Abstract
The enteric nervous system (ENS) is the largest subdivision of the peripheral nervous system and forms a complex circuit of neurons and glia that controls the function of the gastrointestinal (GI) tract. Within this circuit there are multiple subtypes of neurons and glia. Appropriate differentiation of these various cell subtypes is vital for normal ENS and GI function. Studies of the pediatric disorder Hirschprung’s Disease (HSCR) have provided a number of important insights into the mechanisms and molecules involved in ENS development, however there are numerous other GI disorders that potentially may result from defects in development/differentiation of only a subset of ENS neurons or glia. Our understanding of the mechanisms and molecules involved in this process is far from complete. Critically, it is unclear at what point the fates of enteric neural crest cells (ENCCs) become committed to a specific subtype cell fate and how these cell fate choices are made. We will review our current understanding of ENS differentiation and highlight key questions that need to be addressed in order to gain a more complete understanding of this biological process.
Keywords: Neural Crest, ENS, Differentiation, Transcriptional Regulation
Introduction
The enteric nervous system (ENS) is the largest subdivision of the peripheral nervous system. The ENS is responsible for regulating peristalsis, blood flow, and water and electrolyte transport in the gut (1, 2). ENS development is a coordinated process in which neural crest derived ENS precursors must migrate from specific axial locations to and then along the gut. Subsequently they differentiate into the various types of neurons and glia that make up the ENS. Defects in ENS precursor migration, proliferation and differentiation have been shown to lead to hypo and aganglionosis phenotypes in several model systems. In human’s intestinal aganglionosis, when present in newborns, leads to the gastrointestinal (GI) disorder Hirschprung’s Disease (HSCR) (3–7)
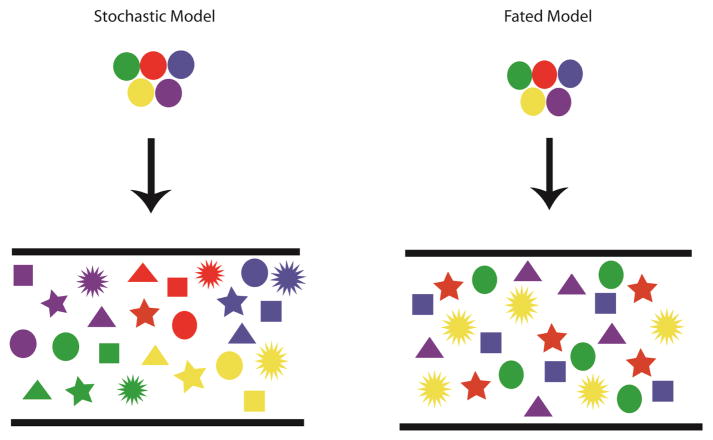
Differentiation of the enteric neural crest derived cells (ENCCs) is one of the key processes in the formation of a fully functional ENS. While studies have begun to elucidate the complexities behind this process there are still significant gaps in our understanding. The ENS is a complex system that is made up of up to 17 different subtypes of neurons (1). How this diversity in neuronal subtypes is generated is one the central unanswered questions in the field. Several studies have shown that ENS neurons and glia can be traced back to specific axial populations of neural crest cells but it is unclear exactly when the fate of these neural crest cells becomes determined to generate ENCCs (4, 8–11). It is also unclear at what stage during ENS development these ENCCs become fated to generate a specific subtype of enteric neuron or glia. Potentially, this could be a stochastic process only occurring when ENCCs migrate along the gut and is completely dependent on the ENCCs’ final location within the GI tract. At the other extreme, individual ENCCs could be fated to become specific subtypes during the formation of the ENCC population in the pre-migratory/pre-enteric neural crest (Figure 1).
Figure 1.
Models of ENCC Differentiation. There are two models that could explain the differentiation of the ENCCs into the various subtypes in the gut. The first is a stochastic model in which the fate of the ENCCs is not specified until they reach the gut. In this model any kind of ENS cell subtype can come from any ENCC depending on where it ends up in the gut. In the fated model ENCCs are fated to become specific subtypes early in development and cells derived from one specific ENCC are all fated to become a specific subtype. The different colored shapes indicate different subtypes of enteric neurons and glia.
Understanding the processes and mechanisms that regulate differentiation of the various ENS subtypes will not only help us to understand the development of the ENS but may also help us gain a better understanding of the pathologies of various GI disorders. While human aganglionosis disorders have been extensively studied, pathological analysis of patients has been limited to looking for the presence or absence of neurons in the gut. Because there are a large number of GI motility disorders that present in the clinic with no obvious underlying cause, it is quite plausible that some of these conditions result from the absence/loss of a specific subset of ENS neurons or glia (12). A better understanding of the mechanisms involved in the development of subtypes of enteric neurons and glia may give significant insights into the etiologies of some currently unexplained GI motility disorders.
Early ENS Specification of the Neural Crest
One major unanswered question in ENS development is how early during embryogenesis does ENS specification occur? The key event appears to be the formation of specific axial populations of neural crest cells (NCCs). Classic chick-quail chimera studies indicate that cells within the vagal neural crest are sufficient to form most of the ENS (13, 14). In the zebrafish model system, the ENS is completely formed from vagal neural crest cells, however in mammals and chicks the ENS is formed not only from the vagal cells but is also derived in part from sacral neural crest cells (15–18). These vagal and sacral neural crest cells appear to develop in a semi-cell autonomous manner indicating there is some level of specification that occurs early on during neural crest formation. When vagal and sacral neural crest cells were reciprocally transplanted to the other axial location, transplanted cells went on to form structures appropriate for their new axial location. However sacral crest cells were not as efficient at generating ENS neurons and glia as vagal crest cells (8, 9). Similarly, when vagal NCCs were transplanted to the sacral region the transplanted cells followed the normal migration route of sacral cells to the gut but did so earlier and in a much greater number (10). It appears that while there is some flexibility in the axial origin of the neural crest that gives rise to the ENCCs, vagal NCCs are the preferential axial source of NCCs for the ENS and a critical number of NCCs are necessary for normal formation of the ENS (19). When the number of vagal NCC derived ENCC precursors is reduced the rate of ENCC migration along the gut proceeds at a much slower rate (20). This may be due to a lack of cell-cell contact between the low numbers of ENCCs (20). Mathematical modeling of the process of ENCC colonization of the gut suggests that proliferation differences between the different axial neural crest populations determine their ability to generate a complete ENS (21). These models show that cranial neural crest have a spatially determined proliferative advantage in forming an ENS in comparison to trunk neural crest (21). Given these results, it is clear that distinct populations of NCCs are specified to be ENCCs within the vagal neural crest and these cells are needed to correctly populate the gut with neurons and glia.
