Abstract
The subgranular zone of the hippocampal formation gives rise to new neurons that populate the dentate gyrus throughout life. Cells in the hippocampus exhibit rhythmic clock gene expression and the circadian clock is known to regulate the cycle of cell division in other areas of the body. These facts suggest that the circadian clock may regulate adult neurogenesis in the hippocampus as well. In the present study, neurogenesis in the hippocampal subgranular zone was examined in arrhythmic Bmal1 knockout (-KO) mice and their rhythmic heterozygous and wildtype littermates. Proliferation and survival of newly generated subgranular zone cells were examined using bromodeoxyuridine labelling, while pyknosis (a measure of cell death) and hippocampal volume were examined in cresyl violet stained sections. There was no significant difference in cellular proliferation between any of the groups, yet survival of proliferating cells, 6 weeks after the bromodeoxyuridine injection, was significantly greater in the BMAL1-KO animals. The number of pyknotic cells was significantly decreased in Bmal1-KO animals, yet hippocampal volume remained the same across genotypes. These findings suggest that while a functional circadian clock is not necessary for normal proliferation of neuronal precursor cells, the normal pruning of newly generated neurons in the hippocampus may require a functional circadian clock.
Introduction
Proliferation of neural stem cells persists into adulthood in the subventricular and subgranular zones (SGZ) of the mammalian brain [1], [2]. The SGZ gives rise to new granule cells in the dentate gyrus (DG) of the hippocampus. Rates of proliferation and survival of these new neurons can be modulated by a number of factors, including the richness of environment, exercise, learning, sleep, stress, and levels of glucocorticoids [3]–[9]. Many of these factors are under circadian control, suggesting that the circadian clock may influence proliferation and survival of neurons formed during adulthood [10]. For example, manipulation of the cortisol circadian rhythm both enhances clock gene expression and suppresses mitosis in the DG [4]. Furthermore, the circadian clock may be involved in regulating the cell cycle in the SGZ, as disruption of the molecular clock leads to aberrant proliferation [11].
The master circadian clock is located in the suprachiasmatic nucleus (SCN) [12]. Circadian rhythms arise from autoregulatory transcription/translation feedback loops within individual cells. Specifically, a dimer of the CLOCK and BMAL1 proteins binds to an E-box element in the promoter regions of the Period genes Per1 and Per2 and the Cryptochrome genes Cry1 and Cry2 to initiate transcription. The protein products of the Per and Cry genes dimerize and translocate to the nucleus, where they inhibit the activity of CLOCK and BMAL1, thereby turning off their own expression. Gradual degradation of PER and CRY proteins over the night eventually releases CLOCK and BMAL1 from inhibition, allowing transcription to resume the following day [13]. Transgenic animals lacking either both Period genes or both Crypotochrome genes are arrhythmic [14], [15]. Mice lacking Bmal1 are the only single-gene knockout (-KO) model that reliably leads to arrhythmicity in overt locomotor rhythms as well as cessation of oscillations of clock gene expression in the SCN [16].
Expression of these clock genes is not limited to the master pacemaker in the SCN; oscillators can be found elsewhere in the brain and body. Of these extra-SCN brain regions that express the various clock genes, the granule cells of the DG and pyramidal cells of CA1 and CA3 in the hippocampus have the highest level of expression of Per1, Per2, Clock [17]–[19] and Bmal1 [20], and expression of these genes in the hippocampus is rhythmic [21]. Furthermore, Per2 is expressed in proliferating cells in the SGZ, and the Per2brdm1 mutation leads to enhanced proliferation [22]. In other tissues, a functional circadian clock is necessary for normal mitotic cell division [23], [24].
Given the role of the circadian clock in regulating the cell cycle, and the presence of clock genes in the hippocampus, it is possible that a functional circadian clock is essential for normal patterns of proliferation and survival of neurons born during adulthood. Using arrhythmic Bmal1-KO mice and littermate controls, we examined cell proliferation in the SGZ, and survival and apoptotic cell death in granule cell layer (GCL) of the hippocampus.
Materials and Methods
Animals
Male animals were generated from an in-lab breeding program founded by heterozygous breeders from Jackson Laboratories, MA, USA (strain: B6.129-Arntltm1Bra/J). Heterozygous breeders were paired to produce wildtype (WT, +/+, n = 11), heterozygous (Het, +/−, n = 10), and KO (−/−, n = 11) animals for proliferation and survival studies. A further 9 animals (WT n = 2, Het n = 3, KO n = 4) were produced for behavioral recordings. Mice were weaned at 21–22 days of age, and were housed with same sex littermates in a colony room with a 12∶12 light/dark cycle (lights on at 7 AM). Animals were provided food and water ad libitum and all procedures were performed in accordance with the Canadian Council on Animal Care guidelines, and were approved by the Life and Environmental Sciences Animal Care Committee at the University of Calgary (Protocol # AC12-0003). All attempts were made to minimize pain and discomfort.
