Summary
Objectives
The PI3k/Akt pathway has been associated with development and progression of bladder tumors, with most studies focused on papillary or muscle invasive tumors. We sought to characterize the expression patterns of the PI3K/Akt pathway in a large cohort of high-risk pre-invasive carcinoma in situ tumors of the bladder. Or goal was to understand whether PI3K/Akt pathway alterations associated with CIS resemble earlier or later stage bladder cancers.
Material and Methods
We evaluated tissue specimens from 97 patients with carcinoma in situ of the bladder, of which 14 had a concomitant papillary tumor. All patients were treated with intravesical BCG. All specimens were characterized for PTEN, p-AKT, and p-S6 immunoreactivity. Markers where evaluated for percentage and intensity of staining and were scored using a 0-3+ grading system.
Results
PTEN staining was noted as least intense in 67% of tumor specimens and 22% of normal urothelium. PAkt and p-S6 had intense staining in 77% and 90% of tumor specimens versus 44% and 68% in normal tissue, respectively. Low intensity staining for PTEN at 12 months correlated with higher recurrence risk (p = 0.026).
Conclusion
We describe a large cohort of carcinoma in situ bladder tumors with decreased staining intensity of PTEN and increased staining intensity of p-AKT and p-S6, similar to high grade and stage papillary tumors. Low intensity staining of PTEN at 12 months was associated with an increased risk of recurrence.
Keywords: Bladder cancer, PTEN, carcinoma in situ
INTRODUCTION
At initial diagnosis, 75% of patients with UC will present with non muscle-invasive disease.[1] While many of these patients will experience a recurrence of their bladder cancer, a smaller percentage will progress to an aggressive and invasive phenotype. Identification of biological markers that differentiate the more aggressive UCs from other phenotypes is currently a topic of great interest.[2] One possibility for identifying aggressive tumors is to recognize genomic variations that differentiate them from less aggressive tumors, which may also allow for more efficient treatment.
PTEN, a tumor suppressor, is required for the regulation of the PI3K/Akt pathway. PTEN functions as a tumor suppressor by hydrolyzing the 3-phosphate of PIP3, which converts it to its inactive form (PIP2) and leads to inhibition of its downstream effects.[3-5] When every element of the PTEN/PI3K/Akt pathway is in perfect harmony, it regulates cell growth, proliferation, and survival.[6, 7] Inactivating mutations or deletions of PTEN lead to activation of Akt, which in return regulates multiple downstream cellular survival and growth responses, including S6, that are associated with a number of human tumors including UCs.[8-14] Bladder tumors that have aquired abnormalities within the PI3K/Akt pathway are associated with a more aggressive phenotype and poor survival rates. Furthermore, loss of PTEN has been associated with increasing grade and stage of papillary tumors. [15, 16]
However, very little has been reported on the effects of PTEN loss on earlier stage bladder cancer, particularly CIS.[17, 18] By IHC staining of PTEN, p-Akt, and p-S6 in tumor and normal tissue, we attempted to characterize the PTEN/PI3K/Akt-related phenotype of CIS of the bladder.
MATERIALS AND METHODS
After obtaining institutional review board approval for this retrospective study, we evaluated 97 patients with CIS of the bladder at our institution between 1986 and 2008. Patients were included if they had primary CIS or CIS associated with NMIUC (Ta or T1). Each patient underwent maximal TUR of the bladder tumor with macroscopic complete removal of all papillary or T1 disease, and pathologic confirmation was achieved by two pathologists specializing in genitourinary oncology (LLG and HAA). A patient whose tumor included Ta or T1 components was eligible, but only the CIS components of the tumor were stained for evaluation, which were away from the papillary tumor. In cases with both components on the same slide, evaluating he stains focused on the CIS component of the tumor. Following TUR, all patients completed a 6 cycle induction course of 81 mg of intravesical (Connaught strain) BCG. None of the patients in our study were treated with maintenance BCG.
