our knowledge, this is the first study to formally assess whether risk preference for therapy is one of the potential explanations of the lower use among Blacks of more effective, though more risky, therapy for a chronic disabling disease. Our study adds to the under-researched field of racial/cultural differences in risk/benefit perception (37) and suggests an important influence of these differences on care-seeking and health disparities.
We found a contrasting risk preference result for Blacks versus Whites. Risk seeking and risk aversion for gains and losses have been well studied. Kahnemann-Tversky’s important Prospect Theory is based on several standard studies showing that people as a whole tend to seek certainty in potential gains (i.e., risk-aversion in a gain-frame) and avoid certainty in potential losses (i.e., risk-seeking in a loss-frame). Additionally, Prospect Theory asserts that people are overly swayed by very unlikely but potentially disastrous results (38).
However, many “standard” studies of risk preferences leading to Prospect Theory, and many studies since, have been conducted in university students and/or faculty (39, 40). Further, studies of patients on this research topic, and all research in general, have mostly examined well-educated and higher income subpopulations (41), whereas minorities have been underrepresented (42). This omission of subpopulations is important, since previous literature has shown that one subculture’s medical beliefs and behaviors may not be concordant with another subculture’s beliefs, or with those of biomedicine. Discrepant models of health and illness may lead to discrepant patient preferences, as well as diminished effectiveness of communication during the clinical visit (43).
In our study, the preferences of Blacks predicted they would be less risk-seeking for gains than White patients when offered a more effective but more risky therapy. Why did Black patients choose not to seek the gain of therapy from a more effective therapy for a chronic disabling disease? We theorize one explanation is that Blacks have learned “cultural risk aversion for gains”. Cultural risk aversion for gains is based either on learned distrust of the healthcare system (44) or on low expectations of the healthcare system by a subculture (45) that arises when a cultural subgroup has watched significant gains in lifespan, economic prosperity, and power of the larger culture, but has not themselves experienced parallel gains, even though they live in the same country/larger culture (46). In that case, the disadvantaged subgroup would doubt their own likelihood of experiencing any proposed gain offered to them that is also offered to the larger culture. Similarly, an excluded, poor, or socially isolated subculture, who perceives oppression even from the medical establishment, could as a group be more risk averse for medical losses as well as medical gains. Thus, risking the loss of their existing state of health, no matter how small, might loom large compared to a potential gain in health from a risky procedure or therapy.
There are several limitations of this study. Consecutive RA patients were recruited, and the participants may not be representative of other community-based samples. Participants were fairly well educated, and it is possible that results could differ in a predominantly non- or low literate population. We collected few variables indicative of socioeconomic status and future work should examine the influence of a broader spectrum of these markers including health literacy. The decision to classify subjects who refused to list income and had private insurance, in an income bracket of > $40,000 was made a priori and has not been validated. In addition, we did not include out-of-pocket costs in the ACA questionnaire as they differed markedly between insurance plans. The study was conducted in patients with prevalent disease currently taking medications, since recruiting treatment naïve patients with new onset disease would not have been feasible. We did not include physician characteristics or descriptors of the patient-physician relationship (such as trust) in our study. While our methods do not replicate decision-making in clinical practice, the approach used in this study allowed us to conclude that the observed findings were due to differences at the patient and not provider level.
Given these results, physicians should confirm that patients have accurate expectations regarding the natural history and treatment of their disease, and ensure that patient preferences are based on an informed assessment of the pros and cons related to available treatment options. Improved awareness and understanding of how RA patients weigh treatment risks and benefits will hopefully improve uptake, adherence and outcomes.
Acknowledgments
Dr. Fraenkel is supported by the K23 Award AR048826-01 A1. Dr. Smith is supported by NHLBI 1U10HL083732, by National Center on Minority Health and Health Disparities 5P60MD002256, by NHLBI 1R25HL092622, and by NHLBI 1U54HL090516.
We would like to thank Drs. Shoba Wani, Brian Walitt, Paul DeMarco, Lawrence Schwartz, Lenore Buckley, Christopher Wise, Neal Roberts, George Moxley, Francoise Mullen, and DeLona Norton for assisting with recruitment. In addition, we are indebted to all the patients who participated in the study for their time and interest.
Appendix A. Standardized Descriptions
Subcutaneous Injection
An injection given right under the skin, like an insulin injection. You can give it yourself or have someone else do it. It can be given at home or in a clinic.
Intravenous Infusion
This means the medicine will be given to you through a needle placed in a vein in your arm. It is given by a nurse in a clinic. It will take about 2 hours to give you the full dose of medicine.
Remission
This means that you do not have any joint pain, swelling or stiffness, but you still need to continue to take your medications.
Reversible Side Effects
The arthritis medication can cause mild or moderate nausea and vomiting (you sometimes feel a little queasy and vomit about once a day). The nausea and vomiting go away after the dose of the medication is lowered or if necessary when the medication is stopped.
Liver Damage
The arthritis medication can cause liver damage. People with liver damage may become tired, weak, and lose their appetite. Many patients don’t get other symptoms, but in some, the liver damage gets worse, and can cause yellow skin, intense itching, and bloating of the stomach.