Genotyping
Animals were genotyped according to the protocol of Bunger et al., [16] using multiplex PCR with one forward primer (CCACCAAGCCCAGCAACTCA) and two reverse primers, one specific to the portion of the Bmal1 gene that is deleted in the KO animals (ATTCGGCCCCCTATCTTCTG) and the other specific to the Neomycin gene that replaces the deleted portion of the Bmal1 gene in the engineered gene (TCGCCTTCTATCGCCTTCTTGACG). The first reverse primer amplifies a 400 bp band corresponding to the wild-type allele, while the second reverse primer amplifies a 600 bp band. PCR was performed on genomic DNA from tail biopsies for 40 cycles of 95°C, 15 s: 60°C, 15 s: 72°C, 1 min in 1× PCR buffer (Sigma-Aldrich) containing 3.5 mM MgCl2.
Behavior
To confirm that Bmal1-KO mice were arrhythmic, animals (WT n = 2, Het n = 3, KO n = 4) were housed in cages equipped with running wheels to record circadian patterns of wheel-running behavior as has been described previously [25]. Animals were housed in a light-dark cycle for about 4 weeks, before being transferred to constant darkness for a further four weeks. A Chi-square periodogram was used to confirm the presence or absence of a significant circadian rhythm over the last 16 days in constant darkness. The power of the chi-square statistic for the dominant period was used for analysis, and if no significant rhythm was detected, a power of zero was assigned.
Proliferation
Animals were injected intraperitoneally (i.p.) with 50 mg/kg bromodeoxyuridine (BrdU) (Sigma-Aldrich) on postnatal day 60 at midday. 24 hours later they were deeply anesthetized with sodium pentobarbital (240 mg/kg) and perfused transcardially with phosphate buffered saline (PBS) followed by 4% paraformaldehyde. Brains were postfixed in 4% paraformaldehyde for 24 hours, followed by at least 24 hours in 20% sucrose in PBS. Four series of 35 µm sections were collected throughout the extent of the DG using a cryostat.
Immunohistochemistry procedures were based on Antle and colleagues [26]. All washes were at room temperature unless otherwise noted. A diaminobenzidine procedure was used to assess proliferation as follows: tissue was rinsed in Tris-buffered saline (TBS) then in 0.5% H2O2 in 0.1%TBS for 10 minutes, then in 50% formamide/2X saline-sodium citrate for 2 hours at 65°C, then rinsed for 15 minutes in 2X saline-sodium citrate, then a 30 minute incubation in 2N HCl at 37°C. Sections then had a 10 minute 0.1 M borate buffer (pH 8.5) rinse. TBS rinses preceded 1-hour incubation in TBS-Plus (3% Normal rabbit serum solution of TBS and Triton X-100). Sections were incubated for 48 hours in sheep anti-BrdU (1∶1000, in TBS-Plus, AB1893, Abcam, ON, Canada) at 4°C. Next, TBS rinses preceded 1-hour incubation in biotinylated rabbit anti-sheep antibody (1∶200, Vector Labs, ON, Canada). TBS rinses preceded 1-hour avidin-biotin complex incubation (ABC elite, Vector Labs). TBS rinses preceded a 3-minute reaction in 25 ml TBS containing 0.05% diaminobenzidine (DAB), 0.02% NiCl2, and 80 µl of 30% H2O2. Sections were dehydrated in an alcohol series, mounted on slides, and coverslipped using Permount. The number of nuclei stained for BrdU in the SGZ was counted on thirteen sections. As this represented only every 4th section, this final count was multiplied by 4 to provide an estimate of the total number of BrdU-labeled cells.
Survival
Mice were injected with BrdU (100 mg/kg i.p.) at midday. Six weeks post-injection they were perfused and brain tissue was collected as described above. Immunohistochemistry was similar to that described above except for the following differences for immunofluorescence. The tissue was incubated in 10% normal goat serum, followed by 24 hours in TBS-Plus containing rat anti-BrdU (1∶200, Abd Serotech, Raleigh, NC, USA), and mouse anti-NeuN (1∶2000, Millipore, Billerica, MA, USA). Secondary antibody (1∶500, Alexa Fluor 488-labelled goat-anti-mouse, biotin-labelled goat anti-rat, Jackson ImmunoResearch, PA, USA) incubations were for 24 hours, and the biotin was labeled using a 1-hour Streptavidin Alexa Fluor 594 (1∶500, Life Technologies Inc., Burlington, ON, Canada) incubation. TBS rinses preceded mounting tissue on gel-coated slides. After brief air drying, tissue was coverslipped with Krystalon. BrdU-labelled cells were counted in all sections and the total was multiplied by 4 to obtain an estimate of total numbers expected in the entire DG. Using a Nikon C1si Spectral Confocal microscope, BrdU/NeuN co-localization was assessed across the rostral-caudal extent of the DG. A cell was considered positive for BrdU/NeuN if a nuclear signal from both the 488 nm and 561 nm lasers co-localized in the x-, y-, and z-axes.
