Abstract
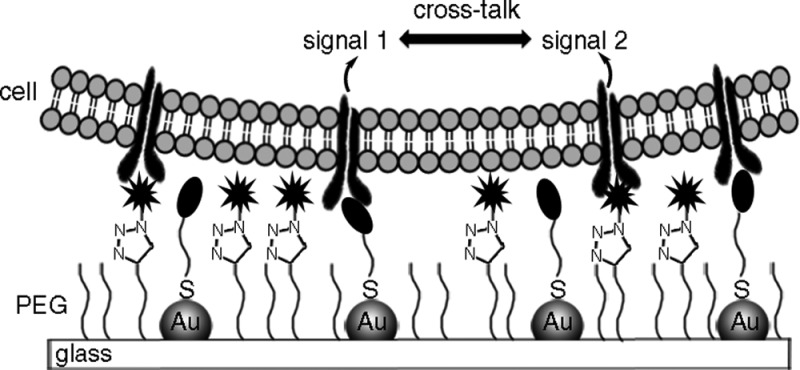
The presentation of biologically active molecules at interfaces has made it possible to investigate the responses of cells to individual molecules in their matrix at a given density and spacing. However, more sophisticated methods are needed to create model surfaces that present more than one molecule in a controlled manner in order to mimic at least partially the complexity given in natural environments. Herein, we present dual-functionalized surfaces combining quasi-hexagonally arranged gold nanoparticles with defined spacings and a newly developed PEG-alkyne coating to functionalize the glass in the intermediate space. The PEG-alkyne coating provides an inert background for cell interactions but can be modified orthogonally to the gold nanoparticles with numerous azides, including spectroscopically active molecules, peptides, and biotin at controlled densities by the copper(I)-catalyzed azide alkyne click reaction. The simultaneous presentation of cRGD on the gold nanoparticles with 100 nm spacing and synergy peptide PHSRN in the space between has a striking effect on REF cell adhesion; cells adhere, spread, and form mature focal adhesions on the dual-functionalized surfaces, whereas cells cannot adhere on either monofunctional surface. Combining these orthogonal functionalization methods creates a new platform to study precisely the crosstalk and synergy between different signaling molecules and clustering effects in ligand–receptor interactions.
1. Introduction
The specific functionalization of substrates with bioactive molecules such as adhesion peptides, growth factors, and other signaling molecules is important in understanding cell–material interactions and can make it possible to control the response of a cell to its surroundings. The complex interactions of the cell with the extracellular matrix (ECM) and the crosstalk between different signaling molecules highlight the need to develop well-defined substrates that can be specifically functionalized with multiple ligands at defined densities and geometric arrangements.1 Recently, materials that can be functionalized orthogonally with two different bioactive molecules including polymer-based hydrogels,2 thin polymer films,3 supported lipid bilayers,4 self-assembled monolayers (SAMs) of thiols on gold,5,6 and SAMs of silanes on silicon or glass7 have been the focus, and some were used to investigate the crosstalk between signaling events in cells.8,9
Cell-adhesion tripeptide motif RGD (arginine-glycine-aspartic acid), present in many ECM proteins as a ligand for integrins, is one of the most frequently used ligands to promote cell attachment to artificial materials.10−14 The binding of integrins to RGD motifs results in the clustering of integrins and the recruitment of intracellular proteins to form stable focal contacts and also influences other signaling events.15 RGD-mediated adhesion can be further promoted by using a cyclic form of RGD, cRGD,16 and adding other peptides derived from ECM proteins such as synergy peptide PHSRN (proline-histidine-serine-arginine-asparagine) from the ninth type III domain in fibronectin.17−19 Correspondingly, various materials dual-functionalized with the RGD motif and the synergy peptide have been used to study cell adhesion.4,20,21
In addition to the composition and specific immobilization of bioactive molecules, the spacing and density of the ligands influence the cell’s response. Previously, we established block copolymer micellar nanolithography (BCML)22,23 as a powerful and versatile method of preparing quasi-hexagonally arranged gold nanoparticles on glass substrates to control the spacing between ligands immobilized on the gold nanoparticles with thiol chemistry. The functionalized gold particles with a diameter of <8 nm are so small that only a single integrin can bind to one particle. In addition, covalent or electrostatic coatings of poly(ethylene glycols) (PEGs) were used successfully to minimize nonspecific interactions between cells and the glass.8,24,25 Previous studies on cell adhesion to cRGD-functionalized quasi-hexagonally arranged gold nanoparticles on glass surfaces with a nonspecific-adhesion-preventing PEG2000 layer in the intermediate space reveal that a spacing of the nanoparticles, and therefore of the integrin ligands, of ≤58 nm is needed for cell rat embryonic fibroblast (REF) adhesion and spreading.26−28
In this work, we introduce a route to functionalize the above-described gold nanostructured glass surfaces with a second molecule through a new PEG-alkyne coating for the space between the gold nanoparticles. The PEG-alkyne contains a terminal alkyne functional group that can be modified with the copper(I)-catalyzed azide alkyne cycloaddition (CuAAC), also known as the click reaction, orthogonal to the gold nanoparticles. Click reactions are used for the biofunctionalization of various materials due to their mild reaction conditions, bio-orthogonality, and high conversion efficiency and stereoselectivity.29−31 Here, we show that the PEG-alkyne coating passivates the surface against nonspecific interactions and that peptides as well as small molecules with an azide functional group can be conveniently attached. The density of the biomolecule introduced at the PEG-alkyne can be easily adjusted by forming mixed SAMs with an inert PEG2000, and we examine REF cell adhesion on cRGD-modified PEG-alkyne layers at different densities. Cell adhesion studies on bifunctional nanopatterns, where the PEG-alkyne is modified with synergy peptide PHSRN and the gold nanoparticles are modified with cRGD, reveal a striking effect of dual functionalization on integrin clustering in cell adhesion. The dual-functionalized surfaces presented here offer a new platform for studying the role of ligand and receptor spacing in systems where there is crosstalk between two different signaling molecules.
2. Materials and Methods
Synthesis of (CH3CH2O)3Si-PEG3000-alkyne
(CH3CH2O)3Si-PEG3000-alkyne (PEG-alkyne in the following) is synthesized analogously to (CH3CH2O)3Si-PEG2000-OCHH3 (PEG2000) as described previously.25 To a suspension of 1 equiv of amino-terminated PEG (0.30 mmol H2N-PEG3000-alkyne, Iris Biotech, Marktredwitz, Germany) in 4 to 5 mL of DMF under an argon atmosphere, 1.1 equiv of of 3-(triethoxysilyl)-propylisocyanate is added, and after stirring for 24 h at room temperature, the solution is cooled to 0 °C. An excess of diethyl ether is added, and the suspension is stirred for 1 h at 0 °C before filtering off the precipitate, washing it with cold diethyl ether, and drying under vacuum yields PEG-alkyne as a white powder. 1H NMR [300 MHz, CDCl3] δ 6.33 (s, 1H, CH2–C(=O)–NH), 5.60 (br s, 2H, NH–C(=O)–NH), 3.89–3.28 (m, 298H; O–CH2 and N–CH2), 3.15 (br s, 2H, Si–CH2–CH2–CH2), 2.51 (m, 2H, C(=O)–CH2–CH2), 2.39 (m, 2H, C(=O)–CH2–CH2), 2.01 (t, 4J = 2.6 Hz, 1H, CH), 1.63 (br s, 2H, Si–CH2–CH2), 1.22 (t, 3J = 7.0 Hz, 9H, CH3), 0.65 (br s, 2H, Si–CH2).
