Abstract
Our previous studies have demonstrated that genetic deletion of the Muc2 gene causes colorectal cancers in mice. The current study further showed that at the early stage (<3 months) the Muc2 knockout mice spontaneously developed chronic inflammation in colon and rectum, similar pathological features as human colitis; and at the late stage (>3 months) the mice exhibited colorectal cancer, including a unique phenotype of rectal prolapsed (rectal severe inflammation and adenocarcinoma). Thus, the age of 3 months might be the key point of the transition from chronic inflammation to cancer. To determine the mechanisms of the malignant transformation, we conducted miRNA array on the colonic epithelial cells from the 3-month Muc2 −/− and +/+ mice. MicroRNA profiling showed differential expression of miRNAs (i.e. lower or higher expression enrichments) in Muc2 −/− mice. 15 of them were validated by quantitative PCR. Based on relevance to cytokine and cancer, 4 miRNAs (miR-138, miR-145, miR-146a, and miR-150) were validate and were found significantly downregulated in human colitis and colorectal cancer tissues. The network of the targets of these miRNAs was characterized, and interestedly, miRNA-associated cytokines were significantly increased in Muc2 −/−mice. This is the first to reveal the importance of aberrant expression of miRNAs in dynamically transformation from chronic colitis to colitis-associated cancer. These findings shed light on revealing the mechanisms of chronic colitis malignant transformation.
Introduction
Colorectal cancer (CRC) is the third common malignant disease and the second leading causes of cancer-related death [1]. Similar as other malignancies, genetic factors contribute a lot to CRC formation, but, only about 20% of CRC cases can be genetically attributed to familiar history [2]–[4]. In fact, most of sporadic CRC are strongly linked to environmental factors, by which the most often mutations in adenomatous polyposis coli (APC) tumor suppressor gene lead to destruction of Apc/GSK3β/Axin complex and activation of Wnt/β-catenin pathway [2], [5], [6] Aberrant activation of Wnt/β-catenin pathway not only promotes proliferation of intestinal epithelial cells but also induces their arrest as they move towards the end of the crypt and prevent shedding or apoptosis of transformed cells. The canonical “genetic pathway to colorectal cancer” has been well studies. The emerging mechanisms of colorectal cancer formation by environmental factors are associated with chronic inflammation, named colitis-associated colorectal cancer (CAC) [7]–[10] With the changes of diets and life-style in China, CRC incidence rate increases rapidly than any other cancers in recent years [11], and quite amount of CRC cases are linked to chronic inflammatory bowel disease (IBD). It is acceptable that CAC is preceded by clinically detectable IBD [8], [12], such as Crohn’s disease (CD) or ulcerative colitis (UC). Epidemiology and clinical studies have suggested that UC increases CAC risk by up to 18–20%, while CD by up to 8% after 30 years of active disease [13]–[15]. In mouse models, single injection of carcinogen azoxymethane (AOM) leads to multiple colonic tumors, only when coupled with chronic colitis induced by dextran sodium sulfate (DSS), while when inflammation is absent it takes multiple injections of AOM and longer time for tumor formation [16], [17]. These clinical and experimental observations clearly pinpoint CAC as classical inflammation-driven cancer. However, unlike Apc/Wnt/β-catenin pathway, the mechanisms underlying colitis-associated cancer, and particularly, of colitis malignant transformation, are largely unclear, majorly due to lack of an appropriate model for dynamical investigation.
Intestinal epithelia are protected by a layer of mucin secreted by goblet cells against mechanical and chemical injuries, potent causes of inflammation, and the most abundant secreted intestinal mucin is encoded by the Muc2 gene [18]. Previous studies have shown that decreased number of goblet cells and reduced Muc2 expression is commonly observed in ulcerative colitis and colorectal carcinoma [19]. The importance of MUC2 in intestinal homeostasis is reflected by alterations of cell proliferation, migration and apoptosis in the mouse intestine upon genetic deletion of the Muc2 gene [20], and most importantly, Muc2-deficiency mice (Muc2 −/− mice) spontaneously develop small and large intestinal, and rectal, tumors [20]. Mechanistic studies have shown that tumorigenesis is associated with activation of chronic inflammation, and is not associated with Wnt/β-catenin signaling [20]. However, the inflammation increased intestinal tumorigenesis in Apc mutant mice by introducing Muc2 deficiency to the mice [21], and loss of cyclin dependent kinase inhibitor p21WAF1 enhanced intestinal tumor formation in Muc2 −/− mice [22], Our current study further showed that Muc2 deficiency mice spontaneously develop chronic colitis at their early age (<3 moths), whose histopathology was similar to ulcerative colitis in patients. After 3 months, the Muc2 −/− mice develop colonic and rectal tumors. Therefore, the age of 3 months might be the key point at which chronic colitis progresses to colorectal cancer, i.e. colitis malignant transformation, and the Muc2 mice could be one of the best engineered models of colitis-associated cancer to dynastically study the mechanism of malignant transformation of chronic colitis.
To reveal the molecular mechanisms of colitis malignant transformation, we isolated colonic epithelial cells from the Muc2 −/− mice and conducted miRNA profiling. We found differential expression of miRNAs at the key point of malignant transformation. Some miRNAs were characterized in human colitis and colorectal cancer tissues. Interestingly, the downregulated miRNAs were consistent with the alterations in mice, and linked to the increases of cytokines, suggesting the epigenetic alterations may play critical roles during colitis malignant transformation.
Materials and Methods
Ethics Statement:
The animal care and use were approved by the Institutional Animal Care and Use Committee of Xinxiang Medical University and University of Illinois at Chicago, and human samples collection and use were approved by the Institutional Review Board of Xinxiang Medical University. All patients gave informed consent in written.
Muc2 Mouse Model and Pathology Characterization:
As reported previously [20]–[22], the Muc2 +/− mice were backcrossed to generate Muc2 −/− and Muc2 +/+ mice, and 10 mice per group were fed with standard rodent chow diet for 3 months or 6 month. At the endpoints, the mice were sacrificed, entire gastrointestinal tract was opened and washed with cold PBS and fixed in 10% buffered formalin. The tissues were embedded in paraffin, sectioned and stained for histopathology characterization.
Muc2 Mouse Colonic Epithelia Cells Collection, mRNA Analysis, and miRNA Profiling:
Using the published protocol by us [23]–[25], mouse colonic epithelial cells were collected from 3-month aged Muc2 +/+ and Muc2 −/− mice, respectively. Four mice from each group were used. The total RNAs were extracted using Trizol reagent (Invitrogen, Carlsbad, CA) for cytokine mRNA analysis and miRNA array analysis. The quality and quantity of the RNA was determined using Bioanalyzer and Gel electrophoresis. Cytokine mRNA levels were analyzed using q-RT-PCR. The primers used for mouse cytokine analysis were listed in Table S1.
The miRNA array was performed in the Genomic Facility of University of Chicago (Chicago, Illinois). Affymetrix GeneChip miRNA Arrays version 3.0 was used for miRNA profile. In brief, 200 ng of total RNA were labeled using FlashTag Biotin HSR Labeling Kit according manufacturer’s protocol (Affymatrix), and about 130 ul of Affymatrix hybridization cocktail buffer (FS450-002) were used for about 18 hours according to the protocol (Affymatrix). The array was then scanned using Affymatrix GeneChip Scanner 3000. The raw data was processed with Expression Console 1.2.0.20, data value was defined using Log Expression Signal - RMA-DABG. The miRNAs with a fold change >2.0 or <0.5 and a t-test value <0.01 were selected as differentially expressed miRNAs. The detailed experimental design, detailed protocol and data analysis could be accessed at Gene Expression Omnibus (GEO) (Access # GSE56577).
