Abstract
Background
The clinical and prognostic significance of CD133 in non-small-cell lung cancer (NSCLC) remains controversial. To clarify a precise determinant of the clinical significance of CD133, we conducted a systematic review and meta-analysis to evaluate the association of CD133 with prognosis and clinicopathological features of NSCLC patients.
Methods
The electronic and manual searches were performed through the database of Pubmed, Medline, Web of Science, Scopus, and Chinese CNKI (from January 1, 1982 to January 1, 2014) for titles and abstracts by using the following keywords: “CD133”, “ac133” or “Prominin-1”, and “lung cancer” to identify the studies eligible for our analysis. Meta-analysis was performed by using Review Manager 5.0 and the outcomes included the overall survival and various clinicopathological features.
Results
A total of 23 studies were finally included, and our results showed that CD133 level was significantly correlated with the overall survival (OR = 2.25, 95% CI: 1.24–4.07, P = 0.008) of NSCLC patients but not with the disease free survival (OR = 1.33, 95% CI = 0.77–2.30, P = 0.31). With respect to clinicopathological features, CD133 level was positively correlated with lymph node metastasis (OR = 1.99, 95%CI = 1.06–3.74, P = 0.03), but not correlated with the histological classification (OR = 1.00, 95%CI = 0.81–1.23, P = 0.99(ac), OR = 0.87, 95%CI = 0.61–1.24, P = 0.45(sc)), or differentiation (OR = 0.94, 95%CI 0.53–1.68, Z = 0.20, P = 0.84 random-effect) of NSCLC patients.
Conclusion
High level of CD133 expression trends to correlate with a worse prognosis and a higher rate of lymph node metastasis in NSCLC patients, revealing CD133 as a potential pathological prognostic marker for NSCLC patients.
Introduction
Lung cancer is the first leading cause of death worldwide. Because of its high incidence and recurrence rate, the 5-years overall survival has been estimated at nearly 10% [1]. According to the theory of cancer stem cells (CSCs), tumors can be recognized as a result of abnormal organogenesis driven by a small subpopulation of cancer cells that are able to be self-renewal and to produce the heterogeneous lineages of cancer cells [2]–[4]. The cell surface marker CD133, also known as prominin-1, is widely used as a CSCs marker in various tumors, including leukemia, lung cancer, colon cancer and brain cancer [5]–[10]. Furthermore, its prognostic and clinicopathological values in the above-mentioned cancers have been widely studied [11]–[14].
Up to date, controversy exists concerning the correlation between CD133 and prognostic value with respect to Non-Small-Cell Lung Cancer (NSCLC) [1], [15]. Some studies reported that high expression of CD133 was correlated with unfavorable prognosis [1], [11], which were hardly concurred by others [16], [17]. Here, we performed a systematic review and meta-analysis in the published literatures to clarify whether the expression of CD133 was associated with the clinicopathological features and prognosis of NSCLC patients.