It is clear that the spatial organization of the neural crest is important for determining the eventual fate of the ENCCs. One key anatomical structure that affects the early specification of specific vagal neural crest derivatives is the dorsal aorta. Expression of BMP4 and 7 from the dorsal aorta induces the expression of pro-sympathetic neural genes. Inhibition of BMP expression from the dorsal aorta prevents sympathetic neuron formation (22, 23). The expression of BMP by the dorsal aorta gives rise to a concentration gradient of BMP ligand in this region and this means that as neural crest cells migrate towards the gut they are exposed to varying BMP concentrations. As a result, NCCs may acquire different cell fates within the ventrally migrating stream depending on the length of time and the concentration of BMP ligand to which they are exposed to during their migration. Similarly, there is a gradient of Wnt expression extending from the neural tube laterally. NCCs that express β-catenin, a down stream signaling component of the canonical signaling pathway, at high levels have been shown to form sensory neurons (24, 25). While these concentration gradients have been well studied for sympathetic neurons their specific affects on ENCCs are less clear and needs further study.
The previously discussed embryological chick-quail chimera studies suggest that there is some level of fate determination that is occurring early on in the formation of the neural crest but the molecular basis behind this fate determination is unclear. At the earliest stage of neural crest specification, the transcription factors FoxD3 and Sox10 are expressed by the neural crest at the stage when it arises from the neuroepithelium (26, 27). Functionally, FoxD3 has been shown to be important in the early selection of neuronal cell fates as opposed to non-neuronal cell fates within the neural crest (27). Sox10 is also expressed throughout neural crest development in the vagal and sacral regions and continues to be expressed in ENCCs when they reach the gut and begin migrating along it (11, 16). Sox10 along with Pax3 induce expression of the tyrosine kinase RET, a key gene in ENS development and an early ENCC marker that has been shown to be the primary gene associated with HSCR (16, 28–30). RET’s initial function is to promote the survival of ENCCs, acting as the signal transducing component of the GDNF receptor along with its co-receptor GFR(alpha)1 (31, 32). Phox2b is another early marker whose expression is dependent on Sox10 (33, 34). Not only is Phox2b Sox10-dependent, but it also is expressed by ENCCs throughout their migration along the gut (34). Phox2b also appears to be important for the expression of RET as well as for the expression of the basic helix-loop-helix (bHLH) transcription factors Ascl1 and Hand2 (33, 35). Furthermore, in addition to the previously mentioned transcription factors, Hox genes are involved in determining the fate of neural crest cells. Studies have shown that the different axial populations of NCCs express different Hox genes, dependent on their axial origin, and this NCC Hox gene expression affects their cell fate (36, 37). Vagal neural crest cells express HoxB3 and this expression may play a role in determining the ENCC fate for a subset of these cells (38).
Several of the identified transcription factors are expressed in both the pre-migratory and migratory vagal neural crest precursors, his suggests that these factors potentially have an important role in ENCC/ENS fate determination. However, the full complement of transcription factors that are responsible for the selection of ENS precursor cell fate as opposed to other neural crest cell derivatives has not been elucidated. In addition, while these transcription factors are required for ENS development, it is not clear whether any of these genes confers a specific ENCC cell fate for an individual NCC or whether their function is simply to confer a commitment to a neural/glia cell fate.
Post Neural Crest Differentiation Control
Once the ENCCs begin to enter the gut their environment changes significantly and the signals they receive become even more important for maintaining their proliferative potential and for determining their eventual ENS cell fate. In vitro studies have shown that sympathetic neuroblasts take on enteric characteristics when cultured with gut monolayers while enteric neuroblasts take on sympathetic characteristics when cultured with dorsal aorta monolayers (39). This clearly demonstrates the importance of environmental signals in regulating ENS differentiation (39). As the ENCCs enter the gut, they continue to express Sox10, RET, and Phox2b (11, 16, 31–33). Sox10 is critically important for maintaining the progenitor state of ENCCs and it appears that in mice Sox10 expressing cells maintain neurogenic potential into adulthood (40, 41). Sox10 also influences the expression of several other proteins shown to be involved in ENCC development including Ascl1, a bHLH transcription factor that actually represses Sox10 expression and promotes expression of pro-neural genes (26). This Sox10 driven expression of Ascl1 appears to be modulated by the notch pathway as a component of the notch signaling pathway, Hes1, represses Ascl1 expression (39). As a result, notch signaling is potentially important in maintaining the ENCCs progenitor potential at least for a subset of ENCCs though this requires further investigation (42, 43).
Sox10 also influences the expression of the G-Protein coupled receptor EDNRB, which has been shown to be important for complete ENCC colonization of the gut (44–46). EDNRB and its ligand endothelin-3 (ET-3), seem to prevent neuronal differentiation in ENCCs and helps maintain their potential to colonize the rest of the gut (45, 47, 48). Another gene that appears to prevent neuronal differentiation in favor of proliferation is sonic hedgehog (shh). shh is expressed in the developing gut endoderm. shh modulates ENCC responsiveness to GDNF promoting cell proliferation and migration while attenuating/inhibiting neuronal differentiation (49, 50).
Many of the genes that have been shown to be critical to ENS development appear to be expressed throughout this developmental process. Interestingly, many of these signaling pathways involved early in neural crest development often have additional/alternate functions later in ENCC specification. One example is the BMP family of proteins, specifically BMP4. BMP4 is involved in differentiation of neural crest-derived cells into neurons in vitro (51–53). It appears that ENCCs have a dose dependent response to BMP signaling, as low concentrations of BMP promote an ENCC to stay in an undifferentiated proliferative state while high concentrations promote neurogenesis (52, 53). One way in which BMP may influence neurogenesis is through its interaction with Ascl1 (54, 55). BMP2 decreases the stability of Ascl1 leading to an inhibition of certain pro-neural genes (56–58). Ascl1 appears to promote the expression of the transcription factor Phox2a and together these two transcription factors promote the expression of a subset of pro-neural genes. Previous studies looking at autonomic nervous system development have shown Ascl1 couples the expression of general neuronal markers with subtype specific markers (59, 60). Ascl1 is also critically important for the development of esophageal neurons as Ascl1−/− mice have perturbed gangliogenesis in the esophagus (61). This suggests that other basic helix-loop-helix (bHLH) pro-neural transcription factors are also involved in ENS development (62). One potential candidate bHLH transcription factor, expressed in ENCCs, is Hand2, whose expression is also regulated by BMP in vitro (63). Furthermore Hand2 has been shown to regulate expression and function of phox2a and phox2b genes in the development of sympathetic nervous system (SNS) and couples neurogenesis and cell type gene expression in the SNS (64–66).
