Abstract
Mounting evidence suggests that a more extensive surgical resection is associated with an improved life expectancy for both low-grade and high-grade glioma patients. However, radiographically complete resections are not often achieved in many cases due to the lack of sensitivity and specificity of current neurosurgical guidance techniques at the margins of diffuse infiltrative gliomas. Intraoperative fluorescence imaging offers the potential to improve the extent of resection and to investigate the possible benefits of resecting beyond the radiographic margins. Here, we provide a review of wide-field and high-resolution fluorescence-imaging strategies that are being developed for neurosurgical guidance, with a focus on emerging imaging technologies and clinically viable contrast agents. The strengths and weaknesses of these approaches will be discussed, as well as issues that are being addressed to translate these technologies into the standard of care.
Keywords: Fluorescence-image guided surgery, gliomas, extent of resection, intraoperative high-resolution confocal microscopy, 5-ALA-induced PpIX
INTRODUCTION
Glioma resection in neurosurgery currently relies upon visual observation of tissues with low-power (low-resolution) surgical microscopes and neuronavigation based on magnetic resonance imaging (MRI). In recent years, MR-based neuronavigation and intraoperative MRI, coupled with intraoperative mapping techniques and functional MRI, have enabled improved radiographic and functional outcomes, yet still present major drawbacks. In particular, because the margins of gliomas are diffuse and ill-defined, the boundaries indicated by T1-weighted Gadolinium-enhanced MRI (for high-grade gliomas), or T2-weighted hyperintensity (for low-grade gliomas) are ambiguous and do not necessarily correspond to biological metrics such as histopathological grade or tumor-cell density. In addition, brain shift is a major impediment for neuronavigation-based resections. While intraoperative MRI allows for periodic image acquisition during an operation to mitigate brain-shift artifacts, this technology is time-consuming, expensive, and does not circumvent the aforementioned limitations of conventional structural imaging.
For the neurosurgical oncologist, the balance of maximal cytoreduction and functional preservation remain central challenges, as the process of determining an ideal resection margin suffers from insufficient and unreliable visual, tactile, and radiographic data. Although considerable controversy persists regarding the clinical benefits of more extensive extent of resection for low- and high-grade gliomas, mounting literature continues to support this approach.1,2 While an analysis of glioma extent of resection is not within the scope of this technology-focused review, quantitative intraoperative imaging could provide the evidence base necessary to determine an optimal resection threshold that maximizes patient outcomes. For example, it may be possible to evaluate the benefits of resection margins that are beyond, or independent of, the radiographic margins (i.e., “supramarginal resection”).3,4 Furthermore, such technologies could allow clinical researchers to investigate the correlation between qualitative radiographic margins and quantitative biological metrics such as tumor cell density.
In light of the continued need for improved image-guidance technologies to investigate and improve glioma resection, there is rising interest in optical imaging for neurosurgical guidance. Here, we describe wide-field optical image-guidance technologies currently under clinical and preclinical development, including imaging hardware and clinically viable contrast agents. Our discussion is organized into the following sections: (1) wide-field fluorescence image-guided surgery (FIGS), which is approved in some parts of Europe and under investigation in the United States; (2) fluorescence contrast agents, the majority of which are still in preclinical development; and (3) high-resolution intraoperative imaging technologies in preclinical and first-in-human stages of development.
WIDE-FIELD FLUORESCENCE IMAGING
Wide-field fluorescence imaging refers to low-power surgical microscopy in which full image fields are acquired continuously through the use of an eyepiece, and/or at a rapid frame rate with a digital detector array (CMOS or CCD camera). The field of view of a wide-field system typically ranges from tens to hundreds of millimeters along any given lateral dimension, with a spatial resolution of tens to hundreds of microns. Here, wide-field systems are assumed to be low-resolution imaging systems as opposed to high-resolution imaging systems that are capable of clearly visualizing individual cells (<10 micron resolution). The most popular commercially available surgical microscopes for wide-field fluorescence image-guided surgery FIGS have been the Zeiss Pentero™ BLUE400 and the Leica FL400 microscopes, both utilized in numerous clinical studies and approved for routine use in the United States and Europe. In recent years, there have been numerous studies demonstrating the benefits of FIGS for the resection of high-grade gliomas,5–9 the overwhelming majority of which have utilized a specific contrast agent known as 5-aminolevulinic acid (5-ALA).For example, a landmark phase-III study in Europe by Stummer et al. demonstrated that the use of FIGS with 5-ALA contrast resulted in higher rates of GTR (65% vs. 36%) and 6-month progression-free survival (41% vs. 21.1%) compared to control patients.7 Although this study was not powered to assess overall survival, nor were surgeons permitted to use intraoperative neuronavigation during tumor resection, intraoperative 5-ALA fluorescence has since emerged as an important new adjunct for high-grade glioma surgeries.5,6,8 An in-depth discussion of 5-ALA and other contrast agents will be provided later in this article.