PEGylation of SiO2 Surfaces
All glass coverslips are cleaned for 1 h in freshly prepared piranha solution (3:1 H2SO4/H2O2 (30%)), rinsed thoroughly with water, sonicated three times for 3 min in fresh water, and dried in a stream of nitrogen. The silicon dioxide-coated QCM sensors are cleaned by immersing them in an aqueous sodium dodecyl sulfate solution (3%) for 1 h, rinsing thoroughly with water, and drying in a stream of nitrogen before treatment with oxygen plasma (TePla 100-E, 0.4 mbar, 150 W, 45 min). The quasi-hexagonal gold nanoparticles on glass substrates are prepared as described before using block polymer micelle nanolithography (BCML),22,23 and the surfaces are activated with oxygen plasma (TePla 100-E, 0.4 mbar, 150 W, 10 min). For the PEGylation reaction, substrates are immersed in a solution of 0.25 mM PEG (with the appropriate PEG-alkyne to PEG2000 ratio) and 25 μM dry triethylamine in dry toluene (dried over molecular sieves (3 Å)) and held at 80 °C overnight under a nitrogen atmosphere. The substrates are rinsed with ethyl acetate, sonicated for 5 min in ethyl acetate, rinsed with methanol, sonicated for 5 min in methanol, rinsed again with methanol, and dried in a stream of nitrogen.
General Protocol for CuAAC on Surfaces
For the click reaction with different azide-containing molecules, substrates are incubated with 150–250 μL of a freshly prepared aqueous reaction solution with 100 mM l-ascorbic acid, 100 mM Tris HCl (pH 8.5), 150 μM azide-R, and 1 mM CuSO4 for 2 h in a moisture chamber. Samples are washed with water or buffer solution and dried in a stream of nitrogen. Azides used in this study include 5/6-carboxyrhodamine 110-PEG3-azide (fluorophore-azide) for the measurements of surface fluorescence, 3-azidomethyl-5-iodopyridine for XPS measurements, K(N3)GGNGEPRGDTYRAYK(fluorescein)GG for the α-chymotrypsin assay, azide-PEG3-biotin (biotin-azide) for functionalization with streptavidin, Nα,Nα-bis(carboxymethyl)-l-azido-lysine hydrochloride (azide-NTA) for Cu XPS measurements, and cyc(RGDfE)K(N3) (azide-cRGD) and K(N3)PHSRN for cell adhesion studies.
Fluorescence Microscopy
Fluorescence imaging is carried out with an Olympus IX inverted microscope (Olympus, Hamburg, Germany) using a Delta Vision RT system (Applied Precision Inc., Issaquah, WA, USA) equipped with a cooled CCD camera (Photometrics, Kew, Australia), and processing is controlled by Resolve3D (Applied Precision Inc., Issaquah, WA, USA). Images are acquired using either a 10× (Neofluor 10×/0.3 phase contrast, Carl Zeiss, Jena, Germany) air lens or a 60× (PlanApo 60x/1.4 Olympus, Hamburg, Germany) oil-immersion lens. Fluorescence imaging of dual fluorescently labeled surfaces is carried out with a Leica DM6000B microscope (Leica Microsystems, Wetzlar, Germany) and a Leica DFC 365 FX camera. Image acquisition is performed with a 10× (HC PL APO 10×/0.40 Leica, Wetzlar, Germany) air lens, and processing is controlled by an LAS AF 3.1.0.
Chymotrypsin Assay
The ligand density on PEG-alkyne-modified surfaces is quantified by adopting a method established by Barber et. al.32 Briefly, glass substrates coated with varying mol % of PEG-alkyne are modified by the click reaction with a fluorescently labeled peptide, K(N3)GGNGEPRGDTYRAYK(fluorescein)GG, which contains a chymotrypsin digestion site. The coating on the surfaces is enzymatically digested with α-chymotrypsin by treating the substrate with 1.25 μg/mL α-chymotrypsin in 30 mM Tris HCl (pH 8.00), 50 mM CaCl2, and 10 μM HCl for 16 h at 30 °C. The surfaces that have been enzymatically digested and the untreated samples are mounted with Mowiol, and the fluorescence intensity on the surfaces is measured. The fluorescence intensity of the solution above the digested surface is measured with a microplate reader (Infinite M2000 microplate reader, TECAN, Männedorf, Switzerland), and the peptide concentration in solution is calculated by using solutions of the fluorescently labeled peptide with known concentrations
QCM (Quartz-Crystal Microbalance) Measurements
All QCM measurements are performed with a Q-Sense E4 System and sensors from Q-Sense (Västra Frölunda, Sweden). A flow rate of 10 μL/min and a temperature of 25 °C are used throughout all experiments.
To investigate nonspecific protein absorption on PEG-alkyne-modified QCM sensors, they are equilibrated with PBS and then exposed to increasing concentrations of BSA (0.01, 0.1, 1, and 5% BSA in PBS) for 15 min each, followed by 5 min rinsing steps with PBS.
QCM sensors, modified with different mol % of PEG-alkyne and subsequently clicked with an azide-PEG3-biotin conjugate, are also first equilibrated with PBS and then incubated with streptavidin in PBS (5 μg/mL) for 1 h. Subsequently, the sensors are rinsed again with PBS for about 30 min to remove unbound streptavidin.
Dual Functionalization of Gold Nanopatterned Surfaces with PEG-Alkyne
Gold nanopatterned surfaces are prepared by BCML22,23 as previously described with average particle spacings of 54 ±12 nm for the labeling with two different fluorophores and 100 ±13 nm for the cell experiments with synergy peptide PHSRN. For the former, a gold-loaded polymer micelle solution of 4 mg/mL polystyrene(110 000)-b-poly-2-vinylpyridine(70 500) (Polymer Source, Quebec, Canada) in o-xylene with a tetrachloroauric acid to vinylpyridine monomer ratio of 0.3 is used, and for the latter 2 mg/mL polystyrene(216 000)-b-poly-2-vinylpyridine(60 000) (Polymer Source, Quebec, Canada) in toluene with a tetrachloroauric acid to vinylpyridine monomer ratio of 0.3 is used. The surfaces are PEGylated following the procedure described above.
Labeling of Gold-Nanopatterned PEG-Alkyne Surfaces with Two Different Fluorophores
Azide-PEG3-biotin is clicked to a pure PEG-alkyne monolayer, and the surface is incubated with 250 μL of HS-C11-(EG)3-NTA (HS-NTA) (250 μM) and NiCl2 (250 μM) in Tris-NaCl buffer for 1 h at room temperature in a moisture chamber. After washing with Tris-NaCl buffer for about 5 min, the surface is incubated with 250 μL of Atto 565-streptavidin (5 μg/mL in Tris-NaCl buffer) for 1 h and washed once more. Finally, the surfaces are incubated with 250 μL of His6-eGFP (10 μM) in Tris-NaCl buffer for 1 h, washed with Tris-NaCl buffer, and mounted with Mowiol.
Dual Functionalization of Gold-Nanopatterned PEG-Alkyne Surfaces with cRGD and PHSRN
Gold nanopatterned surfaces coated with 10 mol % PEG-alkyne are clicked with the synergy peptide K(N3)PHSRN as described above. Subsequently, surfaces are incubated with c[RGDfK(3-mercaptopropionyl-aminohexanoic acid)] (cRGD-SH) (25 μM) in water for 2 h and then washed thoroughly with PBS three times for 10 min each.
Cell Culture and Immunofluorescence Staining
REF WT (rat embryonic fibroblasts, wild type) and REF YFP-pax (rat embryonic fibroblasts expressing YFP-paxillin) (kindly provided by B. Geiger, The Weizmann Institute of Science, Israel) are cultured in DMEM supplemented with 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin-streptamycin at 37 °C and 5% CO2. Cells are cultured in serum-free medium the previous day and during the experiments. Surfaces used in cell experiments are treated with 70% ethanol for 5 min at room temperature and/or washed three times with sterile PBS. Cells are seeded onto the samples at a density of ca. 5000 cells/cm2 and incubated for 4 h at 37 °C and 5% CO2. Cells are fixed with 4% paraformaldehyde (PFA) in PBS (pH 7.4) for 20 min at room temperature and washed several times with PBS. For permeabilization, samples are treated with 0.1 vol % Triton X-100 in PBS for 5 min at room temperature, followed by blocking with 1% BSA in PBS for about 10–30 min. Where appropriate, samples are incubated with mouse antivinculin IgG (1:200 dilution) and rabbit antipaxillin IgG (1:200 dilution) in PBS with 1% BSA for 1 h at room temperature. Following a washing step with PBS, cells are labeled with Alexa Fluor 488 goat antimouse IgG (5 μg/mL), Alexa Fluor 647 goat antirabbit IgG (5 μg/mL), and/or TRITC-conjugated phalloidin (2 μg/mL) in PBS with 1% BSA for 1 h. After a further washing step with PBS, samples are mounted in Mowiol containing DAPI (1 μg/mL).