Another gene that appears to have a bimodal role in ENS development is FoxD3. While the ENS glial population comes from the same ENCC precursor pool as the ENS neurons, in mice glial development lags behind the development of the neurons as glial markers are not seen in the early migrating ENCC chains as opposed to neuronal markers, which are observed (67–69). This is not the case however in the chick where ENS neuronal and glial differentiation occurs concurrently in the migrating chains of ENCC (70). FoxD3 has been shown to promote gliogenesis in the ENS, as well as influence proliferation and neural patterning (71). Notch signaling and the bHLH transcription factor Hand2 also affect the ENS glial cell development (72). However Hand2 appears to indirectly affect glial development, because the glial phenotype seen in Hand2−/− mice results from an overall reduced size of the initial ENCC progenitor pool rather than any specific effect on gliogenesis cell fate determination (73). Sox10 also is expressed in glia cells into adulthood allowing these cells to maintain their neurogenic potential (41, 74) These results clearly indicate that there are several regulatory pathways that function to maintain the cell fate potential in the ENCCs. However, it is less clear when these cells become further committed to a specific enteric neuron subtype or glial cell fate and how the switch from proliferating ENCC to committed neural or glial precursor is regulated.
Subtype Specification
Depending on the species, differentiation of ENCCs begins at different times during the migration process. In zebrafish, there appears to be two main waves of neuronal differentiation that occur at 72 and 96 hours post fertilization (hpf), respectively(75). This brings up an interesting problem as at 72hpf the ENCCs have migrated along the length of the gut, but a subset continue to proliferate and another group needs to circumferentially migrate around the gut to completely populate it. This means that only a specific subset of cells differentiate in this initial wave. While differentiation does not begin until after the anterior posterior migration along the gut is completed in zebrafish, mouse ENCCs in the anterior portion of the gut begin to differentiate before ENCCs have migrated to the posterior end of the gut (69). In both fish and mice, differentiation of all neuronal and glial subtypes does not occur at the same time. This indicates that ENCCs must be temporally restricted in the cell types to which they can give rise.
While there are specific waves of differentiation seen in the ENCCs of model organisms pan-neuronal markers appear much earlier in ENS development. These markers begin to appear as early as E10.5 in mice and in zebrafish between 24 and 48 hpf (76–78). These early cells are electrically active as early as E11.5 in mice, indicating that these cells have begun exhibiting both the molecular and physiological characteristics of neurons (79).
While certain pan-neuronal markers begin to show up early in ENS development the presence of specific markers for nitronergic (nNOS), serotonergic (5-HT), cholinergic (ChAT, VAChT), dopaminergic (DBH, TH) neurons appear at varying times during ENS formation (75, 80–85). Similarly various other neuronal and pan-neuronal markers (IkCa, CGRP, calbindin, calretinin, VIP, substance P) appear at varying times in ENS development (Figure 2) (80–82, 84, 85). The earliest expressed neuronal cell type specific marker in mice and zebrafish is nNOS, appearing around E11.5 in mice and between 48 and 72hpf in zebrafish (75, 80). Calbindin and IkCa channels also appear at E11.5 in mice but many other differentiation markers are absent (80). Substance P, VIP, and 5-HT neurons appear around E14 in mice while CGRP is not present until E17 (81, 82). Molecules involved in the synthesis of acetylcholine are present in mice between E10–12 but the ChAT and VAChT markers themselves do not appear until around E18.5 (83). Similarly in zebrafish expression of the markers for VIP, calbindin, CGRP, 5-HT and others do not appear until later in development between 72 and 96hpf (75, 84).
Figure 2.
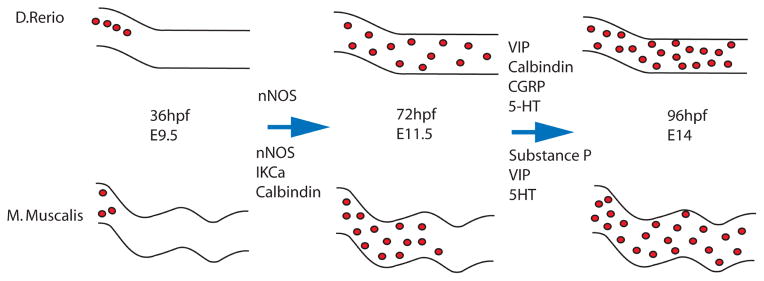
Schematic diagram of ENCCs populating the guts of D. Rerio and M. Muscalis. ENCCs enter the gut at 36hpf and E9.5 in mouse and zebrafish respectively. nNOS appears in zebrafish between 48–72hpf and around E11.5 in mice. IKCa and calbindin also appear in mice around E11.5. at 72hpf ENCCs have populated the posterior of the gut in zebrafish and a first wave of differentiation has occurred while ENCCs are still migrating to the posterior in mice. A 96hpf the second differentiation wave has occurred in zebrafish while at E14 ENCCs have populated the posterior gut in mice. Between 72 and 96hpf VIP, calbindin, CGRP, and 5HT appear in zebrafish. Substance P, VIP, and 5HT appear at E14 in mice while CGRP doesn’t appear until much later at E17. ChaT and VAChT also do not appear until later in mouse development at E18.5.