Mouse miRNA Validation using quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR):
Based on the degrees of changed miRNA levels from the miRNA array profile, 6 of the most upregulated and 9 of the most downregulated miRNAs were validated using qRT-PCR. The reverse transcription (RT) primers and forward primers and probes used for the miRNA validation were listed in Table S2. The universal Taqman probe and universal reverse primer for qRT-PCR were purchased from Integrated DNA Technologies (IDT) (Table S2).
Total RNA from mouse colonic epithelia was polyadenylated with Poly(A) Polymerase Tailing Kit (Epicentre). Briefly, 10 µl of reaction including 1 µg of RNA, 1 µl of 10x reaction buffer, 1 µl of 10 mM ATP and 1 unit of Poly(A) polymerase was incubated at 37°C for 30 minutes, followed by enzyme inactivation at 65°C for 5 min and then put on ice. After polyadenylation, reverse transcription was performed in a 10 µl reaction containing 1 µl of the polyadenylation reaction product, 1 µl of 0.5 µM RT primer, 0.5 µl of 10 mM dNTP, 1 µl of AMV 10x reaction buffer, and 50 units of AMV High Performance Reverse Transcriptase (Promega, Madison, WI). The reaction was incubated at 42°C for 60 min, and then terminated by heating at 70°C for 10 min. RT products were amplified and detected using a S-Poly(T) method, as reported by us [26]. A 20 µl PCR reaction contains 2 µl of RT products (4-fold dilution), 10 µl of 2x GoTaq® Hot Start Colorless Master Mix (Promega, Madison, WI), 0.2 µM forward primer, 0.2 µM universal reverse primer, and 0.25 µM universal Taqman probe. The PCR reaction was performed at 95°C for 30 s, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. The qRT-PCR results were analyzed as reported by us[27]–[29]. The snoRNA202 was used as internal control.
Human Samples Collection:
Sixteen paires of human colorectal cancer tissues, colitis tissues, and their adjacent normal colon mucosa, were collected from November, 2012 through October, 2013, from the First Affiliated Hospital and the Affiliated Xinxiang Central Hospital, Xinxiang Medical University. Portion of the samples were snapped into liquid nitrogen and then stored at −80°C for RNA extraction and for qRT-PCR analysis. All patients gave informed consent in written. The sample collection and use was approved by the Institutional Review Board of Xinxiang Medical University.
RNA extraction and qRT-PCR for mRNA analysis for human samples were similar as described above for the analysis in mouse colonic epithelial cells. The primers and probes used for miRNA analysis were listed Table S2. SNORD 44 was used as internal control.
miRNA Targets Identification, Biological Functions Categorization, and Network Analysis:
To identify the targets of the miRNAs, we used online software - David (http://david.abcc.ncifcrf.gov/) and a predicted list of conserved miRNA target genes obtained from targetscan (http://www.targetscan.org/), starBase (http://starbase.sysu.edu.cn/), Tarbase (http://microrna.gr/tarbase/), and miRbase (http://mirbase.org/index.shtml). The biological functions of the miRNAs were categorized by Gene Oncology (GO). Potent targets network and signaling were proposed using KEGG (http://www.kegg.jp) and Ensembl (http://www.ensembl.org) web tools.
Results
Histopathology of the Muc2 Mouse Model of Colitis-associated Cancer:
Previous work have demonstrated that targeted gene knockout of the Muc2 gene caused tumor formation in entire gastrointestinal tract, including duodenum, colon and rectum[20], and the Muc2 −/− mice are susceptible to DSS-induced inflammatory bowl diseases[30]. In this study, we found that at 3 months or earlier, the Muc2 −/− mice spontaneously developed chronic inflammation in colon and rectum, accompanying with a few tumors in these sites (Figure 1). As shown in Figure 1A, the colon mucosa lacked of goblet cells and exhibited the features of ulcerative colitis, such as superficial erosion and intensive infiltrations of inflammatory cells throughout the mucosa, submucosa and even muscle layer of the colon, which was similar as observed in human ulcerative colitis. The chronic colitis was accompanied by an adenoma. At the age of 6 months, Muc2 −/− mice developed tumors at colon and rectum, as reported [20], but severe inflammation was still observed in intra-tumors and extra-tumors (Figure 1B). A unique phenotype was that Muc2 −/− mice developed rectal prolapses (Figure 1C) that has never been reported previously. Histopathologically, the prolapse was adenocarcinoma with severe inflammation at the rectums (Figure 1D), displaying superficial erosion, inflammatory cells infiltration and cancer cell invasion. In addition, the severity of inflammatory cell infiltration was associated with the severity of rectal prolase, but was not associated with cancer cell invasion and differentiation in the rectum (data not shown).
Figure 1. Muc2 −/− mice exhibited chronic inflammation and cancers in colon and rectum.
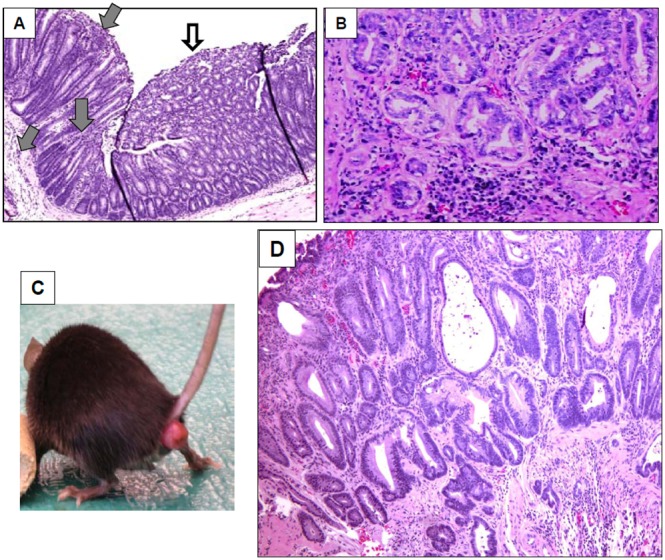
A, Colitis and an adenoma in mouse colon at age of 3 months. Gray arrows pointed severe infiltration of inflammatory cells, white arrow pointed an adenoma. B, colonic adenocarcinoma with inflammatory cell infiltration at age of 6 months. C, Rectal prolapsed at 6 months. D, Histopathology of the rectal prolapse, showing cancer cell invasion and severe inflammation in the rectum (age of 6 months).
miRNA Profiling Revealed Differential Expression of miRNA in Muc2−/− Mice
Recent studies have suggested the important roles of miRNAs in carcinogenesis. To investigate whether aberrant expression of miRNAs are involved in colitis malignant transformation, we isolated the colonic epithelial cells from 3-month old mice (4 Muc2 −/− mice and 4 Muc2 +/+ mice) and conducted miRNA profiling. It is known that the interaction between stromal and epithelial cells plays important role in driving colitis-associated colorectal cancer (CAC). Muc2 was overexpressed in colonic epithelial cells, and genetic deficiency of this gene is sufficient to cause colitis and colorectal cancer, exerting the importance of Muc2 in the development of colitis and CAC. To determine the roles of aberrantly expressed miRNAs resulted from Muc2 absence in initiating colitis and facilitating colorectal cancer transformation, we used colonic epithelial cells instead of stromal cells or entire colon tissues for miRNA array. MiRNA profiling analysis showed differential levels of miRNAs, among them 20 miRNAs were significantly downregulated and 71 miRNAs were significantly upregulated (Table 1) in Muc2 −/− mice, in comparison with Muc2 +/+ mice (change fold >2 or <0.5; T<0.01, p value<0.05, q value<0.05). As showing in Table S3 and Table S4, most of the miRNAs have been reported to regulate their targets and play critical roles in cancer initiation, progression and metastasis, in different tissues and cells. While, biological functions of some miRNAs are not clear and warrant further investigation.