Surveillance for all patients included physical exam, urine cytology, and cystoscopy every 3 months for the first year. All patients except for 10 and 9 at 6 months and 12 months presented for followup, respectively. Recurrences were defined by histopathologically confirmed UC, by biopsy or resection, or a positive urinary cytology. Progression was defined as the development of T1 or T2 (muscle-invasive) disease. At the treating physician’s discretion, patients with recurrence or progression were managed with a second course of intravesical BCG or by surgical intervention with either repeat TUR alone or RC.
IHC analysis was performed on formalin-fixed paraffin-embedded pretreatment tumor samples. Validated markers of PI3K/Akt pathway activation were studied, including PTEN (Mab) clone 6H2.1, (EDTA, target retrieval solution PH9, S2368 from Dako), for control we used genetically proven endometrial carcinoma with PTEN deletion, p-AKT (Ser 473(736E11) (Mab) rabbit from Cell Signaling, (Citrate buffer PH6), for control we used cell lines known to over express pAkt and p-S6 Ribosomal protein(Ser 240/244) (Pab), IHC was performed on Ventana discovery XT, CC1 stand, dilution 1:200, for positive control, normal tonsil as recommended by the manufacturer.
For each marker, immunoreactivity was assessed for the percentage of tumor cells expressing the marker, and the intensity of the staining was graded from 0 to 3+ (0 negative; 1+ weak; 2+ medium; 3+ strong). The stains were similarly assessed in the adjacent non-neoplastic urothelium, whenever it was present in the sample. The slides were reviewed by 2 pathologists together to reach a score for each stain. There was no attempt to address interobserver variability for this study. To minimize bias, both pathologists were blinded to recurrence data. The entire tissue section was examined and the intensity given to each tumor was the result of the overall assessment of the stain.
To determine the association between PTEN intensity and response at 12 months we used Fisher’s exact test, and Spearman’s rank correlation test was used to correlate changes in staining for each marker. All analyses were done using Stata 12.0 (Stata Corp., College Station, TX).
RESULTS
The baseline demographics of the entire cohort of 97 patients are shown in Table 1. Overall, 79 patients (81%) were male and the median age at the time of presentation was 76 years old (IQR 70–82).
Table 1. Demographics and treatment response in patients treated for CIS of the bladder (n = 97).
| Patient characteristics | Median (IQR) or frequency (%) |
|---|---|
| Age at initiation of BCG (years) | 76 (70–82) |
| Male | 79 (81%) |
| Pathology | |
| pTis alone | 76 (78.5%) |
| pTis and pTa | 14 (14.5%) |
| pTis and pT1 | 7 (7%) |
| Radical cystectomy | |
| Radical cystectomy within a year | 12 (12%) |
| Time to radical cystectomy (months) | 5 (4–8) |
| Responders | |
| 6 months (n = 97) | 57 (59%) |
| 12 months (n = 97) | 51 (53%) |
The observed frequencies and intensities of staining of PTEN, p-Akt, and p-S6 for CIS tumor specimens and their corresponding normal tissues are shown in Table 2. Overall, PTEN staining was present in 91% (86/94) of tumor and 96% (74/77) of normal tissue samples. Least intense (1+) PTEN staining pattern was seen in 67% (63/94) of CIS samples compared to only 22% (17/77) of cells in the adjacent non-neoplastic tissue. Intense (grade 2 and 3) p-Akt staining was present in 77% (73/95) of tumor specimens compared to only 44% (29/66) in the adjacent non-neoplastic tissue. Intense (grade 2 and 3) p-S6 staining was present in 90% (84/93) of tumor specimens but only in 68% (53/78) of normal tissue. The median percentage of PTEN staining was 60% (IQR 40–80), while in adjacent non-neoplastic tissue the median percentage was 80% (IQR 70–80). In contrast, p-AKT and p-S6 showed more staining in tumor (70% [IQR 40–80] and 60% [IQR 40–70], respectively) when compared to adjacent non-neoplastic tissue (50% [IQR 30–60] and 40% [IQR 30–50], respectively). (Figure 1) The Spearman rank correlation between PTEN and p-S6 was 0.01 (p >0.9) and between PTEN and p-AKT was 0.04 (p = 0.7).