While it is clear that the markers of these different subtypes begin to appear at different times, it is less clearly understood how a particular subtype is specified in the ENS. So far only a few genes and signaling pathways have been shown to have a specific role in the specification of a specific ENS neuronal subtype. One of these genes, Ascl1, appears to regulate 5-HT ENS neuronal differentiation, as this type of neuron is particularly affected in Ascl1−/− mice (62). However Ascl1 does not appear to be solely responsible for 5-HT ENS neuron development as not all Ascl1 expressing ENCC/ENS neurons are 5-HT positive (86). 5-HT neurons, along with calretenin expressing neurons, are also influenced by norepinephrine transporter (NET) as NET −/− mice have reduced numbers of these neuronal subtypes (87). Neutrophin-3 (NT-3) appears to affect submucosal intrinsic primary afferent neurons expressing CGRP because there are fewer of these neurons in TrkC −/− mice, which is the receptor for NT-3 (88). Hand2 −/− mice also have a complete loss of nNOS and VIP enteric neurons and a significant reduction in the number of calretnin and ChAT enteric neurons (73, 89). It also appears that nNOS formation is influenced by neural activity as ENCCs exposed to tetanus toxin to block neural activity by SNARE mediated vesicle fusion caused a decrease in the number of nNOS enteric neurons(80). Interestingly GDNF-RET signaling can also influence subtype specification as increasing GDNF expression later in ENS development can alter the numbers of certain neuronal subtypes including nNOS neurons (90–92). This may be a result of the influence GDNF has determining when ENCC precursors leave the cell cycle. Different ENS neuronal subtypes arise at varying time points so by altering the stage at which ENCC leave the cell cycle will influence the respective proportions of different ENS neuronal subtypes (91). Clearly some ENS neuronal subtypes develop later than others so it is possible that the earlier born neurons influence the development of later developing neurons. Furthermore 5-HT expression in the ENS seems to influence the development of dopaminergic, GABAergic, CGRP expressing, and late born nitregic enteric neurons but it is not clear whether 5-HT directly influences their differentiation or it is simply important for their development/survival (93). Together these results indicate that it is a combination of extrinsic and intrinsic signals that lead to ENCC/ENS neuron and glial cell fate determination and differentiation.
Further insights into ENS cell subtype specification can be gained from examining specification in other parts of the peripheral nervous system. Sympathetic neuron expression of DBH is influenced by a combination of factors including Phox2a and Hand2 (94–96). Similarly, Phox2a and Hand2 influence sympathetic neuron expression of TH, however TH neurons respond differently to protein kinase A (PKA) activity as compared to DBH neurons. In TH neurons Phox2a and PKA act independently but in DBH neurons Phox2a and PKA synergistically (94, 97). Finally the cholinergic markers ChAT and VAChT in the parasympathetic system appear to be influenced by the expression of PKA as well as the transcription factor REST (80, 98).
It is clear from looking at studies into subtype specification in other neuronal systems that the formation of these subtypes is a complicated process involving the interaction of multiple transcription factors and signaling pathways. To fully understand the specific molecular combination of signals and factors that influence terminal cell fate specification in the ENS will require the generation of more conditional knockout and over-expression animal models. In addition, a better understanding of the precise lineage relationship between the different ENS neuronal and glial subtypes is still needed if we are to get a more complete understanding of this process. Given that different subtype markers appear at different times it is also possible that the different ENS neuron and glia subtypes may become specified at different times and that ENS development is some combination of the two models presented in Figure 1. Recent technological advances such as the development of the brainbow lineage reporter system will potentially allow us to gain a much clearer understanding of the lineage relationship within the developing ENS (99, 100).
Perspectives
The ENS is a complex, dynamic circuit of neurons and glia that are necessary for normal healthy digestion. Any errors in ENS formation can have drastic consequences for the development of an individual, as evidenced by the aganglionosis phenotype seen in Hirschprung’s disease. Furthermore we have barely begun to understand the neuronal basis of many GI disorders. The complex circuit that is the ENS is made up of multiple different subtypes of neurons, each of which are necessary for normal ENS function. Should the proper equilibrium of neuronal subtypes not form there is a risk the whole circuit will not work properly. While we can characterize the aganglionosis phenotype in HSCR comparatively easily, detecting GI disorders that affect only a specific subpopulation of ENS neurons and glia is far more challenging. Clinically the loss/reduction in number of a specific ENS neuronal subtype potentially could be the cause of many GI disorders that as of present have no known cellular basis.
While our understanding of the differentiation and development of the ENS has grown greatly over the past few years, there is still much that still needs to be determined. As we have pointed out in this review, while the appearance of subtypes has been well characterized the actual molecular basis for how this specification process occurs both cellularly and mechanistically is not well understood. How early does enteric neuron and glia subtype specification begin? When are ENCCs committed to a specific neuronal subtype fate? Is it extrinsic or intrinsic factors that determine a specific subtype specification? All of these questions and more will need to be answered in order to begin to gain a more complete understanding of the complex process of ENS formation.
Acknowledgments
We would like to thank Andreas Fritz, Stephen L’Hernault and Shanthi Srivanth for their comments on this manuscript.
