Abstract
Rationale
Growth arrest DNA damage inducible alpha (GADD45a) is a stress-induced gene we have shown to participate in the pathophysiology of ventilator-induced lung injury (VILI) via regulation of mechanical stress-induced Akt ubiquitination and phosphorylation. The regulation of GADD45a expression by mechanical stress and its relationship with acute lung injury (ALI) susceptibility and severity, however, remains unknown.
Objectives
We examined mechanical stress-dependent regulatory elements (MSRE) in the GADD45a promoter and the contribution of promoter polymorphisms in GADD45a expression and ALI susceptibility.
Methods and Results
Initial studies in GADD45a knockout and heterozygous mice confirmed the relationship of GADD45a gene dose to VILI severity. Human lung endothelial cells (EC) transfected with a luciferase vector containing the full length GADD45a promoter sequence (−771 to +223) demonstrated a >4 fold increase in GADD45a expression in response to 18% cyclic stretch (CS, 4 h) compared to static controls while specific promoter regions harboring CS-dependent MSRE were identified using vectors containing serial deletion constructs of the GADD45a promoter. In silico analyses of GADD45a promoter region (−371 to −133) revealed a potential binding site for specificity protein 1 (SP1), a finding supported by confirmed SP1 binding with the GADD45a promoter and by the significant attenuation of CS-dependent GADD45a promoter activity in response to SP1 silencing. Separately, case-control association studies revealed a significant association of a GADD45a promoter SNP at −589 (rs581000, G>C) with reduced ALI susceptibility. Subsequently, we found allelic variation of this SNP is associated with both differential GADD45a expression in mechanically stressed EC (18% CS, 4 h) and differential binding site of interferon regulatory factor 7 (IRF7) at this site.
Conclusion
These results strongly support a functional role for GADD45a in ALI/VILI and identify a specific gene variant that confers risk for ALI.
Introduction
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome, are complex disorders that are precipitated by the interplay of both environmental factors (such as mechanical ventilation) and genetic factors. Several case-control association studies have identified specific single nucleotide polymorphisms (SNPs) that contribute to ALI susceptibility and survival [1]–[3]. In this regard, we have previously employed preclinical models of ALI and global gene expression profiling to identify several ALI candidate genes, including GADD45a, and ALI-associated SNPs [4]–[8]. As these studies have yielded important insights into ALI pathobiology and implicated specific genetic variants associated with ALI risk and severity, further research may ultimately lead to novel therapeutic targets that bring personalized medicine to the fore in strategies aimed at treating or preventing ALI.
Growth arrest DNA damage inducible alpha (GADD45a) is a stress-induced gene which we previously reported to be significantly upregulated in multi-species pre-clinical models of ventilator-induced lung injury (VILI) [9]. We subsequently reported that mice lacking GADD45a gene (GADD45a −/−) demonstrated significantly increased VILI susceptibility [10] and linked this observation to effects of GADD45a depletion on the differential ubiquitination of Akt resulting in both increased proteasomal degradation of Akt and decreased Akt phosphorylation in response to mechanical stress [11]. However, the regulation of GADD45a expression in response to mechanical stress and the association of GADD45a genetic variants with ALI/VILI susceptibility are largely unknown.
In the present study, we hypothesized the existence of GADD45a SNPs that are associated with functional effects on promoter activity and GADD45a expression levels as well as ALI susceptibility. We relied on complementary approaches including the use of GADD45a promoter deletion constructs in endothelial cells (EC) subjected to cyclic stretch (CS) to determine regions harboring mechanical stress response elements (MSRE) followed by ALI case-control association studies focused on specific promoter regions of interest to identify GADD45a SNPs that are associated with both functional effects on GADD45a promoter activity in response to mechanical stress and ALI clinically. Our results provide evidence for ALI/VILI susceptibility conferred by specific GADD45a genetic variants that further supports an important role for GADD45a in susceptibility to inflammatory lung injury.
Materials and Methods
EC Culture and in vitro Cyclic Stretch
Human pulmonary artery endothelial cells (EC) (Lonza, US-Allendale, NJ) were plated onto BioFlex silicone elastomer six-well plates coated with type I collagen and were cultured in endothelial growth medium (EGM-2) containing 10% FBS (Lonza, US-Allendale, NJ) in 5% CO2 at 37°C and 95% humidity to achieve contact-inhibited monolayers. For mechanical stress studies, BioFlex plates were placed on a Flexcell Strain Tension System (FX-3000, Flexcell International, Hillsborough, NC) kept in a 5% CO2 incubator at 37°C and 95% humidity. Plates were stretched to produce either 5% or 18% elongation at a frequency of 0.5 Hz, 30 cycles/min. As we have previously reported, 18% cyclic stretch (CS) corresponds to pathologically relevant levels of mechanical stress that result in phenotypic EC monolayer changes, increased susceptibility to barrier-disruptive agonists, but with preserved monolayer integrity even after prolonged exposure (48 h) [12].
GADD45a Promoter Vector and Molecular Cloning
GADD45a promoter cloned into pSGG luciferase vector was purchased from SwitchGear Genomics (S119097, Menlo Park, CA). In silico analysis and gene sequencing of the vector confirmed 1008 bp insert (−771 to +237) spanning regions of the GADD45a promoter and exon 1, 80 bp away from the transcription start site. Primers were then designed at every 200 bp of the GADD45a promoter sequence (Table S1) and amplified PCR fragments of 806, 606, 368, and 172 bp sizes were cloned into an empty pSGG luciferase vector to generate 5′ serial deletion constructs of GADD45a promoter. Cloning results were confirmed by DNA sequencing using primers (forward: TCCATCAAAACAAAACGAAACAA and reverse: CCGTCTTCGAGTGGGTAGAATG) sequences provided by SwitchGear Genomics (Menlo Park, CA).
Dual Luciferase Reporter Gene Assay
EC were co-transfected with GADD45a plasmid constructs containing firefly luciferase reporter (1 µg) and TK renilla vector (20 ng) using Fugene HD transfection reagent (Promega, Madison, WI, USA). Dual luciferase activity was measured using Luciferase Assay reagent II and Stop & Glo reagent (Promega, Madison, WI) according to the manufacturer’s protocol. Normalized luciferase activity was expressed as ratio of firefly and renilla luciferases activities.
Site-directed Mutagenesis
A point mutation was created in the GADD45a promoter sequence (at −589) to create promoter SNP rs581000 (G>C) using primer sequences (5′-acaaacgggttggtttttcttttttcagcttccaaccct-3′ and 5′-agggttggaagctgaaaaaagaaaaaccaacccgtttgt-3′) obtained by quick change primer design software and using QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Lara, CA) according to manufacturer’s protocol. Mutagenesis was verified by DNA sequencing.
In silico Analysis
The core and matrix similarity of TF binding sites in the GADD45a promoter region were evaluated using Genomatix software (http://www.genomatix.de).
