Abstract
BACKGROUND
The intervention of advanced prostate cancer (PCa) in patients has been commonly depending on androgen deprivation therapy. Despite of tremendous research efforts, however, molecular mechanisms on AR regulation remain poorly understood, particularly for castration resistant prostate cancer (CRPC). Targeting AR and associated factors is considered an effective strategy in PCa treatment.
METHODS
Human prostate cancer cells were used in this study. Manipulations of Skp2 expression were achieved by Skp2 shRNA/siRNA or overexpression of plasmids. Dual luciferase reporter assay was applied for AR activity assessment. Western blot, ubiquitination assay, immunoprecipitation, and immunofluorescence were applied to detect the proteins.
RESULTS
Our results demonstrated that Skp2 directly involves the regulation of AR expression through ubiquitination-mediated degradation. Skp2 interacted with AR protein in PCa cells, and enforced expression of Skp2 resulted in a decreased level and activity of AR. By contrast, Skp2 knockdown increased the protein accumulation and activity of AR. Importantly, changes of AR contributed by Skp2 led to subsequent alterations of PSA level in PCa cells. AR ubiquitination was significantly increased upon Skp2 overexpression but greatly reduced upon Skp2 knockdown. AR mutant at K847R abrogated Skp2-mediated ubiquitination of AR. NVP-BEZ235, a dual PI3K/mTOR inhibitor, remarkably inhibited Skp2 level with a striking elevation of AR.
CONCLUSIONS
The results indicate that Skp2 is an E3 ligase for proteasome-dependent AR degradation, and K847 on AR is the recognition site for Skp2-mediated ubiquitination. Our findings reveal an essential role of Skp2 in AR signaling.
Keywords: Skp2, androgen receptor (AR), ubiquitination, prostate cancer (PCa)
INTRODUCTION
Prostate cancer (PCa) remains the second leading cause of cancer-related deaths among men in the U.S. and Western countries. Androgen deprivation therapy (ADT) is the standard chemotherapy for patients with advanced and metastatic PCa since it was first introduced by Huggins and Hodges [1], yet many patients suffered the relapse after a short period of remission. Therefore, we need to gain a more comprehensive and in-depth understanding of the mechanisms on the initiation, development, and progression of PCa in order to develop efficient chemotherapies. Androgen receptor (AR) plays an important role on the proliferation of prostate cells as well as the abnormal etiology in prostate tissue [2]. Meanwhile, AR as a nuclear receptor and transcription factor directly contributes to regulations of many genes essential for PCa. As a result, targeting AR or AR-associated factors is an effective strategy to control PCa in patients. Recently, several E3 ligases have been reported to contribute to AR modifications at post-translational level. For example, MDM2 E3 ubiquitin ligase is required in the Akt-mediated ubiquitination and degradation of AR [3]. RNF6 E3 ubiquitin ligase induces AR ubiquitination to promote AR transcriptional activity [4]. More recently, Siah2 E3 ubiquitin ligase selectively targets a pool of NCOR1-bound and transcriptionally-inactive AR for ubiquitin-dependent degradation, which thereby promoting expression of AR target genes [5]. These reports indicated that AR functions can be synergistically regulated by various E3 ubiquitin ligases during PCa development, suggesting the complexity of AR signaling pathways in PCa. Ubiquitination is a process in which the ubiquitin molecule is linked to their specific sites of substrates in single or chains to alter the function of target proteins [6,7]. Ubiquitination is a multi-step enzymatic process that sequentially starts with E1 ubiquitin activating enzyme, follows by E2 conjugating enzyme and finishes at E3 ubiquitin ligase [8,9] to maintain normal cellular homeostasis in eukaryotic cells [10,11]. Although a large number of E3 ubiquitin ligases have been reported, the regulation of AR at post-translational modifications is still elusive.
Skp2 (S-phase kinase-associated protein 2) is an E3 ubiquitin ligase for p27, a cell cycle inhibitor [12], and its overexpression is frequently found in a variety of advanced human cancers [13–15], including PCa. AR is reported to act as an upstream regulator of Skp2 through blocking D-box-dependent protein degradation [16]. Paradoxically, AR is the target of several E3 ubiquitin ligases for either protein degradation or function promotion. The phenomenon on Skp2 upregulation and the differentiated activation of AR in PCa prompted us to investigate if Skp2 regulates AR to contribute to AR-targeted genes and eventually PCa. Therefore, we hypothesized that Skp2 is a novel E3 ubiquitin ligase that targets AR for ubiquitination. Since literature reported that Akt phosphorylates both AR and Skp2 [17,18], we intended to minimize the effect of Akt in this study. To investigate whether AR regulation by Skp2 is Akt dependent, we applied BEZ235, a dual PI3K/mTOR inhibitor, to abolish Akt phosphorylation in PCa cells and further assessed the efficacy of BEZ235 on targeted signaling pathways in PCa cell lines to explore a novel avenue for chemotherapeutic strategy of PCa [19,20]. In this study, we discovered that Skp2 is an E3 ubiquitin ligase for AR degradation, and revealed a possible mechanism on AR regulation in advanced PCa. Furthermore, we demonstrated the efficacy of BEZ235 on Skp2 inhibition in PCa cells, and results may warrant further investigations on optimized combination therapies on PCa.
MATERIALS ANDMETHODS
Cell Culture, Reagents and Antibodies
LNCaP, 22Rv1, and PC3 cells were purchased from ATCC and C4-2B cells were from M.D. Anderson. LNCaP, C4-2B, DU145, and 22Rv1 cells were cultured in RPMI 1460, and PC3 cells were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin in a moist chamber at 37°C with 5% CO2. BEZ235 (LC Laboratories, Woburn, MA). Cycloheximide, MG132, anti-Flag (F1804-200UG) and DMSO (Sigma, St. Louis, MO), A/G plus agarose beads (Santa Cruz, Dallas, TX), Ni-NTA agarose beads (Life Technologies, Grand Island, NY), Skp2 siRNA oligos (Cell Signaling, Danvers, MA) were purchased. Anti-AR, anti-Skp2, anti-p27, anti-Ub, anti-c-Myc (A-14, sc-789) (Santa Cruz), anti-Akt/phosphor-Akt, anti-pS6 (Cell Signaling) were used in our study.
SiRNA andshRNA
Skp2 siRNA or control oligos at 10 nM was transfected into prostate cancer cells using Lipofectamine 2000 (Life technologies). After 48 hr, cells were washed with cold PBS and lysed with RIPA buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, and 1 mM EDTA). Cell lysates were subjected to Western blot analysis with indicated antibodies. Stable knockdown of Skp2 in prostate cancer cells was obtained using lentivirus. In brief, pLKO.1- Skp2-shRNA or scrambled plasmids were co-transfected with psPAX2, and pMD2.G plasmids into 293T cells at 70% confluence. After 48 hr, viral supernatants were collected, filtrated, and transferred to cells grown in six-well plates at 80% confluence. Infected cells were replaced with flesh medium, and after 48 hr then selected with puromycin at 2 μg/ ml for 7 days. Sequences for Skp2 shRNA or scrambled shRNA are as following: shRNA1 CCGGGCC TAAGCTAAATCGAGAGAACT GAGTTCTC TC GAT TTAGCTTAGGCTTTTTG; shRNA2 CCGGGATAG TGTCATGCTAAAGAATCTCG AGATTCT TTAGCA TGACACTATCTTTTTG; scrambled sequence CCGGA TGAGTCAACG CGAATACAGACT CGAGTCTGT ATTCGCGTTGACTCATTTTTTTG.