Table 2. Frequency and intensity of staining for markers PTEN, p-Akt, and p-S6 in CIS tumor specimens and adjacent normal tissue.
| Markers | Staining intensity |
Frequency | |
|---|---|---|---|
|
| |||
| Tumor (n = 97) |
Normal (n = 97) |
||
| PTEN | |||
| 0 | 8 (8%) | 2 (2%) | |
| 1 | 63 (65%) | 17 (18%) | |
| 2 | 17 (18%) | 42 (43%) | |
| 3 | 6 (6%) | 16 (16%) | |
| – | 3 (3%) | 20 (21%) | |
|
| |||
| p-Akt | |||
| 0 | 5 (5%) | 1 (1%) | |
| 1 | 17 (18%) | 36 (37%) | |
| 2 | 38 (39%) | 28 (29%) | |
| 3 | 35 (36%) | 1 (1%) | |
| – | 2 (2%) | 31 (32%) | |
|
| |||
| p-S6 | |||
| 0 | 3 (3%) | 0 (0%) | |
| 1 | 6 (6%) | 25 (26%) | |
| 2 | 19 (20%) | 38 (39%) | |
| 3 | 65 (67%) | 15 (15%) | |
| – | 4 (4%) | 19 (20%) | |
|
| |||
|
Median staining (IQR)
|
|||
| PTEN | 60% (40–80) | 80% (70–80) | |
| p-Akt | 70% (40–80) | 50% (30–60) | |
| p-S6 | 60% (40–70) | 40% (30–50) | |
–, sample not available; IQR, interquartile range.
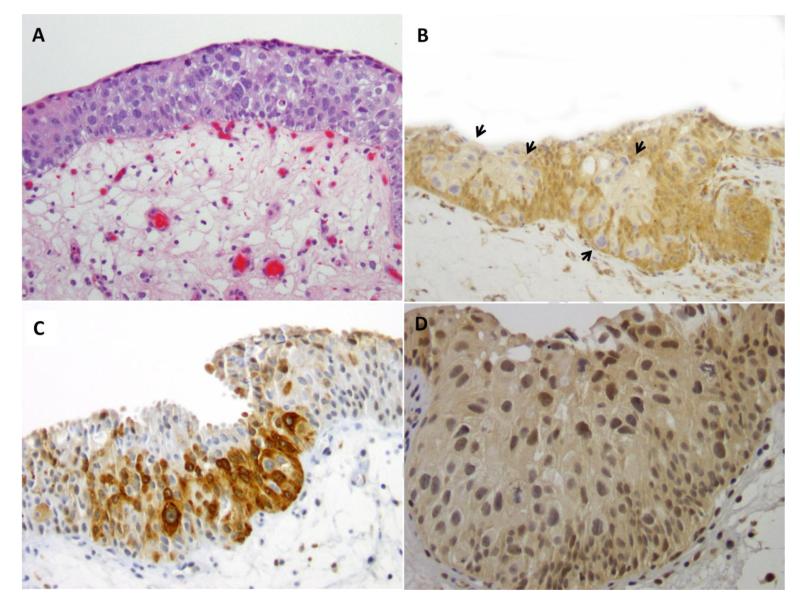
Figure 1.
A (H&E). Urothelial carcinoma in situ composed of large and pleomorphic tumor cells. Mitotic figures present. B (PTEN). Loss of PTEN expression (0 intensity) in tumor cells (arrows) compared to adjacent non-neoplastic urothelium. C (p-S6). Tumor cells with strong expression (3+ intensity) of p-S6 compared to adjacent non-neoplastic urothelium. D (p-Akt). Increased expression of p-Akt (2+ intensity) in tumor cells.
Table 3 demonstrates the association between PTEN IHC staining and recurrence at 6 and 12 months. Staining intensity with PTEN did not show a significant association with disease recurrence at 6 months (all p >0.088). At 12 months a significantly higher recurrence risk was observed in patients with tumors demonstrating low intensity or no PTEN staining (p = 0.026). Furthermore, when grouping PTEN staining as low intensity (0–2+) and high intensity (3+), low staining intensity continued to be associated with a higher recurrence rate at 12 months (p = 0.040). No differences were found in 12-month recurrence rates based on p-Akt (all p >0.8) and p-S6 (all p >0.6) staining intensity or when comparing low intensity (grade 0–2) versus high intensity staining (grade 3) (all p >0.5).