Apoptosis Analysis and Hippocampal Volume
One series of sections from the animals used for the survival of BrdU labelled cells was stained with cresyl violet to examine differences in numbers of pyknotic/apoptotic cells in the DG. A pyknotic cell was defined as one in which the nucleus exhibited the following features: strong and homogeneous staining, condensed chromatin and fragmentation of the nucleus [27]. The same series of tissue was also utilized to determine GCL volume using the Cavalieri method [28], [29]. Briefly, images of brain sections containing the DG were taken using a Zeiss Axioskop 2 microscope attached to a Qimaging camera using a 5X (NA 0.25) objective. ImageJ (http://rsbweb.nih.gov/ij/) was employed to position a random, systematic sampling grid over each image. An area per point of 0.01 mm2 was deemed appropriate, and the total number of points in contact with the GCL was counted in each section. The number of contact points per section was multiplied by the area associated with each point (0.01 mm2), the section cut thickness (35 µm), and the section sampling fraction (4). The resulting numbers were then summed to provide an estimate of total GCL volume.
Statistical Analysis
All cell counting was performed blind to genotype. All data were analyzed using one-way analysis of variance (SigmaStat, v 2.03; Systat Software, Inc, IL, USA) with Tukey post hoc tests.
Results
Behavior
Using a Chi-square periodogram, Bmal1-KO mice had either no detectable rhythm (n = 2, Figure 1) or a rhythm outside the circadian range (19.0 h and 11.5 h, both with low power). Bmal1-KO mice had significantly lower power in their Chi-square periodograms (F(2,8) = 9.291, p = 0.015) than either wildtype or heterozygous animals.
Figure 1. Homozygous Bmal1-KO mice are arrhythmic.
Representative wheel-running activity charts (actograms) from Bmal1 (+/+), (+/−) and (−/−) animals. Every horizontal line represents 2 days of activity, with subsequent days plotted to the right of and below the previous day. Vertical deflections from the horizontal represent 10 minute bins in which wheel revolutions were detected, with the height of the deflection being proportional to the number of revolutions. The animals were housed in a light/dark cycle for the first 4 weeks (darkness indicated by grey shading) and were then transferred into constant darkness for another 4 weeks. Wildtype (left) and heterozygous (middle) animals had normal circadian patterns of activity in both the light/dark cycle as well as in constant darkness. The Bmal1-KO mice were arrhythmic throughout. Animals in this circadian activity assessment were different than those used in the subsequent proliferation/survival experiments.
Proliferation
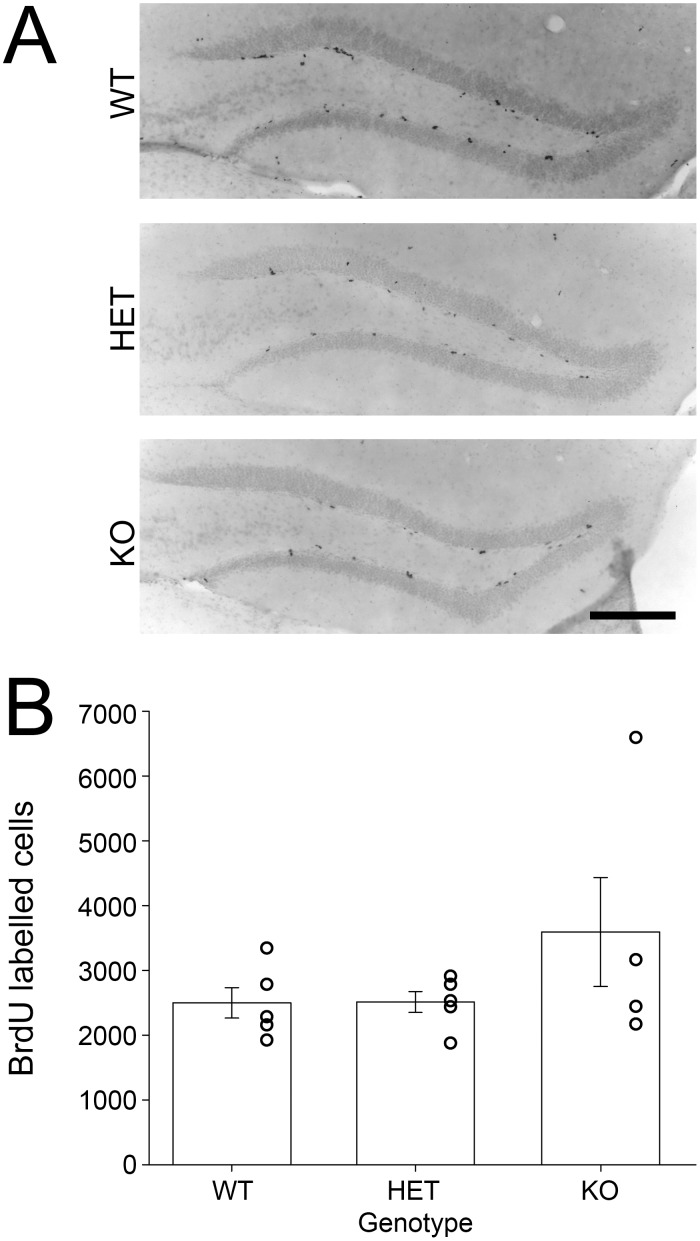
There were no significant differences between genotypes in the number of BrdU-positive cells 24 hours after the BrdU injection (F(2,13) = 1.289, p = 0.314, Figure 2). A single Bmal1-KO animal had exceptionally high number of BrdU-labelled cells, leading to a high amount of variance, and a slight increase in the mean number of newly generated cells in the Bmal1-KO group. All other Bmal1-KO animals had virtually the same number of BrdU labeled cells as did wildtype and heterozygous animals.