All images are analyzed with ImageJ 1.45s (http://imagej.nih.gov/ij). The number of cells per mm2 is based on the number of nuclei in the DAPI channel, and the cell area is quantified from images of the actin staining. The errors in the number of cells per mm2 are given as the standard deviation of at least three independent experiments. The cell area is shown as a box plot, where each box is defined by the first and third quartiles of the data, the line in the box represents the median, x represents the mean, and the whiskers represent the 5th and 95th percentiles with outliers not shown. The statistical significance is evaluated using a nonparametric Mann–Whitney U test.
3. Results and Discussion
PEG-Alkyne Film Formation on Nanostructured Glass Surfaces
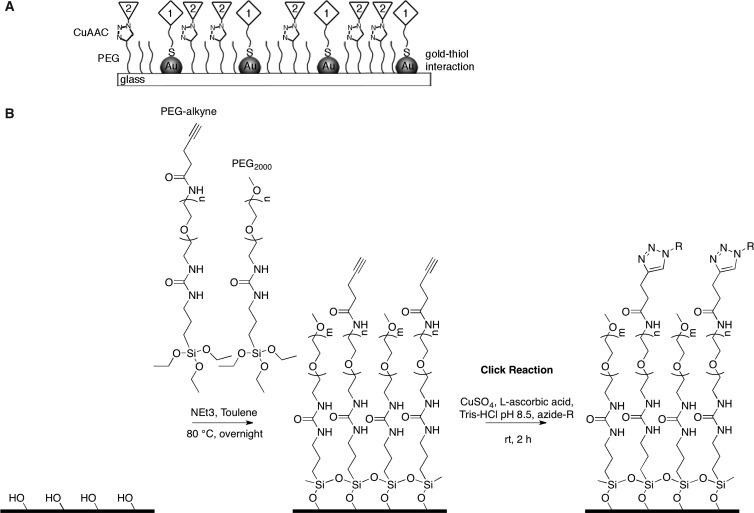
For the preparation of the dual-functionalized gold nanostructured surfaces presented in this study, we take advantage of the unique and orthogonal chemistries of glass–silane, gold–thiol, and the click reaction (Figure 1A). The clickable PEG-alkyne silane is coupled covalently with inert PEG2000 silane to the glass substrates, forming a self-assembled monolayer (SAM) (Figure 1B). In a second step, a molecule of choice with an azide group can be bound to the PEG-alkyne molecules via CuAAC, and in a final step, the gold nanoparticles can be modified with thiol-group-containing ligands. The newly developed PEG-alkyne functionalization offers several advantages: (i) the desired molecule is linked to the PEG layer in an oriented way, stereoselectively forming a 1,4-disubstituted 1,2,3-triazole; (ii) the PEG itself acts as a passivating layer between the substrate and the introduced functionality, preventing nonspecific adsorption; (iii) a large variety of different azides can be used to modify the layer depending on the desired application by the click reaction; and (iv) the density of the clickable PEG-alkyne on the surface can be adjusted by mixing the clickable PEG-alkyne with an inert PEG2000 in different ratios in the PEGylation reaction.
Figure 1.
(A) Schematic illustration of the dual-functionalized surfaces. The quasi-hexagonally arranged gold nanoparticles are functionalized with gold thiol chemistry (1), and the space between the particles is functionalized with copper(I)-catalyzed azide alkyne cycloaddition (CuAAC), also known as the click reaction, on PEG-alkyne (2). (B) Immobilization of silane-terminated PEG-alkyne and PEG2000 on glass substrates and the subsequent functionalization of PEG-alkyne with azides by using the click reaction.
Functionalization of PEG-Alkyne Films by Cooper(I)-Catalyzed Azide Alkyne Cycloaddition
To first demonstrate that PEG-alkyne coated surfaces can be efficiently modified with azides and that the modification density can be tuned, surfaces with various ratios of PEG-alkyne to PEG2000 (mole % PEG-alkyne = 0, 25, 50, 75 and 100) are clicked with an azide-group-containing small fluorescent molecule, 5/6-carboxyrhodamine 110-PEG3-azide, and the fluorescence on the surface is quantified. As seen in the fluorescence measurements, the number of immobilized fluorophores correlates directly with the ratio of PEG-alkyne to PEG2000 in the PEGylation reaction, and the signal increases with increasing mol % of PEG-alkyne and shows saturation with increasing PEG-alkyne content (Supporting Information, Figure S1). To exclude the possibility that the click reaction is incomplete at high surface densities under the reaction conditions, surfaces with pure PEG-alkyne are first reacted with the nonfluorescent biotin-azide and then reacted again with a fresh reaction solution containing the small molecule fluorophore-azide (Supporting Information, Figure S2). Comparing the fluorescence intensity on this surface with a surface that is solely modified with the small molecule fluorophore-azide demonstrates that 99% of the modifiable surface alkynes are already modified after the first reaction (assuming a linear relationship between fluorescence intensity on the surface and immobilized fluorophore). Additionally, the modification of the PEG monolayer at the surface with the click reaction is confirmed by XPS using 3-azidomethyl-5-iodopyridine as the coupling reagent in order to use the iodine as an XPS marker. We observe that the I 5d signal intensity depends linearly on the PEG-alkyne content of the film and is not observed on the PEG2000-coated surfaces, while for the C 1s signal a larger shoulder at lower binding energy is observed with increasing PEG-alkyne content after the click reaction (Supporting Information, Figure S3A,B). XPS measurements in the N 1s region on a pure PEG-alkyne surface that is modified with the small molecule 3-azidomethyl-5-iodopyridine shows a shoulder at higher binding energy indicating the formation of the triazole ring33 on the surface compared to unmodified surfaces (Supporting Information, Figure S3C). The N 1s signal is fitted for four different nitrogen species (398.9, 399.7, 401.4, and 402.5 eV) and from the relative areas of the peaks the conversion percentage of the surface alkynes is calculated to be about 74%. At the same time, the Si 2p signals for surfaces of diverse PEG compositions show approximately the same intensity (Supporting Information, Figure S3D), demonstrating that the thickness of the PEG layer is nearly the same for all compositions, about 2.15 nm as determined in an earlier study for pure PEG2000 films.25 Furthermore, the modification density for different PEG-alkyne compositions is quantified in more detail by coupling a peptide with an α-chymotrypsin enzymatic digestion site between an azide and a fluorescent group, K(N3)GGNGEPRGDTYRAYK(fluorescein)GG, to the PEG films. As observed with the azide-containing small fluorescent molecule (Supporting Information, Figure S1), the fluorescence intensity on the surface correlates with the mol % of PEG-alkyne used in the PEGylation reaction, i.e., with the extent of immobilized fluorescently labeled peptide, and also shows saturation with increasing PEG-alkyne (Figure 2). Subsequently, these surfaces are treated with α-chymotrypsin, thereby releasing the fluorophore-bearing part of the peptide into the reaction solution. The fluorescence in solution is measured, and the concentration is quantified using digested fluorescently labeled peptide solutions of known concentrations to calculate the peptide density on the surface before the digestion with chymotrypsin. Additionally, after the enzymatic digestion, the fluorescence signal on the surfaces is at background level for different mol % of PEG-alkyne, showing that the peptide digestion is quantitative. These results show that we can adjust the modification density from 0 to 38.4 pmol/cm2 by increasing the relative PEG-alkyne concentration in the PEGylation reaction. The fluorescence intensity on the surfaces shows saturation with rising mol % of PEG-alkyne, which could be due to either steric hindrance at high functionalization densities or local quenching caused by a high surface concentration of the dye.