Materials and Methods
Literature Search Strategy
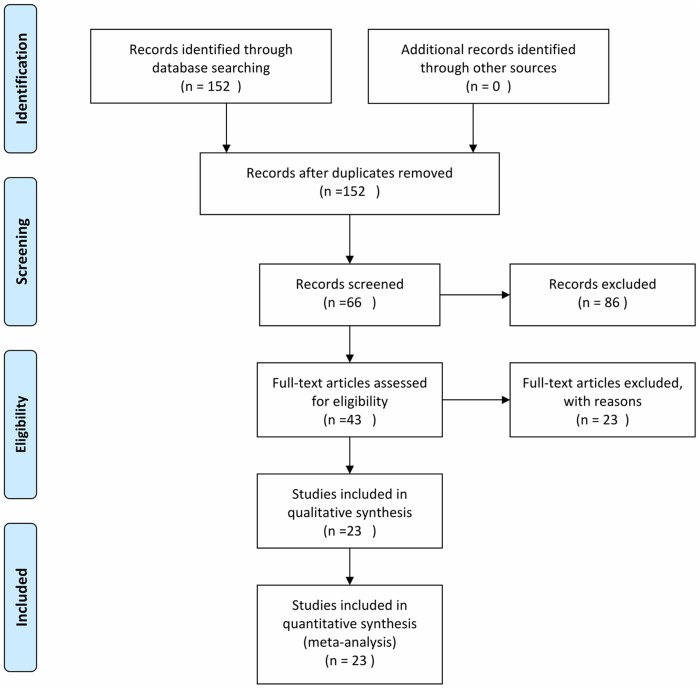
We adapted the Cochrane Central Register of Controlled Trials, and performed a comprehensive publication search through the Pubmed, Medline, Web of Science, Scopus, and Chinese CNKI database by using the following search strings: “CD133”, “ac133” or “Prominin-1”, and “lung cancer” from January 1, 1982 to January 1, 2014. Titles and abstracts were reviewed to identify reports which examined the association of CD133 level with clinical outcomes, such as overall survival (OS), disease free survival (DFS), and clinicopathological features including age, histology, differentiation and lymph node metastasis in NSCLC patients (Figure 1).
Figure 1. Flow chart for selection of studies.
Selection Criteria
The criteria for inclusion were listed as follows: (1) studies dealing with NSCLC patients (excluding the small cell lung cancer patients); (2) articles concerning about the association of CD133 level with either prognostic values or clinicopathological features; (3) the expression of CD133 was detected on cancer tissue, rather than in the serum or any other kinds of specimens; (4) the pathological method is the golden standard for diagnosis of lung cancer; (5) articles providing sufficient data to allow the estimation of an odds ration (OR) of OS, DFS and clinicopathological features; (6) articles with no limitations on language or the minimum number of patients of every single study. Reviews, comments, duplicated studies, and irrelevant articles were excluded (Figure 1).
Data Extraction
The valuable data including author, publication year, country of study, number of patients, detection method, TNM stage, preoperative therapy, clinicopathological features, the number of patients whose cancer tissue was positive for CD133 and the corresponding percentages were extracted from the included papers and illustrated in Table 1. Discrepancies were resolved by discussion among three authors in cases of conflicting evaluations.
Table 1. General characteristics of included studies.
| Studies | Year | Country | NO. of Patients | Technique | Cutoff values | TNM | Pre-operate therapy | No. of CD133 Positve (%) |
| Herpel E [26] | 2011 | Germany | 86 | IHC | ≥20% | I/II | NO | 13(15%) |
| Pirozzi G [27] | 2013 | Italy | 45 | FC/IHC/PCR | ≥10% | I–III | NO | 12(26.7%) |
| Gottschling S [16] | 2013 | Germany | 55 | IHC | ≥10% | I/II | NO | 10(18%) |
| Shien K [28] | 2012 | Japan | 50 | IHC | >1% | N2–N3 | YES | 15(30%) |
| Woo T [5] | 2011 | Japan | 177 | IHC | ≥17.5% | I | NO | 81(45.8%) |
| Mizugaki.H [1] | 2013 | Japan | 161 | IHC | – | I–IV | NO | 124(77%) |
| Alamgeer M [8] | 2013 | Australia | 205 | IHC | ≥5% | I | NO | 104(50.7%) |
| Xu YH [29] | 2010 | China | 102 | IHC | ≥10% | I–IV | Not mentioned | 51(50%) |
| Salnikov AV [15] | 2009 | Germany | 81 | IHC | ≥20% | I–III | Not mentioned | 51(63%) |
| Li F [30] | 2011 | China | 145 | IHC | >1% | I | NO | 46(31.7%) |
| Okudela K [2] | 2012 | Japan | 177 | IHC | ≥17.5% | I | NO | 81(45.8%) |
| Chen JR [25] | 2010 | China | 65 | IHC | ≥10% | I–III | NO | 45(69.2%) |
| Wei YP [24] | 2008 | China | 77 | IHC | ≥10% | I–IV | Not mentioned | 40(51.9%) |
| Wu S [31] | 2012 | China | 305 | IHC | >1% | II–IV | Not mentioned | 149(48.9%) |