Table 3. Immunohistochemical analysis of PTEN in tumor cells.
| At 6 months | ||||||||
|---|---|---|---|---|---|---|---|---|
| Evidence of disease |
PTEN intensity in tumor cells |
P value* | PTEN intensity in tumor cells |
P value* | ||||
| 0 | 1 | 2 | 3 | 0–2 | 3 | |||
| Yes (N = 31) |
4 (13%) |
20 (65%) |
7 (23%) |
0 (0%) |
31 (100%) |
0 (0%) |
||
| No (N = 56) |
2 (4%) |
39 (70%) |
9 (16%) |
6 (11%) |
50 (89%) |
6 (11%) |
||
| Total (N = 87) |
6 (7%) |
59 (68%) |
16 (18%) |
6 (7%) |
0.10 | 81 (93%) |
6 (7%) |
0.085 |
| At 12 months | ||||||||
|---|---|---|---|---|---|---|---|---|
| Evidence of disease |
PTEN intensity in tumor cells |
P value* | PTEN intensity in tumor cells |
P value* | ||||
| 0 | 1 | 2 | 3 | 0–2 | 3 | |||
| Yes (N = 37) |
6 (16%) |
22 (59%) |
9 (24%) |
0 (0%) |
37 (100%) |
0 (0%) |
||
| No (N = 51) |
2 (4%) |
35 (69%) |
8 (16%) |
6 (12%) |
45 (88%) |
6 (12%) |
||
| Total (N = 88) |
8 (9%) |
57 (65%) |
17 (19%) |
6 (7%) |
0.026 | 82 (93%) |
6 (7%) |
0.040 |
P value was calculated using Fisher’s exact test.
Of the 97 patients evaluated solitary CIS was present in 76 patients; 14 patients had a concomitant diagnosis of CIS with pTa, and 7 had CIS and pT1. All patients treated had high-grade histology and 47 (48%) had multifocal disease. Only 9 patients (9%) had previously been treated with intravesical BCG.
At 6 and 12 months, 57 (59%) and 51 (53%) of patients were disease free, respectively. At 6 and 12 months follow up, 17 (17.5%) and 14 (14.5%) patients, respectively, had a positive voided urinary cytology. At 6 months, 22 patients underwent a TUR and 14 (63%) had tumor present, at 12 months, 19 patients underwent a TUR and 12 (63%) had a malignancy. Of the 14 patients with a tumor at 6 months, 7 had pTis, 2 had pTa, and 5 had pT1 (2 with concomitant pTis). At 12 months, 9 patients showed pTis, 2 had pTa and 1 had a tumor of unknown pathology. Twelve patients (12%) underwent RC within a median of 5 months (IQR 4–8), because of disease progression or BCG failure.
DISCUSSION
UC can be classified into two separate tumor pathways i.e., tumors with a low potential for invasion and progression and those with a high potential for invasion and metastasis. Genomic variation has been hypothesized as an explanation for the different capacities of these two tumor types[19] with PTEN loss as a possible factor driving urothelial progenitor cells towards potentially invasive tumors.[2]
Furthermore, activation of the PI3K/Akt pathway in NMIUC has been shown to lead to aggressive UC, with increased rates of progression and reduced cancer-specific survival.[15] Additionally, low-intensity PTEN staining has been associated with progression from NMIUC to MIUC.
We report a detailed IHC characterization of the PI3K/Akt pathway in CIS of the bladder, which adds new insight given the paucity of published literature on CIS IHC expression profiles. In our series of CIS tumors we demonstrate a predominantly low intensity of PTEN IHC staining. Only 24% of our tumor specimens demonstrated moderate to intense PTEN staining, with 73% showing low intensity or no staining. Similarly, in a cohort of 161 patients with NMIUC, which included 7 patients with CIS of the bladder, were analyzed for PTEN staining with 5 (71%) showing low-intensity staining. Lack of PTEN IHC staining was also associated with high-grade and high-stage papillary UC but not with recurrence or progression. Furthermore, decreased PTEN staining was more likely found in CIS (71%) compared to T1 (64%) and Ta (44%) disease (p = 0.037). Our series identifies similar findings of low PTEN staining (73%) as seen with high stage and grade papillary tumors (64% T1).[18] Our much larger cohort of CIS patients showed similar low-intensity staining of PTEN and we were able to identify a significant correlation with recurrence at 12 months following BCG therapy. Moreover, when comparing patients with and without recurrence at 12 months, we found a higher incidence of recurrence in patients who had CIS tumors with low intensity or no staining of PTEN. Of the 36 patients who had recurrence at 12 months, all 36 (100%) had CIS tumors with low intensity or no staining of PTEN.