Proliferation and Invasion Assay
PC3, C4-2B cells and their corresponding Skp2 knockdown stable cells lines were used for the assays. In proliferation assay, cells were starved overnight and then seeded into a 24-well plate in triplicates, and cell numbers were determined on Day 0, 3, 6, 9 using trypan blue method. The averages were obtained to generate the growth curve, and the assay was repeated three times. The scratch assay was performed in 60-mm dishes, a scratch was made using a sterile 10 μl pipet tip followed with PBS wash after cells reached confluence, and images were taken at 0, 24, and 48 hr. Invasion assay was performed using 8 μm, 24-well size cell culture inserts (BD Biosciences), cells were starved overnight and then suspended at the density of 1 × 105/ml in serum-free DMEM, 1 × 104 cells were added on the top of matrigel (2 mg/ml, BD) in inserts, and the bottom chambers contained 600 μl of DMEM with 10% FBS. After cells were permitted to migrate for 20 hr, inserts were washed with PBS and were fixed in 3.7% formalin for 10 min, followed with 0.5% crystal violet staining. After the non-invaded cells on the top of transwell were removed with cotton swabs, invaded cells were counted from three fields per treatment under microscope.
Western Blot Analysis, Immunoprecipitation, and Ubiquitination Assay
Cell lysates of equal amounts of proteins were subjected to 8–12% SDS–PAGE analysis and immunobloting procedures as described previously [21]. For immunoprecipitation, cells were transfected with empty vector, pCMV3.1-3xFlag-AR, pcDNA3-Myc-Skp2, or pLKO.1-Skp2-shRNA expressing plasmids. After 24–48 hr, cells were harvested in RIPA buffer (1× PBS, pH 7.4, 2 mM EDTA, 1% Triton X-100, 0.5% or 0.25% sodium deoxycholate, and protease inhibitor), and cell lysates were sonicated briefly and centrifuged at 14,000 for 20 min at 4°C. The supernatant was precleared with 100 μl of protein A/G plus agarose beads, and then immunoprecipitated with 3 μg of antibodies, while normal IgG (either rabbit or mouse) (Sigma) was used as a negative control. The mixture of the supernatant and antibodies was incubated with 100 μl of Protein A/G plus agarose beads at 4°C for 16 hr. After precipitation, the beads were washed three times with PBS and then added with 100 μl of 2× sample buffer (1:1). Boiled beads were used for standard immunobloting. For in vivo ubiquitination assay, pCI-His-Ub, Flag-tagged AR, and Myc-tagged Skp2, (or shRNA-Skp2) plasmids were transfected into 293T cells for 48 hr. MG-132 at 20 μM was added at last 3 hr, cells were harvested in lysis buffer (6 M guanidine-HCl, 0.1 mM Na2HPO4/NaH2PO4, 10 mM imidazole), briefly sonicated and centrifuged at 14,000 rpm for 30 min at 4°C. Ni-NTA beads (75 μl) was added to protein lysates for 3 hr, beads were washed and eluted, and the elution was subjected to Western blot analysis.
Semi-quantitative RT-PCR and Site-Directed Mutagenesis
Total RNAs were extracted from cells using TRIZOL reagent, 5 μg of total RNA were used to prepare cDNAs using SuperScript III first strand synthesis kit (Invitrogen). Semi-quantitative RT-PCR was performed with a Bio-Rad CFX96 Real-time system in triplicates, in a 20 μl reaction volume consisting of 10 μl of 2× SYBR Green PCR Master Mix (Applied Biosystems), 2 μl of cDNAs, and 0.5 μM of each primer per sample. Following forward and reverse primers were used: AR, forward 5′-GACCAGATGG CTGTCA-TCA-3′, reverse 5′-GGAACCACCAACTGCTTAGC-3′; Skp2, forward 5′-ATAAGCTTATGCACAGGAAGCA-CCCTCCAGG-3′, reverse 5′-AAGAATTCTCATAGAC-AACGGGCTTTTGC-3′; Actin, forward 5′-GCTATCCA-GGCTGTGCTATC-3′, reverse 5′-TGTCACG CACGA-TTTCC-3′. An aliquot of each sample was used as PCR templates to obtain the threshold cycle (Ct). The level of target mRNA was determined by comparative CT method (ΔΔCt). The relative fold change of target mRNA expression was presented as a ratio of the level of target mRNA in treated cells to that in untreated cells. For site-directed mutagenesis, pCMV3.1-3xFlag-tagged AR plasmid was used as the template to generate the AR mutants by using site-directed mutagenesis kit according to manufacturer’s instruction (Agilent Technologies). The primer sequences are: for AR K845R, forward 5′-CTCGATCGTATCATTGCATGCAGAAGAAAAAATCC CACATCCTGCTC-3′, reverse 5′-GAGCAGGATGTG GGA TTTTTTCTTCTGCATGCAATGATACGATCGA G-3′; for AR K847, forward 5′-CTCGATC GTATCATTG-CATGCAAAAGAAGAAATCCCACATCCTGC TC-3′, reverse 5′-GAGCAGGA TGTGGGATTTCT TCTTTTG-CATGCAATGATACGATCGAG-3′; for AR K845R-K847R, 5′-CTCGATCGTATCATTGCATGCAGA AGA-AGAAATCCCACATCCTGCTC-3′, reverse 5′-GAGC-AGGATGTGGGATTTTTTCTTCTGCATGCAATGATA-CGATCG AG-3′.
Immunofluorescence (IF)
For IF, 293T cells, grown in 10% charcoal-stripped FBS (Invitrogen) on cover slips in six-well plates, were transfected with 2 μg of pCMV-c-Myc, pCMV3.1-3xFlag, pcDNA3-Myc-Skp2 or pCMV3.1-3xFlag-AR for 48 hr. After treated with 10 nM DHT for 30 min, cells were fixed with 4% paraformaldehyde and followed with primary antibodies for 1 hr and secondary antibodies (donkey anti-rabbit Alexa Fluor 568 and/or goat anti-mouse IgG Alexa Fluor 488 (Invitrogen, 1:1,000) for 30 min. Nuclei are counterstained with DAPI (Invitrogen) at a final concentration of 1 μg/ml for 10 min [21]. In C4-2B cells, cells grown on cover slips were fixed with 4% paraformaldehyde and followed by incubation with anti-AR, and anti-Skp2 at 1:200 for overnight, secondary antibodies were applied as aforementioned.