Figure 2.
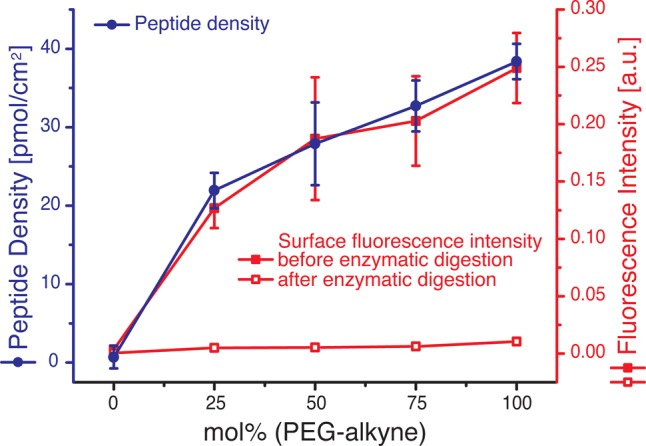
Click reactions on PEG-alkyne modified surfaces with various PEG-alkyne mol % fractions. Determination of the coupled peptide density with different ratios of PEG-alkyne and PEG2000 by enzymatic digestion through α-chymotrypsin. The peptide density is quantified by fluorescence intensity measurements in solution after the release of a formerly surface-bound fluorophore-bearing part of the peptide (filled circles, left y axis, blue). In addition, fluorescence intensities versus the mol % of PEG-alkyne on the surfaces before (filled squares, right y axis, red) and after enzymatic digestion (empty squares, right y axis, red) are illustrated. The error bars are the standard deviations of at least three independent experiments.
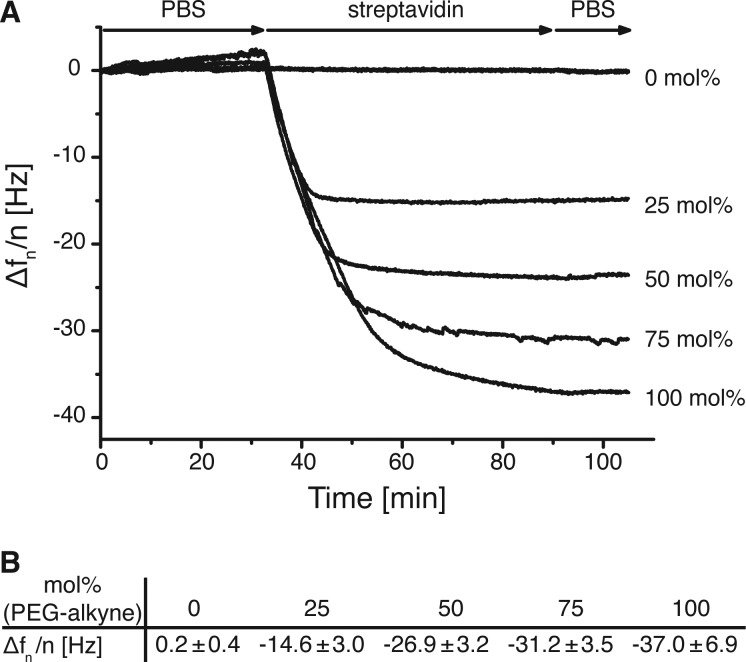
The PEG-alkyne coating allows for the attachment of many different azides, including spectroscopically active small molecules and peptides, as shown above. Another frequently used method for the immobilization of a broad class of biotinylated molecules is based on the stable interaction between biotin and streptavidin,11 and this interactions has already been used to fabricate dual functionalized nanopaterned surfaces similar to the ones described here.8,9 This interaction can also be used to further broaden the scope of possible modifications of the PEG-alkyne surfaces by first clicking a biotin-azide, which can subsequently react with streptavidin and further biotinylated molecules. The binding of streptavidin to PEG-alkyne-coated surfaces modified with biotin-azide is shown for different PEG compositions (Figure 3). While the PEG2000-coated QCM sensor, which is subjected to the click reaction conditions, does not show any streptavidin adsorption, the biotin-modified PEG-alkyne QCM sensors bind different amounts of streptavidin depending on the PEG-alkyne mol %.
Figure 3.
(A) Exemplary QCM curves of the fifth overtone showing the binding of streptavidin to biotinylated PEG monolayers. QCM crystals are PEGylated with different mol % of clickable PEG-alkyne and clicked with biotin-azide prior to the measurements. The QCM crystals are equilibrated with PBS for 30 min, incubated with 5 μg/mL streptavidin in PBS for 1 h, and subsequently rinsed with PBS for 30 min to remove unbound streptavidin. (B) Frequency change for surfaces of different compositions after streptavidin binding. The errors are the standard deviation from at least three independent experiments.
Passivating Properties of PEG-Alkyne Films
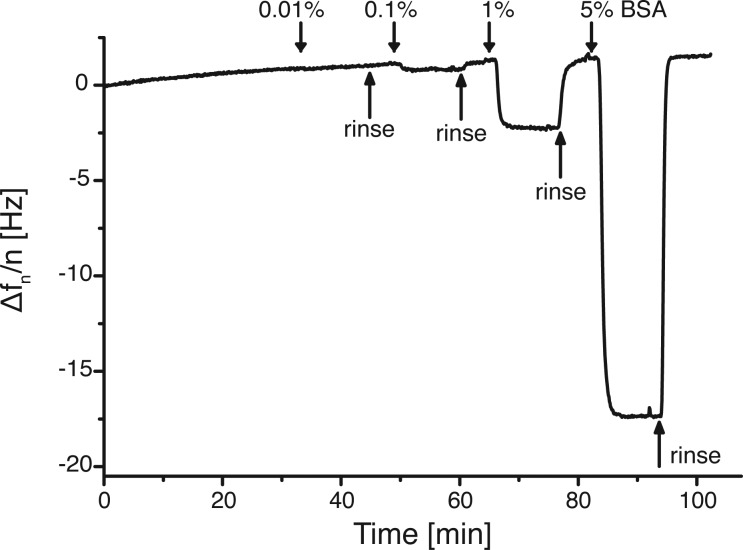
Besides control over the functionalization density and versatile modification possibilities of the PEG-alkyne, its protein-repellent properties and its passivating characteristics against nonspecific protein adsorption are evaluated. For this, increasing concentrations of BSA are passed over PEG-alkyne-modified QCM sensors, and changes in frequency are monitored on the QCM (Figure 4). While with increasing concentrations of BSA more distinct frequency changes are temporarily observed, the immediate return to the initial frequency with PBS washes clearly shows that the protein is not adsorbed and that these frequency changes are caused only by significant changes in the medium’s viscosity due to the high protein concentrations. The results for PEG-alkyne monolayers are similar to the QCM curves observed for PEG2000, which we reported in an earlier study.25
Figure 4.
QCM curve illustrating the frequency change upon addition of different amounts of BSA in PBS for a PEG-alkyne-coated SiO2 sensor surface. The 11th overtone is presented.
To verify that the copper ions utilized catalytically in the click reaction do not adsorb significantly to the surfaces and cause cell toxicity, the remaining copper on the surfaces is investigated by XPS (Supporting Information, Figure S4). While we are not able to detect any copper signal on PEGylated surfaces before and after subjecting them to click reaction conditions without any azide, a very low Cu signal is detected on surfaces that are modified with cRGD or NTA. A PEG-alkyne monolayer modified with an NTA/Cu2+ complex, for which a clear Cu XPS signal is observed, is used as a positive control. From the XPS measurements, we concluded that only very low concentrations of copper remain on the surfaces after the click reaction and that these copper ions are of minor importance for subsequent cell adhesion.