Electrophoretic Mobility Shift Assay (EMSA)
EMSA was performed using a LightShift Chemiluminescent EMSA Kit (Thermo Scientific, Asheville, NC, USA) and biotin-labeled synthetic oligonucleotides carried either the G or C allele of the SNP rs581000 (−589) or the GADD45a promoter region (−191 to −140) with SP1 binding sites. Non-biotin-labeled synthetic oligonucleotides with the consensus sequences for IRF7 and SP1 were used as competitors. Nuclear cells extracts from HeLa and HL60 cells (Promega, Madison, WI) were incubated with biotin-labeled oligonucleotides (20 fmol) in presence of IRF7 and SP1 competitor respectively and eletrophoresed on 6% Novex DNA retardation gels (Invitrogen, Carlsbad, CA, USA) as described elsewhere [13]. The images were obtained by chemiluminescence according to the manufacturer’s instructions.
Transfection of siRNA
EC were transfected with SP1 siRNA (100 nM, Dharmacon Thermo Scientific, Pittsburgh, PA) or non-specific, scrambled sequence RNA using transfection reagent siPORT Amine (Ambion, Austin, TX, USA) in serum-free conditions according to the manufacturer’s protocol. siRNA and plasmid co-transfection was performed using Lipojet (SignaGen Laboratories, Rockville, MD) according to the manufacturer’s protocol.
Western Blotting
Total proteins were extracted using NP-40 lysis buffer (50 mM TrisHCl pH 7.4, 150 mM NaCl, 1% NP-40, and 5 mM ethylenediaminetetraacetic acid) supplemented with 40 mM sodium fluoride, 0.1 M sodium orthovanadate, 0.2 mM phenylmethylsulfonyl fluoride, 10 mM N’ ethyl malamide, and protease and phosphatase inhibitor cocktail (Calbiochem, San Diego, CA). Protein concentration was measured using bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL) and Western blotting was performed using standard protocols using SP1 antibody (Cell Signaling, Danvers, MA).
Study Populations and Demographics
The association of GADD45a SNPs with ALI was studied in case-control samples from Chicago, IL comprised of African American (AA) and European American (EA) subjects. A detailed description of the population studied is provided in Table 1 and in our previous report [5]. A total of 208 unrelated severe sepsis cases (137 EA and 71 AA) including 114 with ALI (74 EA and 40 AA) and 368 healthy population-based controls subjects (186 EA and 182 AA) were collected. Control subjects from this group reported a negative personal and first-degree family history of sepsis or ALI. Ancestry was defined as at least three grandparents of either AA or EA ancestry. Severe sepsis and ALI were defined according to the American-European Consensus Criteria [14] and the Society of Critical Care Medicine consensus statement [15]. Additionally, samples from the Canary Islands, Spain that included 95 population-based controls and 80 severe sepsis cases including 66 with ALI were also studied [6]. Spanish cases were admitted to an ICU within 24 h of a diagnosis of severe sepsis. Further details about regarding each study population is available in Table 1. To account for population stratification, the association study was performed in AA, EA and Spanish individuals separately. A total of 93, 96 and 20 ancestry informative markers were previously genotyped by our group in EA, AA and Spanish subjects, respectively, which suggested limited influence of population stratification on association results within each study sample [5], [6]. The study was approved by the University of Illinois Institutional Review Board and was in accordance with Helsinki rules.
Table 1. Population characteristics of Chicago and Spanish samples studied.
| Characteristics | Sepsis+ALI | ALI | ||||
| AA | EA | Spanish | AA | EA | Spanish | |
| Sample Size | 71 | 137 | 80 | 40 | 74 | 66 |
| Gender # | 31/40 | 71/66 | 58/22 | 19/21 | 41/33 | 54/12 |
| Age * | 54.9±19.3 | 61.2±15.8 | 65.2±12.3 | 54.6±15.3 | 57.7±16.9 | 58.4±12.2 |
| APACHE II * | 27.1±7.0 | 28.4±7.6 | 22.4±5.8 | 28.0±7.7 | 29.7±7.4 | 20.1±5.4 |
| Survival % | 62.7 | 64.1 | 56.1 | 52.4 | 62.1 | 52.8 |
| Smoking (%) | 37.7 | 38.8 | 30.4 | 34.2 | 40.3 | 29.5 |
| Diabetes (%) | 20.0 | 19.7 | 21.4 | 16.7 | 19.0 | 18.6 |
| Renal Failure (%) | 29.3 | 19.0 | – | 21.4 | 19.0 | – |
| Characteristics | Controls | |||||
| AA | EA | Spanish | ||||
| Sample Size | 182 | 186 | 95 | |||
| Gender # | 90/92 | 84/102 | 53/42 | |||
| Age * | 56.6±17.4 | 55.6±14.4 | 48.9±12.3 | |||
| APACHE II * | NA | NA | NA | |||
| Survival % | NA | NA | NA | |||
| Smoking (%) | – | – | 30.5 | |||
| Diabetes (%) | 0.0 | 0.0 | 9.5 | |||
| Renal Failure (%) | 0.0 | 0.0 | – | |||
*expressed as mean ± SD.
APACHE II: Acute Physiology and Chronic Health Evaluation.
# Male/Female.
- Data not available.
Human GADD45a Gene Resequencing
DNA samples of 30 randomly selected ALI cases (15 AA and 15 EA) from the Chicago study were sequenced for GADD45a SNP discovery. DNA sequencing and polymorphism identification were performed by Polymorphic DNA Technologies (Alameda, CA) according to established guidelines. The genomic sequence NM_001924.3 was used as the reference sequence.
Genotyping
Genotyping was conducted using the iPLEX Gold Platform (Sequenom, San Diego, CA) according to the manufacturer’s protocol. Briefly, iPLEX assays were scanned by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and individual SNP genotype calls were automatically generated with Sequenom TYPER 3.4 software. Genotyping was validated by the TaqMan allelic discrimination assay (Life Technologies, Carlsbad, CA). TaqMan genotyping was performed using a 7900HT Fast Real-Time PCR System (Life Technologies, Carlsbad, CA), with automated calls generated by SDS software based on discriminating plots (95% confidence). Genotyping was blind to case-control status and ethnic background of the samples.
Preclinical Model of VILI
Male 8 to 12 week-old wildtype (WT) C57BL/6 (Jackson Laboratory, Bar Harbor, ME), GADD45a−/− (a gift of Dr. Michael O’Reilly, University of Rochester and Dr. Albert Fornace, Brigham and Women’s Hospital) and GADD45a +/− (GADD45a −/− crossed with C57BL/6) were subjected to mechanical ventilation (Harvard Apparatus, Holliston, MA) with tidal volumes of 40 ml/kg, 65 breaths per minute for 4 h as we have previously described [11]. All the GADD45a −/− and GADD45a +/− mice were genotyped by PCR as described elsewhere [16]. All animal experiments were approved by the Animal Care and Use Committees of the University of Illinois at Chicago.
Statistical Analysis
Individual SNP association testing with ALI and odds ratio estimates with 95% confidence intervals (CI) was performed using SNPstats software assuming two genetic models, additive and dominant [17]. To account for the multiple-testing, raw p values from tests in each cohort were adjusted using the Benjamini-Horchberg procedure [18]. Departures from Hardy-Weinberg equilibrium were tested by means of exact test [19]. Haploview software was used to study the correlation and linkage disequilibrium (LD) between SNPs [20]. The multiple-marker selection algorithm haplotype r2 included in TagIT 3.03 software was used to select a set of SNPs (tSNPs) [21]. Student t-test was used for comparison of promoter activity and protein expression between different groups. Statistical significance was set at a p value of less than 0.05.