| Cortes-Dericks, L [32] | 2012 | Switzerland | 64 | PCR | – | I–III | NO | 63(98%) |
| Bertolini.G [33] | 2009 | Italy | 60 | IHC/FC | ≥5% | I–IV | – | 14(23.8%) |
| Lin XY [34] | 2009 | China | 54 | IHC | >1% | – | – | 27(50%) |
| Zhang HZ [35] | 2007 | China | 77 | IHC | ≥5% | – | NO | 40(51.9%) |
| Janikova M [9] | 2010 | Czech | 121 | TMA/IHC | ≥10% | – | – | 23(19%) |
| Sullivan JP [36] | 2010 | USA | 207 | TMA/IHC | – | I | – | 56(27%) |
| Li LD [37] | 2013 | China | 50 | IHC | – | I–IV | NO | 43(85.7%) |
| Moreira AL [38] | 2010 | USA | 85 | IHC | – | I–III | –– | 18(21.6%) |
| Tirino V [17] | 2009 | Italy | 89 | IHC | – | I–IV | NO | 64(72%) |
Statistical Analysis
This study was reported in accordance to the PRISMA-statement (Checklist S1). Statistical analysis was performed by Review Manager 5.0 software. The data was analyzed by means of SPSS version 13.0 (SPSS Inc; Chicago, USA). P<0.05 was considered as statistical significance. To minimize heterogeneity, confounding factors, effects of selection, and ascertainment biases across studies, the data extracted from eligible studies were in accordance with the following criteria: (1) the DFS and OS data that were extracted from each eligible study were separately used in the independent analysis; (2) if the study was applied with both univariate analysis and multivariate analysis, the multivariate analysis data were used. Heterogeneity across studies was evaluated by the Q test and P-values (Cochrans Q test, P-value>0.05 or I 2>50% indicated the existence of heterogeneity across studies). Fixed or Random model was used depending on heterogeneity analysis. ORs and RRs were calculated by a fixed-effects model if P-value>0.05, otherwise a random effect model was used.
Results
Included Studies
Twenty-three studies including 2538 patients met with our criteria in this meta-analysis. The main features of each eligible study were extracted (Table 1). About one-third of the studies dealt with patients with early stage (stage I) of NSCLC, and the rest of the studies was focused on the patients with stage from I to III/IV. Thirteen studies reported that no pre-operative therapy was performed on patients and one study dealing with N2/N3 patients mentioned that pre-operative therapy was applied, while the others had no relevant reports at all. Eleven included studies indicated DFS, 14 studies had OS, and 20 studies reported clinicopathological details. Among the 23 studies, four studies reported neither OS nor DFS, but presented clinicopathological features. In addition, another three articles reported either OS or DFS, but without clinicopathological data.
Meta Analysis for OS in NSCLC
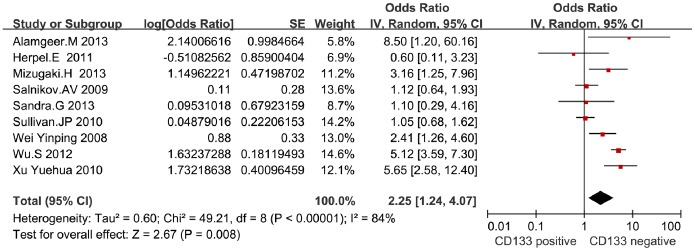
Data of 3-year and 5-year OS extracted from 11 eligible studies were included in the meta-analysis. Since the heterogeneity was significant (I 2 = 84%, P = 0.00001), a random-effect model was used to calculate the OR of OS in NSCLC patients. Meta-analysis found that the patients whose cancer tissue was positive for CD133 expression showed a worse OS than those with negative ones (OR = 2.25, 95% CI: 1.24–4.07, P = 0.008, random model), suggesting that CD133 could be an independent prognostic factor in NSCLC patients (Figure 2).