Another type of PEG coating for glass that is commonly used to prevent nonspecific interactions is PLL-g-PEG (poly-l-lysine-graft-poly(ethylene glycol)), which physisorbs on glass based on electrostatic interactions. To date, a number of variants of PLL-g-PEGs with biotin,8 azide,30 or peptide14 modification to introduce specific interactions have been reported. The here-presented PEG-alkyne thus provides a complementary platform for glass functionalization in cases where a covalent attachment to the glass is desirable and for cases where the azide is on the molecule to be attached.
Integrin-Mediated Cell Adhesion to cRGD on PEG-Alkyne Surfaces
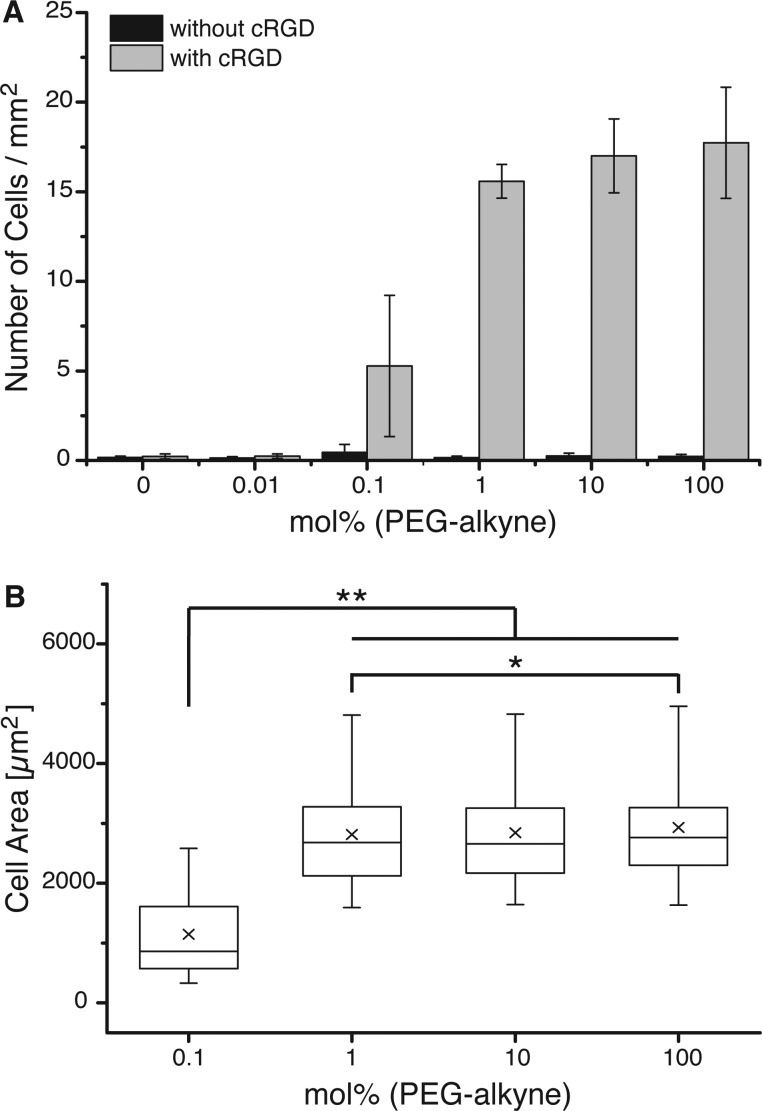
The protein-repellent properties of PEG-alkyne monolayers in combination with the possibility to attach specific chemical modifications at controlled densities make this an attractive platform to study specific cell interactions. As a model system, we investigate the adhesion of fibroblasts to glass surfaces with varying PEG-alkyne densities before and after functionalization with cRGD. Surfaces with 0, 0.01, 0.1, 1, 10, and 100 mol % PEG-alkyne are functionalized with azide-cRGD in the presence of the copper catalyst. An equal number of rat embryonic fibroblasts expressing YFP-paxillin (REF YFP-pax) are seeded on each surface before and after the click reaction. The number of adherent cells as well as their spreading area after 4 h is quantified as characteristics of cell-adhesion behavior. Before the click reaction, almost no cells adhere to the nonfunctionalized PEG monolayers of different PEG-alkyne/PEG2000 ratios, indicating good inertness against nonspecific adhesion (Figure 5A). The number of adherent cells on the cRGD-functionalized PEG-alkyne monolayers significantly depends on the mol % of PEG-alkyne, i.e., on the cRGD density. Some cells adhere to surfaces with 0.1 mol % PEG-alkyne, but cells are not able to adhere to surfaces with even lower PEG-alkyne densities. On the other hand, at a PEG-alkyne density of 1 mol % or higher the coating provides sufficient anchor points for cell adhesion and the number of adherent cells increases only slightly with additional PEG-alkyne content in the film. The spreading area of the cells shows a similar trend to the number of cells that adhere on the surfaces; the spreading area of cells on 0.1 mol % PEG-alkyne surfaces is only half as large as the spreading area of cells on 1 mol %, 10 mol %, and pure PEG-alkyne coatings (Figure 5B). Therefore, we conclude that 1 mol % PEG-alkyne with cRGD modification is sufficient to ensure stable cell adhesion and spreading for REF YFP-pax.
Figure 5.
(A) Number of cells per mm2 and (B) spreading area of REF YFP-pax on PEG monolayers with different mol % of PEG-alkyne functionalized with cyclic RGD. Data are evaluated after 4 h of adhesion by means of fluorescence staining of cell nuclei (DAPI) and actin (phalloidin-TRITC). *p < 0.003 and **p < 0.0001.
There are numerous reports of cell adhesion on various RGD-modified substrates with varying densities that emphasize how sensitive the cell behavior is to both the cell type and the ligand presentation.11−14,29,34 Chemically well-defined SAMs on gold surfaces are used extensively to study cell adhesion as they can provide an inert background and can be tailored specifically with a number of signaling molecules. In cell adhesion studies on SAMs on gold, Hudalla et al. show that 0.1 mol % RGD modification in the SAM is sufficient for human mesenchymal stem cells (hMSC) to spread and that with increased RGD concentrations the number of cells that adhere to the film as well as their spreading area increases.34 Likewise, in a study looking at the adhesion behavior of HeLa cells on PLL-g-PEG-RGD-coated glass surfaces, cell adhesion is observed starting at 52 nm average RGD spacing and maximal attachment was observed at 10 nm average RGD spacing.12 Our results with SAMs on glass surfaces show quite parallel behavior to these observations; surfaces with 0.1 mol % cRGD modification are sufficient to provide cell attachment, and weak spreading and a higher number of adherent cells with a twice as large spreading area is observed with cRGD densities above 1 mol %. On the basis of the peptide density on the surface (as determined with the chymotrypsin digestion assay using the density for the 25 mol % PEG-alkyne surface), the average distance between two cRGDs is about 47 and 15 nm at 0.1 and 1 mol % PEG-alkyne, respectively. Results of previous studies from our group on gold nanopatterned surfaces show that a cRDG spacing of 58 nm or lower is required for the stable adhesion and spreading of REF cells.26,27 This slight difference can be due to the statistical distribution of the cRGD-functionalized PEG-alkyne molecules in the SAMs and better control over the spatial cRGD modification on the nanostructured surfaces.
Orthogonal Fluorophore Labeling of Gold Nanostructures and PEG-Alkyne Coated Glass Surfaces
To obtain dual-functionalized surfaces, we prepare quasi-hexagonal gold nanopatterns by BCML on glass surfaces and coat the glass between the nanoparticles with the clickable PEG-alkyne. The gold nanoparticles and the PEG-alkyne can be orthogonally functionalized by gold–thiol chemistry and the click reaction, respectively (Figure 6A). To demonstrate this, we couple two different fluorescently labeled molecules to the PEG-alkyne and the gold nanoparticles; the PEG-alkyne is clicked with a biotin-azide following the binding of an Atto 565 fluorescently labeled streptavidin, and the gold nanoparticles are modified with an NTA-thiol conjugate, which is subsequently bound to a His6-eGFP using NTA/Ni2+ chemistry. The orthogonal functionalization can be visualized under a fluorescence microscope on a surface that is partially nanostructured. As can be seen in the line profiles, the fluorophore coupled to the PEG-alkyne layer, visualized in the red channel, is distributed homogeneously over the whole sample, but the fluorescent protein attached to the gold nanoparticles, visualized in the green channel, is present only in the nanostructured area (Figure 6B).