Results
GADD45a Expression Levels and Susceptibility to Ventilator-induced Lung Injury (VILI)
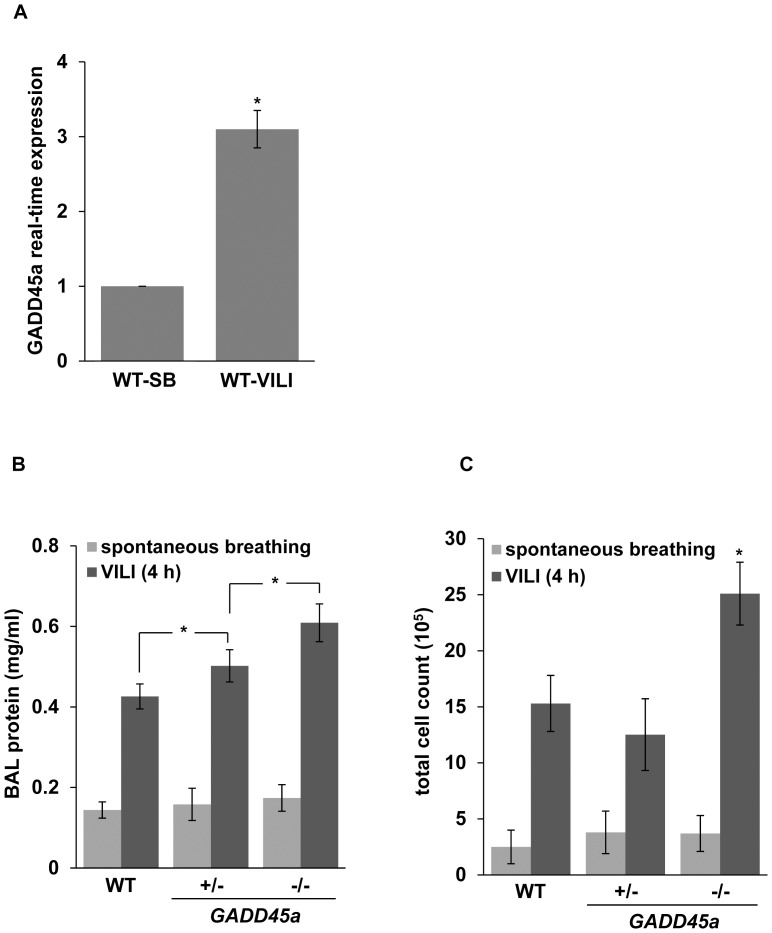
We previously identified GADD45a as a significantly upregulated gene in multi-species pre-clinical models of VILI and reported increased VILI susceptibility in GADD45a knockout (GADD45a −/−) mice [10], [11]. To extend these earlier studies, we initially assessed GADD45a mRNA levels by real-time PCR in spontaneously breathing (SB) and VILI-challenged (VT 40 ml/kg, 4 h) wildtype (WT) mice and confirmed significantly increased GADD45a mRNA levels in response to mechanical stress (Figure 1A). Next, to confirm that variable GADD45a expression levels are associated with variable susceptibility and severity of ALI in vivo, WT, GADD45a heterozygous mice (GADD45a +/−) and GADD45a −/− mice were subjected to VILI challenge (VT 40 ml/kg, 4 h) and BAL fluid was collected for analysis. In response to VILI challenge, these studies confirmed that heterozygous GADD45a +/− mice exhibit an intermediate phenotype with significantly increased BAL protein levels compared to WT mice and but significantly decreased BAL protein levels compared to GADD45a −/− mice (Figure 1B). BAL total cell counts were notable for a significant increase in VILI-challenged GADD45a −/− mice compared to both WT and GADD45a +/− animals while there were no significant differences noted between WT and GADD45a +/− animals (Figure 1C). No changes were observed between groups of spontaneously breathing animals. These results support a critical role for GADD45a expression in murine VILI response.
Figure 1. GADD45a expression and mechanical stress-induced murine lung injury.
(A) GADD45a mRNA levels from lung homogenates of wildtype (WT) mice subjected to high tidal volume mechanical ventilation (VT = 40 ml/kg, 4 h) were significantly increased in comparison to spontaneously breathing (SB) control mice (n = 3/group, *p<0.05). (B) WT, GADD45a heterozygous (GADD45a +/−), and GADD45a knockout (GADD45a−/−) mice were subjected to high tidal volume mechanical ventilation (VT 40 ml/kg, 4 h) and BAL fluid was collected for analyses. BAL fluid total protein levels were significantly higher in GADD45a +/− mice compared to WT and significantly less than GADD45a−/− mice. SB animals showed no difference (n = 3/group, *p<0.05). (C) Total cell counts in BAL fluid from VILI-challenged GADD45a−/− mice were significantly increased compared to both GADD45a+/− and WT animals while there was no difference was observed between WT and GADD45a +/− animals after VILI challenge (n = 3/group, *p<0.05).
GADD45a Promoter Activity in Response to Mechanical Stress
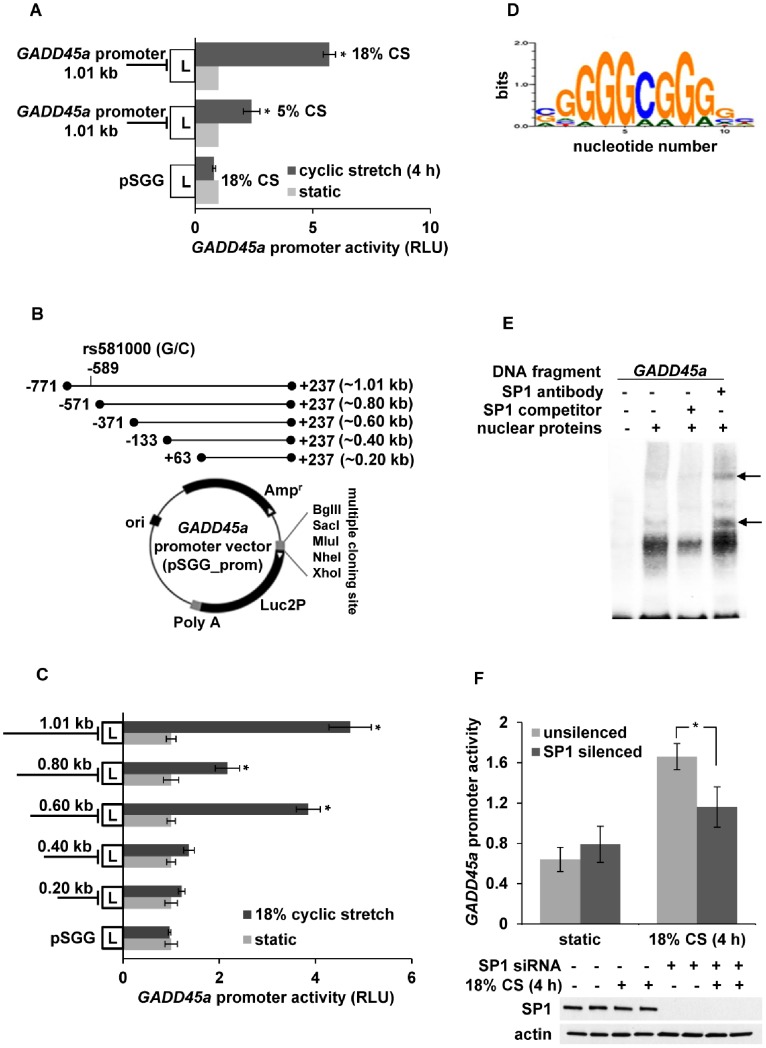
To assess the effect of mechanical stress on human GADD45a promoter activity, we exposed human pulmonary artery endothelial cells (EC) transfected with pSGG luciferase vector containing a human GADD45a promoter sequence (−771 to +237, 1.01 kb) to either 5% or 18% cyclic stretch (CS, 4h). Compared to static controls, 5% and 18% CS induced ∼2.5- and ∼6-fold increases in luciferase activity, respectively, confirming GADD45a promoter regulation by mechanical stress (Figure 2A).