Figure 2. Bmal1-KO do not exhibit proliferation differences in the sub granular zone of the dentate gyrus.
A) Photomicrographs of DAB labelled BrdU positive cells in Bmal1 (+/+), (+/−) and (−/−) SGZ of the DG, 24 hours after an i.p. injection of BrdU. Scale bar = 200 µm. B) Number of cells labelled with BrdU throughout the extent of the hippocampus, calculated by counting cells in every fourth slice and multiplying by 4. Differences between genotypes were not significant.
Survival
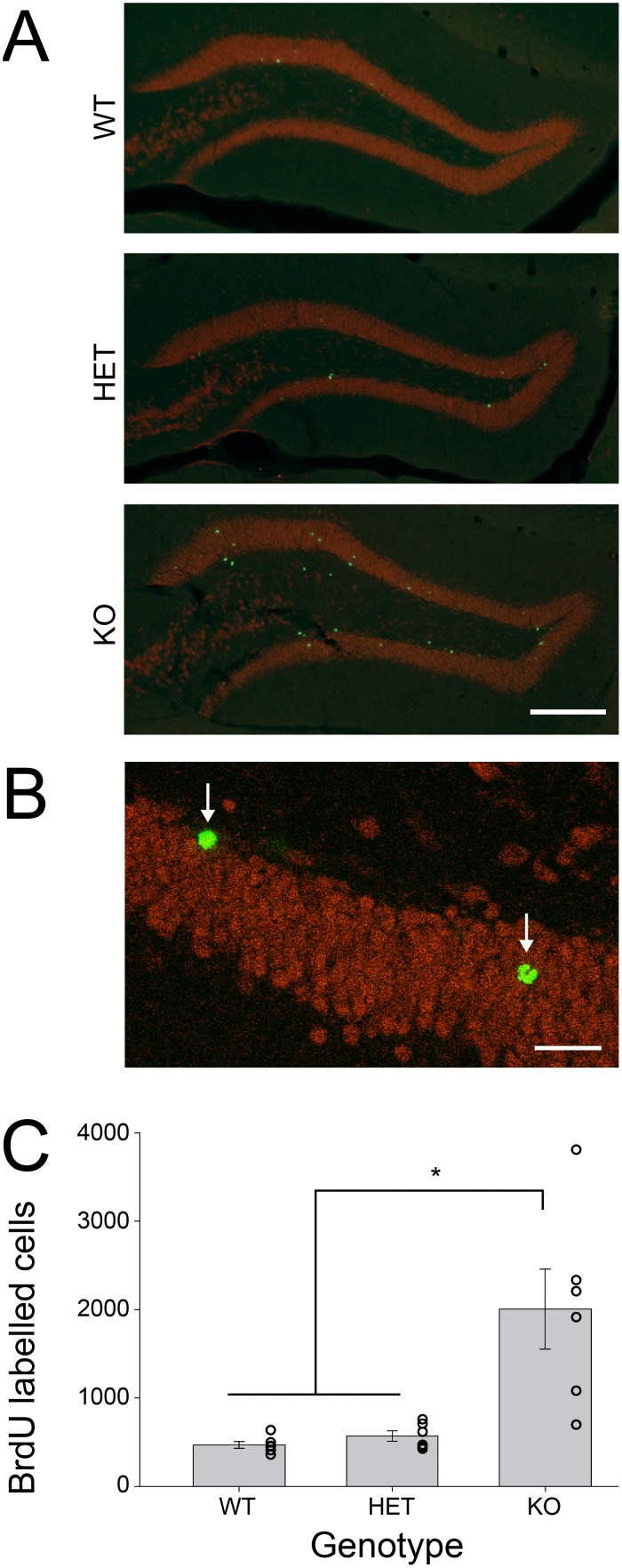
Bmal1-KO mice had significantly more BrdU labelled cells 6 weeks after BrdU injection (F (2,15) = 10.882, p = 0.001, Figure 3). The number of BrdU positive cells did not differ between wildtype and heterozygous animals (p = 0.959). Confocal analysis revealed that the majority of BrdU-labeled cells were NeuN positive (WT = 94.5±2.1%; Het = 90.2±3.06%; KO = 93.07±2.1%) and there was no difference between the genotypes (F (2,15) = 0.800, p = 0.46).
Figure 3. Bmal1-KO exhibit enhanced survival of newly generated cells in the dentate gyrus relative to heterozygous and wildtype mice.
A) Representative photomicrographs of BrdU (green) and NeuN (red) immunoreactivity in Bmal1 (+/+), (+/−), and (−/−) mice 6 weeks after an i.p. injection of BrdU. Scale bar = 200 µm. B) High-magnification confocal image of NeuN/BrdU labeled cells. Scale bar = 10 µm. C) Total number of BrdU labelled cells throughout the hippocampus, calculated by counting cells in every fourth slice and multiplying by 4. *p<0.05.