Lung Damage
The arthritis medication can cause lung problems that cause a dry cough, shortness of breath, and fever. Patients with this side effect need to be admitted to the hospital for treatment with oxygen and intravenous medications (steroids by vein). Treatment takes an average of two weeks.
Risk of Tuberculosis (TB)
Before starting the infusion medication, patients will be examined for TB with a skin test. If this test is positive, you will take a medication for 9 months and this medication will decrease the risk of TB becoming active.
Heart Failure
Patients with heart failure have shortness of breath during activity or, sometimes, even without activity. It occurs when the heart doesn’t pump as well as it should. Heart failure can cause fluid to build up in the legs and lungs.
Neurologic Disease
There have been rare cases where people taking the medication have developed disorders that affected their nervous system. Signs that can indicate that you might have a problem include: changes in your vision, weakness in your arms and/or legs, and numbness or tingling in any part of the body.
Risk of Cancer
Theoretical risk of cancer means that because the medication affects the immune system, it has the potential to increase cancer risk with long-term use. An increased risk has not been shown in studies of this drug in comparison with patients with RA not on medications, but the studies have followed patients for less than 5 years. If the medication does turn out to increase the risk of cancer after long-term use, the risk might be 1/1000.
Appendix B. Examples of ACA Questions
Rating Question
If two medications were acceptable in all other ways, how important would this difference be?
Paired-Comparison Question
Contributor Information
Florina Constantinescu, Virginia Commonwealth University.
Suzanne Goucher, Washington Hospital Center.
Arthur Weinstein, Georgetown University, Washington Hospital Center.
Wally Smith, Virginia Commonwealth University.
Liana Fraenkel, Yale University School of Medicine; Clinical Epidemiology Research Center, VA CT Healthcare System, West Haven, CT.
References
- 1.Braverman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research. JAMA. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 2.Byrne MM, Souchek J, Richardson M, Suarez-Almazor M. Racial/ethnic differences in preferences for total knee replacement surgery. J Clin Epidemiol. 2006;59:1078–86. doi: 10.1016/j.jclinepi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African-American, Hispanic, and White primary care patients. Med Care. 2003;41:479–89. doi: 10.1097/01.MLR.0000053228.58042.E4. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim SA. Racial/ethnic variations in physician recommendations for cardiac revascularization. Am J Public Health. 2003;93:1689–93. doi: 10.2105/ajph.93.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long JA, Chang VW, Ibrahim SA, Asch DA. Update on the health disparities literature. Ann Intern Med. 2004;141:805–12. doi: 10.7326/0003-4819-141-10-200411160-00013. [DOI] [PubMed] [Google Scholar]
- 6.Miranda J, Cooper LA. Disparities in care for depression among primary care patients. J Gen Intern Med. 2004;19:120–6. doi: 10.1111/j.1525-1497.2004.30272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez-Almazor ME. Ethnic variation in knee replacement: patient preferences or uninformed disparity? Arch Intern Med. 2005;165:1117–24. doi: 10.1001/archinte.165.10.1117. [DOI] [PubMed] [Google Scholar]
- 8.Berrios-Rivera JP, Johnson ML, Huston DP, Suarez-Almazor ME. Disparities in the use of biologic therapies for the treatment of rheumatoid arthritis. Arthritis Rheum. 2006;54:S347. [Google Scholar]
- 9.Bruce B, Fries JF, Murtagh KN. Health status disparities in ethnic minority patients with rheumatoid arthritis: a cross-sectional study. J Rheumatol. 2007;34:1475–9. [PubMed] [Google Scholar]
- 10.Constantinescue F, Goucher S, Weinstein A, Fraenkel L. Racial disparities in treatment preferences for rheumatoid arthritis. Med Care. doi: 10.1097/MLR.0b013e31818af829. in press; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh J, Cuttler L, Shin M, Silvers JB, Neuhauser D. Medical decision-making and the patient: understanding preference patterns for growth hormone therapy using conjoint analysis. Med Care. 1998;36:AS31–45. doi: 10.1097/00005650-199808001-00005. [DOI] [PubMed] [Google Scholar]
- 12.Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res. 2002;37:1681–705. doi: 10.1111/1475-6773.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–3. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraenkel L, Bogardus S, Wittink DR. Understanding patient preferences for the treatment of lupus nephritis with adaptive conjoint analysis. Med Care. 2001;39:1203–16. doi: 10.1097/00005650-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Breedveld F, Emery P, Keystone E, et al. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004;63:149–55. doi: 10.1136/ard.2003.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 17.Smolen JS, Van der Heijde D, St Clair EW, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006;54:702–10. doi: 10.1002/art.21678. [DOI] [PubMed] [Google Scholar]
- 18.van der Heijde DLK, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–74. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RM. Adaptive Conjoint Analysis. Sawtooth Software Conference Proceedings; 1987. pp. 253–65. [Google Scholar]