References
- 1.Furness JCM. The Enteric Nervous system. Glasgow: Churchill Livingstone; 1987. [Google Scholar]
- 2.Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. The American journal of physiology. 1996;271(2 Pt 1):G223–30. doi: 10.1152/ajpgi.1996.271.2.G223. Epub 1996/08/01. [DOI] [PubMed] [Google Scholar]
- 3.Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 2. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2002;5(4):329–49. doi: 10.1007/s10024-002-0002-4. Epub 2002/05/23. [DOI] [PubMed] [Google Scholar]
- 4.Kusafuka T, Puri P. Genetic aspects of Hirschsprung’s disease. Seminars in pediatric surgery. 1998;7(3):148–55. doi: 10.1016/s1055-8586(98)70010-1. Epub 1998/08/27. [DOI] [PubMed] [Google Scholar]
- 5.Newgreen D, Young HM. Enteric nervous system: development and developmental disturbances--part 1. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2002;5(3):224–47. doi: 10.1007/s10024-001-0142-y. Epub 2002/05/15. [DOI] [PubMed] [Google Scholar]
- 6.Puri P, Ohshiro K, Wester T. Hirschsprung’s disease: a search for etiology. Seminars in pediatric surgery. 1998;7(3):140–7. doi: 10.1016/s1055-8586(98)70009-5. Epub 1998/08/27. [DOI] [PubMed] [Google Scholar]
- 7.Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Current opinion in pediatrics. 2000;12(6):610–7. doi: 10.1097/00008480-200012000-00017. Epub 2000/12/06. [DOI] [PubMed] [Google Scholar]
- 8.Erickson CA, Goins TL. Sacral neural crest cell migration to the gut is dependent upon the migratory environment and not cell-autonomous migratory properties. Developmental biology. 2000;219(1):79–97. doi: 10.1006/dbio.1999.9597. Epub 2000/03/15. [DOI] [PubMed] [Google Scholar]
- 9.Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Developmental Biology. 1974;41(1):162–84. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- 10.Burns AJ, Delalande JM, Le Douarin NM. In ovo transplantation of enteric nervous system precursors from vagal to sacral neural crest results in extensive hindgut colonisation. Development. 2002;129(12):2785–96. doi: 10.1242/dev.129.12.2785. Epub 2002/06/07. [DOI] [PubMed] [Google Scholar]
- 11.Southard-Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nature genetics. 1998;18(1):60–4. doi: 10.1038/ng0198-60. Epub 1998/01/13. [DOI] [PubMed] [Google Scholar]
- 12.Bassotti G, Villanacci V, Bellomi A, Fante R, Cadei M, Vicenzi L, et al. An assessment of enteric nervous system and estroprogestinic receptors in obstructed defecation associated with rectal intussusception. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2012;24(3):e155–61. doi: 10.1111/j.1365-2982.2011.01850.x. Epub 2011/12/23. [DOI] [PubMed] [Google Scholar]
- 13.Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. The Journal of comparative neurology. 1954;101(2):515–41. doi: 10.1002/cne.901010212. Epub 1954/10/01. [DOI] [PubMed] [Google Scholar]
- 14.Le Douarin N. A biological cell labeling technique and its use in expermental embryology. Developmental biology. 1973;30(1):217–22. doi: 10.1016/0012-1606(73)90061-4. Epub 1973/01/01. [DOI] [PubMed] [Google Scholar]
- 15.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. Journal of embryology and experimental morphology. 1973;30(1):31–48. Epub 1973/08/01. [PubMed] [Google Scholar]
- 16.Anderson RB, Stewart AL, Young HM. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell and tissue research. 2006;323(1):11–25. doi: 10.1007/s00441-005-0047-6. Epub 2005/09/01. [DOI] [PubMed] [Google Scholar]
- 17.Kapur RP. Colonization of the murine hindgut by sacral crest-derived neural precursors: experimental support for an evolutionarily conserved model. Developmental biology. 2000;227(1):146–55. doi: 10.1006/dbio.2000.9886. Epub 2000/11/15. [DOI] [PubMed] [Google Scholar]
- 18.Serbedzija GN, Burgan S, Fraser SE, Bronner-Fraser M. Vital dye labelling demonstrates a sacral neural crest contribution to the enteric nervous system of chick and mouse embryos. Development. 1991;111(4):857–66. doi: 10.1242/dev.111.4.857. Epub 1991/04/01. [DOI] [PubMed] [Google Scholar]
- 19.Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135(9):1681–91. doi: 10.1242/dev.017418. Epub 2008/04/04. [DOI] [PubMed] [Google Scholar]
- 20.Young HM, Bergner AJ, Anderson RB, Enomoto H, Milbrandt J, Newgreen DF, et al. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Developmental biology. 2004;270(2):455–73. doi: 10.1016/j.ydbio.2004.03.015. Epub 2004/06/09. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Brinas IM, Binder BJ, Landman KA, Newgreen DF. Neural crest regionalisation for enteric nervous system formation: implications for Hirschsprung’s disease and stem cell therapy. Developmental biology. 2010;339(2):280–94. doi: 10.1016/j.ydbio.2009.12.014. Epub 2010/01/20. [DOI] [PubMed] [Google Scholar]
- 22.Morikawa Y, Zehir A, Maska E, Deng C, Schneider MD, Mishina Y, et al. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development. 2009;136(21):3575–84. doi: 10.1242/dev.038133. Epub 2009/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reissmann E, Ernsberger U, Francis-West PH, Rueger D, Brickell PM, Rohrer H. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 1996;122(7):2079–88. doi: 10.1242/dev.122.7.2079. Epub 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 24.Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, et al. Lineage-specific requirements of beta-catenin in neural crest development. The Journal of cell biology. 2002;159(5):867–80. doi: 10.1083/jcb.200209039. Epub 2002/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HY, Kleber M, Hari L, Brault V, Suter U, Taketo MM, et al. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303(5660):1020–3. doi: 10.1126/science.1091611. Epub 2004/01/13. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38(1):17–31. doi: 10.1016/s0896-6273(03)00163-6. Epub 2003/04/15. [DOI] [PubMed] [Google Scholar]
- 27.Kos R, Reedy MV, Johnson RL, Erickson CA. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128(8):1467–79. doi: 10.1242/dev.128.8.1467. Epub 2001/03/23. [DOI] [PubMed] [Google Scholar]
- 28.Lang D, Epstein JA. Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer. Human molecular genetics. 2003;12(8):937–45. doi: 10.1093/hmg/ddg107. Epub 2003/04/02. [DOI] [PubMed] [Google Scholar]