Table 1. Down- and up-regulated miRNAs in Muc2−/− mouse colonic epithelial cells profiled by miRNA array.
| miRNAs | Chromosome location | Mean (fold changes) | T-Test (<0.01) |
| Down-regulated | |||
| mmu-miR-3096b-3p | – | 0.053013368 | 0.000804092 |
| mmu-miR-3096-3p | – | 0.082183923 | 0.000431388 |
| mmu-miR-146a | chr11: 43374397–43374461 [−] | 0.196486214 | 0.005127109 |
| mmu-miR-138 | chr8: 94324311–94324381 [+] | 0.247414164 | 0.005127109 |
| mmu-miR-1949 | chr18: 35554567–35554636 [+] | 0.247414164 | 0.001366367 |
| mmu-miR-5123 | chr4: 40850056–40850138 [−] | 0.338563887 | 0.009803996 |
| mmu-miR-132 | chr11: 75173682–75173747 [+] | 0.362863789 | 0.001008516 |
| mmu-miR-5099 | chr12: 36816205–36816278 [+] | 0.375009747 | 0.001860521 |
| mmu-miR-1a-1-star | chr2: 180389048–180389124 [+] | 0.382226577 | 0.001695487 |
| mmu-miR-24-2-star | chr8: 84208815–84208921 [+] | 0.396392068 | 0.001922905 |
| mmu-miR-21 | chr11: 86584067–86584158 [−] | 0.397768242 | 0.001673221 |
| mmu-miR-147 | chr2: 122640803–122640881 [+] | 0.401229615 | 0.000804292 |
| mmu-miR-5112 | chr18: 82720281–82720340 [+] | 0.409660302 | 0.002989666 |
| mmu-miR-677-star | chr10: 128085286–128085363 [+] | 0.423372656 | 0.002245339 |
| mmu-miR-150 | chr7: 45121757–45121821 [+] | 0.433769344 | 0.009595381 |
| mmu-miR-27a | chr8: 84208672–84208758 [+] | 0.470576115 | 0.000890949 |
| mmu-miR-196b-star | chr6: 52230081–52230165 [−] | 0.48380581 | 0.006710287 |
| mmu-miR-23a | chr8: 84208518–84208592 [+] | 0.498270131 | 0.001669314 |
| mmu-miR-145 | chr18: 61647825–61647894 [−] | 0.498570581 | 0.001129314 |
| mmu-miR-5105 | – | 0.49933846 | 0.000289965 |
| Up-regulated | |||
| mmu-miR-466g | chr2: 10514595–10514674 [+] | 2.0139111 | 0.000615992 |
| mmu-miR-327 | chr14: 44947443–44947511 [−] | 2.056227653 | 0.003244991 |
| mmu-miR-466m-3p | chr2: 10466663–10466746 [+] | 2.077718207 | 0.00355787 |
| mmu-miR-466f-3p | – | 2.084931522 | 0.003418186 |
| mmu-miR-1931 | chr10: 93162785–93162903 [+] | 2.088547565 | 0.000362476 |
| mmu-miR-1894-3p | chr17: 35917889–35917969 [+] | 2.095798477 | 0.002599441 |
| mmu-miR-3470b | chr16: 44013852–44013977 [+] | 2.110375908 | 0.006057193 |
| mmu-miR-1896 | chr13: 21445160–21445240 [+] | 2.199994627 | 0.004807219 |
| mmu-miR-3093-3p | chr3: 88215171–88215257 [+] | 2.230705407 | 0.004988571 |
| mmu-miR-195-star | chr11: 70235042–70235135 [+] | 2.281527432 | 0.006474418 |
| mmu-miR-204-star | chr19: 22750605–22750672 [+] | 2.317388619 | 0.004938058 |
| mmu-miR-125a-3p | chr17: 17830812–17830879 [+] | 2.321407829 | 0.002948655 |
| mmu-miR-669f-3p | chr2: 10467229–10467349 [+] | 2.325434009 | 0.002838976 |
| mmu-miR-16-1-star | chr14: 61631880–61631972 [−] | 2.374296321 | 0.008688873 |
| mmu-miR-466i-3p | chr13: 17747473–17747593 [+] | 2.411615655 | 0.002526037 |
| mmu-miR-1943 | chr15: 79375228–79375300 [−] | 2.428389769 | 0.002672958 |
| mmu-miR-1967 | chr8: 124022641–124022722 [+] | 2.441046876 | 0.005668811 |
| mmu-miR-150-star | chr7: 45121757–45121821 [+] | 2.470837274 | 0.008970092 |
| mmu-miR-383 | chr8: 38252133–38252202 [−] | 2.505328877 | 5.02E-05 |
| mmu-miR-669p-star | chr2: 10489116–10489202 [+] | 2.549121255 | 0.003814622 |
| mmu-miR-877 | chr17: 35960730–35960814 [−] | 2.553542375 | 0.008090848 |
| mmu-miR-92a-2-star | chrX: 52741838–52741928 [−] | 2.611719574 | 0.004892665 |
| mmu-miR-669c-star | chr2: 10509296–10509404 [+] | 2.634446716 | 0.000446431 |
| mmu-miR-5133 | chr9: 62122518–62122594 [−] | 2.643592852 | 0.001549712 |
| mmu-miR-3113-star | chrX: 151859562–151859637 [+] | 2.727350278 | 0.001069083 |
| mmu-miR-3082-5p | chr17: 25831365–25831428 [−] | 2.74156561 | 0.001627678 |
| mmu-miR-466i-5p | chr13: 17747473–17747593 [+] | 2.784657705 | 0.005962544 |
| mmu-miR-574-5p | chr5: 64970318–64970395 [+] | 2.789487333 | 0.001972081 |
| mmu-miR-3474 | chr2: 158638583–158638640 [+] | 2.923101651 | 0.004805635 |
| mmu-miR-466h-3p | chr2: 10514891–10514971 [+] | 2.933249925 | 0.001659026 |
| mmu-miR-5120 | chr4: 44607491–44607568 [−] | 3.010493495 | 0.003511011 |