Pyknosis and Hippocampal Volume
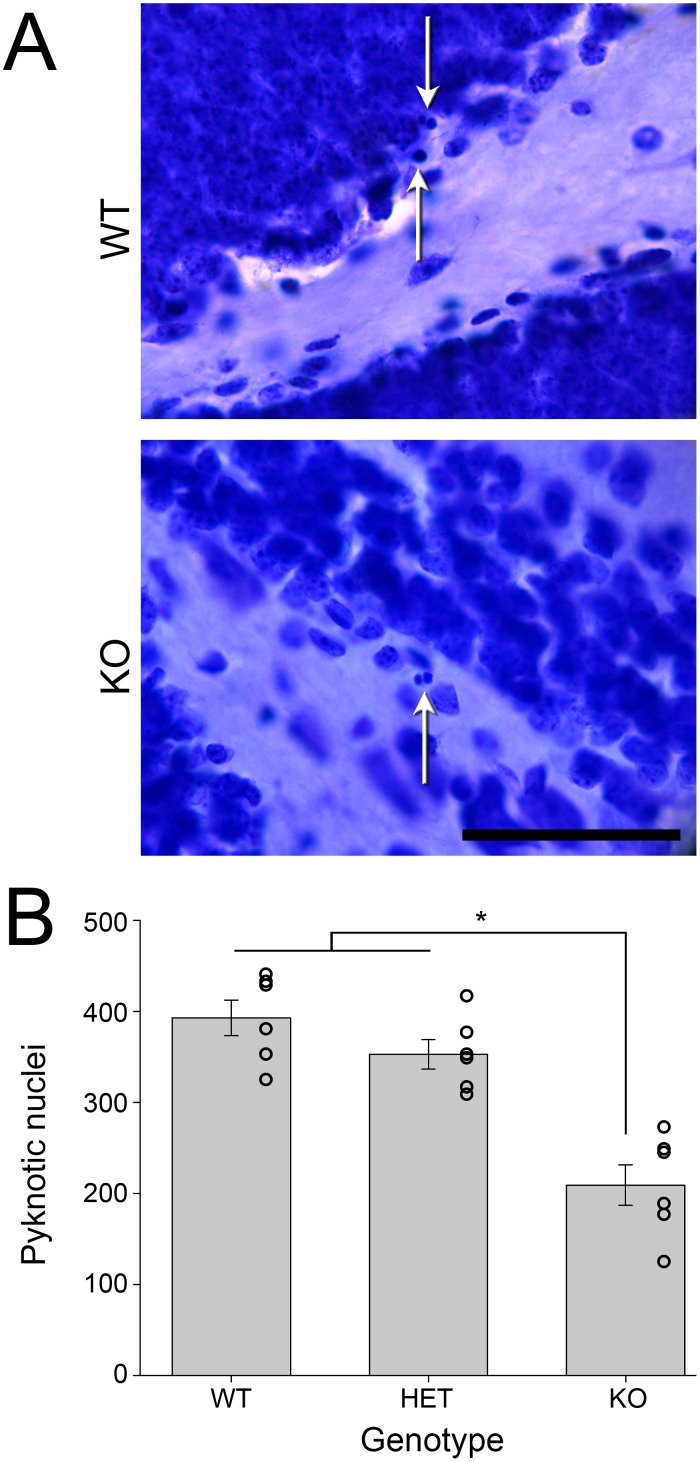
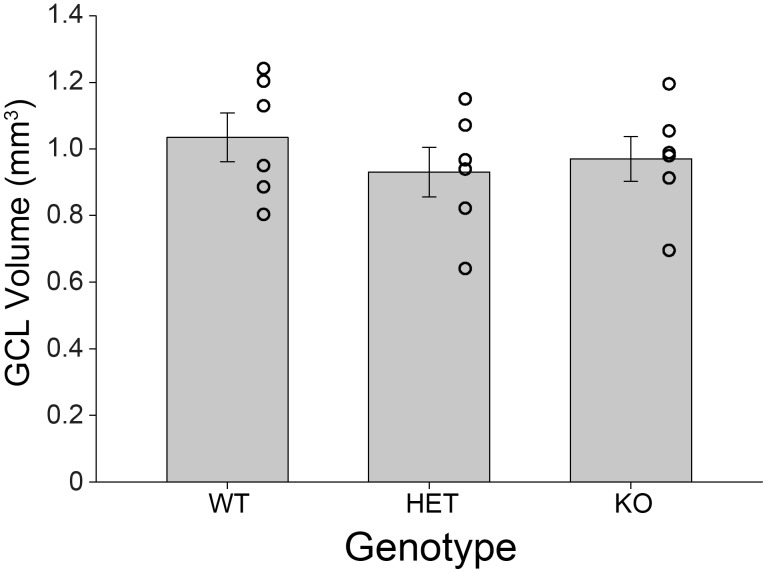
Bmal1-KO animals had significantly fewer pyknotic cells in the DG than either the wildtype or heterozygous animals (F (2,17) = 24.115, p<0.001, Figure 4). There was no difference between wildtype and heterozygous animals (p = 0.349). The GCL volume did not differ across genotypes (F (2,17) = 0.534, p = 0.597, Figure 5).