Figure 2. Meta-analysis of overall survival between CD133 positive and CD133 negative in NSCLC patients.
Meta Analysis on DFS in NSCLC
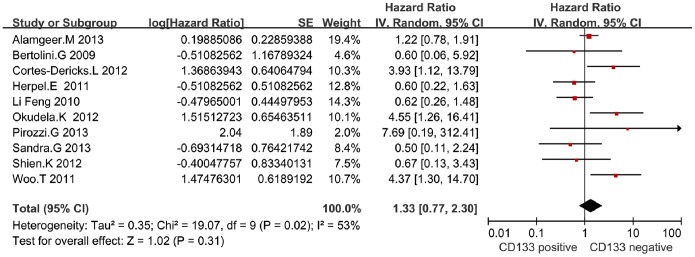
Data on DFS were also extracted from ten studies. A random-effect model (I 2 = 53%, P = 0.02) was used to calculate the OR of DFS in NSCLC patients. Meta-analysis showed that no difference existed between CD133-positive patients and CD133-negative ones (OR = 1.33, 95% CI = 0.77–2.30, P = 0.31, random model). (Figure 3).
Figure 3. Meta-analysis of disease free survival between CD133 positive and CD133 negative in NSCLC patients.
CD133 Expression and Clinicopathological Parameters
Nineteen studies provided the information of various clinicopathological parameters and their correlation with CD133 positive expression was summarized in Table 2. Twelve studies had the statistical data on age of patients. However, none of them reported the association between age and CD133 positive expression. Fifteen studies demonstrated that there was no correlation between CD133 expression and gender. In addition, among nine included studies, only one study showed that smoking was positively correlated with CD133 expression in NSCLC patients.
Table 2. Narrative review of the association between clinicopathological parameters and CD133 positive expression with respect to non-small-cell-lung cancer patients.
| Items | Significant correlation (P<0.05) | Non-significant correlation (P≥0.05) |
| Age | – | [1], [15], [24], [17], [26], [27], [28], [29], [30], [31], [32], [33] |
| Gender | – | [1], [8], [15], [24], [17], [26], [27], [28], [29], [30], [31], [32], [33], [36], [37] |
| Smoking | [36] | [1], [8], [24], [30], [29], [28], [32], [33] |
| Histology | [8], [29], [32], [33], [36], [38] | [1], [15], [24], [17], [25], [26], [27], [28], [30], [31], [35] |
| Differentiation | [29], [31], [34], [38] | [1], [26], [37], [24], [25], [35] |
| Lymph node metastasis | [25], [31], [34], [35] | [8], [17], [26], [32], [36] |