Figure 2. GADD45a promoter activity and functional promoter region in response to mechanical stress.
(A) Human pulmonary artery endothelial cells (EC) were plated on Bioflex stretch plates and transfected with a full-length GADD45a promoter vector followed by cyclic stretch (CS, 5% or 18%) for 4h. Dual luciferase reporter assay revealed about significant increases in GADD45a promoter activity proportional to the degree of CS (n = 3/condition, * p<0.05 compared to respective static controls). (B) Schematic representation of full-length and deletion fragments of GADD45a promoter and empty pSGG reporter vector. Promoter fragments with 200 bp serial deletions were PCR amplified and cloned into empty pSGG vector to generate GADD45a promoter constructs. (C) EC were transfected with GADD45a promoter deletion constructs followed by 18% CS (4 h). Dual luciferase reporter assay revealed ∼4 fold increase in reporter activity in both full-length (1.01 kb) and 0.6 kb (−371 to +237) GADD45a promoter fragment in response to 18% CS cells compared to respective static controls. Comparatively, 18% CS-induced luciferase activity was significantly decreased in 0.8 kb, 0.4 kb and 0.2 kb fragments. (D) In silico analysis of GADD45a promoter region −371 to −133 by Genomatix predicted binding sites for transcription factor SP1. The sequence logo graphically represents the SP1 consensus sequence. The relative height of each base within each stack represents relative frequency of the corresponding base at that position. Highly conserved positions are represented by higher stacks of base symbols A, T, G, C. (E) The binding of SP1 with the GADD45a promoter was detected by EMSA using biotin-labeled GADD45a promoter fragment and HL60 nuclear extract in the presence or absence of a non-labeled SP1 competitor and antibodies specific for SP1. DNA-protein interaction was characterized by complex formation upon the addition of nuclear proteins, which was blocked in the presence of an SP1 competitor. Addition of SP1 antibody altered the mobility of the complex, characterized by a super-shift of DNA-protein complex (arrow). (F) EC co-transfected with SP1 siRNA and a full-length GADD45a promoter vector followed by 18% CS (4 h) exhibited significantly attenuated GADD45a promoter activity compared to unsilenced cells transfected with the full-length GADD45a promoter vector alone (n = 3/condition, * p<0.05). Silencing was confirmed by Western blotting (representative blots shown).
To identify the potential GADD45a promoter regions harboring mechanical stress response elements, EC were transfected with either a full length GADD45a promoter vector or one of the four promoter deletion vectors (Figure 2B) prior to exposure to excessive mechanical stress (18% CS, 4 h). These studies revealed no significant increase in mechanical stress-induced GADD45a promoter activity with the fragments representing the proximal promoter nucelotides +63 to +237 (0.2 kb) or −133 to +237 (0.4 kb) (Figure 2C). However, luciferase activity was significantly increased with the promoter fragment from −371 to +237 (0.6 kb), indicating the presence of MSRE in the region of nucelotides −371 to −133. However, further extension distally to include nucleotides −571 to +237 (0.8 kb) was associated with a significant decrease in promoter activity in response to CS indicating elements that repress transcription in this region. Notably, the full length promoter (−771 to +237; 1.01 kb) overrode these effects and restored mechanical stress-dependent GADD45a promoter activity (Figure 2C), suggesting presence of cis-regulatory elements in this region (−771 to −571). No effects were observed with any of the vectors under static conditions.
Next, using Genomatix software we performed the in silico analysis of the GADD45a promoter region from −371 to −133, which predicted binding sites (Figure 2D) for specificity protein 1 (SP1), a transcription factor ubiquitously expressed in all mammalian cells [22], at positions −232, −202, −177 of the GADD45a promoter. To validate this finding, we performed EMSA with nuclear extracts from HL60 cells (human promyelocytic leukemia cells) in the presence or absence of a non-biotin-labeled SP1 competitor and antibodies specific for SP1. These experiments identified DNA-protein interaction by the complex formed between the biotin-labeled GADD45a promoter fragment (range: −191 to −140 and length: 51mer) and HL60 nuclear proteins that was abrogated in the presence of the SP1 competitor (Figure 2E). To confirm the presence of SP1 in the DNA-protein complex, antibodies against SP1 were added to the binding reaction prior to adding the probe at 4°C. A significant shift in the DNA-protein complex was observed consistent with an alteration in the mobility of the complex due to binding of SP1 antibody with the DNA-protein complex (Figure 2E). Moreover, silencing SP1 in EC transfected with the full-length GADD45a promoter vector significantly reduced 18% CS-induced GADD45a promoter activity compared to unsilenced control cells (Figure 2F). Taken together, these studies indicate SP1 is an important regulator of EC GADD45a expression induced by excessive mechanical stress.
GADD45a Genetic Variants and Association of Promoter SNP rs581000 with Acute Lung Injury (ALI)
Resequencing of the GADD45a gene and 2 kb of upstream and downstream regulatory elements identified 31 variations (29 SNPs and 2 indels) including two variants not previously identified (Table 2). Seven GADD45a tagging SNPs (tSNPs) with minor allele frequency (MAF) >0.10 were genotyped in both Chicago and Spanish samples (Table 2). A case–control association study was performed in the Chicago cohort, comprised of 208 severe sepsis cases including 114 with ALI (137 EA and 71 AA) and 368 healthy controls (186 EA and 182 AA), and in the Spanish cohort comprised of 80 severe sepsis cases, 66 with ALI, and 95 healthy controls. The population characteristics of the cohorts studied is provided in Table 1. The assessment of ancestry informative markers suggested no significant difference in the genetic background of cases and controls in these cohorts [5], [6]. Additionally, to account for population stratification, the association study was performed separately in AA, EA and Spanish individuals.