Dual Luciferase Reporter Assay
Cells were grown in 24-well plates and transfected with plasmids (AR, Myc-Skp2, shSkp2) along with 2.5 ng of pRL-CMV Renilla reporter and 200 ng ARR2-PB-Luc plasmid for 48 hr, and pcDNA3.1 was used to balance the total plasmid DNA. After 6 hr, the medium was replaced with fresh medium in 10% charcoal stripped FBS (cFBS) for 24 hr. After incubation in DHT at 10 nM for additional 24 hr, cells were harvested for luciferase assay according to manufacturer’s protocol (Promega). The luciferase activities relative to the untreated control were determined, and results are shown as means (±SD) of triplicates [21].
Statistical Analysis
All statistical analyses were conducted using a Student’s t-test. Statistical significance was determined by two tail analysis of variance and the values of P <0.05 were considered statistically significant.
RESULTS
Skp2 Knockdown Upregulates AR Protein Expression in PCa Cells
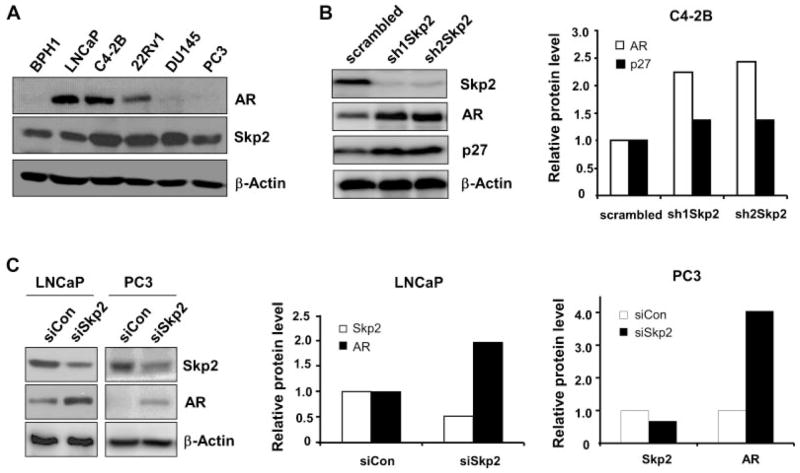
To investigate if Skp2 plays an important role on the regulation of AR protein in PCa cells, we examined the protein levels of Skp2 and AR in PCa cell lines. As shown, Skp2 was detected in all cell lines, while AR was only found in LNCaP, C4-2B, and 22Rv1 but not in DU145 and PC3 PCa cell lines as well as in BPH-1, a non-tumorigenesis prostate cell line (Fig. 1A). Since C4-2B cells are positive on both Skp2 and AR, we decided to knock down Skp2 in this cell line using short hairpin RNA (shRNA) approach. Western blot analysis demonstrated that Skp2 level was significantly reduced by shRNA approach, together with an elevation of p27 protein. Surprisingly, we found that Skp2 knockdown resulted in a striking elevation of AR protein level in C4-2B cells, as compared to the control (Fig. 1B). Quantification analysis indicated that Skp2 knockdown resulted in a more than twofold increase of AR protein as compared to the controls. In order to verify this observation, we performed Skp2 knockdown in other PCa cell lines with small interfering RNA (siRNA) or shRNA approach. Our results showed that AR protein levels were dramatically increased upon Skp2 knockdown in LNCaP and 22Rv1 PCa cell lines (Fig. 1C and Supplementary Fig. S3A). Surprisingly, Skp2 knockdown remarkably led to a restoration of AR protein in PC3 and DU145 cells (Fig. 1C and Supplementary Fig. S3A), two PCa cell lines negative for AR protein expression but positive with AR mRNA [22]. Skp2 as a proto-oncogene is overexpressed in many cancers, so we evaluated the biological effects of Skp2 knockdown on the proliferation of PCa cells. As shown, Skp2 knockdown significantly decreased the growth and the migration rate of prostate cancer cells as compared with that of controls (Supplementary Fig. S1A–D). Together, our results revealed the essential roles of Skp2 on AR regulation and the cell proliferation in PCa cells.
Fig. 1.
Skp2 knockdown upregulates AR protein level. A: Protein levels of AR and Skp2 in prostate cancer cells. B: Skp2 knockdown upregulates AR protein level in C4-2B cells. Skp2 was knocked down by shRNA, and scrambled sequence as control. C: Skp2 knockdown upregulates AR protein in LNCaP and PC3 cells. Skp2 was knocked down by siRNA, or control. Indicated antibodies were used to detect corresponding proteins, and β-actin was used as loading control. The intensities of protein bands were quantified by densitometry using ImageJ software.
Skp2 Knockdown Upregulates AR Activity at Post-Translational Level
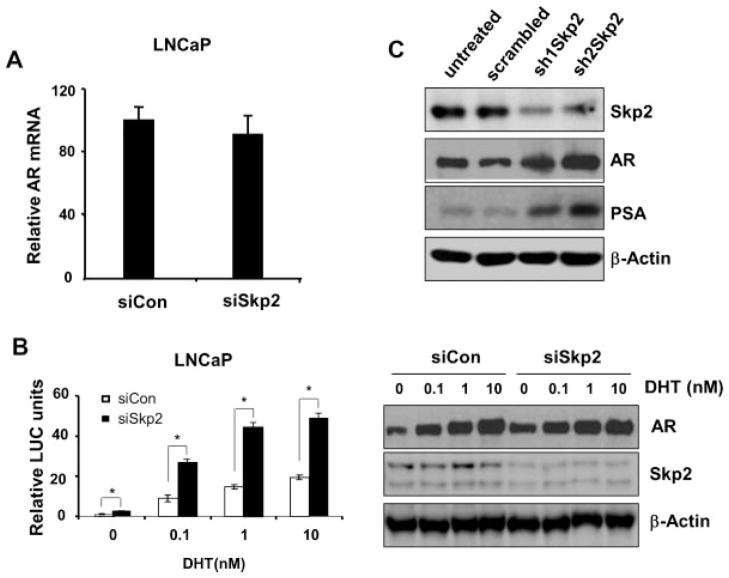
To understand the molecular mechanisms leading to the upregulation of AR protein upon Skp2 knockdown, we first aimed at the transcription level of AR. Semi-quantitative RT-PCR analysis showed that AR mRNA level upon Skp2 knockdown in cells was comparable to that of in the control (Fig. 2A), indicating that AR changes upon Skp2 knockdown were not occurred at the mRNA level. Then we turned our efforts to investigate the function and activities of AR protein. As the elevation of functional AR protein is correlated with the increased activities of AR, we hypothesized that the accumulation of AR protein by Skp2 knockdown would result in an increase of AR activities in PCa cells. To test this possibility, we knocked down Skp2 in LNCaP cells using siRNA first and then transfected ARR2-probasin promoter-luciferase (ARR2PB-Luc) reporter plasmids. After treated with DHT (5-α-dihydrotestosterone), cells were lysed for the reporter assay. Remarkably, our results showed that AR activities were significantly increased in LNCaP cells upon Skp2 knockdown (Fig. 2B). Quantification analysis revealed that AR activities were elevated twofold as compared to controls, which is consistent with the changes of AR protein (Fig. 1C). Interestingly, AR activities were elevated upon Skp2 knockdown in various concentrations of DHT. However, Western blot results using same lysates showed that AR protein levels in Skp2 knockdown cells was much higher in the absence of DHT treatment compared with that of control cells (Fig. 2B, Right). Our results indicated that Skp2 knockdown in LNCaP cells did not generate an additional increase of AR protein levels when cells were treated with DHT. To further validate our findings on Skp2-mediated AR regulation, we decided to examine PSA, a downstream target gene of AR and its protein expression is positively regulated by AR activity in PCa cells. Indeed, Western blot analysis demonstrated that PSA level in LNCaP cells was markedly elevated upon Skp2 knockdown as compared to the controls, which is in line with AR protein level (Fig. 2C). These findings encouraged us to investigate whether Skp2 knockdown can generate a similar consequence on PSA in DU145 cells, a PCa cell line negative for AR. Surprisingly, as demonstrated by Western blot, Skp2 knockdown in DU145 cells by siRNA resulted in a detectable level of AR protein as compared to the control (Supplementary Fig. S3A). These results indicate that the restored AR protein is executing its transcriptional function in PCa cells. Together, we have shown that Skp2 plays an essential role on the regulation of AR protein level and its target gene-PSA in PCa cells.