- 20.Fries JF, Spitz PW, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 21.Smedley BD, Stith AY, Nelson AR. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2002. [PubMed] [Google Scholar]
- 22.Affairs CoEaJ. Black-white disparities in health care. JAMA. 1990;263:2344–6. doi: 10.1001/jama.1990.03440170066038. [DOI] [PubMed] [Google Scholar]
- 23.Andrulis DP. Access to care is the centerpiece in the elimination of socioeconomic disparities in health. Ann Intern Med. 1998;129:412–6. doi: 10.7326/0003-4819-129-5-199809010-00012. [DOI] [PubMed] [Google Scholar]
- 24.Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(Suppl 1):108–45. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- 25.Hargraves JL, Hadley J. The contribution of insurance coverage and community resources to reducing racial/ethnic disparities in access to care. Health Serv Res. 2003;38:809–29. doi: 10.1111/1475-6773.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American College of Physicians. Racial and ethnic disparities in health care: a position paper of the American College of Physicians. Ann Intern Med. 2004;141:226–32. doi: 10.7326/0003-4819-141-3-200408030-00015. [DOI] [PubMed] [Google Scholar]
- 27.Lavizzo-Mourey R, Lumpkin JR. From unequal treatment to quality care. Ann Intern Med. 2004;141:221. doi: 10.7326/0003-4819-141-3-200408030-00011. [DOI] [PubMed] [Google Scholar]
- 28.Coffey RM, Andrews RM, Moy E. Racial, ethnic, and socioeconomic disparities in estimates of AHRQ patient safety indicators. Med Care. 2005;43(3 Suppl):I48–57. doi: 10.1097/00005650-200503001-00008. [DOI] [PubMed] [Google Scholar]
- 29.Smith WR, Betancourt JR, Wynia MK, et al. Recommendations for teaching about racial and ethnic disparities in health and health care. Ann Intern Med. 2007;147:654–65. doi: 10.7326/0003-4819-147-9-200711060-00010. [DOI] [PubMed] [Google Scholar]
- 30.Pincus T, Esther R, DeWalt DA, Callahan LF. Social conditions and self-management are more powerful determinants of health than access to care. Ann Intern Med. 1998;129:406–11. doi: 10.7326/0003-4819-129-5-199809010-00011. [DOI] [PubMed] [Google Scholar]
- 31.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–84. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 32.Kressin NR. Racial differences in cardiac catheterization as a function of patients’ beliefs. Am J Public Health. 2004;94:2091–7. doi: 10.2105/ajph.94.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong K, Hughes-Halbert C, Asch DA. Patient preferences can be misleading as explanations for racial disparities in health care. Arch Intern Med. 2006;166:950–4. doi: 10.1001/archinte.166.9.950. [DOI] [PubMed] [Google Scholar]
- 34.Whittle J, Conigliaro J, Good CB, Joswiak M. Do patient preferences contribute to racial differences in cardiovascular procedure use? J Gen Intern Med. 1997;12:267–73. doi: 10.1046/j.1525-1497.1997.012005267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341:1661–9. doi: 10.1056/NEJM199911253412206. [DOI] [PubMed] [Google Scholar]
- 36.Katz JN. Patient preferences and health disparities. JAMA. 2001;286:1506–9. doi: 10.1001/jama.286.12.1506. [DOI] [PubMed] [Google Scholar]
- 37.Chrisman NJ. The health seeking process: an approach to the natural history of illness. Cult Med Psychiatry. 1977;1:351–77. doi: 10.1007/BF00116243. [DOI] [PubMed] [Google Scholar]
- 38.Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–91. [Google Scholar]
- 39.Inukai K, Takahashi T. Distinct neuropsychological processes may mediate decision-making under uncertainty with known and unknown probability in gain and loss frames. Med Hypotheses. 2006;67:283–6. doi: 10.1016/j.mehy.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology. 2005;182:508–15. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- 41.Olson EA, Hoopera CJ, Collinsa P, Luciana M. Adolescents’ performance on delay and probability discounting tasks: Contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Pers Indiv Differ. 2007;43:1886–97. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durant RW, Davis RB, St George DM, Williams IC, Blumenthal C, Corbie-Smith GM. Participation in research studies: factors associated with failing to meet minority recruitment goals. Ann Epidemiol. 2007;17:634–42. doi: 10.1016/j.annepidem.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pachter LM. Culture and clinical care. Folk illness beliefs and behaviors and their implications for health care delivery. JAMA. 1994;271:690–4. doi: 10.1001/jama.271.9.690. [DOI] [PubMed] [Google Scholar]
- 44.Dovidio JF, Penner LA, Albrecht TL, Norton WE, Gaertner SL, Shelton JN. Disparities and distrust: The implications of psychological processes for understanding racial disparities in health and health care. Soc Sci Med. 2008;67:478–86. doi: 10.1016/j.socscimed.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Dayton E, Zhan C, Sangl J, Darby C, Moy E. Racial and ethnic differences in patient assessments of interactions with providers: disparities or measurement biases? Am J Med Qual. 2006;21:109–14. doi: 10.1177/1062860605285164. [DOI] [PubMed] [Google Scholar]
- 46.Byrd WM, Clayton LA. An American health dilemma: a medical history of African Americans and the problem of race. New York: Routlege; 2000. [Google Scholar]