Figure 6.
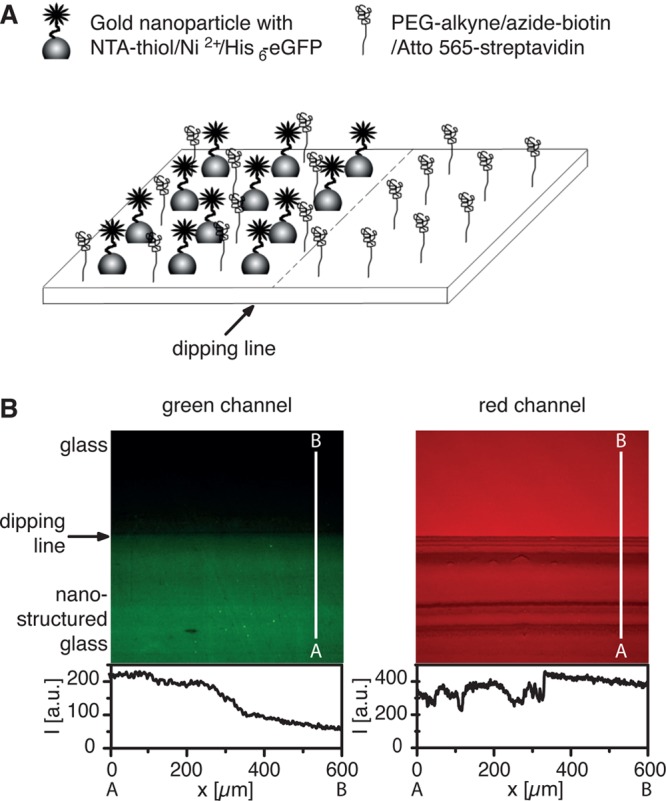
(A) Scheme for the dual functionalization of partially nanostructured and PEG-alkyne coated surfaces. (B) Fluorescent images of dual-functionalized gold nanopatterns and PEG-alkyne on glass surfaces. The PEG-alkyne monolayer is first modified with a biotin-azide via the click reaction and then coupled to Atto 565-labeled streptavidin. The gold nanoparticles are modified with an NTA-thiol conjugate and coupled to a His6-tagged eGFP through NTA/Ni2+ chemistry. As can be seen in the line profiles from A to B, the PEG-alkyne coupled fluorophore, visualized in the red channel, is distributed homogeneously over the sample (right) whereas the fluorophore linked to gold dots, visualized in the green channel, is present only in the nanostructured area (left).
Effect of the Synergy Peptide PHSRN on Integrin-Mediated Cell Adhesion to cRGD
The dual functionalization of gold nanoparticles and PEG-alkyne on glass surfaces allows for the presentation of two different bioactive molecules to the cell not only with a defined chemical composition but also with a defined spatial organization. This enables us to study both the mutual influence that these signaling molecules have on the cellular response and the role of the spacing of signaling molecules. The peptide PHSRN derived from the ninth type III domain in fibronectin is also known as the synergy peptide17 because it enhances cell spreading in the presence of the RGD motif.4,20,21 Here, we investigate how the additional presentation of synergy peptide PHSRN affects the integrin-mediated adhesion of REF (rat embryonic fibroblasts) cells through cRGD motifs. In earlier work from our group, we showed that REF cells cannot adhere to surfaces when the spacing of the gold nanoparticles is larger than 85 nm because the integrin clusters required for focal adhesions cannot form.26 Here, gold nanopatterned surfaces with 100 nm spacing (Supporting Information, Figure S5) are functionalized with synergy peptide K(N3)PHSRN between particles at PEG-alkyne (10 mol % PEG-alkyne) by the click reaction and with cRGD at the gold nanoparticles by gold thiol chemistry (Figure 7A). For comparison, control surfaces with just one of the two modifications are also used, and an equal number of REF WT cells are seeded on all three surfaces. After 4 h, the density of cells that adhered on the surfaces and their spreading area are quantified, and focal adhesions are looked at by vinculin and paxilin staining to evaluate the cell adhesion to the surfaces. Although REF WT cells do not adhere on substrates functionalized solely with synergy peptide PHSRN and only very few cells adhere to the surfaces solely functionalized with the cRGD at these low densities, REF WT cells do adhere well on substrates presenting both signaling molecules, cRGD and PHSRN (Figure 7B). Parallel to this observation, cells have a smaller spreading area on substrates that are functionalized only with cRGD compared to surfaces functionalized with both cRGD and PHSRN (Figure 7C). The spreading area observed for cells on the dual-functionalized surfaces is also similar to the spreading areas observed on homogeneous cRGD-modified surfaces at high densities in Figure 5B. Cells on surfaces presenting both signaling molecules also develop mature focal adhesions as can be observed by the vinculin and paxilin staining and the well-developed actin fibers characteristic of adhered fibroblasts (Figure 7D). On the other hand, such mature focal contacts cannot be observed in cells on substrates functionalized only with cRGD. Thus, using the developed dual-functionalized substrates, it can be demonstrated that the presence of the synergy peptide influences the focal adhesion assembly significantly and that each individual signaling molecule is not sufficient to mediate cell adhesion at the given densities.
Figure 7.
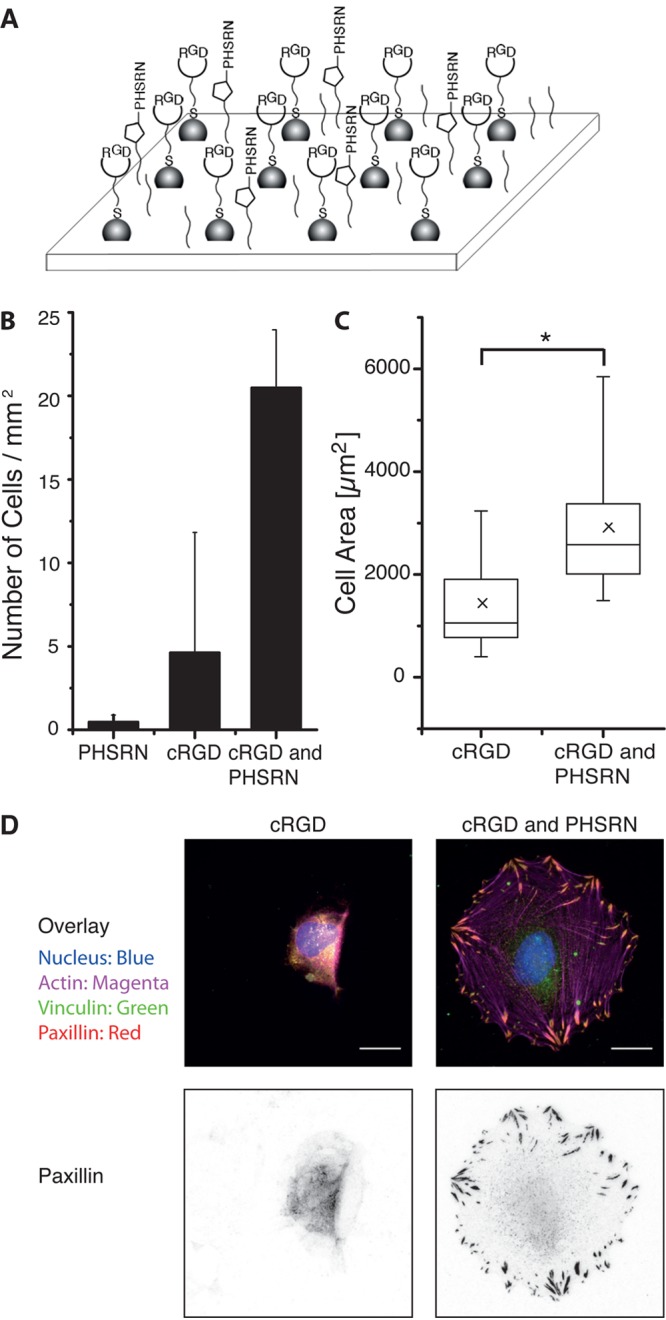
(A) Scheme for the dual functionalization with adhesion peptide cRGD and synergy peptide PHSRN. (B) Density of adherent REF WT cells on substrates with gold nanoparticles and PEG-alkyne (10 mol %) functionalized with adhesion peptide cRGD and/or synergy peptide PHSRN. The average distance of the gold nanoparticles is 100 nm. (C) The spreading area of cells on cRGD- or cRGD- and PHSRN-functionalized surfaces. (*p < 0.0001) (D) Fluorescent images of adherent cells on substrates functionalized with cRGD or cRGD and PHSRN. The nucleus is shown in blue, actin in magenta, vinculin in green, and paxillin in red. The vinculin and paxilin stains colocalize. In particular, the inverted paxilin staining (right) demonstrates the formation of mature focal adhesions on the bifunctional surfaces. The scale bar is 20 μm.