Fig. 2.
Skp2 regulates AR activity at posttranslational level. A: Skp2 knockdown does not significantly change AR mRNA level in LNCaP cells. P = 0.34 > 0.05. Skp2 knockdown was achieved by siRNA or control. Total RNA isolation and semi-quantitative RT-PCR were performed in standard protocols, and data were analyzed using comparative CT method (ΔΔCt). B: Skp2 knockdown increases AR activities. Leftpanel, Skp2 knockdown was achieved by siRNA or control, pRL-CMV, and ARR2PB-Luc plasmid were co-transfected for 48 hr. After grown in Charcoal-stripped FBS, cells were treated with DHT 24 hr before harvest. Right panel, cell lysates were subjected to SDS–PAGE and membrane blots were probed with AR and Skp2 antibodies, and β-actin was used as loading control. C: Skp2 knockdown increases PSA level in LNCaP cells. Skp2 knockdown was achieved by shRNA or scrambled sequence as control. Immunoblots showed that increased AR and PSA proteins upon Skp2 knockdown.
Overexpression of Skp2 Downregulates AR Protein Expression and Activity in PCa Cells
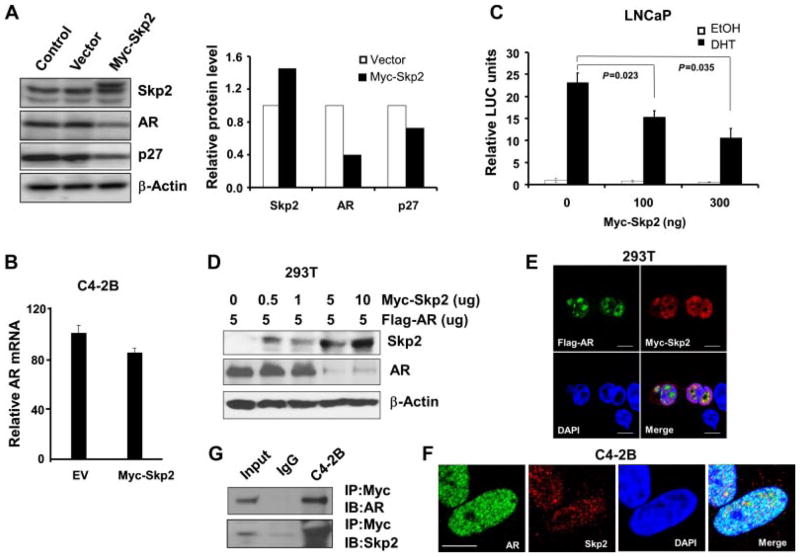
We reasoned that Skp2 overexpression would decrease AR protein in PCa cells. To test this, we overexpressed Myc-tagged Skp2 in C4-2B cells and then checked the change of AR protein level. As predicted, overexpression of Skp2 indeed resulted in a decrease of AR protein level by around 50%, which was accompanied with a decrease of p27 protein level (Fig. 3A). Semi-quantitative RT-PCR results showed that mRNA level of AR upon Skp2 overexpression was comparable to the control (Fig. 3B), indicating that Skp2 may mediate AR at the post-transcription level. To understand the effects of Skp2 overexpression on AR activities, we co-transfected Skp2 and ARR2-PB-Luc reporter plasmids into LNCaP cells and then followed by 10 nM DHT treatment. The results showed that Skp2 inhibited endogenous activities of AR in LNCaP cells in a dose-dependent manner upon DHT induction (Fig. 3C). In order to exclude the interruption of the endogenous AR on Skp2-mediated AR reduction, we overexpressed a constant level of Flag-AR and increasing levels of Myc-Skp2 in 293T cells. Western blot analysis revealed that AR protein levels indeed decreased with an increased expression levels of Skp2 (Fig. 3D), indicating that Skp2 reduces AR protein endogenously and exogenously in a dose-dependent manner. To understand whether Skp2 interacts with AR in cells, we co-transfected Flag-AR and Myc-Skp2 plasmids into 293T cells for 48 hr and then stimulated with 10 nM DHT for 30 min before fixation. Immunofluorescence (IF) staining results showed a co-localization of Skp2 and AR proteins in nuclei of cells (Fig. 3E), while no co-localization was found in these controls (Supplementary Fig. S3D). We further assessed the endogenous interaction of AR and Skp2 using C4-2B cells, and our results showed AR protein is indeed co-localized with Skp2 protein in PCa cells (Fig. 3F). We then performed immunoprecipitation [5] to understand the physical contact of Skp2 and AR proteins in C4-2B cells. Our results showed that AR and Skp2 indeed have a physical contact in PCa cells at both exogenous and endogenous levels (Fig. 3G and Supplementary Fig. S3C).
Fig. 3.