| mmu-miR-467d-star | chr2: 10507630–10507714 [+] | 3.08977118 | 0.004977926 |
| mmu-miR-546 | chr10: 126998440–126998560 [+] | 3.116658319 | 0.000267697 |
| mmu-miR-615-5p | chr15: 103014910–103015001 [+] | 3.165646144 | 0.001906752 |
| mmu-miR-466j | chr10: 60960723–60960844 [+] | 3.193193545 | 0.00044491 |
| mmu-miR-466h-5p | chr2: 10514891–10514971 [+] | 3.215403963 | 0.003518948 |
| mmu-miR-1962 | chr7: 135566162–135566282 [+] | 3.305801273 | 0.000199144 |
| mmu-miR-296-3p | chr2: 174267047–174267125 [−] | 3.369419364 | 0.002478334 |
| mmu-miR-667-star | chr12: 109720006–109720097 [+] | 3.369419364 | 0.005918912 |
| mmu-miR-135a-1-star | chr9: 106154124–106154213 [+] | 3.392855529 | 0.002717844 |
| mmu-miR-669f-5p | chr2: 10467229–10467349 [+] | 3.422380103 | 0.005286193 |
| mmu-miR-5110 | chr11: 85760616–85760702 [−] | 3.452161599 | 0.00719303 |
| mmu-miR-5122 | chr4: 133369776–133369864 [+] | 3.464146636 | 0.001568357 |
| mmu-miR-3075 | chr14: 25534439–25534523 [+] | 3.512504321 | 0.000262538 |
| mmu-miR-1930-star | chr10: 77641224–77641307 [+] | 3.543070076 | 0.000277333 |
| mmu-miR-328-star | chr8: 105308364–105308460 [−] | 3.555370725 | 0.006676725 |
| mmu-miR-669c | chr2: 10509296–10509404 [+] | 3.573901815 | 0.000129374 |
| mmu-miR-3064-5p | chr11: 106782693–106782759 [−] | 3.586309503 | 0.001181755 |
| mmu-miR-129-5p | chr6: 29022619–29022691 [+] | 3.61751751 | 0.000610483 |
| mmu-miR-466f | chr2: 10466944–10467037 [+] | 3.642679334 | 0.001960095 |
| mmu-miR-92b-star | chr3: 89227116–89227198 [−] | 3.655325801 | 0.000962434 |
| mmu-miR-3473 | chrX: 162874918–162874995 [−] | 3.738604865 | 0.005536237 |
| mmu-miR-211-star | chr7: 64205806–64205911 [+] | 3.837056477 | 0.00152237 |
| mmu-miR-760-3p | chr3: 122293585–122293703 [−] | 3.9244759 | 0.002240405 |
| mmu-miR-669n | chr3: 115979902–115979955 [+] | 4.17709513 | 0.009992096 |
| mmu-miR-704 | chr6: 47803576–47803652 [−] | 4.25748073 | 0.002715333 |
| mmu-miR-3470a | chr6: 83090311–83090387 [−] | 4.399989253 | 0.002512862 |
| mmu-miR-1966 | chr8: 105615466–105615573 [+] | 4.407620464 | 0.000453366 |
| mmu-miR-323-5p | chr12: 109712508–109712593 [+] | 4.563054863 | 0.00046665 |
| mmu-miR-3104-5p | chr7: 141992179–141992241 [+] | 4.667014663 | 0.000569568 |
| mmu-miR-3102-star | chr7: 100882306–100882409 [−] | 4.699476317 | 0.001673908 |
| mmu-miR-3081-star | chr16: 44558046–44558129 [−] | 4.83159658 | 0.000792041 |
| mmu-miR-5130 | chr14: 102982549–102982632 [−] | 5.445256466 | 0.001466623 |
| mmu-miR-3472 | − | 6.158162767 | 0.006037372 |
| mmu-miR-3077-star | chr14: 57798424–57798487 [+] | 6.531887757 | 0.008639499 |
| mmu-miR-1982-star | chr10: 80828797–80828870 [+] | 6.680703355 | 0.006823859 |
| mmu-miR-5135 | chr12: 76533134–76533212 [−] | 7.727490631 | 0.004441672 |
| mmu-miR-705 | chr6: 85336292–85336373 [−] | 7.8489518 | 0.000703155 |
| mmu-miR-5132 | chrX: 74023528–74023598 [−] | 11.95879399 | 0.006610448 |
| mmu-miR-5115 | − | 16.16722314 | 0.008489482 |
| mmu-miR-5102 | − | 21.00265778 | 0.003512181 |
Mouse miRNAs Validation by qRT-PCR:
To evaluate the accuracy of the profiled miRNAs alteration in mouse colonic epithelial cells, we selected 15 most relevant miRNAs for validation using qRT-PCR. The 6 upregulated miRNAs (mmu-miR-5132-5p, mmu-miR-3104-5p, mmu-miR-669c-5p, mmu-miR-705, mmu-miR-760-3p, mmu-miR-1962) and the 9 downregulated miRNAs (mmu-miR-146a, mmu-miR-138, mmu-miR-5123, mmu-miR-196b, mmu-miR-5099, mmu-miR-150, mmu-miR-145, mmu-miR-27a, mmu-miR-23a) chosen for validation were also based on their target genes predicted, whose functions are well relevant to inflammation and cancer. As shown in Figure 2, the changes of miRNA assayed by qRT-PCR were consistent with the changes profiled by miRNA array analysis.
Figure 2. The aberrantly expressed miRNAs were validated in Muc2 −/− mice colonic epithelial cells, in comparison with Muc2 +/+ mice.
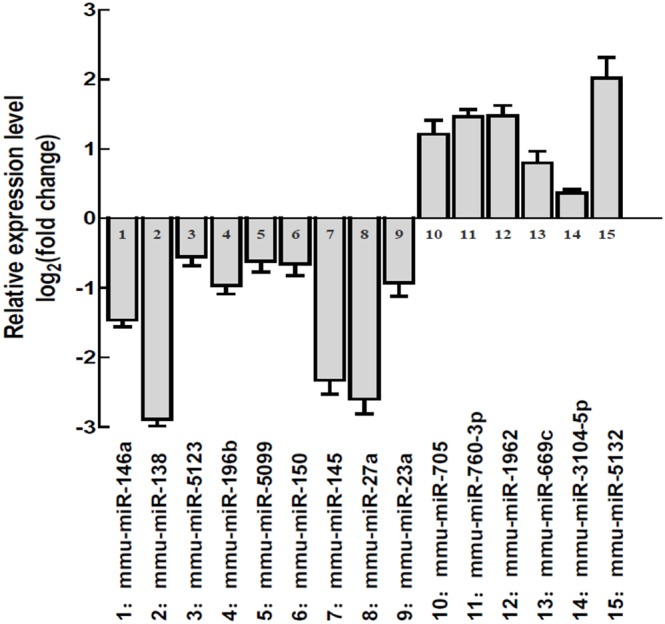
Four mice at age of 3 months from each genotype group were sacrificed and colonic epithelial cells were isolated for RNA extraction and qRT-PCR analysis. The columns stood for Mean+/−SD.