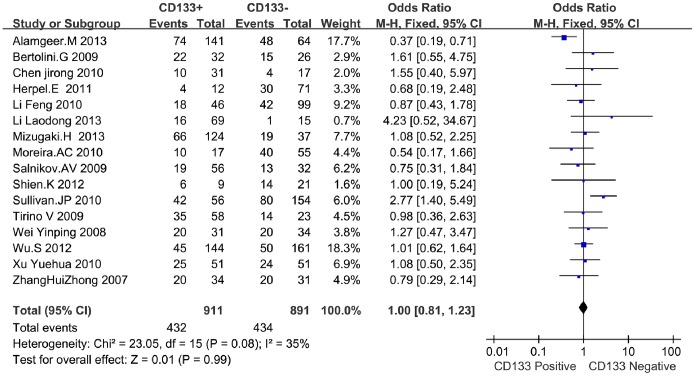
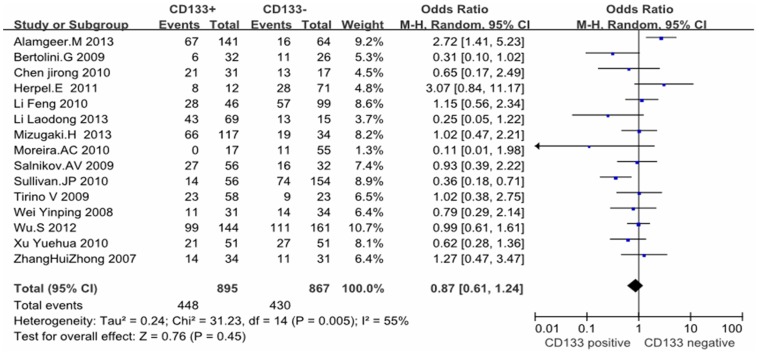
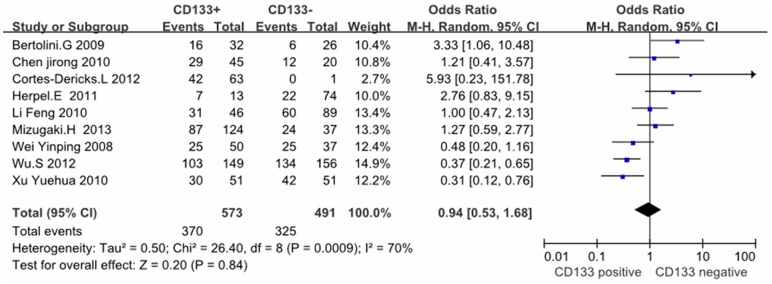
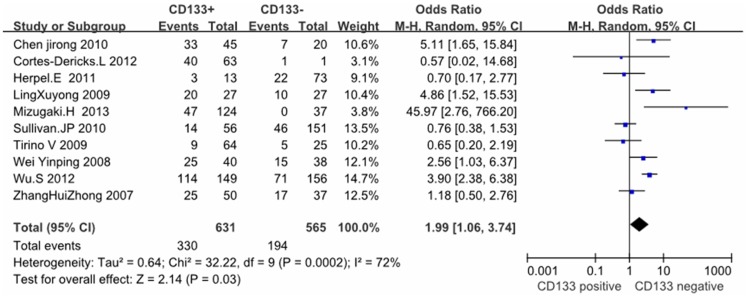
Except these above-mentioned parameters, controversies also existed on the correlation among histology, differentiation, lymph node metastasis and CD133 expression in these included studies. Seventeen studies evaluated the relationship between CD133 expression and histological types in NSCLC patients. The pooled OR was 1.00, (95% CI = 0.81–1.23, P = 0.99, fixed effect) (Figure 4) and 0.87 (95%CI = 0.61–1.24, P = 0.45, random-effect) (Figure 5), suggesting that CD133 expression was not associated with the histology of adenocarcinoma or squamous carcinoma. In addition, no correlation was found between the expression of CD133 and the differentiation of NSCLC (OR = 0.94, 95%CI = 0.53–1.68, Z = 0.20, P = 0.84, random-effect) (Figure 6). Notably, the meta-analysis suggested that CD133 expression was positively correlated with lymph node metastasis (OR = 1.99, 95%CI = 1.06–3.74, P = 0.03, random-effect) (Figure 7).
Figure 4. Forrest plot of odds ratios for the association of CD133 expression with adenocarcinoma in NSCLC patients.
Figure 5. Forrest plot of odds ratios for the association of CD133 expression with squamous carcinoma in NSCLC patients.
Figure 6. Meta-analysis of correlation between CD133 expression and differentiation in NSCLC patients.
Figure 7. Meta-analysis of correlation between CD133 expression and lymph node metastasis in NSCLC patients.
Discussion
Up to date, clinically approved biomarkers have been found to guide treatment and predict outcomes in several solid tumors, and several studies have demonstrated that CD133 is a specific cell surface marker of CSCs [1], [2], [18]–[20]. However, the clinical value of CD133 remains controversial in several solid tumors including lung cancer [6]. In this meta-analysis study, we revealed that high expression of CD133 was positively correlated with the poor prognosis of NSCLC patients.