Skp2 overexpression downregulates the protein level and activity of AR. A: Enforced expression of Skp2 downregulates AR protein level in C4-2B cells. Cells were transfected with Myc-tagged Skp2 plasmids for 48 hr, and cell lysates were subjected to Western blot analysis with indicated antibodies. B: Skp2 expression does not significantly decrease AR mRNA level in C4-2B cells. P = 0.3 > 0.05, comparing the effects of Skp2 overexpression with the control. Total RNA was isolated from cells 48 hr after transfected with Myc-tagged Skp2, and then semi-quantitative RT-PCR was performed, and data were analyzed using comparative CT method (ΔΔCt). C: Enforced expression of Skp2 decreases AR activity in LNCaP cells. Cells were co-transfected by Myc-tagged Skp2, pRL-CMV and ARR2PB-Luc plasmids for 48 hr. Cells were androgen-starved and treated with 10 nM of DHT 24 hr before harvest. D: AR protein level is downregulated by Skp2 in a dose-dependent manner. 293T cells were co-transfected with Flag-AR and increasing amounts of Myc-tagged Skp2 for 48 hr. E: Co-localization of Skp2 and AR in cells. 293T cells were co-transfected with Myc-Skp2 and Flag-AR plasmids (Myc with Flag, Myc-Skp2 with Flag, and Myc with Flag-AR as controls) for 48 hr, and DHTat10 nM was added 30 min before harvest. Cells were probed with rabbit anti-Flag and mouse anti-Myc at 4°C for overnight and then corresponding secondary antibodies at room temperature for1hr. F: Endogenous localization of AR and Skp2 in C4-2B cells. DHTat10 nM was added to cultured cells 30 min before IF staining. G:Skp2 binds with AR. C4-2B cells were co-transfected with Myc-Skp2 and Flag-AR, and cell extracts were immunoprecipitated with anti-Myc antibody and immunoblotted with anti-AR antibody andanti-Skp2antibody.
Skp2 Induces AR Degradation Via Ubiquitination
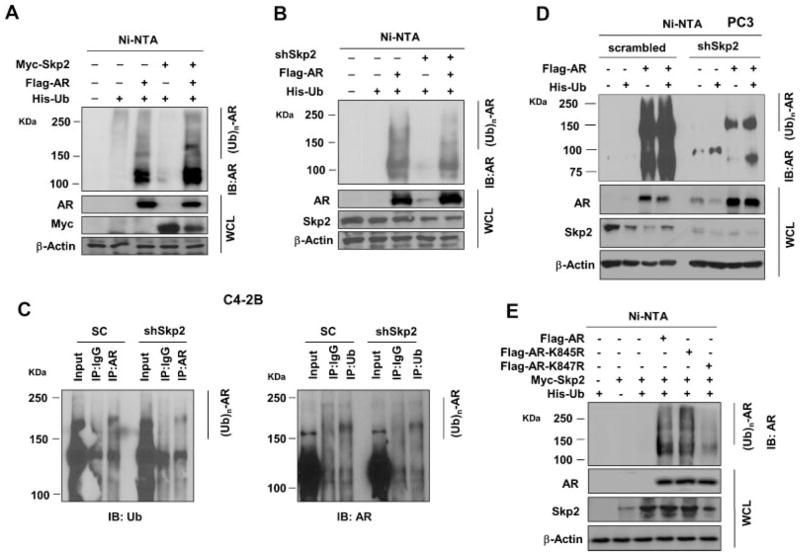
Skp2 is an E3 ligase for targeted proteins to contribute to proteasomal-mediated degradation through ubiquitination [23]. Our findings above led us to postulate that Skp2 may be an E3 ligase for AR and thus lead to the ubiquitin-dependent proteasomal degradation of AR. To test this hypothesis, we transfected 293T cells with His-Ub, Flag-AR, and Myc-Skp2 (or shSkp2) plasmids, and ubiquitinated ARs were subjected to SDS–PAGE analysis using anti-AR antibody [24]. Our results showed that AR ubiquitination increased by Skp2 overexpression as compared to the control, whereas AR ubiquitination decreased by Skp2 knockdown (Fig. 4A, B). These lines of evidence suggested that Skp2 regulates AR through ubiquitin-mediated proteasomal degradation. Next, we wished to know whether the molecular machinery of Skp2-mediated AR regulation still holds true in PCa cells. To this end, we chose two different PCa cell lines, C4-2B cells with a detectable level of endogenous AR protein and PC3 cells without detectable AR protein. We first used C4-2B cells with Skp2 knockdown to perform IP to see the impact of Skp2 loss on endogenous ubiquitination of AR. The results showed that AR ubiquitination was decreased by Skp2 knockdown as compared to the control (Fig. 4C), which is consistent with AR elevation in Fig. 1B. We then used PC3 cells with Skp2 knockdown (PC3-shSkp2) to see the impact of Skp2 knockdown on exogenous ubiquitination of AR. As expected, ubiquitinated AR was dramatically reduced in PC3-shSkp2 cells than that in the control (Fig. 4D), which is in agreement with the result in 293T cells (Fig. 4B). Surprisingly, we found that Skp2 knockdown by shRNA in PC3 cells not only resulted in the restoration of AR expression but also a decrease of AR ubiquitination (Fig. 4D), suggesting that AR ubiquitination may occur through other E3 ligases [1]. Literature showed that lysine residues 845 (K845) and 847 (K847) on AR are the two most important sites for ubiquitintaion [4]. To examine if K845 and K847 on AR are the ubiquitination sites for Skp2, we generated AR mutants by replacing lysine with arginine, K845R and K847R, through site-directed mutagenesis. We performed the ubiquitination assay in these two AR mutants, and found that AR mutation on K847 (not K845) greatly reduced the capability of AR interaction with ubiquitin, while AR mutation on K845 did not reduce AR ubiquitination as compared to AR-WT (Fig. 4E). Therefore, our discoveries provided a novel molecular mechanism on Skp2-mediated AR regulation through ubiquitination in PCa cells.
Fig. 4.
Skp2 mediates AR degradation via ubiquitination. A, B: Skp2 positively regulates AR ubiquitination. 293T cells were transfected with Myc-Skp2 (or shSkp2), Flag-AR and His-Ub plasmids for 48 hr, MG132 at 20 μM was added 3 hr before harvest. Ni-NTA beads were incubated with cell lysates, and the elution was subjected to immunoblotting analysis. C: Skp2 knockdown decreases endogenous ubiquitinated ARinC4-2B cells. C4-2B cells with Skp2 knockdown or scrambled sequence were used for immunoprecipitation with anti-AR or anti-ubiquitin antibody, normal rabbit IgG or mouse IgG were used as a control. Immunoprecipitated proteins were subjected to immunoblotting with anti-ubiquitin or anti-AR antibody. D: Skp2 knockdown restores AR expression and decreases A Rubiquitination in PC3 cells. AR and His-Ub were transfected into PC3 cells with Skp2 knockdown or control. The ubiquitination assay was performed as (A). E: AR mutation K847R abrogates Skp2-mediated AR ubiquitination. AR wild type, AR K845R or K847R mutants were transfected into 293T cells along with His-Ub, Myc-Skp2, and ubiquitination assay was performed as described previously.