Synergy peptide PHSRN derived from the ninth type III domain in fibronectin enhances cell spreading on RGD-functionalized substrates but rarely supports cell adhesion on its own and cannot support cell spreading.18,19 It is still controversial if PHSRN and RGD bind synergistically to the same integrin receptor or if the two peptides bind competitively to the same binding site. Recent work on dual-functionalized materials with these two peptides immobilized statistically has shown that the total density of the peptides and the ratio of the two peptides determine if a synergistic or a competitive effect is observed.4,20 The PHSRN peptide synergistically enhances the cell adhesion when present at equal concentrations with RGD, but at high RGD densities, the presence of the synergy peptide has a negative effect on cell adhesion. While these and other studies with RGD-/PHSRN-modified surfaces suggest a cross-talk between these two adhesion peptides, the functionalization is statistically distributed and the spacing between the RGD ligands is not controlled. In our study, we show that the synergy peptide not only allows the adhesion and spreading of cells on surfaces with low cRGD densities but also changes the spatial requirements for integrin binding to the cRGD. When using the gold nanopatterns, the clustering of the integrin is determined by the spacing of the gold nanoparticles because the size of the nanoparticle (<8 nm) allows for only one integrin to bind to one gold nanoparticle. In accordance with observations in earlier studies, REF cells are not able to adhere to surfaces with 100 nm particle spacing where the gold nanoparticles are modified with cRGD and the space in between the particles is passivated with PEG2000. When we use the same modified surfaces and click the synergy peptide to a film containing 10 mol % PEG-alkyne, the REF cells adhere well to the surface, spread, and also form mature focal adhesions. Thus, we conclude that in the presence of the synergy peptide, cells can form stable focal contacts even on substrates where the spacing between integrin ligand cRGD molecules is 100 nm. In future studies, we intend to study integrin clustering in the presence of different synergy peptide concentrations and particle spacings (and corresponding cRGD densities) in greater detail. This platform is not limited to the study of cell adhesion and can be used to study the crosstalk between cell–matrix, cell–cell, and cell–growth factor signaling. The effect of ligand spacing for a signaling molecule immobilized on the gold nanoparticles can be investigated while making the space between the particles adhesive, or the effect of an immobilized signaling molecule on adhesion receptor clustering can be investigated by placing the adhesion molecule on the gold nanoparticles.
4. Conclusions
We establish here a new platform to make dual-functionalized surfaces with defined spatial arrangements to study cell adhesion. For this, we develop a surface coating, PEG-alkyne, for glass substrates that can be conveniently modified with a large number of azide-containing molecules including spectroscopically active small molecules, peptides, and biotin by the click reaction. The modification density can be tuned from 0 to 38.4 pmol/cm2 by forming mixed films with a nonfunctional analogue, PEG2000. The resulting PEG-alkyne coatings are inert against nonspecific protein adsorption and nonspecific cell adhesion. The coating is suited to study specific cell responses to varying signaling molecule densities, as shown in adhesion studies with REF YFP-pax cells. These cells start to adhere to surfaces when the 0.1 mol % PEG film is modified with adhesion peptide cRGD and the number of adherent cells and spreading areas reach saturation at modification densities higher than 1 mol %. The dual-functionalized surfaces are generated by combining the PEG-alkyne coating with hexagonally arranged gold nanoparticle arrays on glass. The surfaces can be orthogonally functionalized with two molecules: the first molecule is introduced by the click reaction, and the second molecule, by gold thiol chemistry. On surfaces presenting adhesion peptide cRGD attached to the gold nanoparticles with 100 nm spacing and synergy peptide PHSRN bound to 10 mol % PEG-alkyne, REF cells adhere and spread. In contrast, cells do not adhere to surfaces where only one of the two modifications is present. Therefore, we can conclude that the focal adhesion assembly is altered in the presence of the synergy peptide. The dual-functionalized surfaces can also be used to investigate the crosstalk and spatial requirements for processes with two signaling molecules, which involve adhesion and another signaling event by either presenting the adhesion molecule on the nanoparticles and the second signaling molecule in the space between the nanoparticles or vice versa.
Acknowledgments
We thank the Max-Planck Society for financial support. S.V.W. thanks the Alexander von Humboldt Foundation for a postdoctoral fellowship. Part of this research was supported by contract research Glykobiologie/Glykomik of the Baden-Württemberg Stiftung. J.P.S. is the Weston visiting professor at the Weizmann Institute of Science. The research group is part of excellence cluster CellNetworks at the University of Heidelberg. We thank Dr. A. Cavalcanti-Adam, Dr. C. Cobley, Y. Schön, and M. Danner for support. This work was part of the ERC Advanced Grant SynAd under grant agreement no. 294852-SynAd.