- 29.Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122(1):349–58. doi: 10.1242/dev.122.1.349. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 30.Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119(4):1005–17. doi: 10.1242/dev.119.4.1005. Epub 1993/12/01. [DOI] [PubMed] [Google Scholar]
- 31.Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, et al. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126(12):2785–97. doi: 10.1242/dev.126.12.2785. Epub 1999/05/20. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi M, Matsuoka I. Enhancement of sympathetic neuron survival by synergistic action of NT3 and GDNF. Neuroreport. 2000;11(11):2541–5. doi: 10.1097/00001756-200008030-00039. Epub 2000/08/16. [DOI] [PubMed] [Google Scholar]
- 33.Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399(6734):366–70. doi: 10.1038/20700. Epub 1999/06/09. [DOI] [PubMed] [Google Scholar]
- 34.Elworthy S, Pinto JP, Pettifer A, Cancela ML, Kelsh RN. Phox2b function in the enteric nervous system is conserved in zebrafish and is sox10-dependent. Mechanisms of development. 2005;122(5):659–69. doi: 10.1016/j.mod.2004.12.008. Epub 2005/04/09. [DOI] [PubMed] [Google Scholar]
- 35.Vincentz JW, VanDusen NJ, Fleming AB, Rubart M, Firulli BA, Howard MJ, et al. A Phox2- and Hand2-dependent Hand1 cis-regulatory element reveals a unique gene dosage requirement for Hand2 during sympathetic neurogenesis. J Neurosci. 2012;32(6):2110–20. doi: 10.1523/JNEUROSCI.3584-11.2012. Epub 2012/02/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung H, Lacombe J, Mazzoni EO, Liem KF, Jr, Grinstein J, Mahony S, et al. Global control of motor neuron topography mediated by the repressive actions of a single hox gene. Neuron. 2010;67(5):781–96. doi: 10.1016/j.neuron.2010.08.008. Epub 2010/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber L, Ferdin M, Holzmann J, Stubbusch J, Rohrer H. HoxB8 in noradrenergic specification and differentiation of the autonomic nervous system. Developmental biology. 2012;363(1):219–33. doi: 10.1016/j.ydbio.2011.12.026. Epub 2012/01/13. [DOI] [PubMed] [Google Scholar]
- 38.Chan KK, Chen YS, Yau TO, Fu M, Lui VC, Tam PK, et al. Hoxb3 vagal neural crest-specific enhancer element for controlling enteric nervous system development. Developmental dynamics: an official publication of the American Association of Anatomists. 2005;233(2):473–83. doi: 10.1002/dvdy.20347. Epub 2005/03/16. [DOI] [PubMed] [Google Scholar]
- 39.Pisano JM, Birren SJ. Restriction of developmental potential during divergence of the enteric and sympathetic neuronal lineages. Development. 1999;126(13):2855–68. doi: 10.1242/dev.126.13.2855. Epub 1999/06/08. [DOI] [PubMed] [Google Scholar]
- 40.Paratore C, Eichenberger C, Suter U, Sommer L. Sox10 haploinsufficiency affects maintenance of progenitor cells in a mouse model of Hirschsprung disease. Human molecular genetics. 2002;11(24):3075–85. doi: 10.1093/hmg/11.24.3075. Epub 2002/11/06. [DOI] [PubMed] [Google Scholar]
- 41.Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. The Journal of clinical investigation. 2011;121(9):3412–24. doi: 10.1172/JCI58200. Epub 2011/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, et al. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(10):5355–60. doi: 10.1073/pnas.94.10.5355. Epub 1997/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.YOYS Notch signaling is required for the maintenance of enteric neural crest progenitors. Development. 2008;135(21):3555–65. doi: 10.1242/dev.022319. [DOI] [PubMed] [Google Scholar]
- 44.Zhu L, Lee HO, Jordan CS, Cantrell VA, Southard-Smith EM, Shin MK. Spatiotemporal regulation of endothelin receptor-B by SOX10 in neural crest-derived enteric neuron precursors. Nature genetics. 2004;36(7):732–7. doi: 10.1038/ng1371. Epub 2004/06/01. [DOI] [PubMed] [Google Scholar]
- 45.Bondurand N, Natarajan D, Barlow A, Thapar N, Pachnis V. Maintenance of mammalian enteric nervous system progenitors by SOX10 and endothelin 3 signalling. Development. 2006;133(10):2075–86. doi: 10.1242/dev.02375. Epub 2006/04/21. [DOI] [PubMed] [Google Scholar]
- 46.Kruger GM, Mosher JT, Tsai YH, Yeager KJ, Iwashita T, Gariepy CE, et al. Temporally distinct requirements for endothelin receptor B in the generation and migration of gut neural crest stem cells. Neuron. 2003;40(5):917–29. doi: 10.1016/s0896-6273(03)00727-x. Epub 2003/12/09. [DOI] [PubMed] [Google Scholar]
- 47.Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Developmental biology. 1998;197(1):93–105. doi: 10.1006/dbio.1998.8876. Epub 1998/06/13. [DOI] [PubMed] [Google Scholar]
- 48.Wu JJ, Chen JX, Rothman TP, Gershon MD. Inhibition of in vitro enteric neuronal development by endothelin-3: mediation by endothelin B receptors. Development. 1999;126(6):1161–73. doi: 10.1242/dev.126.6.1161. Epub 1999/02/18. [DOI] [PubMed] [Google Scholar]
- 49.Reichenbach B, Delalande JM, Kolmogorova E, Prier A, Nguyen T, Smith CM, et al. Endoderm-derived Sonic hedgehog and mesoderm Hand2 expression are required for enteric nervous system development in zebrafish. Developmental biology. 2008;318(1):52–64. doi: 10.1016/j.ydbio.2008.02.061. Epub 2008/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu M, Lui VC, Sham MH, Pachnis V, Tam PK. Sonic hedgehog regulates the proliferation, differentiation, and migration of enteric neural crest cells in gut. The Journal of cell biology. 2004;166(5):673–84. doi: 10.1083/jcb.200401077. Epub 2004/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizuseki K, Sakamoto T, Watanabe K, Muguruma K, Ikeya M, Nishiyama A, et al. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):5828–33. doi: 10.1073/pnas.1037282100. Epub 2003/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chalazonitis A, D’Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, et al. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(17):4266–82. doi: 10.1523/JNEUROSCI.3688-03.2004. Epub 2004/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gajavelli S, Wood PM, Pennica D, Whittemore SR, Tsoulfas P. BMP signaling initiates a neural crest differentiation program in embryonic rat CNS stem cells. Experimental neurology. 2004;188(2):205–23. doi: 10.1016/j.expneurol.2004.03.026. Epub 2004/07/13. [DOI] [PubMed] [Google Scholar]