Skp2 Regulates AR Expression Independent of Akt/mTOR Pathway
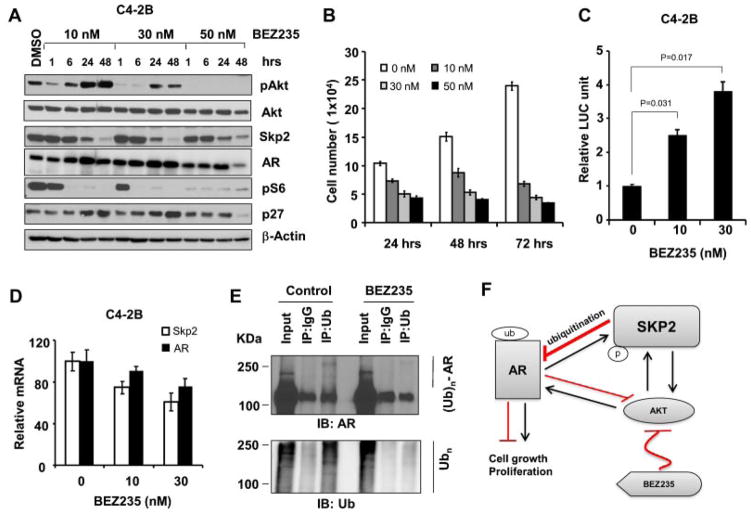
Aberrant activations of Akt and mTOR signaling pathways play essential roles in PCa through the regulation of cell survival, proliferation, and metabolism [25–27]. As both Skp2 and AR are phosphorylated by the hyperactivation of Akt, we then explored the role of Akt/mTOR pathway on Skp2-mediated regulation of AR in PCa. We chose BEZ235, a small molecule inhibitor of phosphatidylinositol 3-kinase (PI3K)/ mTOR to evaluate its impact on Skp2 and AR interaction. As expected, BEZ235 treatment in C4-2B cells completely abolished mTORC1 activity as indicated by pS6 after 1 hr exposure (Fig. 5A). In addition, BEZ235 completely blocked the activation of pAkt when treated at 50 nM, but pAkt inhibition was short when cells were treated at lower concentrations such as 30 nM or 10 nM (Fig. 5A). Surprisingly, BEZ235 treatment resulted in a striking inhibition of Skp2 protein level in 24–48 hr under three different concentrations. More importantly, AR protein level increased when Skp2 decreased after BEZ235 treatment for 24 hr in both pAkt inhibited and activated conditions. These results suggested that Skp2 regulates AR in an Akt/ mTOR-independent manner in PCa cells. To confirm this observation in other PCa cells, we treated LNCaP, PC3, and 22Rv1 cells with BEZ235, and our results demonstrated that Skp2 reduction and AR up-regulation indeed occurred concurrently (Supplementary Fig. S2A, C). We then assessed the biological effects of BEZ235 treatment, and found that Skp2 inhibition by BEZ235 was correlated with a significant inhibition of cell proliferation in C4-2B cells (Fig. 5B). In consistent with elevated AR protein levels by BEZ235 (Fig. 5A), AR activities were also increased by treating with BEZ235 compared with the control (Fig. 5C). Semi-quantitative RT-PCR results revealed that AR mRNA levels in BEZ235-treated cells were comparable to the control, indicating the elevated AR was not caused by increased transcription of AR (Fig. 5D, S2B). We then reasoned that BEZ235 treatment increased AR through reducing the Skp2-mediated ubiquitination of AR. The immunoprecipitation results showed that ubiquitinated AR proteins were reduced by BEZ235 treatment in C4-2B cells (Fig. 5E, Upper).
Fig. 5.
Skp2 inhibition correlates with elevated AR protein by BEZ235. A: BEZ235 decreases Skp2 and increases AR protein expression inC4-2B cells. Cells were treated with BEZ235 at various doses. Cell lysates were subjected to Westernblot analysis with indicated antibodies. B: BEZ235 inhibits the proliferation of C4-2B cells. Cells were treated with BEZ235 for various periods of time, and a representative experiment was repeated in triplicate. C: BEZ235 increases AR activity. After C4-2B cells were treated with BEZ for 24 hr, luciferase assay was performed following manufacture’s instruction. D: BEZ235 does not significantly affect AR mRNA level. After C4-2B cells were treated with BEZ235 for 24 hr, semi-quantitative RT-PCR was performed and data were analyzed using comparative CT method (ΔΔCt). E: BEZ235 decreases ubiquitinated AR. Cell lysates from C4-2B cells treated with BEZ235 or vehicle control were immunoprecipitated with anti-Ub, and precipitated proteins were subjected to western blot analysis with anti-AR and anti-Ub antibodies. F: A schematic model on Skp2 and AR signaling pathways in prostate cancer. BEZ235 suppresses Akt via the inhibition of PI3K and mTOR, and Akt has direct impact on AR up-regulation. Akt also phosphorylates Skp2 to prevent it from degradation. Elevated Skp2, in turn, reduces AR through poly-ubiquitination.
DISCUSSION
Skp2, a member of the F-box protein family, is an E3 ubiquitin ligase responsible for the proteasomal degradation of several tumor suppressors, including p27 and FOXO1 [12,28]. Skp2 overexpression is frequently observed in a variety of human cancers, including PCa, and its oncogenic role has been reported in many cancers [15]. Our study is the first report to reveal a novel role of Skp2 in regulation of AR through ubiquitin-mediated degradation in PCa.
AR plays an essential role in the growth, proliferation, and differentiation of prostate cells, but aberrant elevation of AR drives the initiation, development, and progression of PCa [29]. Androgen ablation is recommended for patients with advanced PCa, but relapses were observed in many cases and patients eventually died of castration resistant prostate cancer (CRPC) [2]. Cancerous cells proliferate in microenvironments partial-dependent or independent of androgen signaling for survival and progression in CRPC [29,30]. Decoding signaling pathways governed by AR and its cofactors could lead to the development of novel chemotherapies on PCa control. Literature reports that AR is regulated by E3 ligases through proteasomal degradation in PCa cells through various mechanisms. For example, the ubiquitin-mediated degradation of AR by MDM2 or Siah2 depends on its phosphorylated or inactive status in cells [31,32]. Yet, transcriptional activities and specificities of AR are dramatically induced, not decreased, by RNF6-mediatd ubiquitination [4], indicating the complexities of AR ubiquitination in PCa. In this study, we applied overexpression and knockdown approaches to demonstrate that Skp2 mediates AR protein levels through regulation of AR ubiquitination in PCa cell lines. Our collective evidence implied that Skp2 is an E3 ligase that regulates AR level through ubiquitin-mediated proteasomal degradation in PCa cells. Furthermore, Skp2-mediated AR degradation occurs in an Akt/ mTOR-independent manner, highlighting its unique role in PCa but different from MDM2 and Siah2.