CD133 is a five-transmembrane cell surface glycoprotein, and its gene is specifically located on chromosome 4p15, a region that contains genes related to mature organ homoeostasis, tumorigenesis and cancer progression [19]. Previous studies have determined that CD133-positive cancer cells possess CSCs phenotype, which contributes to the self-renewal and tumorigenic capabilities [21]–[23]. However, controversies remain regarding to whether the correlation of CD133 expression with either poor prognosis or the clinicopathological parameters in NSCLC patients exists [16], [17], [24], [25].
Although our study revealed the positive correlation between CSCs marker CD133 and patients’ survival, CD133 itself as a biomarker has its limitations on predicting prognosis and clinicopathological parameters in patients. Firstly, overall survival (OS) and disease free survival (DFS) were determined from unadjusted RRs in the published papers, and RRs from the survival curves might be less reliable those that from direct analysis of variance. Ideally, measurements should be directly obtained from the statistical data in published papers and then adjusted by using other prognostic factors. Secondly, the condition of patients included in this study was not normalized. Seven included studies were focused on the patients in the early stage, while others dealt with the stage I to IV or the advanced cancer patients. Twelve studies included in this analysis reported that patients did not receive any chemotherapy before surgery and one study targeting at N2–N3 patients reported that neoadjuvant chemotherapy was applied before surgery while others lacked details. Thirdly, the cutoff values of CD133 expression in different papers were variable. Lastly,the OR of each study is generally small and the conclusion might be affected by one or two reports with large ORs. All of these factors might partly influence the significance of CD133 expression in the survival and the clinicopathological analysis.
In summary, the present meta-analysis indicates that CD133 expression is associated with a poor OS and a high rate of lymph node metastasis and no correlation exists between CD133 expression and DFS or other common clinicopathological parameters such as histology and tumor differentiation. CD133 may be a potential prognostic marker and useful therapeutic target in lung cancer, and co-detection of CD133 with other CSC markers including ALDH1A1 and OCT4 may be more valuable and helpful in clinical application in NSCLC patients.
Supporting Information
PRISMA checklist.
(DOC)
Acknowledgments
We thank Dr. Xia Zhang from Institute of Pathology and Southwest Cancer Center, Southwest Hospital, Third Military Medical University and Key Laboratory of Tumor Immunopathology, Ministry of Education of China, for critical review of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data is available in the paper and in the supplementary files.
Funding Statement
This work was supported by grants from the National Basic Research Program of China (973 Program, Grant No. 2010CB529403), National Natural Science Foundations of China (Grant No. 81272598). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mizugaki H, Sakakibara-Konishi J, Kikuchi J, Moriya J, Hatanaka KC, et al. (2013) CD133 expression: a potential prognostic marker for non-small cell lung cancers. Int J Clin Oncol. [DOI] [PubMed]
- 2. Okudela K, Woo T, Mitsui H, Tajiri M, Masuda M, et al. (2012) Expression of the potential cancer stem cell markers, CD133, CD44, ALDH1, and beta-catenin, in primary lung adenocarcinoma–their prognostic significance. Pathol Int 62: 792–801. [DOI] [PubMed] [Google Scholar]
- 3. Pine SR, Marshall B, Varticovski L (2008) Lung cancer stem cells. Dis Markers 24: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu C, Xie D, Yu SC, Yang XJ, He LR, et al. (2013) beta-Catenin/POU5F1/SOX2 Transcription Factor Complex Mediates IGF-I Receptor Signaling and Predicts Poor Prognosis in Lung Adenocarcinoma. Cancer Research 73: 3181–3189. [DOI] [PubMed] [Google Scholar]