Table 2. Summary of SNPs in GADD45a gene.
| SNP ID | Positiona | Locationb | Allele | Frequ ency AAc | Frequency EAc | Tagging SNP |
| rs1511686 | 68,148,921 | c.-318-1939 | T>A | 0.13 | 0.00 | |
| – | 68,149,189 | c.-318-1671 | G>A | 0.00 | 0.03 | |
| rs115517134 | 68,149,321 | c.-318-1539 | A>G | 0.03 | 0.00 | |
| rs188178283 | 68,150,258 | c.-318-602 | G>T | 0.00 | 0.03 | |
| rs581000 | 68,150,271 | c.-318-589 | G>C | 0.37 | 0.13 | ** |
| rs3783456 | 68,150,622 | c.-318-238 | A>G | 0.03 | 0.00 | |
| rs3783457 | 68,150,673 | c.-318-187 | C>A | 0.03 | 0.00 | |
| – | 68,150,681 | c.-318-179 | C>G | 0.03 | 0.00 | |
| rs1397946 | 68,150,898 | c.-280 | A>T | 0.13 | 0.00 | ** |
| rs3783460 | 68,150,931 | c.-247 | T>G | 0.03 | 0.00 | |
| rs3783462 | 68,151,227 | c.44+6 | T>A | 0.03 | 0.00 | |
| rs3783465 | 68,151,645 | c.45-63 | G>A | 0.03 | 0.00 | |
| rs3783466 | 68,151,685 | c.45-23 | C>T | 0.17 | 0.13 | |
| rs2759219 | 68,151,980 | c.147-53 | G>T | 0.3 | 0.13 | ** |
| rs149179509 | 68,152,171 | c.285 | G>A | 0.00 | 0.03 | |
| rs3783468 | 68,152,363 | c.384+93 | G>A | 0.23 | 0.57 | ** |
| rs3783469 | 68,152,386 | c.384+116 | T>C | 0.03 | 0.13 | ** |
| rs681673 | 68,152,388 | c.384+118 | C>T | 0.5 | 0.27 | |
| rs532446 | 68,152,438 | c.384+168 | T>C | 0.5 | 0.27 | ** |
| rs3783472 | 68,152,558 | c.384+288 | G>C | 0.03 | 0.00 | |
| rs143275022 | 68,152,736 | c.384+466 | del T | 0.00 | 0.03 | |
| rs3171012 | 68,153,206 | c.385-138 | T>C | 0.17 | 0.13 | ** |
| rs3046000 | 68,153,271–68,153,279 | c.385-73_385-65 | del (9nt) | 0.1 | 0.00 | ** |
| rs3783478# | 68,153,451 | c.492 | A>G | 0.13 | 0.00 | ** |
| rs3783479 | 68,153,484 | c.*27 | C>T | 0.03 | 0.00 | |
| rs607375 | 68,154,086 | c. * 564+65 | C>G | 0.4 | 0.27 | ** |
| rs3783482 | 68,154,204 | c.*564+183 | C>T | 0.03 | 0.00 | |
| rs3783483 | 68,154,208 | c.*564+187 | G>A | 0.07 | 0.00 | ** |
| rs606470 | 68,154,299 | c. * 564+278 | T>C | 0.23 | 0.03 | ** |
| rs77985416 | 68,154,373 | c.*564+352 | G>A | 0.03 | 0.00 | |
| Rs78379425 | 68,154,466 | c.*564+445 | G>A | 0.03 | 0.00 |
Position on chromosome 1 according to NCBI Build 37.
Location relative to reference sequence NM_001924.3.
Represents minor allele frequency in gene resequencing data in 15 African American (AA) and 15 European American (EA).
Boldface highlights SNPs genotyped in both Chicago and Spanish study.
** Represents tagging SNP.
* Represents 3′UTR region.
# represents synonymous SNP (Glu164Glu).
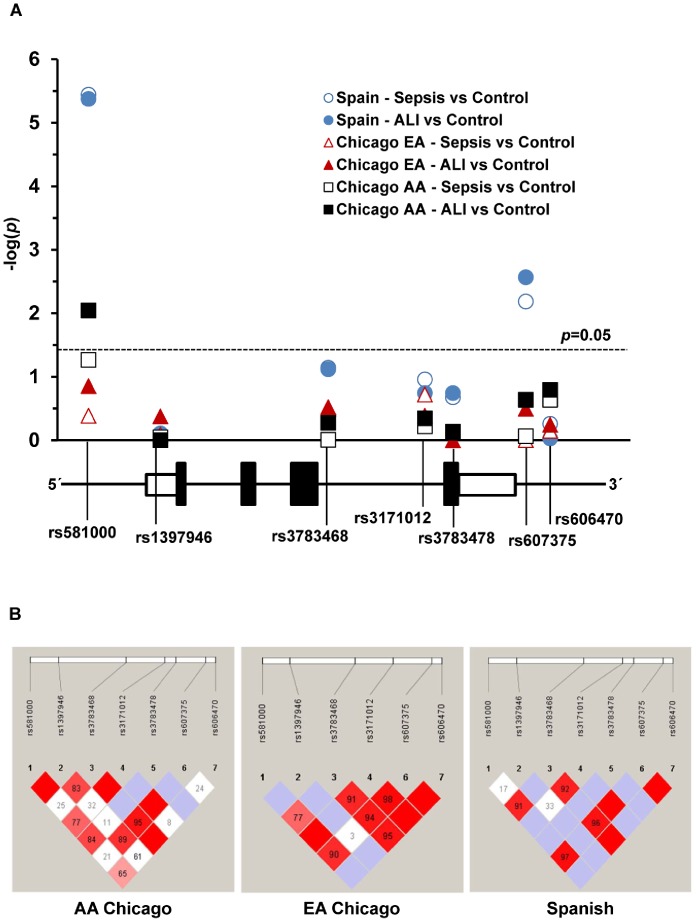
These studies identified a significant association of the GADD45a promoter SNP rs581000 with allele C reducing the risk of both sepsis and ALI in AAs from Chicago (p = 0.009, adjusted p = 0.05 under a dominant model) and in Spanish subjects (p = 4.20E-06, adjusted p value = 0.00003 under a dominant model, FDR <1%) (Figure 3A and Table 3). However, in EA from Chicago, the association did not reach to a significant level. Additionally, none of the other tSNPs resulted in significant association with sepsis/ALI in EAs or AAs subjects from Chicago. In Spanish subjects, reduced sepsis/ALI susceptibility was also significantly associated with the intergenic SNP rs607375 (p = 0.0027, adjusted p = 0.009 under a dominant model, FDR <1%) (Figure 3A). Moreover, the promoter SNP rs581000 demonstrated nearly complete LD with the intergenic SNP rs607375 in the Spanish cohort (LD; D′ = 0.97) and EAs from the Chicago cohort (LD; D′ = 0.90) (Figure 3B). In AAs, the promoter SNP rs581000 was in complete LD with promoter SNP rs1397946 (D′ = 100) and in partial LD with a synonymous exon SNP rs3783478 (D′ = 84). The Hardy-Weinberg equilibrium was observed in nearly all the comparisons, although an exception was noted for rs581000 in Spanish controls. Notably, the MAF of rs581000 in our Spanish controls (0.28) was comparable to that in a Hispanic reference panel (0.24) from dbSNP [23]. These data strongly implicate an association between GADD45a rs581000 and sepsis/ALI susceptibility.