Our data also demonstrate an increased staining of p-Akt and p-S6 in CIS of the bladder. Several studies have identified the importance of the PI3K/Akt pathway in UC.[16, 20, 21] Sun et al. were able to correlate tumor progression and cancer-specific mortality with high expression of p-AKT and p-S6 for NMIUC treated by transurethral resection, but they did not evaluate any patients with CIS.[15] These findings suggest that expression changes in the PI3K/Akt pathway may occur as early events that, with further damage, may lead to worsening disease. In our evaluation of CIS tumors, we identified 75% of specimens with moderate to intense staining for p-Akt and 87% for p-S6 staining. We were not able to observe a correlation between p-Akt and p-S6 staining in CIS tumors with recurrence at 12 months. Given the complexity within the PI3K/Akt pathway and the numerous feedback pathways found when homeostasis is disturbed we were not surprised by these finding and feel it will be important to study the pathway in more detail paying attention to key regulators of the pathway. Furthermore, increased frequency of low PTEN staining was identified with increased stage and grade of papillary tumors, 13.6% of pTa vs 24.3% of pT2-4 tumors. While the percentage of tumors with low PTEN staining is lower than other reported series, the pattern of increased frequency of low PTEN staining with increased grade and stage of papillary tumors still holds. [15]
Comparing our staining patters to those found in the literature, we could find similar patters as those of high stage invasive bladder tumors. As we move to an error of genetically profiling tumors in helping with treatment decision-making, i.e targeted therapies, this information brings insight into CIS tumors of the bladder. With their similarity of PI3k/Akt pathway staining to invasive bladder tumors this information may help in designing future prospective trials.
The association between PTEN loss and p-AKT activation has been studied in a variety of human cancers, with mixed results. In a series of 86 MIUC samples using IHC staining and a positive correlation between PTEN staining and p-AKT expression was identified.[22] A separate study of 869 UCs, 626 NMIUCs, and 243 MIUCs did not find a significant correlation between PTEN and p-AKT.[15] While both of these studies showed IHC staining of UCs, neither evaluated CIS tumors. In our evaluation of CIS tumors we did not find a significant relationship between PTEN staining intensity and p-AKT and p-S6 staining using Spearman’s rank correlation analysis, further adding to the complexity of the effects associated with PTEN loss in UC.
Our study had several limitations. Within our group of CIS patients a small number of patients few also had concomitant pTa and/or pT1 disease. This group represented a heterogeneous series of high risk groups for recurrence and progression. However, given the small numbers of patients in each of the risk groups, detailed analysis by pathology stage was not informative. Also, although the two pathologists who evaluated all the specimens and graded the staining intensity specialized in genitourinary oncology, the staining intensity scores themselves were not validated. Even with these limitations, however, our series provides IHC characteristics of a large series of CIS specimens for PTEN, p-AKT, and p-S6 phenotype. While we found that low intensity or no PTEN staining of CIS tumors is correlated with higher 12-month recurrence rates, we recognize that many other clinical and pathologic variables not evaluated in this study are likely to also play an important role in the risk of tumor recurrence.
CONCLUSION
Our data demonstrate the IHC characteristics of CIS of the bladder for PTEN, p-AKT, and p-S6 expression in a large cohort of patients. Decreased PTEN staining intensity was associated with an increased recurrence rate at 12 months. Although further validation with a larger external sample set is needed, this study adds to our understanding of the molecular characteristics of CIS lesions of the bladder. We identified similar patterns of staining as previously shown for high grade and advanced stage papillary tumors.