Figure 4. Bmal1-KO exhibit decreased pyknosis, indicative of diminished cell death, in the dentate gyrus relative to heterozygous and wildtype mice.
A) Photomicrograph of cresyl violet stained pyknotic cells, shown by the white arrows. Scale bar = 50 µm. B) Estimates of the total number of GCL pyknotic cells throughout the extent of the hippocampi of Bmal1 (+/+), (+/−), and (−/−) mice, calculated by counting pyknotic cells in every fourth slice and multiplying by 4. *p<0.05.
Figure 5. The volume of the hippocampus does not differ between wildtype, heterozygous and Bmal1-KO mice.
A) Cavalieri estimates of total hippocampal volume in Bmal1 (+/+), (+/−), and (−/−) mice at approximately 100 days of age. Estimates were calculated from the cresyl violet stained tissue series utilized in Figure 3 for pyknotic cell counts. Hippocampal volume estimates were not significantly different across genotypes.
Discussion
The circadian clock has been suggested to regulate the cell cycle in both the brain [11], [22] and periphery [23], [24]. We show here that arrhythmic Bmal1-KO mice exhibit normal levels of proliferation in the SGZ, but enhanced survival relative to wildtype animals 6 weeks after labelling proliferating cells. Given that proliferation is no different, we confirmed a decrease in the number of pyknotic cells in the DG, indicative of decreased apoptosis, consistent with the observation of enhanced survival. Overall, these findings suggest that Bmal1 is necessary for normal apoptotic pruning in the DG.
There are three possible mechanisms by which apoptosis could be impaired in Bmal1-KO mice. First, since circadian clock genes are rhythmically expressed throughout the hippocampal formation [17]–[22], [30]–[33], it is possible that it is the circadian rhythm generated within these cells that is essential for directing apoptosis. In an in vitro cancer model, disrupting the negative limb of the transcription translation feedback loop is associated with enhanced cell death [34], [35] Therefore, it might be expected that disruption Bmal1 (i.e., the positive limb) could lead to lower cell death. Alternatively, cell survival/apoptosis may be affected by SCN-controled rhythms such as glucocorticoid levels or exercise [4], [9], [10]. However, exercise is unlikely to explain the enhanced survival observed, as Bmal1-KO mice exhibit lower, not higher, activity levels [16]. Finally, this may be a case of pleiotropy, where Bmal1’s role in regulating apoptosis may be separate and distinct from its role in regulating circadian rhythms. Many genes involved in growth and apoptosis are regulated by the transcription factor Myc, which binds to E-boxes to regulate gene expression [36]. If BMAL1 competes with Myc for binding to some of these E-box elements then removal of BMAL1 could lead to increased Myc binding to those E-boxes.
While the idea of pleiotropy is intriguing, a number of other studies have suggested that the circadian clock regulates the cell cycle and neurogenesis. There is a circadian rhythm in proliferation [37]. The period of the cell cycle in neural stem/progenitor cells (NSPCs) is approximately a day [38]. Exercise enhances neurogenesis, and the timing of voluntary exercise is controlled by the circadian clock [10]. Disruption of normal sleep wake patterns disrupts neurogenesis [39], although this is independent of disruption of the circadian clock [8]. One study has reported a daily rhythm in proliferation in the hippocampus, with higher proliferation during the day (rest phase), but only in the hilus where proliferation is predominantly gliogenic rather than neurogenic [40]. The hippocampus and the DG express a number of clock genes, including mPer2 [18], [22]. This expression starts when cells are immature proliferating cells, and persists into adulthood. Proliferation is enhanced in mice in which the PAS domain of the mPer2 gene has been deleted (Per2brdm1) [22] although after 3 weeks, the number of surviving neurons in these mice does not differ from wild type controls, owing to enhanced apoptosis [22]. The difference between the present study and those with the Per2brdm1 mice may be due to a couple reasons. First, Per2brdm1 mice are rhythmic, whereas Bmal1-KO mice are not. Alternately, PER2 is on the negative limb of the E-box mediated transcription-translation feedback loop, while BMAL1 constitutes the positive limb of these loops. If neurogenic or apoptotic factors were expressed in part due to activation/inactivation of E-boxes, one might expect the opposite results when disrupting the positive versus the negative limbs of the loop.