Figure 3. GADD45a SNP association with severe sepsis and ALI.
(A) Plots of association P values of tested GADD45a SNPs identified significant association of promoter SNP rs581000 with ALI in AA of Chicago cohort and with both severe sepsis and ALI in Spanish cohort. In Spanish cohort intergenic SNP rs607375 was associated with both severe sepsis and ALI. The dashed line represents a p-value of 0.05 and a schematic of the Gadd45a gene structure below indicates relative position of SNPs tested for association. Black boxes within the gene schematic represent coding exons. White boxes represent the 5′ and 3′ UTR, respectively. (B) Panel represents linkage disequilibrium (LD) patterns across the genotyped SNPs in the two cohorts. High LD is noted between promoter SNP rs581000 and rs607375 in both EAs from the Chicago cohort and in the Spanish cohort. Each diamond of the LD plot represents a pairwise SNP comparison. Numbers and colors in each diamond indicate the magnitude of LD between pairs of SNPs (D′ = 100 corresponds to complete LD denoted by red; D′ = 0 corresponds to absence of LD denoted by white; blue represents an intermediate association).
Table 3. GADD45a promoter SNP rs581000 genotypes in Chicago and Spanish cohorts.
| Chicago study | GG | GC | CC | Frequency (GC+CC) | Odds Ratio (CI) | p value |
| Controls (n = 368) | 215 | 134 | 19 | 0.42 | ||
| Severe sepsis (n = 208) | 139 | 59 | 10 | 0.33 | 0.70 (0.49–0.95) | 0.05 |
| Sepsis+ALI (n = 114) | 76 | 31 | 7 | 0.33 | 0.70 (0.45–1.09) | 0.12 |
| AA Chicago study | GG | GC | CC | Frequency (GC+CC) | Odds Ratio (CI) | p value |
| Controls (n = 182) | 68 | 99 | 15 | 0.63 | ||
| Severe sepsis (n = 71) | 36 | 28 | 7 | 0.49 | 0.58 (0.33–1.01) | 0.05 |
| Sepsis+ALI (n = 40) | 24 | 12 | 4 | 0.40 | 0.40 (0.20–0.80) | 0.009 |
| EA Chicago study | GG | GC | CC | Frequency (GC+CC) | Odds Ratio (CI) | p value |
| Controls (n = 186) | 147 | 35 | 4 | 0.21 | ||
| Severe sepsis (n = 137) | 103 | 31 | 3 | 0.25 | 1.24 (0.74–2.10) | 0.41 |
| Sepsis+ALI (n = 74) | 52 | 19 | 3 | 0.30 | 1.59 (0.87–2.94) | 0.14 |
| Spanish study | GG | GC | CC | Frequency (GC+CC) | Odds Ratio (CI) | p value |
| Controls (n = 95) | 44 | 50 | 1 | 0.54 | ||
| Severe sepsis (n = 80) | 64 | 14 | 2 | 0.20 | 0.22 (0.11–0.43) | 5x10−6 |
| Sepsis+ALI (n = 66) | 54 | 10 | 2 | 0.18 | 0.19 (0.09–0.40) | 6x10−6 |
AA represents African American and EA represents European American in the Chicago study.
Functional Assessment of GADD45a Promoter SNP rs581000
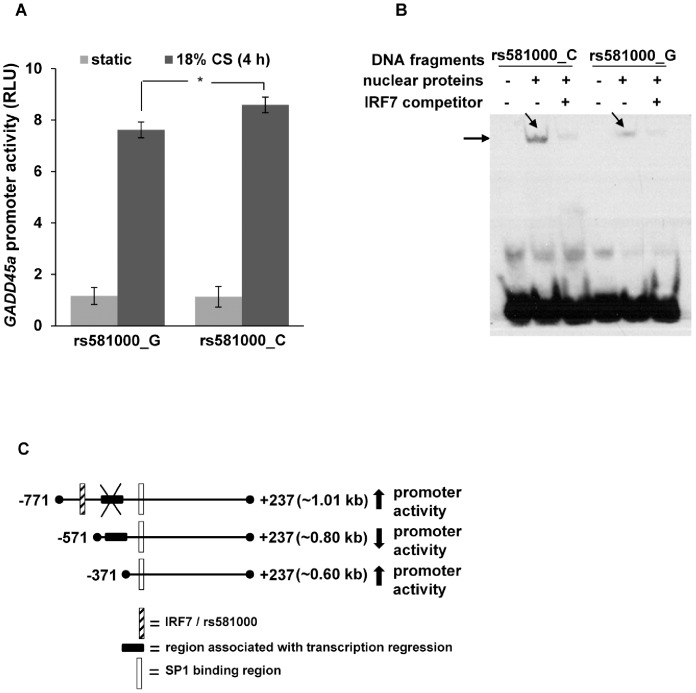
To determine the functional role of the GADD45a promoter SNP rs581000 (G>C), we used site-directed mutagenesis to create a point mutation at position −589 of the full-length (1.01 kb) GADD45a promoter fragment. Luciferase reporter activity was evaluated in EC expressing either the GADD45a vector allele rs581000_C or rs581000_G under static condition and in response to mechanical stress (18% CS, 4h). EC expressing the C allele at −589 (rs581000_C) exhibited significantly increased reporter activity after CS compared to cells expressing the G allele at the same locus (rs581000_G) (Figure 4A). These findings suggest that this allelic variant may correspond to alterations at a specific transcription factor-binding site resulting in variable transcription factor binding and gene expression. In silico analysis using Genomatix predicted that the substitution of G by C at the rs581000 locus results in gain of core sequence for the transcription factor, interferon regulatory factor 7 (IRF7). To investigate the potential contribution of IRF7 to GADD45a expression induced by mechanical stress, we performed EMSA with nuclear extracts from HeLa cells and biotin-labeled oligonucleotide containing the C or G allele (range: −614 to −561) in the presence or absence of competitive non-biotin-labeled oligonucleotides for IRF7. These studies revealed a 3.8 times stronger protein-DNA interaction with allele rs581000_C compared to allele rs581000_G (Figure 4B). This binding affinity of allele rs581000_C was abrogated in the presence of IRF7 competitive elements (Figure 4B) suggesting a significant role for SNP rs581000 in transcriptional regulation of GADD45a via altered binding affinity for IRF7. Collectively, these data support the idea that variable GADD45a expression by excessive mechanical stress is influenced by the cumulative effects of the promoter SNP rs581000, via alterations in DNA binding of IRF7, with overriding effects on an inhibitory region (−571 to −371) and by the additive effect of IRF7 and SP1 (Figure 4C).
Figure 4. Promoter SNP rs581000 effect on mechanical stress-induced GADD45a activity.