One exciting discovery in this study is that Skp2 knockdown restores the protein expression of AR in both PC3 and DU145 PCa cells that are AR negative. However, we noticed that AR restoration upon Skp2 knockdown did not reach the comparable levels of AR proteins in LNCaP or C4-2B cells, which may be contributed by other factors affecting AR level in PC3 and DU145 cells. AR mRNA is present in both PC3 and DU145 cells [33,34], but its level is relatively lower than that in LNCaP or 22Rv1 cells [22]. Our studies showed that AR protein is elevated from the negative to the detectable level in PC3 and DU145 cells upon Skp2 knockdown, and the limited increase of AR indicates that knockdown of Skp2 only reduces one machinery of AR degradations regulated through ubiquitination in these cells. Nevertheless, our study is the first report to show that AR proteins in PC3 and DU145 PCa cells are restored to a detectable level by blocking the proteasomal-degradation machinery (Supplementary Fig. S3A). Previous studies suggested that the difference of AR protein levels between androgen sensitive and insensitive PCa cell lines was caused by their mRNA expression levels or methylation-associated transcription factors [35,36]. Although no major differences on AR gene have been identified among these cells, the mechanisms leading to the aberrant regulations of AR proteins in PC3 and DU145 PCa cells can be more complicated than we expected. We identified that Skp2 is an E3 ligase for AR and plays a pivotal role on the negative regulation of AR protein in PCa cells. Skp2 overexpression resulted in a reduction of AR protein level, and Skp2 knockdown abrogated the inhibitory effect on AR to restore AR protein partially. These results provide convincing explanations on the absence of AR protein but the presence of AR mRNA in these two PCa cell lines.
On the other hand, AR as a transcription factor has been thought an upstream regulator of Skp2 in PCa cells [16]. Paradoxically, we observed that Skp2 knockdown results in the rise of AR levels in cancer cells (Figs. 1B, C and 2C), and shows a great inhibition of the growth and migration (Supplementary Fig. S1A–D). Based on the literature and our studies, Skp2 knockdown causes two major changes on AR signaling: (1) by inhibiting the global activities of glycolysis, metabolism, insulin sensitivity and Akt signaling [37], which dramatically suppressing cell cycles in cells; (2) by decreasing the proteasome-mediated ubiquitination activity that leads to a reduced degradation of AR protein and eventually an increased AR protein level. The overall outcome in cells is driven by the combined impacts of Skp2 knockdown and AR elevation, and Skp2 knockdown exerts more effective inhibition in cell growth due to a reduction of massive multiple essential pathways, which antagonizing the upregulation of cell growth contributed by slightly increased AR. Our evidence, together with previous reports, suggests a possible feedback mechanism on AR-Skp2 signaling pathway at post-translational level.
AR ubiquitination is catalyzed by E3 ubiquitin ligase through two primary sites at K845 and K847, and their mutations greatly reduced the ubiquitination activities of RNF6 [4]. Our results showed that K847R mutant greatly reduced AR ubiquitination activity as compared with WT AR, while K845R mutant failed to alter AR ubiquitination activity. These findings indicate K847 is the major site for Skp2-mediated AR ubiquitination, suggesting that Skp2 executes its unique function on AR ubiquitination different from RNF6 at K845 [4]. It is worth to further investigate how these E3 ligases cooperate to contribute to AR regulation and PCa progression, and mechanisms will be valuable for the development of novel chemotherapies.
Literature reported that Akt phosphorylation plays essential roles on the activities and levels of AR [17,18]. Skp2 deficiency ablates Akt activation in cells [37], but Akt activation also determines oncogenic abilities of Skp2. This notion highlights the critical role of Akt in regulation of Skp2 and AR functions. Suppression of Akt activities and downstream targets by inhibitors would be an appropriate approach for us to evaluate the effects of Akt on Skp2-mediated AR regulation. Therefore, to shed light on in-depth understanding of Skp2-AR interaction in PCa, we decided to bring BEZ235 into our study, BEZ235 has been reported as a potent PI3K inhibitor that antagonizes AKT activity, and that BEZ235 treatmet led to an abnormal elevation of AR in AR positive cells [38]. We applied BEZ235 to our studies for two reasons: (1) to assess the effects of Akt/mTOR inhibition on Skp2 and AR; (2) to explore the effects of BEZ235 on ubiquitination and the potentials of novel treatments for PCa. Given that Akt regulates both AR and Skp2 proteins [16,27], it is logical to detect the down-regulation of Skp2 and AR protein levels after BEZ235 treatment. However, after BEZ235 application, we found that AR protein level was remarkably elevated and Skp2 protein decreased in cells, while mRNA levels of both AR and Skp2 were subsequently decreased (Fig. 5A, C and Supplementary Fig. S2A–C). Elevated AR protein level is regulated at post-transcriptional level upon BEZ235 treatment, implying Skp2-mediated AR degradation exerts more impact on AR protein accumulation than AR mRNA. These results further underscored our finding that Skp2 directly involves AR degradation independent of Akt/ mTOR activation. Recently, literature reveals that Skp2 determines Akt activity. Therefore, knocking down Skp2 will have impact on AR protein accumulation between an increase of AR caused by reduced ubiquitin-mediated AR degradation and a decrease of AR contributed by Akt inactivation (Fig. 5F). Our observation indeed confirmed these two separate events in PCa cells. We also concluded in this study that Skp2-mediated AR degradation can occur independent of Akt activation (Fig. 5A and Supplementary Fig. S2A). The mechanisms to coordinate the balance of Akt and Skp2 signaling in PCa cells are still to be defined. For example, upon BEZ235 (30 nM) treatment in LNCaP cells, pAkt was significantly inhibited at 1 hr, while Skp2 had yet to be affected but AR level was decreased as compared to the control (Supplementary Fig. S2A). Interestingly, AR level became noticeably elevated while Skp2 was significantly decreased at 24 hr, while pAkt was partially inhibited. Moreover, AR becomes noticeably elevated with pAkt abrogation, suggesting pAkt is unlikely to have positive impact on AR elevation under this condition. The inverse correlation between Skp2 and AR indicates the complexities of AR regulation in PCa progression, suggesting that multi-target drug treatments will be ideal in PCa treatment, especially for BEZ235. In summary, we have identified Skp2 E3 ligase that directly involves the AR degradation via ubiquitination. Our findings revealed that Skp2 determines AR protein level independent of Akt/mTOR activation in PCa cells, and provide valuable insights into understanding AR regulation in PCa (Fig. 5F). Therefore, targeting Skp2 combined with other targets will be a novel strategy to control cancers.
Supplementary Material
Acknowledgments
We would like to thank Drs. Simon W. Hayward and LaMonica Stewart for providing prostate cell lines. This study was supported in part by NIH grants: MD004038, MD007586, CA163069, DK055748-13, and TR000445-06. Microscopy experiments and data analysis were performed which is supported in part by NIH grants U54MD007593 and S10RR0254970.