- 54.Lo L, Sommer L, Anderson DJ. MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells. Current biology: CB. 1997;7(6):440–50. doi: 10.1016/s0960-9822(06)00191-6. Epub 1997/06/01. [DOI] [PubMed] [Google Scholar]
- 55.Schneider C, Wicht H, Enderich J, Wegner M, Rohrer H. Bone morphogenetic proteins are required in vivo for the generation of sympathetic neurons. Neuron. 1999;24(4):861–70. doi: 10.1016/s0896-6273(00)81033-8. [DOI] [PubMed] [Google Scholar]
- 56.Vinals F, Reiriz J, Ambrosio S, Bartrons R, Rosa JL, Ventura F. BMP-2 decreases Mash1 stability by increasing Id1 expression. The EMBO journal. 2004;23(17):3527–37. doi: 10.1038/sj.emboj.7600360. Epub 2004/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benavente F, Pinto C, Parada M, Henriquez JP, Osses N. Bone morphogenetic protein 2 inhibits neurite outgrowth of motor neuron-like NSC-34 cells and up-regulates its type II receptor. Journal of neurochemistry. 2012;122(3):594–604. doi: 10.1111/j.1471-4159.2012.07795.x. Epub 2012/05/23. [DOI] [PubMed] [Google Scholar]
- 58.Shou J, Rim PC, Calof AL. BMPs inhibit neurogenesis by a mechanism involving degradation of a transcription factor. Nature neuroscience. 1999;2(4):339–45. doi: 10.1038/7251. Epub 1999/04/16. [DOI] [PubMed] [Google Scholar]
- 59.Lo L, Tiveron MC, Anderson DJ. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development. 1998;125(4):609–20. doi: 10.1242/dev.125.4.609. Epub 1998/04/04. [DOI] [PubMed] [Google Scholar]
- 60.Patzke H, Reissmann E, Stanke M, Bixby JL, Ernsberger U. BMP growth factors and Phox2 transcription factors can induce synaptotagmin I and neurexin I during sympathetic neuron development. Mechanisms of development. 2001;108(1–2):149–59. doi: 10.1016/s0925-4773(01)00503-2. Epub 2001/10/02. [DOI] [PubMed] [Google Scholar]
- 61.Sang Q, Ciampoli D, Greferath U, Sommer L, Young HM. Innervation of the esophagus in mice that lack MASH1. The Journal of comparative neurology. 1999;408(1):1–10. doi: 10.1002/(sici)1096-9861(19990524)408:1<1::aid-cne1>3.0.co;2-4. Epub 1999/05/20. [DOI] [PubMed] [Google Scholar]
- 62.Blaugrund E, Pham TD, Tennyson VM, Lo L, Sommer L, Anderson DJ, et al. Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers and Mash-1-dependence. Development. 1996;122(1):309–20. doi: 10.1242/dev.122.1.309. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 63.Wu X, Howard MJ. Transcripts encoding HAND genes are differentially expressed and regulated by BMP4 and GDNF in developing avian gut. Gene Expr. 2002;10(5–6):279–93. doi: 10.3727/000000002783992361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rychlik JL, Hsieh M, Eiden LE, Lewis EJ. Phox2 and dHAND transcription factors select shared and unique target genes in the noradrenergic cell type. J Mol Neurosci. 2005;27(3):281–92. doi: 10.1385/JMN:27:3:281. Epub 2005/11/11. [DOI] [PubMed] [Google Scholar]
- 65.Xu H, Firulli AB, Zhang X, Howard MJ. HAND2 synergistically enhances transcription of dopamine-beta-hydroxylase in the presence of Phox2a. Dev Biol. 2003;262(1):183–93. doi: 10.1016/s0012-1606(03)00361-0. Epub 2003/09/27. [DOI] [PubMed] [Google Scholar]
- 66.Hendershot TJ, Liu H, Clouthier DE, Shepherd IT, Coppola E, Studer M, et al. Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Dev Biol. 2008;319(2):179–91. doi: 10.1016/j.ydbio.2008.03.036. Epub 2008/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nature reviews Neuroscience. 2007;8(6):466–79. doi: 10.1038/nrn2137. Epub 2007/05/22. [DOI] [PubMed] [Google Scholar]
- 68.Young HM, Turner KN, Bergner AJ. The location and phenotype of proliferating neural-crest-derived cells in the developing mouse gut. Cell and tissue research. 2005;320(1):1–9. doi: 10.1007/s00441-004-1057-5. Epub 2005/02/17. [DOI] [PubMed] [Google Scholar]
- 69.Young HM, Bergner AJ, Muller T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. The Journal of comparative neurology. 2003;456(1):1–11. doi: 10.1002/cne.10448. Epub 2003/01/01. [DOI] [PubMed] [Google Scholar]
- 70.Conner PJ, Focke PJ, Noden DM, Epstein ML. Appearance of neurons and glia with respect to the wavefront during colonization of the avian gut by neural crest cells. Dev Dyn. 2003;226(1):91–8. doi: 10.1002/dvdy.10219. Epub 2003/01/01. [DOI] [PubMed] [Google Scholar]
- 71.Mundell NA, Plank JL, LeGrone AW, Frist AY, Zhu L, Shin MK, et al. Enteric nervous system specific deletion of Foxd3 disrupts glial cell differentiation and activates compensatory enteric progenitors. Developmental biology. 2012;363(2):373–87. doi: 10.1016/j.ydbio.2012.01.003. Epub 2012/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor MK, Yeager K, Morrison SJ. Physiological Notch signaling promotes gliogenesis in the developing peripheral and central nervous systems. Development. 2007;134(13):2435–47. doi: 10.1242/dev.005520. Epub 2007/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei J, Howard MJ. Targeted deletion of Hand2 in enteric neural precursor cells affects its functions in neurogenesis, neurotransmitter specification and gangliogenesis, causing functional aganglionosis. Development. 2011;138(21):4789–800. doi: 10.1242/dev.060053. Epub 2011/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagashimada M, Ohta H, Li C, Nakao K, Uesaka T, Brunet JF, et al. Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression. The Journal of clinical investigation. 2012;122(9):3145–58. doi: 10.1172/JCI63401. Epub 2012/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olden T, Akhtar T, Beckman SA, Wallace KN. Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. Genesis. 2008;46(9):484–98. doi: 10.1002/dvg.20429. [DOI] [PubMed] [Google Scholar]
- 76.Young HM, Ciampoli D, Hsuan J, Canty AJ. Expression of Ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Developmental dynamics: an official publication of the American Association of Anatomists. 1999;216(2):137–52. doi: 10.1002/(SICI)1097-0177(199910)216:2<137::AID-DVDY5>3.0.CO;2-6. Epub 1999/10/27. [DOI] [PubMed] [Google Scholar]
- 77.Baetge G, Gershon MD. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: relationship to the development of enteric neurons. Developmental biology. 1989;132(1):189–211. doi: 10.1016/0012-1606(89)90217-0. Epub 1989/03/01. [DOI] [PubMed] [Google Scholar]