(A) EC were transfected with rs581000_C or rs581000_G vectors and then subjected to 18% CS (4 h). Cells transfected with rs581000_C vector demonstrated significantly increased luciferase reporter activity in response to CS compared to rs581000_G. (B) EMSA was performed with nuclear extracts from HeLa cells and biotin-labeled GADD45a promoter fragments with either the C allele or G allele in the presence or absence of an interferon regulatory factor 7 (IRF7) competitor. Binding affinity of the allelic variants with nuclear proteins is indicated by arrows (arrows = protein-bound DNA). (C) Schematic representation of GADD45a promoter regions with regulatory elements. SNP rs581000 creating cis-regulatory element IRF7 binding site, in the region −771 to −571 are associated with enhancement of promoter activity that overrides putative transcription suppressors in the region −571 to −371 and contributes to regulatory element in the region −371 to −133. Consistent with the deletion of the region −771 to −571 results in significant reduction of promoter activity but is restored by further deletion of region −571 to −371.
Discussion
VILI represents a form of ALI that is precipitated by excessive lung stretch generated by mechanical ventilation. Despite advances in ventilator strategies aimed at reducing the incidence of VILI through the administration of low tidal volumes [24], inflammation, pulmonary edema, and underlying lung disease may yet all contribute to poor compliance that result in an increased risk of injury associated with mechanical ventilation. As ALI remains a common and potentially devastating syndrome with mortality in excess of 35% [25], a better understanding of the underlying pathogenic mechanisms that may lead to the identification of novel therapeutic targets and molecular markers are desperately needed. Along these lines, mounting evidence suggests that ALI/VILI susceptibility and severity are influenced by a complex interplay involving genes and environmental factors [1], [2], [4], [5], [8]. In support of this, we previously reported GADD45a is a novel ALI/VILI candidate gene [10] that affects differential Akt ubiquitination via DNA demethylation of UCHL1, a deubiquitinating enzyme [11]. In the present study, we relied on complementary experimental approaches to characterize GADD45a promoter regulation by mechanical stress and to identify a functional genetic variant of GADD45a associated with altered mechanical stress-induced protein expression that is linked to variable susceptibility to ALI clinically.
The crucial role of GADD45a is strengthened in the current study by evidence of a gene dose effect on the elaboration of lung injury in VILI-challenged GADD45a −/− and GADD45a +/− mice. In addition, we found comparable increases in human EC GADD45a promoter activity in response to cyclic stretch and GADD45a mRNA levels in VILI-challenged WT mice. However, it is important to note that murine and human GADD45a promoters are different in sequence (alignment score 66.7) and thus their regulation by mechanical stress is likely to differ in some respects.
Our combined approach of using GADD45a promoter deletion constructs and ALI-associated promoter SNPs to characterize the CS-dependent regulatory elements identified two transcription factors, SP1 and IRF7, involved in GADD45a expression. Of note, SP1 is a transcription factor expressed in lungs that itself is upregulated in response to cyclic stretch [26] while IRF7 is known to be upregulated in the lungs of mice in response to ALI/VILI [27]. The fact that both of these transcription factors are known to be regulated in contexts relevant to ALI/VILI is particularly intriguing and helps to validate our findings.
The notion that ALI susceptibility and severity may be significantly affected by genetic factors is now well recognized. Among the earliest reports in support of this idea are associations between ALI incidence and specific polymorphisms in the genes encoding surfactant protein B [28] and angiotensin converting enzyme [29]. To date, at least 27 ALI candidate genes have been reported [30] and our group has identified several ALI-associated candidate genes including Type 2 deiodinase [5], MIF [4], myosin light chain kinase (MYLK) [8], pre-B-cell colony-enhancing factor (PBEF), also known as visfatin or nicotinamide phosphoribosyltransferase (NAMPT), [31] and GADD45a [10]. Both PBEF and GADD45a were identified via orthologous gene expression profiling of in vivo models of VILI and in vitro models of increased mechanical stress applied to human lung endothelial cells [9]. Although the function of PBEF is not precisely characterized, PBEF up-regulation was previously reported in human amniotic epithelial cells exposed to mechanical stress [32] and in VILI murine models [33]. Further validation for PBEF as a candidate gene in ALI/VILI was provided by evidence of an association of specific PBEF SNPs with ALI susceptibility and severity [30], [31]. Separately, the up-regulation of GADD45a has been independently verified in murine ALI/VILI [27]. These reports serve to support our candidate gene approach in general and strengthen the case for a functionally important role for GADD45a in ALI/VILI.
We utilized a tagging SNP (tSNP) approach after gene resequencing to study the association of GADD45a gene with sepsis/ALI. These findings identified 31 variations (29 single-base changes and 2 indels), including two variants not previously reported, and identified a significant association of GADD45a promoter SNP rs581000 with reduced ALI susceptibility in AA and Spanish subjects by assuming a dominant model. However, it is unclear whether the observed association is due to a specific SNP or other tSNPs or variants in nearby regions flanking the gene. Of note, SNP rs581000 exhibited LD with intergenic SNP rs607375 in the Spanish and EA study samples which was not observed in the AA study samples. To minimize potential discrepancies in our gene-association study, ancestry informative markers were previously assessed in all three cohorts (EA, AA and Spanish) [5], [6]. In addition, to account for potential bias due to population stratification, the association studies were performed in EA, AA and Spanish individuals separately. Genotyping of GADD45a SNPs in ALI patients revealed significant racial differences in the AA and EA from the Chicago samples. Specifically, rs581000 was associated with ALI in AAs although this was not the case for EAs, a difference supporting the increasing recognition of significant racial disparities in ALI mortality rates [34]. The genotype distribution in this study was consistent with assumptions of Hardy-Weinberg equilibrium, with exception of Spanish controls. Racial ancestry has been reported to affect the frequency of genetic variants due to excess of homozygotes because of inbreeding [35]. However, the minor allele frequency of SNP rs581000 in Spanish controls (0.28) was similar to that in a Hispanic reference panel (0.24) from dbSNP [23].
We found that allelic variation associated with the rs581000 SNP conferred both variable binding of IRF7 to this region of the GADD45a promoter as well as variable GADD45a promoter activity in CS-exposed EC. Maximum reporter activity was observed with the full-length GADD45a promoter fragment carrying the C allele at locus −589, suggesting IRF7 binding overrides transcription repression associated with the region −571 to −371 resulting in the upregulation of GADD45a in response to mechanical stress (Figure 4C). Deletion of either the IRF7 or SP1 binding regions from the GADD45a promoter significantly reduced the promoter activity in response to CS suggesting loss of cis-regulatory regions, MSRE or cooperative transcription factor complexes. However, the negative effects on transcription associated with the region −571 to −371 may indicate the presence of binding sites for specific transcription inhibitors, epigenetic modifiers or silencers in this region (Figure 4C) [13]. These results highlight the prominent functional role of GADD45a promoter SNP rs581000. However, it should be noted that the SNP effect observed cannot completely explain the magnitude of increase observed in promoter activity and it is possible that other SNPs also contribute to GADD45a promoter regulation in response to mechanical stress. To this point, two other SNPs, rs3783456 and rs3783457, located within the same region of SNP rs5810000 were identified by gene resequencing. As rs581000 is a tagging SNP the LD between these SNPs must be high and thus may also be associated with mechanical stress-mediated GADD45a expression and with ALI susceptibility clinically. We were not able to confirm this given the low frequency of these SNPs (Table 1). Accordingly, a full characterization of SNPs associated with GADD45a promoter activity in response to mechanical stress is an important area of further investigation.