Aberrant Expression of miRNAs in Human Colitis, Colorectal Tumor and Adjacent Normal Mucosa
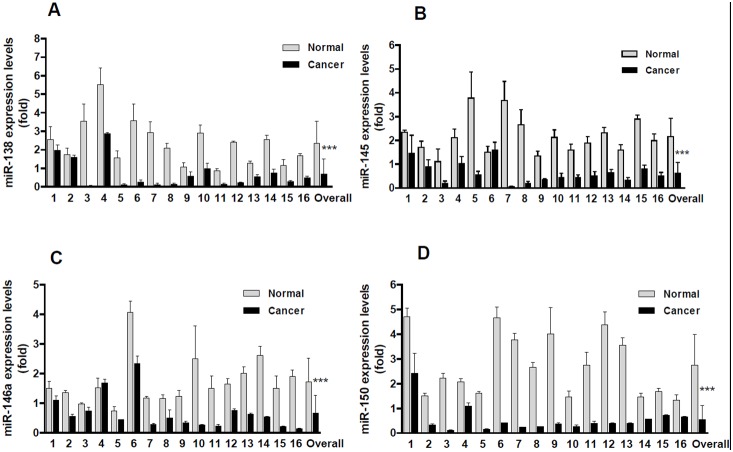
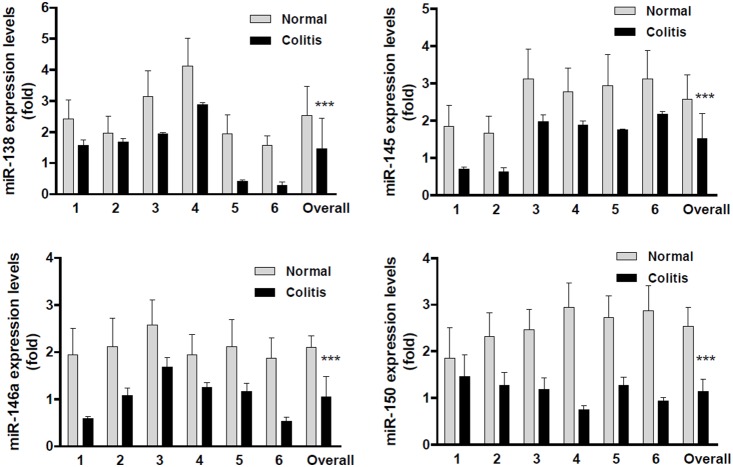
To determine whether the aberrantly expressed miRNAs have clinical significance, we chose 4 miRNAs for analysis in human colorectal cancer and colitis tissues. As shown in Figure 3, in overall, the expression level of miR-138, miR-145, miR-146a and miR-150 were downregulated by approximately 3.37, 3.39, 2.56 and 4.99 fold in colorectal cancers than those in the matched adjacent normal mucosa (p<0.0001). Among them, all the 16 colorectal cancers showed downregulated miR-138 and miR-150 levels (Figures 3A and 3D), and 15 out of the 16 colorectal cancers showed lower miR-145 and miR-146a expression levels than normal control (Figures 3B and 3C). Only one patient (Sample 6) showed upregulation of miR-145, but the upregulation was not significant (Figure 3B, p>0.05). Interestingly, the downregulation of these miRNAs were also observed in human chronic colitis tissues (Figures 4, p<0.0001, compared to the normal mucosa). Although the observations were obtained from small sized samples, the trends of significant downregulation of miRNAs (miR-138, 145, 146a and miR-150) strongly suggested their clinical importance of linkage to chronic colitis and colitis-associated colorectal cancer, indirectly indicating their potential biological functions of involving in colitis malignant transformation. In fact, the functions of these miRNAs on tumor suppression are being under investigation using manipulated cell culture system in vitro and tumor-bearing nude mice in vivo.
Figure 3. The downregulated miRNAs were confirmed in human colorectal cancers.
A, miR-138 was significantly downregulated in colorectal cancers. B, miR-145 was significantly downregulated in colorectal cancers. C, miR-146a was significantly downregulated in colorectal cancers. D, miR-150 was significantly downregulated in colorectal cancers. Each column stood for one patient, total 16 patients. The Overall stood for the Mean of the miRNAs in all patients. (***p<0.0001, compared to adjacent normal mucosa).
Figure 4. The downregulated miRNAs were confirmed in human colitis.
A, miR-138 was significantly downregulated in colitis. B, miR-145 was significantly downregulated in colitis. C, miR-146a was significantly downregulated in colitis. D, miR-150 was significantly downregulated in colitis. Each column stood for one patient, total 6 patients. The Overall stood for the Mean of the miRNAs in all patients. (***p<0.0001, compared to adjacent normal mucosa).
miRNA Target Network Characterization:
Since the changed miRNAs had great significance in Muc2 −/− mice and human colitis malignant transformation, and the selected miRNA showed downregulation in colitis and colorectal cancers, we next elucidated whether there were any common targets of these miRNAs. Unfortunately, no common inflammation- and cancer-associated targets for all of the 4 miRNAs (miR-138, 145, 146a and miR-150) were identified using miRNA target prediction tools. However, we did find some common targets of any 3 or 2 of the 4 miRNAs. As shown in Figure 5 and Table 2, there were 21, 13 and 25 common targets between miR-138 and miR-145, miR-146a and miR-150, respectively; there were 16 and 15 common between miR-145 and miR-146a and miR-150, respectively; and there were 7 common targets between miR-146a and miR-150. Please be noted that CCT3 and PAPPA were the common targets for miR-138, miR-146a and miR-150, and ZHX2 was the common target for miR-138, miR-145 and miR-150. Interestingly, most of these targets are oncogenes. GO term annotation showed that all the targets are involved in cellular physiological process and metabolism, and the regulation of cellular process and regulation of physiological process are the most significantly enriched GO terms. Thus, any dysregulation of these miRNAs and their targets might be sufficient to cause initiation of inflammation and chronic inflammation malignant transformation, studies on any common targets of two or more miRNAs in carcinogenesis is more significant than any targets of a single miRNA.
Figure 5. Numbers of the common targets of the miRNAs (miR-138, 145, 146a and miR-150), and their network.
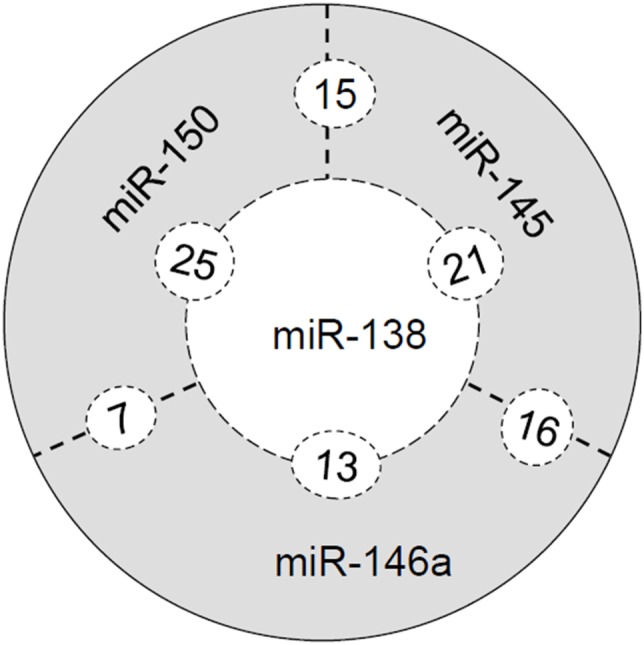
Table 2. Predicted common targets of 2 miRNAs (Names in bold are the common targets for at least 3 miRNAs).