The role of Bmal1 in cell proliferation is equivocal. A recent study reported enhanced proliferation in the SGZ in Bmal1-KO animals, and attributed this to enhanced entry and diminished exit from the cell cycle [11]. The opposite phenomenon is observed with in vitro cell culture models, where over-expression of Bmal1 in has been associated with enhanced proliferation [41] and deletion of Bmal1 leads to delayed proliferation of hepatocytes [23] To further complicate the picture, in the current study we found no effect on proliferation in the Bmal1-KO animals, the same genetic model used by Bouchard-Cannon et al. [11]. A number of differences between these studies could account for the disparate findings. First, Bouchard-Cannon et al. [11] labeled proliferating cells at the light-dark transitions, while we looked at midday. Although these animals are arrhythmic, the light-dark cycle may impose temporal organization on a variety of physiological processes. Furthermore, the ages examined may have contributed to the observed difference; our animals were 60 days of age when treated with BrdU, while those examined by Bouchard-Cannon et al. [11] were only 40 days of age. While proliferation decreases with age, prominent decreases are usually associated with much older animals than were examined in the present study [42], although Bmal1-KO mice do show some characteristics of accelerated aging [43], [44]. Finally, both Bouchard-Cannon et al. [11] and ourselves reported high variability in the proliferation of SGZ cells in Bmal1-KO mice. In our study, this was due to a single animal with high proliferation. Given the high variability and differences in phases, this issue needs to be re-examined more closely with more detailed sampling of clock phases.
While enhanced neurogenesis has generally been associated with improved performance on learning tasks [3], [45], [46], this may not be the case with Bmal1-KO mice. In fact, a recent report demonstrated diminished performance on a radial arm maze task, suggesting that the extra neurons impair performance [11]. As wildtype animals generally only retain a small percentage of newly generated neurons, it has been argued that only those cells that integrate into hippocampal circuits in a coherent fashion are retained, while the remaining are pruned through apoptosis [3], [45], [46]. If that is true, then the diminished cell death observed here might suggest that the newly generated cells cannot be pruned effectively and therefore they may be interfering with proper hippocampal functioning. Alternatively, it is possible that the deficit in learning arises independently of the altered survival of DG neurons. The circadian clock has been implicated in a variety of learning tasks, including both hippocampal-dependent and -independent tasks [47]–[49]. Thus arrhythmic Bmal1-KO mice may have impaired performance on these learning tasks due to their lack of rhythmicity, rather than altered survival of DG neurons. However, this possibility is less likely, as Bmal1-KO mice were only impaired in hippocampal-dependent tasks.
Acknowledgments
The RUN facility at the University of Calgary generously provided the use of the NikonC1si Spectral Confocal microscope.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Please contact the corresponding author (MCA:antlem@ucalgary.ca) for access to the data.
Funding Statement
Funded by the Natural Sciences and Engineering Research Council of Canada (Grant #311874-2010) (http://www.nserc-crsng.gc.ca/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335. [DOI] [PubMed] [Google Scholar]
- 2. Gage FH, Ray J, Fisher LJ (1995) Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci 18: 159–192. [DOI] [PubMed] [Google Scholar]
- 3. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2: 260–265. [DOI] [PubMed] [Google Scholar]
- 4. Gilhooley MJ, Pinnock SB, Herbert J (2011) Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: implications for neurogenesis. Neurosci Lett 489: 177–181. [DOI] [PubMed] [Google Scholar]
- 5. Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LA (2006) Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur J Neurosci 24: 1845–1849. [DOI] [PubMed] [Google Scholar]
- 6. Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D (2009) New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev 13: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mirescu C, Peters JD, Noiman L, Gould E (2006) Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci U S A 103: 19170–19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mueller AD, Mear RJ, Mistlberger RE (2011) Inhibition of hippocampal neurogenesis by sleep deprivation is independent of circadian disruption and melatonin suppression. Neuroscience 193: 170–181. [DOI] [PubMed] [Google Scholar]
- 9. van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270. [DOI] [PubMed] [Google Scholar]
- 10. Holmes MM, Galea LA, Mistlberger RE, Kempermann G (2004) Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res 76: 216–222. [DOI] [PubMed] [Google Scholar]
- 11. Bouchard-Cannon P, Mendoza-Viveros L, Yuen A, Kaern M, Cheng HY (2013) The circadian molecular clock regulates adult hippocampal neurogenesis by controlling the timing of cell-cycle entry and exit. Cell Rep 5: 961–973. [DOI] [PubMed] [Google Scholar]
- 12. Antle MC, Silver R (2005) Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci 28: 145–151. [DOI] [PubMed] [Google Scholar]
- 13. Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35: 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, et al. (2001) Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30: 525–536. [DOI] [PubMed] [Google Scholar]
- 15. van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, et al. (1999) Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398: 627–630. [DOI] [PubMed] [Google Scholar]