In summary, we have identified a strong functional link between GADD45a expression levels and ALI susceptibility and have begun to detail specific genetic variants as well as the mechanisms underlying these observations [11]. It is imperative that our study be subjected to replication for further confirmation of our findings. We speculate that these investigations will yield further insights into ALI/VILI pathogenesis and may ultimately lead to novel therapeutic targets related to GADD45a regulation and signaling.
Supporting Information
Primer sequences for GADD45a 5′ promoter deletion constructs.
(DOCX)
Acknowledgments
The authors are thankful to Ms. Lakshmi Natarajan, Ms. Carrie Evenoski, Mr. Brandon Mapes and Dr. Saad Sammani for their excellent technical assistance.
Funding Statement
These authors have no support or funding to report.
References
- 1. Tejera P, Meyer NJ, Chen F, Feng R, Zhao Y, et al. (2012) Distinct and replicable genetic risk factors for acute respiratory distress syndrome of pulmonary or extrapulmonary origin. J Med Genet 49: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Song Z, Tong C, Sun Z, Shen Y, Yao C, et al. (2010) Genetic variants in the TIRAP gene are associated with increased risk of sepsis-associated acute lung injury. BMC Med Genet 11: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Y, Shao Y, Yu B, Sun L, Lv F (2012) Association of PBEF gene polymorphisms with acute lung injury, sepsis, and pneumonia in a northeastern Chinese population. Clin Chem Lab Med 50: 1917–1922. [DOI] [PubMed] [Google Scholar]
- 4. Gao L, Flores C, Fan-Ma S, Miller EJ, Moitra J, et al. (2007) Macrophage migration inhibitory factor in acute lung injury: expression, biomarker, and associations. Transl Res 150: 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma SF, Xie L, Pino-Yanes M, Sammani S, Wade MS, et al. (2011) Type 2 deiodinase and host responses of sepsis and acute lung injury. Am J Respir Cell Mol Biol 45: 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flores C, Ma SF, Maresso K, Wade MS, Villar J, et al. (2008) IL6 gene-wide haplotype is associated with susceptibility to acute lung injury. Transl Res 152: 11–17. [DOI] [PubMed] [Google Scholar]
- 7. Pino-Yanes M, Ma SF, Sun X, Tejera P, Corrales A, et al. (2011) Interleukin-1 receptor-associated kinase 3 gene associates with susceptibility to acute lung injury. Am J Respir Cell Mol Biol 45: 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao L, Grant A, Halder I, Brower R, Sevransky J, et al. (2006) Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol 34: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grigoryev DN, Ma SF, Irizarry RA, Ye SQ, Quackenbush J, et al. (2004) Orthologous gene-expression profiling in multi-species models: search for candidate genes. Genome Biol 5: R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyer NJ, Huang Y, Singleton PA, Sammani S, Moitra J, et al. (2009) GADD45a is a novel candidate gene in inflammatory lung injury via influences on Akt signaling. Faseb J 23: 1325–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitra S, Sammani S, Wang T, Boone DL, Meyer NJ, et al. (2011) Role of growth arrest and DNA damage-inducible alpha in Akt phosphorylation and ubiquitination after mechanical stress-induced vascular injury. Am J Respir Crit Care Med 184: 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, et al. (2003) Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–797. [DOI] [PubMed] [Google Scholar]
- 13. Han YJ, Ma SF, Wade MS, Flores C, Garcia JG (2012) An intronic MYLK variant associated with inflammatory lung disease regulates promoter activity of the smooth muscle myosin light chain kinase isoform. J Mol Med (Berl) 90: 299–308. [DOI] [PubMed] [Google Scholar]
- 14. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, et al. (1994) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824. [DOI] [PubMed] [Google Scholar]
- 15. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, et al. (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 16. Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, et al. (1999) Genomic instability in Gadd45a-deficient mice. Nat Genet 23: 176–184. [DOI] [PubMed] [Google Scholar]
- 17. Sole X, Guino E, Valls J, Iniesta R, Moreno V (2006) SNPStats: a web tool for the analysis of association studies. Bioinformatics 22: 1928–1929. [DOI] [PubMed] [Google Scholar]
- 18. Benjamini Y HY (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57: 289–300. [Google Scholar]
- 19. Wigginton JE, Cutler DJ, Abecasis GR (2005) A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet 76: 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 21. Weale ME, Depondt C, Macdonald SJ, Smith A, Lai PS, et al. (2003) Selection and evaluation of tagging SNPs in the neuronal-sodium-channel gene SCN1A: implications for linkage-disequilibrium gene mapping. Am J Hum Genet 73: 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao YX, Ramirez MI, Williams MC (2003) Enhanced binding of Sp1/Sp3 transcription factors mediates the hyperoxia-induced increased expression of the lung type I cell gene T1alpha. J Cell Biochem 89: 887–901. [DOI] [PubMed] [Google Scholar]
- 23. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, et al. (2001) dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 29: 308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ney L, Kuebler WM (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med 343: 812–813; author reply 813–814. [PubMed]
- 25. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, et al. (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 26. Seefried L, Mueller-Deubert S, Schwarz T, Lind T, Mentrup B, et al. (2010) A small scale cell culture system to analyze mechanobiology using reporter gene constructs and polyurethane dishes. Eur Cell Mater 20: 344–355. [DOI] [PubMed] [Google Scholar]
- 27. Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, et al. (2005) Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 175: 3369–3376. [DOI] [PubMed] [Google Scholar]
- 28. Lin Z, Pearson C, Chinchilli V, Pietschmann SM, Luo J, et al. (2000) Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS. Clin Genet 58: 181–191. [DOI] [PubMed] [Google Scholar]
- 29. Marshall RP, Webb S, Bellingan GJ, Montgomery HE, Chaudhari B, et al. (2002) Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med 166: 646–650. [DOI] [PubMed] [Google Scholar]
- 30. O′Mahony DS, Glavan BJ, Holden TD, Fong C, Black RA, et al. (2012) Inflammation and immune-related candidate gene associations with acute lung injury susceptibility and severity: a validation study. PLoS One 7: e51104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, et al. (2005) Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med 171: 361–370. [DOI] [PubMed] [Google Scholar]
- 32. Nemeth E, Tashima LS, Yu Z, Bryant-Greenwood GD (2000) Fetal membrane distention: I. Differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. Am J Obstet Gynecol 182: 50–59. [DOI] [PubMed] [Google Scholar]
- 33. Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, et al. (2008) Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med 178: 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moss M, Mannino DM (2002) Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979–1996). Crit Care Med 30: 1679–1685. [DOI] [PubMed] [Google Scholar]
- 35. Mitra S, Desai M, Khatkhatay MI (2006) Vitamin D receptor gene polymorphisms and bone mineral density in post menopausal Indian women. Maturitas 55: 27–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences for GADD45a 5′ promoter deletion constructs.
(DOCX)