| miR-138 and −145 | miR-138 and −146a | miR-145 and −146a | miR-138 and −150 | miR-145 and −150 | miR-150 and −146a |
| BAG1 | BCL11A | ARPC5 | CCT3 | ACBD3 | BZRAP1 |
| CCDC107 | BUD13 | BSG | DHDDS | AMOTL2 | CCT3 |
| CLK3 | CCT3 | C20orf20 | DVL2 | BSN | IRAK1 |
| CPEB4 | CD1B | CD53 | EIF4EBP1 | C1orf177 | NPTN |
| DUSP6 | DMKN | DYX1C1 | ELF2 | CCDC88 | PAPPA |
| FBXO18 | MAT2A | CCPG1 | ERC1 | DYRK1A | SMC3 |
| FMNL2 | MXD4 | FBXL10 | LRRC4 | EIF4B | TRAF2 |
| GGTL3 | PAPPA | GAD1 | MAP3K11 | EIF4EBP2 | |
| GPHN | PDLIM1 | HEMGN | MYBBP1A | MBNL1 | |
| INHBB | PHF1 | HIC2 | NKIRAS2 | NPM3 | |
| LENG8 | PHOX2B | IVNS1ABP | PAPPA | SIRT5 | |
| MAP3K6 | RUNX1T1 | PCBP2 | PFN2 | SMARCD1 | |
| MECP2 | VIM | STC1 | PPARGC1A | STK19 | |
| PHF21A | TLN2 | PTP4A1 | TMEM116 | ||
| PITX3 | VASN | PURB | ZHX2 | ||
| RARA | ZFYVE9 | RAD23B | |||
| RBM41 | RAI17 | ||||
| S100A2 | SEH1L | ||||
| WFDC11 | SUMO1 | ||||
| ZHX2 | TP53INP2 | ||||
| ZMYND11 | TRPS1 | ||||
| UPF2 | |||||
| VEGFB | |||||
| ZBTB | |||||
| ZHX2 |
Cytokines in Muc2−/− Mouse Colonic Epithelial Cells were Up-regulated:
To validate the accuracy of the analysis of the miRNA and their association with cytokine mRNAs, the later are frequently seen in colitis-associated cancer, we determine the alterations of cytokine in mouse colon. As shown in Figure 6, compared to Muc2 +/+ mouse, Muc2 −/− mice showed significant upregulation of cytokines (e.g. IL-6, Cox2, TNF-a, and IL-1β, etc) in colonic epithelial cells from the normal appearing colon mucosa (p<0.01).
Figure 6. The alterations of cytokines in Muc2 mouse colonic epithelial cells.
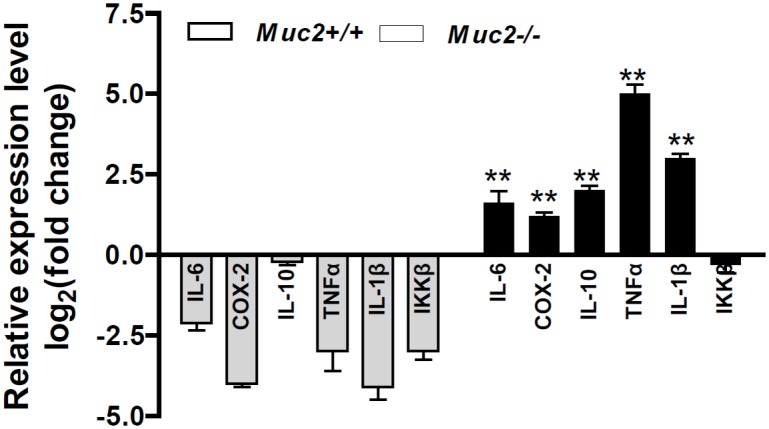
Compared to Muc2 +/+ mice, Muc2 −/− mice showed significant upregulation of cytokines (**p<0.01, compared to Muc2 +/+ mice). Four mice at age of 3 months from each genotype group were sacrificed and colonic epithelial cells were isolated for RNA extraction and qRT-PCR analysis. The columns stood for Mean+/−SD.
Discussion
The current study further characterized a colitis-associated colorectal cancer (CAC) model of Muc2 −/− mice, providing direct evidence that chronic inflammation in colon and rectum could be malignantly transformed, and revealing the potent mechanisms, that was that aberrant miRNA expression in colonic epithelial cells were involved in and might play critical roles during the malignant transformation of chronic colitis by interfering target genes.
Numerous evidence-based studies have demonstrated that chronic colitis is one of the important causes of colorectal cancer, but the underlying mechanisms are not clear, one of the major reasons is lack of spontaneous colitis and colitis-associated colorectal cancer model. Currently, a variety of genetically engineered animal models are available and are very useful for better understanding of the molecular mechanisms underlying the pathogenesis of CAC [31], but pathological features of these models have not been dynamically characterized. The present study characterized the Muc2 mouse model. The Muc2 −/− mice spontaneously develop chronic inflammation in colon and rectum at early stage (<3 months), and after 3 months, the chronic inflammation progress to colorectal cancer. Most importantly, the pathologic features of the chronic inflammation at colon and rectum were similar as human ulcerative colitis, and the aberrantly expressed miRNAs involved in Muc2 −/− mouse colitis malignant transformation were observed in human colitis and colorectal cancer tissues. Another unique feature was the rectal prolapses – rectal cancer with severe inflammation. Therefore, the Muc2 −/− mouse could be the appropriate rodent model of CAC, and could be used as one of the best tools to elucidate the cause of CAC and the underlying molecular mechanisms. The Muc2 −/− mouse could also be used as a mouse model for chemoprevention and therapy for colitis-associated colorectal cancer.
miRNAs have 19–22 nucleotides, are a novel class of small noncoding RNAs that suppress the translation and stability of messenger RNAs (mRNAs) by binding to target mRNAs’ 3′-untranslated regions (3′-UTR)[32], miRNAs have important biological and pathophysical functions of involving in development, cell proliferation, differentiation, apoptosis, inflammation and stress response[33]–[35]. Increasing evidences have suggested that miRNAs are downregulated or upregulated in cancers, acting as tumor suppressors or oncogenes [33], [35], [36], in which the miRNAs play critical roles in tumorigenesis, differentiation, progression (e.g. migration, invasion, angiogenesis, and metastasis) [35]–[37], mainly by interfering with the expression of target genes. Using a miRNA array, we have profiled differential expression of miRNAs in colonic epithelial cells from the Muc2 −/− mice, and these miRNAs are either upregulated as oncogenes or downregulated as tumor suppressors by targeting the genes at the categories of metabolism, cell cycle, differentiation, cell death, DNA replication, homeostasis, signal transduction, response to stimulation, and inflammation, etc. Among the markedly changed miRNAs, the downregulated miRNAs, miR-138, 145, 146a and 150, were validated in both mouse and human tissues, particularly, in human colitis and colorectal cancer tissues, suggesting suppressing roles of miR-138, 145, 146a and 150 in colitis malignant transition via interacting with cytokines and inflammatory factors.