Acknowledgments
Funding: Supported by the Sidney Kimmel Center for Prostate and Urologic Cancer and the Michael and Zea Wiener Foundation. Dr. Sfakianos is a research fellow in urologic oncology supported by NIH T32-CA82088.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dinney CP, McConkey DJ, Millikan RE, et al. Focus on bladder cancer. Cancer cell. 2004;6(2):111–116. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, Matos T, Cordon-Cardo C. Molecular pathways of urothelial development and bladder tumorigenesis. Urologic oncology. 2010;28(4):401–408. doi: 10.1016/j.urolonc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 4.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. Journal of clinical oncology. 2004;22(14):2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 5.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annual review of biochemistry. 2001;70:247–279. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 6.Ching CB, Hansel DE. Expanding therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway. Laboratory investigation. 2010;90(10):1406–1414. doi: 10.1038/labinvest.2010.133. [DOI] [PubMed] [Google Scholar]
- 7.Strimpakos AS, Karapanagiotou EM, Saif MW, Syrigos KN. The role of mTOR in the management of solid tumors: an overview. Cancer treatment reviews. 2009;35(2):148–159. doi: 10.1016/j.ctrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Aveyard JS, Skilleter A, Habuchi T, Knowles MA. Somatic mutation of PTEN in bladder carcinoma. British journal of cancer. 1999;80(5-6):904–908. doi: 10.1038/sj.bjc.6690439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuruta H, Kishimoto H, Sasaki T, H, et al. Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer research. 2006;66(17):8389–8396. doi: 10.1158/0008-5472.CAN-05-4627. [DOI] [PubMed] [Google Scholar]
- 10.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133(3):403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 12.Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. Journal of the National Cancer Institute. 1999;91(22):1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 13.Saal LH, Gruvberger-Saal SK, Persson C, L, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nature genetics. 2008;40(1):102–107. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer cell. 2003;4(4):257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 15.Sun CH, Chang YH, Pan CC. Activation of the PI3K/Akt/mTOR pathway correlates with tumour progression and reduced survival in patients with urothelial carcinoma of the urinary bladder. Histopathology. 2011;58(7):1054–1063. doi: 10.1111/j.1365-2559.2011.03856.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Choi SK, Ro JY. Overexpression of DJ-1 and HSP90alpha, and loss of PTEN associated with invasive urothelial carcinoma of urinary bladder: Possible prognostic markers. Oncology letters. 2012;3(3):507–512. doi: 10.3892/ol.2011.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Beltran A, Jimenez RE, Montironi R, et al. Flat urothelial carcinoma in situ of the bladder with glandular differentiation. Human pathology. 2011;42(11):1653–1659. doi: 10.1016/j.humpath.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Han KS, Jeong IG, Joung JY, et al. Clinical value of PTEN in patients with superficial bladder cancer. Urologia internationalis. 2008;80(3):264–269. doi: 10.1159/000127338. [DOI] [PubMed] [Google Scholar]
- 19.Goebell PJ, Knowles MA. Bladder cancer or bladder cancers? Genetically distinct malignant conditions of the urothelium. Urologic oncology. 2010;28(4):409–428. doi: 10.1016/j.urolonc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Korkolopoulou P, Levidou G, Trigka EA, et al. A comprehensive immunohistochemical and molecular approach to the PI3K/AKT/mTOR (phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene/mammalian target of rapamycin) pathway in bladder urothelial carcinoma. BJU international. 2012;110:E1237–1248. doi: 10.1111/j.1464-410X.2012.11569.x. [DOI] [PubMed] [Google Scholar]
- 21.Schultz L, Albadine R, Hicks J, et al. Expression status and prognostic significance of mammalian target of rapamycin pathway members in urothelial carcinoma of urinary bladder after cystectomy. Cancer. 2010;116(23):5517–5526. doi: 10.1002/cncr.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mundhenk J, Hennenlotter J, Zug L, et al. Evidence for PTEN-independent Akt activation and Akt-independent p27(Kip1) expression in advanced bladder cancer. Oncology letters. 2011;2(6):1089–1093. doi: 10.3892/ol.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]