- 78.Bisgrove BW, Raible DW, Walter V, Eisen JS, Grunwald DJ. Expression of c-ret in the zebrafish embryo: potential roles in motoneuronal development. Journal of neurobiology. 1997;33(6):749–68. Epub 1997/11/22. [PubMed] [Google Scholar]
- 79.Hao MM, Boesmans W, Van den Abbeel V, Jennings EA, Bornstein JC, Young HM, et al. Early emergence of neural activity in the developing mouse enteric nervous system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(43):15352–61. doi: 10.1523/JNEUROSCI.3053-11.2011. Epub 2011/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hao MM, Moore RE, Roberts RR, Nguyen T, Furness JB, Anderson RB, et al. The role of neural activity in the migration and differentiation of enteric neuron precursors. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2010;22(5):e127–37. doi: 10.1111/j.1365-2982.2009.01462.x. Epub 2010/01/20. [DOI] [PubMed] [Google Scholar]
- 81.Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. The Journal of comparative neurology. 1989;285(2):262–73. doi: 10.1002/cne.902850208. Epub 1989/07/08. [DOI] [PubMed] [Google Scholar]
- 82.Rothman TP, Nilaver G, Gershon MD. Colonization of the developing murine enteric nervous system and subsequent phenotypic expression by the precursors of peptidergic neurons. The Journal of comparative neurology. 1984;225(1):13–23. doi: 10.1002/cne.902250103. Epub 1984/05/01. [DOI] [PubMed] [Google Scholar]
- 83.Rothman TP, Gershon MD. Phenotypic expression in the developing murine enteric nervous system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1982;2(3):381–93. doi: 10.1523/JNEUROSCI.02-03-00381.1982. Epub 1982/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olsson C, Holmberg A, Holmgren S. Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut. The Journal of comparative neurology. 2008;508(5):756–70. doi: 10.1002/cne.21705. Epub 2008/04/09. [DOI] [PubMed] [Google Scholar]
- 85.Uyttebroek L, Shepherd IT, Harrisson F, Hubens G, Blust R, Timmermans JP, et al. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio) The Journal of comparative neurology. 2010;518(21):4419–38. doi: 10.1002/cne.22464. Epub 2010/09/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell and tissue research. 2008;334(2):147–61. doi: 10.1007/s00441-008-0684-7. Epub 2008/10/16. [DOI] [PubMed] [Google Scholar]
- 87.Li Z, Caron MG, Blakely RD, Margolis KG, Gershon MD. Dependence of serotonergic and other nonadrenergic enteric neurons on norepinephrine transporter expression. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(49):16730–40. doi: 10.1523/JNEUROSCI.2276-10.2010. Epub 2010/12/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chalazonitis APT, Rothman TP, DiStefano PS, Bothwell M, Blair-Flynn J, Tessarollo L, Gershon MD. Neurotrophin-3 is required for the survival-differentiation of subsets of developing enteric neurons. J Neurosci. 2001;21(15):5620–36. doi: 10.1523/JNEUROSCI.21-15-05620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.D’Autreaux F, Margolis KG, Roberts J, Stevanovic K, Mawe G, Li Z, et al. Expression level of Hand2 affects specification of enteric neurons and gastrointestinal function in mice. Gastroenterology. 2011;141(2):576–87. 87 e1–6. doi: 10.1053/j.gastro.2011.04.059. Epub 2011/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan H, Bergner AJ, Enomoto H, Milbrandt J, Newgreen DF, Young HM. Neural cells in the esophagus respond to glial cell line-derived neurotrophic factor and neurturin, and are RET-dependent. Developmental biology. 2004;272(1):118–33. doi: 10.1016/j.ydbio.2004.04.025. Epub 2004/07/10. [DOI] [PubMed] [Google Scholar]
- 91.Wang H, Hughes I, Planer W, Parsadanian A, Grider JR, Vohra BP, et al. The timing and location of glial cell line-derived neurotrophic factor expression determine enteric nervous system structure and function. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(4):1523–38. doi: 10.1523/JNEUROSCI.3861-09.2010. Epub 2010/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uesaka T, Enomoto H. Neural precursor death is central to the pathogenesis of intestinal aganglionosis in Ret hypomorphic mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(15):5211–8. doi: 10.1523/JNEUROSCI.6244-09.2010. Epub 2010/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(24):8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. Epub 2011/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu H, Firulli AB, Zhang X, Howard MJ. HAND2 synergistically enhances transcription of dopamine-beta-hydroxylase in the presence of Phox2a. Developmental biology. 2003;262(1):183–93. doi: 10.1016/s0012-1606(03)00361-0. Epub 2003/09/27. [DOI] [PubMed] [Google Scholar]
- 95.Swanson DJ, Adachi M, Lewis EJ. The homeodomain protein Arix promotes protein kinase A-dependent activation of the dopamine beta-hydroxylase promoter through multiple elements and interaction with the coactivator cAMP-response element-binding protein-binding protein. The Journal of biological chemistry. 2000;275(4):2911–23. doi: 10.1074/jbc.275.4.2911. Epub 2000/01/25. [DOI] [PubMed] [Google Scholar]
- 96.Kim HS, Seo H, Yang C, Brunet JF, Kim KS. Noradrenergic-specific transcription of the dopamine beta-hydroxylase gene requires synergy of multiple cis-acting elements including at least two Phox2a-binding sites. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18(20):8247–60. doi: 10.1523/JNEUROSCI.18-20-08247.1998. Epub 1998/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Developmental biology. 2005;277(2):271–86. doi: 10.1016/j.ydbio.2004.09.034. Epub 2004/12/25. [DOI] [PubMed] [Google Scholar]
- 98.Timmusk T, Palm K, Lendahl U, Metsis M. Brain-derived neurotrophic factor expression in vivo is under the control of neuron-restrictive silencer element. The Journal of biological chemistry. 1999;274(2):1078–84. Epub 1999/01/05. [PubMed] [Google Scholar]
- 99.Pan YA, Livet J, Sanes JR, Lichtman JW, Schier AF. Multicolor Brainbow imaging in zebrafish. Cold Spring Harbor protocols. 2011;2011(1) doi: 10.1101/pdb.prot5546. pdbprot5546. Epub 2011/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450(7166):56–62. doi: 10.1038/nature06293. Epub 2007/11/02. [DOI] [PubMed] [Google Scholar]