- 16. Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, et al. (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shieh KR (2003) Distribution of the rhythm-related genes rPERIOD1, rPERIOD2, and rCLOCK, in the rat brain. Neuroscience 118: 831–843. [DOI] [PubMed] [Google Scholar]
- 18. Segall LA, Amir S (2010) Glucocorticoid regulation of clock gene expression in the mammalian limbic forebrain. J Mol Neurosci 42: 168–175. [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, et al. (2001) Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. J Comp Neurol 430: 518–532. [PubMed] [Google Scholar]
- 20. Namihira M, Honma S, Abe H, Tanahashi Y, Ikeda M, et al. (1999) Daily variation and light responsiveness of mammalian clock gene, Clock and BMAL1, transcripts in the pineal body and different areas of brain in rats. Neuroscience Letters 267: 69–72. [DOI] [PubMed] [Google Scholar]
- 21. Jilg A, Lesny S, Peruzki N, Schwegler H, Selbach O, et al. (2010) Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus 20: 377–388. [DOI] [PubMed] [Google Scholar]
- 22. Borgs L, Beukelaers P, Vandenbosch R, Nguyen L, Moonen G, et al. (2009) Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci 10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F (2008) The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem 283: 4535–4542. [DOI] [PubMed] [Google Scholar]
- 24. Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, et al. (2003) Control mechanism of the circadian clock for timing of cell division in vivo. Science 302: 255–259. [DOI] [PubMed] [Google Scholar]
- 25. Smith VM, Sterniczuk R, Phillips CI, Antle MC (2008) Altered photic and non-photic phase shifts in 5-HT(1A) receptor knockout mice. Neuroscience 157: 513–523. [DOI] [PubMed] [Google Scholar]
- 26. Antle MC, LeSauter J, Silver R (2005) Neurogenesis and ontogeny of specific cell phenotypes within the hamster suprachiasmatic nucleus. Brain Res Dev Brain Res 157: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP (2010) Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging 31: 151–161. [DOI] [PubMed] [Google Scholar]
- 28. Gundersen HJ, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147: 229–263. [DOI] [PubMed] [Google Scholar]
- 29. Schmitz C, Hof PR (2005) Design-based stereology in neuroscience. Neuroscience 130: 813–831. [DOI] [PubMed] [Google Scholar]
- 30. Abe H, Honma S, Ohtsu H, Honma K (2004) Circadian rhythms in behavior and clock gene expressions in the brain of mice lacking histidine decarboxylase. Brain Res Mol Brain Res 124: 178–187. [DOI] [PubMed] [Google Scholar]
- 31. Fonzo LS, Golini RS, Delgado SM, Ponce IT, Bonomi MR, et al. (2009) Temporal patterns of lipoperoxidation and antioxidant enzymes are modified in the hippocampus of vitamin A-deficient rats. Hippocampus 19: 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Golini RS, Delgado SM, Navigatore Fonzo LS, Ponce IT, Lacoste MG, et al. (2012) Daily patterns of clock and cognition-related factors are modified in the hippocampus of vitamin A-deficient rats. Hippocampus 22: 1720–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang LM, Dragich JM, Kudo T, Odom IH, Welsh DK, et al. (2009) Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro 1. [DOI] [PMC free article] [PubMed]
- 34. Lee JH, Sancar A (2011) Circadian clock disruption improves the efficacy of chemotherapy through p73-mediated apoptosis. Proc Natl Acad Sci U S A 108: 10668–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee JH, Sancar A (2011) Regulation of apoptosis by the circadian clock through NF-kappaB signaling. Proc Natl Acad Sci U S A 108: 12036–12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thompson EB (1998) The many roles of c-Myc in apoptosis. Annu Rev Physiol 60: 575–600. [DOI] [PubMed] [Google Scholar]
- 37. Smith JM, Hechtman A, Swann J (2010) Fluctuations in cellular proliferation across the light/dark cycle in the subgranular zone of the dentate gyrus in the adult male Syrian hamster. Neurosci Lett 473: 192–195. [DOI] [PubMed] [Google Scholar]
- 38. Cameron HA, McKay RD (2001) Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435: 406–417. [DOI] [PubMed] [Google Scholar]
- 39. Mueller AD, Pollock MS, Lieblich SE, Epp JR, Galea LA, et al. (2008) Sleep deprivation can inhibit adult hippocampal neurogenesis independent of adrenal stress hormones. Am J Physiol Regul Integr Comp Physiol 294: R1693–1703. [DOI] [PubMed] [Google Scholar]
- 40. Kochman LJ, Weber ET, Fornal CA, Jacobs BL (2006) Circadian variation in mouse hippocampal cell proliferation. Neurosci Lett 406: 256–259. [DOI] [PubMed] [Google Scholar]
- 41. Lin F, Chen Y, Li X, Zhao Q, Tan Z (2013) Over-expression of circadian clock gene Bmal1 affects proliferation and the canonical Wnt pathway in NIH-3T3 cells. Cell Biochem Funct 31: 166–172. [DOI] [PubMed] [Google Scholar]
- 42. Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16: 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, et al. (2005) Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis 41: 122–132. [DOI] [PubMed] [Google Scholar]
- 44. Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20: 1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marin-Burgin A, Schinder AF (2012) Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res 227: 391–399. [DOI] [PubMed] [Google Scholar]
- 47. Cain SW, Chou T, Ralph MR (2004) Circadian modulation of performance on an aversion-based place learning task in hamsters. Behav Brain Res 150: 201–205. [DOI] [PubMed] [Google Scholar]
- 48. Cain SW, Ralph MR (2009) Circadian modulation of conditioned place avoidance in hamsters does not require the suprachiasmatic nucleus. Neurobiol Learn Mem 91: 81–84. [DOI] [PubMed] [Google Scholar]
- 49. Valentinuzzi VS, Menna-Barreto L, Xavier GF (2004) Effect of circadian phase on performance of rats in the Morris water maze task. J Biol Rhythms 19: 312–324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Please contact the corresponding author (MCA:antlem@ucalgary.ca) for access to the data.