Supporting Information Available
Materials and experimental details on XPS. Surface fluorescence intensity and I, N, C, Si, and Cu XPS measurements on PEG-alkyne surfaces. Fluorescence intensity data on the reaction efficiency of CuAAC. SEM image of gold nanopatterns. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Geiger B.; Spatz J. P.; Bershadsky A. D. Environmental Sensing Through Focal Adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [DOI] [PubMed] [Google Scholar]
- Azagarsamy M. A.; Anseth K. S. Bioorthogonal Click Chemistry: an Indispensable Tool to Create Multifaceted Cell Culture Scaffolds. ACS Macro Lett. 2013, 2, 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F.; Zheng J.; Yu J.; Zhou J.; Becker M. L. Cascading “Triclick” Functionalization of Poly(Caprolactone) Thin Films Quantified via a Quartz Crystal Microbalance. Biomacromolecules 2013, 14, 2857–2865. [DOI] [PubMed] [Google Scholar]
- Ochsenhirt S. E.; Kokkoli E.; McCarthy J. B.; Tirrell M. Effect of RGD Secondary Structure and the Synergy Site PHSRN on Cell Adhesion, Spreading and Specific Integrin Engagement. Biomaterials 2006, 27, 3863–3874. [DOI] [PubMed] [Google Scholar]
- Hudalla G. A.; Murphy W. L. Chemically Well-Defined Self-Assembled Monolayers for Cell Culture: Toward Mimicking the Natural ECM. Soft Matter 2011, 7, 9561–9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudalla G. A.; Murphy W. L. Immobilization of Peptides with Distinct Biological Activities Onto Stem Cell Culture Substrates Using Orthogonal Chemistries. Langmuir 2010, 26, 6449–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.; Zheng J.; Amond E. F.; Stafford C. M.; Becker M. L. Facile Fabrication of “Dual Click” One- and Two-Dimensional Orthogonal Peptide Concentration Gradients. Biomacromolecules 2013, 14, 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcassian D.; Depoil D.; Rudnicka D.; Liu M.; Davis D. M.; Dustin M. L.; Dunlop I. E. Nanoscale Ligand Spacing Influences Receptor Triggering in T Cells and NK Cells. Nano Lett. 2013, 13, 5608–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Depoil D.; Palma M.; Sheetz M. P.; Dustin M. L.; Wind S. J. Bifunctional Nanoarrays for Probing the Immune Response at the Single-Molecule Level. J. Vac. Sci. Technol., B 2013, 31, 6F902–1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersel U.; Dahmen C.; Kessler H. RGD Modified Polymers: Biomaterials for Stimulated Cell Adhesion and Beyond. Biomaterials 2003, 24, 4385–4415. [DOI] [PubMed] [Google Scholar]
- Lagunas A.; Comelles J.; Martínez E.; Prats-Alfonso E.; Acosta G. A.; Albericio F.; Samitier J. Cell Adhesion and Focal Contact Formation on Linear RGD Molecular Gradients: Study of Non-Linear Concentration Dependence Effects. Nanomedicine 2012, 8, 432–439. [DOI] [PubMed] [Google Scholar]
- Orgovan N.; Peter B.; Bősze S.; Ramsden J. J.; Szabó B.; Horvath R. Dependence of Cancer Cell Adhesion Kinetics on Integrin Ligand Surface Density Measured by a High-Throughput Label-Free Resonant Waveguide Grating Biosensor. Sci. Rep. 2014, 4, 4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. W.; Hu B.-H.; Delatore S. M.; Garcia A. S.; Messersmith P. B.; Miller W. M. Lipopeptides Incorporated Into Supported Phospholipid Monolayers Have High Specific Activity at Low Incorporation Levels. J. Am. Chem. Soc. 2004, 126, 15223–15230. [DOI] [PubMed] [Google Scholar]
- VandeVondele S.; Voros J.; Hubbell J. A. RGD-Grafted Poly-L-Lysine-Graft-(Polyethylene Glycol) Copolymers Block Non-Specific Protein Adsorption While Promoting Cell Adhesion. Biotechnol. Bioeng. 2003, 82, 784–790. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G.; Ruoslahti E. Integrin Signaling. Science 1999, 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Haubner R.; Gratias R.; Diefenbach B.; Goodman S. L.; Jonczyk A.; Kessler H. Structural and Functional Aspects of RGD-Containing Cyclic Pentapeptides as Highly Potent and Selective Integrin Aνβ3 Antagonists. J. Am. Chem. Soc. 1996, 118, 7461–7472. [Google Scholar]
- Redick S. D.; Settles D. L.; Briscoe G.; Erickson H. P. Defining Fibronectin’s Cell Adhesion Synergy Site by Site-Directed Mutagenesis. J. Cell Biol. 2000, 149, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y.; Mrksich M. The Synergy Peptide PHSRN and the Adhesion Peptide RGD Mediate Cell Adhesion Through a Common Mechanism. Biochemistry 2004, 43, 15811–15821. [DOI] [PubMed] [Google Scholar]
- Eisenberg J. L.; Piper J. L.; Mrksich M. Using Self-Assembled Monolayers to Model Cell Adhesion to the 9th and 10th Type III Domains of Fibronectin. Langmuir 2009, 25, 13942–13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Choi I.; Yeo W.-S. Preparation of Gradient Surfaces by Using a Simple Chemical Reaction and Investigation of Cell Adhesion on a Two-Component Gradient. Chemistry 2013, 19, 5609–5616. [DOI] [PubMed] [Google Scholar]
- Chen X.; Sevilla P.; Aparicio C. Surface Biofunctionalization by Covalent Co-Immobilization of Oligopeptides. Colloids Surf., B 2013, 107, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz J. P.; Mössmer S.; Hartmann C.; Möller M.; Herzog T.; Krieger M.; Boyen H.-G.; Ziemann P.; Kabius B. Ordered Deposition of Inorganic Clusters From Micellar Block Copolymer Films. Langmuir 2000, 16, 407–415. [Google Scholar]
- Lohmüller T.; Aydin D.; Schwieder M.; Morhard C.; Louban I.; Pacholski C.; Spatz J. P. Nanopatterning by Block Copolymer Micelle Nanolithography and Bioinspired Applications. Biointerphases 2011, 6, MR1–MR12. [DOI] [PubMed] [Google Scholar]
- Kenausis G. L.; Vörös J.; Elbert D. L.; Huang N.; Hofer R.; Ruiz-Taylor L.; Textor M.; Hubbell J. A.; Spencer N. D. Poly(L-Lysine)- G-Poly(Ethylene Glycol) Layers on Metal Oxide Surfaces: Attachment Mechanism and Effects of Polymer Architecture on Resistance to Protein Adsorption. J. Phys. Chem. B 2000, 104, 3298–3309. [Google Scholar]
- Blümmel J.; Perschmann N.; Aydin D.; Drinjakovic J.; Surrey T.; Lopez-Garcia M.; Kessler H.; Spatz J. P. Protein Repellent Properties of Covalently Attached PEG Coatings on Nanostructured SiO2-Based Interfaces. Biomaterials 2007, 28, 4739–4747. [DOI] [PubMed] [Google Scholar]
- Arnold M.; Cavalcanti-Adam E. A.; Glass R.; Blümmel J.; Eck W.; Kantlehner M.; Kessler H.; Spatz J. P. Activation of Integrin Function by Nanopatterned Adhesive Interfaces. ChemPhysChem 2004, 5, 383–388. [DOI] [PubMed] [Google Scholar]
- Cavalcanti-Adam E. A.; Micoulet A.; Blümmel J.; Auernheimer J.; Kessler H.; Spatz J. P. Lateral Spacing of Integrin Ligands Influences Cell Spreading and Focal Adhesion Assembly. Eur. J. Cell Biol. 2006, 85, 219–224. [DOI] [PubMed] [Google Scholar]
- Arnold M.; Schwieder M.; Blümmel J.; Cavalcanti-Adam E. A.; Lopez-Garcia M.; Kessler H.; Geiger B.; Spatz J. P. Cell Interactions with Hierarchically Structured Nano-Patterned Adhesive Surfaces. Soft Matter 2009, 5, 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant N. D.; Lavery K. A.; Amis E. J.; Becker M. L. Universal Gradient Substrates for “Click” Biofunctionalization. Adv. Mater. 2007, 19, 965–969. [Google Scholar]
- van Dongen S. F. M.; Maiuri P.; Marie E.; Tribet C.; Piel M. Triggering Cell Adhesion, Migration or Shape Change with a Dynamic Surface Coating. Adv. Mater. 2013, 25, 1687–1691. [DOI] [PubMed] [Google Scholar]
- Kinnane C. R.; Wark K.; Such G. K.; Johnston A. P. R.; Caruso F. Peptide-Functionalized, Low-Biofouling Click Multilayers for Promoting Cell Adhesion and Growth. Small 2009, 5, 444–448. [DOI] [PubMed] [Google Scholar]
- Barber T. A.; Harbers G. M.; Park S.; Gilbert M.; Healy K. E. Ligand Density Characterization of Peptide-Modified Biomaterials. Biomaterials 2005, 26, 6897–6905. [DOI] [PubMed] [Google Scholar]
- Gouget-Laemmel A. C.; Yang J.; Lodhi M. A.; Siriwardena A.; Aureau D.; Boukherroub R.; Chazalviel J. N.; Ozanam F.; Szunerits S. Functionalization of Azide-Terminated Silicon Surfaces with Glycans Using Click Chemistry: XPS and FTIR Study. J. Phys. Chem. C 2012, 117, 368–375. [Google Scholar]
- Hudalla G. A.; Murphy W. L. Using “Click” Chemistry to Prepare SAM Substrates to Study Stem Cell Adhesion. Langmuir 2009, 25, 5737–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.