Previous studies have demonstrated tumor suppressor roles of miR-138 in cancer biology. miR-138 inhibited cancer cell growth and tumorigenesis in non-small cell lung cancer and nasopharyngeal cancer by targeting 3-phosphoinositide-dependent protein kinase-1 (PDK1) and CCND1 [38]–[40]. In colorectal and ovarian cancers, miR-138 suppressed cancer cell migration and metastasis through interfered with TWIST2, SOX4 and HIF1-a [39], [41]. Most recent studies reported that downregulated miR-138 sustained inflammatory factor NF-kB activation and promoted esophageal cancer progression [42], and that miR-138 response to pro-inflammatory cytokines depends on the stabilization of HIF1-α in primary human microvascular endothelial cells [43]. miR-145 is also a tumor suppressor gene. miR145 could target the SOX9/ADAM17 axis and inhibit tumor-initiating cells and IL-6-mediated paracrine effects in head and neck cancer[44]. Moreover, microRNA-145 induces apoptosis with the induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression, targeted oncogene socs7 and regulated interferon-β induction through STAT3 nuclear translocation in bladder cancer cells [45]. Recent studies have reported that TRAIL suppresses chemokine (C-X-C motif) receptor 4 (CXCR4) -mediated human breast cancer cell migration by up-regulating miR-146a expression through NF-κB signaling [46], and that miR-146 regulates epigenetic regulator UHRF1 and modulates gastric cancer invasion and metastasis [47], showing the important roles of miR-146 in inhibiting cancer metastasis by interfering chemokine and epigenetic regulator. As to miR-150, quite a lot of reports have demonstrated its tumor inhibitory function [48]–[51]. While, that miR-150 interacts with cytokines in lymphocyte differentiation and in inflammation has been studied. For instance, in cytotoxic T lymphocytes (CTL), IL-2R and inflammatory signals act through Dicer and miRNAs to control the cytolytic program and CTL differentiation, in which miR-139 and miR-150 are downregulated by inflammation in CTLs, and miR-150 regulates the expression of the IL-2 receptor α-chain (CD25) [52]. In addition, IFN-γ production is significantly increased in the miR-150 knockout mice [53]. Back to our findings, that were, cytokines were significantly increased (Figure 6) and miR-138, 145, 146a and miR-150 were significantly decreased in Muc2 −/− mouse colon and human colitis and colorectal cancer, incorporating with the published observations, strongly support our hypothesis that the cytokine-associated miRNAs, miR-138, 145, 146a and miR-150, play important roles in chronic colitis malignant transformation through interfering with cytokines and inflammatory factors. However, similar as the changes of cytokines as a consequence of colitis in mouse colon, the changes of the miRNAs could also be a consequence chronic colitis and CAC in human colon tissues. It could be possible, but the preliminary data from our ongoing functional studies using manipulated cell culture systems and in vivo nude mouse model have shown individual or synergistical potential of these miRNA (Bao and Yang, unpublished data), confirming tumor suppressing functions of these miRNAs. In addition, the potent resources of the altered miRNAs in human colitis and CRC tissues are not clear and need further investigation, the restults generated from which could clarify the functions cause or effect of these miRNAs in the development of colitis and colorectal cancer.
Our study further identified some common targets of the miR-138, 145, 146a and miR-150 (Table 2 and Figure 5), such as PAPPA (pregnancy-associated plasma protein A), CCT3 (chaperonin containing TCP1, subunit 3) and ZHX2 (zinc fingers and homeoboxes 2), which were the common targets of three miRNAs. These three targets have been reported to be overexpressed in embryonic cells and stem cells, and exert oncogenic functions during cell process and proliferation [54]–[56], which could play synergistic roles with miRNA-regulated cytokine activation to initiate colorectal cancer formation and facilitate colorectal cancer progression. These targets’ expression levels in normal mucosa, colitis and colorectal cancer tissues are not clear, and their biological functions in cancer, are not clear and under investigation. Moreover, it is worthy to point out that tumor formation and progression are controlled by a complex not by a single element, thus, studying on multiple miRNAs and multiple targets, especially on common targets of multiple miRNAs, instead of studying on a single target or single miRNA, are more important, to reveal the causes of cancers and the molecular mechanisms.
Taken above, we have characterized a colitis-associated colorectal cancer model, and revealed the underlying mechanisms that aberrant expressed miRNAs targets cytokines and downstream genes to facilitate colitis malignant transformation. This is the first to reveal the importance of aberrant expression of miRNAs in dynamically transformation from chronic colitis to colitis-associated cancer. These findings shed light on revealing the mechanisms of chronic colitis malignant transformation.
Supporting Information
Primer sequences for the mouse qRT-PCR.
(DOCX)
Primers and Probes for miRNA qRT-PCR.
(XLSX)
Oncogenic miRNAs upregulated in Muc2 −/− mouse colonic epithelial cells profiled by miRNA array.
(DOC)
Tumor suppressor miRNAs downregulated in Muc2 −/− mouse colonic epithelial cells profiled by miRNA array.
(DOC)
Funding Statement
This work was supported in part by the grant from the National Natural Science Foundation of China (grant #91229115 and 81272251), a grant for the Innovative Team of Science and Technology from the Department of Education, Henan Province, China, and Doctor Research Fund (#100820 and 505011) and Startup Fund from Xinxiang Medical University, China. The work was also supported in part by the National College Student Innovative Projects of China (#201310472005 to XL, 201310472012 to BX, and 201310472016 to ZL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA: a cancer journal for clinicians 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 2. Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87: 159–170. [DOI] [PubMed] [Google Scholar]
- 3. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, et al. (2013) Cancer genome landscapes. Science 339: 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rustgi AK (2007) The genetics of hereditary colon cancer. Genes Dev 21: 2525–2538. [DOI] [PubMed] [Google Scholar]
- 5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, et al. (2013) Cancer genome landscapes. Science 339: 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, et al. (1997) Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275: 1787–1790. [DOI] [PubMed] [Google Scholar]
- 7.Terzic J, Grivennikov S, Karin E, Karin M (2010) Inflammation and colon cancer. Gastroenterology 138: 2101–2114 e2105. [DOI] [PubMed]
- 8. Grivennikov SI (2013) Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 35: 229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danese S, Mantovani A (2010) Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene 29: 3313–3323. [DOI] [PubMed] [Google Scholar]
- 10. Trinchieri G (2012) Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol 30: 677–706. [DOI] [PubMed] [Google Scholar]
- 11. Sung JJ, Lau JY, Goh KL, Leung WK (2005) Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol 6: 871–876. [DOI] [PubMed] [Google Scholar]
- 12. Saleh M, Trinchieri G (2011) Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol 11: 9–20. [DOI] [PubMed] [Google Scholar]
- 14. Eaden JA, Abrams KR, Mayberry JF (2001) The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jess T, Rungoe C, Peyrin-Biroulet L (2012) Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol 10: 639–645. [DOI] [PubMed] [Google Scholar]
- 16. Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S (1996) Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut 39: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neufert C, Becker C, Neurath MF (2007) An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc 2: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 18. Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4: 45–60. [DOI] [PubMed] [Google Scholar]