- 5. Woo T, Okudela K, Mitsui H, Yazawa T, Ogawa N, et al. (2011) Prognostic value of CD133 expression in stage I lung adenocarcinomas. International Journal of Clinical and Experimental Pathology 4: 32–42. [PMC free article] [PubMed] [Google Scholar]
- 6. Wang K, Xu J, Zhang J, Huang J (2012) Prognostic role of CD133 expression in colorectal cancer: a meta-analysis. BMC Cancer 12: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen L, Chen XZ, Yang K, Chen ZX, Zhang B, et al. (2013) Prognostic Value of Cancer Stem Cell Marker CD133 Expression in Gastric Cancer: A Systematic Review. Plos One 8. [DOI] [PMC free article] [PubMed]
- 8. Alamgeer M, Ganju V, Szczepny A, Russell PA, Prodanovic Z, et al. (2013) The prognostic significance of aldehyde dehydrogenase 1A1 (ALDH1A1) and CD133 expression in early stage non-small cell lung cancer. Thorax 68: 1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janikova M, Skarda J, Dziechciarkova M, Radova L, Chmelova J, et al. (2010) Identification of Cd133(+)/Nestin+ Putative Cancer Stem Cells in Non-Small Cell Lung Cancer. Biomedical Papers-Olomouc 154: 321–326. [DOI] [PubMed] [Google Scholar]
- 10. Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, et al. (2008) Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res 14: 123–129. [DOI] [PubMed] [Google Scholar]
- 11. Qu H, Li R, Liu Z, Zhang J, Luo R (2013) Prognostic value of cancer stem cell marker CD133 expression in non-small cell lung cancer: a systematic review. Int J Clin Exp Pathol 6: 2644–2650. [PMC free article] [PubMed] [Google Scholar]
- 12. Chen S, Song X, Chen Z, Li X, Li M, et al. (2013) CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PLoS One 8: e56380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen L, Chen XZ, Yang K, Chen ZX, Zhang B, et al. (2013) Prognostic value of cancer stem cell marker CD133 expression in gastric cancer: a systematic review. PLoS One 8: e59154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang K, Chen XZ, Zhang B, Yang C, Chen HN, et al. (2011) Is CD133 a biomarker for cancer stem cells of colorectal cancer and brain tumors? A meta-analysis. Int J Biol Markers 26: 173–180. [DOI] [PubMed] [Google Scholar]
- 15. Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, et al. (2010) CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer 126: 950–958. [DOI] [PubMed] [Google Scholar]
- 16. Gottschling S, Jensen K, Herth FJ, Thomas M, Schnabel PA, et al. (2013) Lack of prognostic significance of neuroendocrine differentiation and stem cell antigen co-expression in resected early-stage non-small cell lung cancer. Anticancer Res 33: 981–990. [PubMed] [Google Scholar]
- 17. Tirino V, Camerlingo R, Franco R, Malanga D, La Rocca A, et al. (2009) The role of CD133 in the identification and characterisation of tumour-initiating cells in non-small-cell lung cancer. Eur J Cardiothorac Surg 36: 446–453. [DOI] [PubMed] [Google Scholar]
- 18. Sterlacci W, Savic S, Fiegl M, Obermann E, Tzankov A (2014) Putative stem cell markers in non-small-cell lung cancer: a clinicopathologic characterization. J Thorac Oncol 9: 41–49. [DOI] [PubMed] [Google Scholar]
- 19. Li Z (2013) CD133: a stem cell biomarker and beyond. Exp Hematol Oncol 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roudi R, Madjd Z, Ebrahimi M, Samani FS, Samadikuchaksaraei A (2013) CD44 and CD24 cannot act as cancer stem cell markers in human lung adenocarcinoma cell line A549. Cell Mol Biol Lett. [DOI] [PMC free article] [PubMed]
- 21. Shi CJ, Gao J, Wang M, Wang X, Tian R, et al. (2011) CD133(+) gallbladder carcinoma cells exhibit self-renewal ability and tumorigenicity. World J Gastroenterol 17: 2965–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, et al. (2010) miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 7: 694–707. [DOI] [PubMed] [Google Scholar]