Footnotes
Conflicts of interest: None.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B. Regulation of androgen action. Vitam Horm. 1999;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- 3.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21(15):4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu K, Shimelis H, Linn DE, Jiang R, Yang X, Sun F, Guo Z, Chen H, Li W, Chen H, Kong X, Melamed J, Fang S, Xiao Z, Veenstra TD, Qiu Y. Regulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitination. Cancer cell. 2009;15(4):270–282. doi: 10.1016/j.ccr.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi J, Tripathi M, Mishra R, Sahgal N, Fazil L, Ettinger S, Placzek WJ, Claps G, Chung LW, Bowtell D, Gleave M, Bhowmick N, Ronai ZA. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer cell. 2013;23(3):332–346. doi: 10.1016/j.ccr.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciechanover A, Heller H, Elias S, Haas AL, Hershko A. ATP-dependent conjugation of reticulocyte proteins with the poly-peptide required for protein degradation. Proc Natl Acad Sci USA. 1980;77(3):1365–1368. doi: 10.1073/pnas.77.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciehanover A, Hod Y, Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978;81(4):1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Maldonado MA. The ubiquitin-proteasome system and its role in inflammatory and autoimmune diseases. Cell Mol Immunol. 2006;3(4):255–261. [PubMed] [Google Scholar]
- 9.Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitinproteasome system. J Biosci. 2006;31(1):137–155. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 10.Hinkson IV, Elias JE. The dynamic state of protein turnover: It’s about time. Trends Cell Biol. 2011;21(5):293–303. doi: 10.1016/j.tcb.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Vicente M, Sovak G, Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40(8–9):622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1(4):193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 13.Li CF, Wang JM, Kang HY, Huang CK, Wang JW, Fang FM, Wang YH, Wu WR, Li SH, Yu SC, Lee JC, Lan J, Shiue YL, Wu LC, Huang HY. Characterization of gene amplification-driven SKP2 overexpression in myxofibrosarcoma: potential implications in tumor progression and therapeutics. Clin Cancer Res. 2012;18(6):1598–1610. doi: 10.1158/1078-0432.CCR-11-3077. [DOI] [PubMed] [Google Scholar]
- 14.Drobnjak M, Melamed J, Taneja S, Melzer K, Wieczorek R, Levinson B, Zeleniuch-Jacquotte A, Polsky D, Ferrara J, Perez-Soler R, Cordon-Cardo C, Pagano M, Osman I. Altered expression of p27 and Skp2 proteins in prostate cancer of African-American patients. Clin Cancer Res. 2003;9(7):2613–2619. [PubMed] [Google Scholar]
- 15.Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, Slingerland J, Krek W. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA. 2001;98(9):5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Sun D, Ji P, Mohler J, Zhu L. An AR-Skp2 pathway for proliferation of androgen-dependent prostate-cancer cells. J Cell Sci. 2008;121(Pt 15):2578–2587. doi: 10.1242/jcs.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha S, Ruoff R, Kahoud N, Franke TF, Logan SK. Androgen receptor levels are upregulated by Akt in prostate cancer. Endocr Relat Cancer. 2011;18(2):245–255. doi: 10.1530/ERC-10-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao D, Inuzuka H, Tseng A, Wei W. Akt finds its new path to regulate cell cycle through modulating Skp2 activity and its destruction by APC/Cdh1. Cell Div. 2009;4:11. doi: 10.1186/1747-1028-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubrovska A, Elliott J, Salamone RJ, Kim S, Aimone LJ, Walker JR, Watson J, Sauveur-Michel M, Garcia-Echeverria C, Cho CY, Reddy VA, Schultz PG. Combination therapy targeting both tumor-initiating and differentiated cell populations in prostate carcinoma. Clin Cancer Res. 2010;16(23):5692–5702. doi: 10.1158/1078-0432.CCR-10-1601. [DOI] [PubMed] [Google Scholar]
- 20.Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, Garcia-Echeverria C, Schultz PG, Reddy VA. The role of PTEN/ Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA. 2009;106(1):268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu W, Xie Y, Ma Y, Matusik RJ, Chen Z. ARF represses androgen receptor transactivation in prostate cancer. Mol Endocrinol. 2013;27(4):635–648. doi: 10.1210/me.2012-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alimirah F, Chen J, Basrawala Z, Xin H, Choubey D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. FEBS Lett. 2006;580(9):2294–2300. doi: 10.1016/j.febslet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 23.Lakshmikanthan V, Zou L, Kim JI, Michal A, Nie Z, Messias NC, Benovic JL, Daaka Y. Identification of betaArrestin2 as a corepressor of androgen receptor signaling in prostate cancer. Proc Natl Acad Sci USA. 2009;106(23):9379–9384. doi: 10.1073/pnas.0900258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan CD, Lum MA, Xu C, Black JD, Wang X. Ubiquitin-dependent regulation of phospho-AKT dynamics by the ubiquitin E3 ligase, NEDD4-1, in the insulin-like growth factor-1 response. J Biol Chem. 2013;288(3):1674–1684. doi: 10.1074/jbc.M112.416339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang WL, Wu CY, Wu J, Lin HK. Regulation of Akt signaling activation by ubiquitination. Cell Cycle. 2010;9(3):487–497. doi: 10.4161/cc.9.3.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt Cell. 2002;111(3):293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 28.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8(6):438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 30.Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12(6):1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- 31.Gaughan L, Logan IR, Neal DE, Robson CN. Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res. 2005;33(1):13–26. doi: 10.1093/nar/gki141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res. 2003;1(14):993–1000. [PubMed] [Google Scholar]
- 33.Edelstein RA, Carr MC, Caesar R, Young M, Atala A, Freeman MR. Detection of human androgen receptor mRNA expression abnormalities by competitive PCR. DNA Cell Biol. 1994;13(3):265–273. doi: 10.1089/dna.1994.13.265. [DOI] [PubMed] [Google Scholar]
- 34.Culig Z, Klocker H, Eberle J, Kaspar F, Hobisch A, Cronauer MV, Bartsch G. DNA sequence of the androgen receptor in prostatic tumor cell lines and tissue specimens assessed by means of the polymerase chain reaction. Prostate. 1993;22(1):11–22. doi: 10.1002/pros.2990220103. [DOI] [PubMed] [Google Scholar]
- 35.Chlenski A, Nakashiro K, Ketels KV, Korovaitseva GI, Oyasu R. Androgen receptor expression in androgen-independent prostate cancer cell lines. Prostate. 2001;47(1):66–75. doi: 10.1002/pros.1048. [DOI] [PubMed] [Google Scholar]
- 36.Tilley WD, Wilson CM, Marcelli M, McPhaul MJ. Androgen receptor gene expression in human prostate carcinoma cell lines. Cancer Res. 1990;50(17):5382–5386. [PubMed] [Google Scholar]
- 37.Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, Huang HY, Tsai KK, Flores LG, Shao Y, Hazle JD, Yu D, Wei W, Sarbassov D, Hung MC, Nakayama KI, Lin HK. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. 2012;149(5):1098–1111. doi: 10.1016/j.cell.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, Scardino PT, Rosen N, Sawyers CL. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer cell. 2011;19(5):575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.