- 19. Andrianifahanana M, Moniaux N, Batra SK (2006) Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta 1765: 189–222. [DOI] [PubMed] [Google Scholar]
- 20. Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, et al. (2002) Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295: 1726–1729. [DOI] [PubMed] [Google Scholar]
- 21. Yang K, Popova NV, Yang WC, Lozonschi I, Tadesse S, et al. (2008) Interaction of Muc2 and Apc on Wnt signaling and in intestinal tumorigenesis: potential role of chronic inflammation. Cancer Res 68: 7313–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang W, Velcich A, Lozonschi I, Liang J, Nicholas C, et al. (2005) Inactivation of p21WAF1/cip1 enhances intestinal tumor formation in Muc2−/− mice. Am J Pathol 166: 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bi X, Tong C, Dockendorff A, Bancroft L, Gallagher L, et al. (2008) Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis 29: 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tong C, Yin Z, Song Z, Dockendorff A, Huang C, et al. (2007) c-Jun NH2-terminal kinase 1 plays a critical role in intestinal homeostasis and tumor suppression. Am J Pathol 171: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mariadason JM, Nicholas C, L’Italien KE, Zhuang M, Smartt HJ, et al. (2005) Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology 128: 1081–1088. [DOI] [PubMed] [Google Scholar]
- 26. Kang K, Zhang X, Liu H, Wang Z, Zhong J, et al. (2012) A novel real-time PCR assay of microRNAs using S-Poly(T), a specific oligo(dT) reverse transcription primer with excellent sensitivity and specificity. PloS one 7: e48536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bi X, Pohl NM, Qian Z, Yang GR, Gou Y, et al. (2012) Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis 33: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fang W, Han A, Bi X, Xiong B, Yang W (2010) Tumor inhibition by sodium selenite is associated with activation of c-Jun NH2-terminal kinase 1 and suppression of beta-catenin signaling. Int J Cancer 127: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fang W, Goldberg ML, Pohl NM, Bi X, Tong C, et al. (2010) Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis 31: 1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, et al. (2006) Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129. [DOI] [PubMed] [Google Scholar]
- 31. Kanneganti M, Mino-Kenudson M, Mizoguchi E (2011) Animal models of colitis-associated carcinogenesis. J Biomed Biotechnol 2011: 342637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 33. Ventura A, Jacks T (2009) MicroRNAs and cancer: short RNAs go a long way. Cell 136: 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farazi TA, Spitzer JI, Morozov P, Tuschl T (2011) miRNAs in human cancer. J Pathol 223: 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Di Leva G, Garofalo M, Croce CM (2014) MicroRNAs in cancer. Annu Rev Pathol 9: 287–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lages E, Ipas H, Guttin A, Nesr H, Berger F, et al. (2012) MicroRNAs: molecular features and role in cancer. Front Biosci (Landmark Ed) 17: 2508–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White NM, Fatoohi E, Metias M, Jung K, Stephan C, et al. (2011) Metastamirs: a stepping stone towards improved cancer management. Nat Rev Clin Oncol 8: 75–84. [DOI] [PubMed] [Google Scholar]
- 38.Ye XW, Yu H, Jin YK, Jing XT, Xu M, et al.. (2014) miR-138 inhibits proliferation by targeting 3-phosphoinositide-dependent protein kinase-1 in non-small cell lung cancer cells. Clin Respir J. [DOI] [PubMed]
- 39. Long L, Huang G, Zhu H, Guo Y, Liu Y, et al. (2013) Down-regulation of miR-138 promotes colorectal cancer metastasis via directly targeting TWIST2. J Transl Med 11: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu X, Lv XB, Wang XP, Sang Y, Xu S, et al. (2012) MiR-138 suppressed nasopharyngeal carcinoma growth and tumorigenesis by targeting the CCND1 oncogene. Cell Cycle 11: 2495–2506. [DOI] [PubMed] [Google Scholar]
- 41. Yeh YM, Chuang CM, Chao KC, Wang LH (2013) MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1alpha. Int J Cancer 133: 867–878. [DOI] [PubMed] [Google Scholar]
- 42. Gong H, Song L, Lin C, Liu A, Lin X, et al. (2013) Downregulation of miR-138 sustains NF-kappaB activation and promotes lipid raft formation in esophageal squamous cell carcinoma. Clin Cancer Res 19: 1083–1093. [DOI] [PubMed] [Google Scholar]
- 43. Sen A, Most P, Peppel K (2014) Induction of microRNA-138 by pro-inflammatory cytokines causes endothelial cell dysfunction. FEBS Lett 588: 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu CC, Tsai LL, Wang ML, Yu CH, Lo WL, et al. (2013) miR145 targets the SOX9/ADAM17 axis to inhibit tumor-initiating cells and IL-6-mediated paracrine effects in head and neck cancer. Cancer Res 73: 3425–3440. [DOI] [PubMed] [Google Scholar]
- 45. Noguchi S, Yamada N, Kumazaki M, Yasui Y, Iwasaki J, et al. (2013) socs7, a target gene of microRNA-145, regulates interferon-beta induction through STAT3 nuclear translocation in bladder cancer cells. Cell death & disease 4: e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang D, Liu D, Gao J, Liu M, Liu S, et al. (2013) TRAIL-induced miR-146a expression suppresses CXCR4-mediated human breast cancer migration. The FEBS journal 280: 3340–3353. [DOI] [PubMed] [Google Scholar]
- 47. Zhou L, Zhao X, Han Y, Lu Y, Shang Y, et al. (2013) Regulation of UHRF1 by miR-146a/b modulates gastric cancer invasion and metastasis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 27: 4929–4939. [DOI] [PubMed] [Google Scholar]
- 48. Bousquet M, Zhuang G, Meng C, Ying W, Cheruku PS, et al. (2013) miR-150 blocks MLL-AF9-associated leukemia through oncogene repression. Mol Cancer Res 11: 912–922. [DOI] [PubMed] [Google Scholar]
- 49. Morris VA, Zhang A, Yang T, Stirewalt DL, Ramamurthy R, et al. (2013) MicroRNA-150 expression induces myeloid differentiation of human acute leukemia cells and normal hematopoietic progenitors. PLoS One 8: e75815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pizzini S, Bisognin A, Mandruzzato S, Biasiolo M, Facciolli A, et al. (2013) Impact of microRNAs on regulatory networks and pathways in human colorectal carcinogenesis and development of metastasis. BMC genomics 14: 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhu X, Lin Z, Du J, Zhou X, Yang L, et al. (2014) Studies on microRNAs that are correlated with the cancer stem cells in chronic myeloid leukemia. Molecular and cellular biochemistry 390: 75–84. [DOI] [PubMed] [Google Scholar]
- 52. Trifari S, Pipkin ME, Bandukwala HS, Aijo T, Bassein J, et al. (2013) MicroRNA-directed program of cytotoxic CD8+ T-cell differentiation. Proceedings of the National Academy of Sciences of the United States of America 110: 18608–18613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zheng Q, Zhou L, Mi QS (2012) MicroRNA miR-150 is involved in Valpha14 invariant NKT cell development and function. J Immunol 188: 2118–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kumar A, Mohan S, Newton J, Rehage M, Tran K, et al. (2005) Pregnancy-associated plasma protein-A regulates myoblast proliferation and differentiation through an insulin-like growth factor-dependent mechanism. The Journal of biological chemistry 280: 37782–37789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boudiaf-Benmammar C, Cresteil T, Melki R (2013) The cytosolic chaperonin CCT/TRiC and cancer cell proliferation. PloS one 8: e60895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kawata H, Yamada K, Shou Z, Mizutani T, Yazawa T, et al. (2003) Zinc-fingers and homeoboxes (ZHX) 2, a novel member of the ZHX family, functions as a transcriptional repressor. The Biochemical journal 373: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences for the mouse qRT-PCR.
(DOCX)
Primers and Probes for miRNA qRT-PCR.
(XLSX)
Oncogenic miRNAs upregulated in Muc2 −/− mouse colonic epithelial cells profiled by miRNA array.
(DOC)
Tumor suppressor miRNAs downregulated in Muc2 −/− mouse colonic epithelial cells profiled by miRNA array.
(DOC)