- 23. Zhang H, Yang N, Sun B, Jiang Y, Hou C, et al. (2014) CD133 positive cells isolated from A549 cell line exhibited high liver metastatic potential. Neoplasma 61: 153–160. [DOI] [PubMed] [Google Scholar]
- 24. Wei YP WW, Hua P (2008) Expression of Tumor Stem Cell Marker CD133 in Non-small Cell Lung Carcinoma and Its Clinical Significance. Journal Of SUN YAT-SEN University(Medical Sciences) 29: 312–316. [Google Scholar]
- 25. Cheng JR, Zumureti WS, Zou J (2010) Expressions of CD133 and CD105 in lung cancer tissue and their clinical significance. Tumor 30: 334–337. [Google Scholar]
- 26. Herpel E, Jensen K, Muley T, Warth A, Schnabel PA, et al. (2011) The cancer stem cell antigens CD133, BCRP1/ABCG2 and CD117/c-KIT are not associated with prognosis in resected early-stage non-small cell lung cancer. Anticancer Res 31: 4491–4500. [PubMed] [Google Scholar]
- 27. Pirozzi G, Tirino V, Camerlingo R, La Rocca A, Martucci N, et al. (2013) Prognostic value of cancer stem cells, epithelial-mesenchymal transition and circulating tumor cells in lung cancer. Oncol Rep 29: 1763–1768. [DOI] [PubMed] [Google Scholar]
- 28. Shien K, Toyooka S, Ichimura K, Soh J, Furukawa M, et al. (2012) Prognostic impact of cancer stem cell-related markers in non-small cell lung cancer patients treated with induction chemoradiotherapy. Lung Cancer 77: 162–167. [DOI] [PubMed] [Google Scholar]
- 29. Xu YH, Zhang GB, Wang JM, Hu HC (2010) B7-H3 and CD133 expression in non-small cell lung cancer and correlation with clinicopathologic factors and prognosis. Saudi Medical Journal 31: 980–986. [PubMed] [Google Scholar]
- 30. Li F, Zeng H, Ying K (2011) The combination of stem cell markers CD133 and ABCG2 predicts relapse in stage I non-small cell lung carcinomas. Med Oncol 28: 1458–1462. [DOI] [PubMed] [Google Scholar]
- 31. Wu S, Yu L, Wang D, Zhou L, Cheng Z, et al. (2012) Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer 12: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cortes-Dericks L, Galetta D, Spaggiari L, Schmid RA, Karoubi G (2012) High expression of octamer-binding transcription factor 4A, prominin-1 and aldehyde dehydrogenase strongly indicates involvement in the initiation of lung adenocarcinoma resulting in shorter disease-free intervals. Eur J Cardiothorac Surg 41: e173–181. [DOI] [PubMed] [Google Scholar]
- 33. Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, et al. (2009) Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A 106: 16281–16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin X, Liu S, Liu N, Yang X, Xu H, et al. (2009) [Expression and Significance of Stem Cell Markers CK19, Notch3, CD133, P75NTR, STRO-1 and ABCG2 in Pulmonary Squamous Carcinomas.]. Zhongguo Fei Ai Za Zhi 12: 316–321. [DOI] [PubMed] [Google Scholar]
- 35. Zhang HZ, Wei YP, Wang M, Wu C, Yang YQ, et al. (2007) [Association of CD133 and endothelin-converting enzyme expressions with prognosis of non-small cell lung carcinoma]. Nan Fang Yi Ke Da Xue Xue Bao 27: 696–699. [PubMed] [Google Scholar]
- 36. Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, et al. (2010) Aldehyde Dehydrogenase Activity Selects for Lung Adenocarcinoma Stem Cells Dependent on Notch Signaling. Cancer Research 70: 9937–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li LD, Yu HN, Wang XY, Zeng JR, Li DY, et al. (2013) Expression of seven stem-cell-associated markers in human airway biopsy specimens obtained via fiberoptic bronchoscopy. Journal of Experimental & Clinical Cancer Research 32. [DOI] [PMC free article] [PubMed]
- 38. Moreira AL, Gonen M, Rekhtman N, Downey RJ (2010) Progenitor stem cell marker expression by pulmonary carcinomas. Modern Pathology 23: 889–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data is available in the paper and in the supplementary files.