Limitations of Wide-Field Fluorescence Image-Guided Surgery
(1) Ambiguity at Margins
As with any wide-field imaging technique, it is not possible to quantitatively or reproducibly define a margin for gliomas. Wide-field visualization of 5-ALA-induced PpIX contrast is effective at revealing regions of bulk tumor (deep red), but fluorescence intensities decay near the margins (lighter pink) and vanish entirely as the tumor cell density continues to decline. On histopathological examination, however, all three territories demonstrate glioma cell infiltration.10
(2) Working Distance and Angle
It is necessary to maintain a constant working distance and a perpendicular angle between the wide-field microscope and the tissue for optimal fluorescence visualization. Tumor cavities are often out-of-range for the fixed-working-distance fluorescence microscopes used for FIGS. In addition, sidewalls are difficult to visualize because of their steep angle with respect to the illumination source and optics. Finally, tumors located within sulci and behind bends are inaccessible without a miniature imaging probe.8
(3) Sensitivity
Wide-field FIGS with 5-ALA remains ineffective for LGGs, as it does not produce visible fluorescence for >95% of low-grade tumors (Fig. 1B). In a few cases, heterogeneous fluorescence has been noted in focal areas of anaplastic transformation. However, the vast majority of LGGs are invisible with 5-ALA.9,11–17 Interestingly, PpIX fluorescence can be measured ex vivo in LGG tissue following 5-ALA administration.11,12 In these analyses, the resultant fluorescence intensity of the tumor tissue is significantly higher than in similarly treated normal tissue12 and increases with both tumor grade and proliferative index.10,11,18
Figure 1.
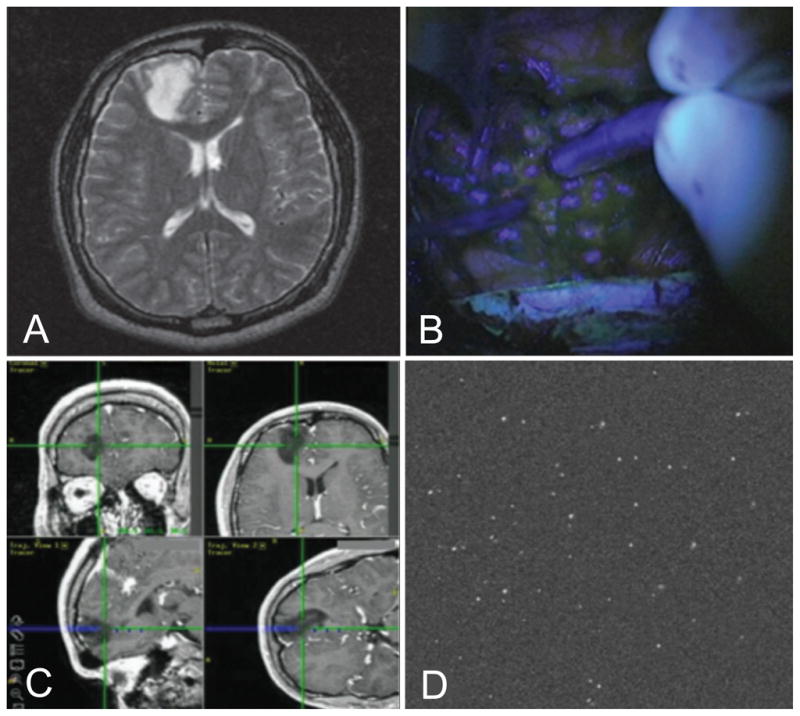
(A) MRI of low-grade glioma. (B) Wide-field FIGS with undetectable fluorescence. (C) Intraoperative neuronavigation showing the location of a confocal microscope within the tumor cavity. (D) Multiple fluorescent cells (> 30) are visualized within the 450 × 450 μm field of view of a miniature confocal microscope.13
(4) Quantification
A central challenge to fluorescence imaging is the accurate quantification of contrast agents at each resolvable tissue location. This task is often complicated by background and variations in tissue optical properties that can affect the amount of optical signal that is detected from each tissue location (image pixel). Advanced FIGS systems are now utilizing multispectral imaging to correct for tissue optical properties and the nonspecific accumulation of molecular contrast agents.19–21 These methods typically utilize ratiometric imaging (normalization), either between fluorescence measurements19–22 or a combination of fluorescence and light attenuation images.23,24 The SurgOptix T3-platform©, currently in phase II clinical trials, is based on one such multispectral imaging technology in which three camera channels are used to simultaneously image color images (conventional reflected light), fluorescence, and light absorption.23,24 By doing so, as shown in Figs. 2D – 2F from a preclinical mouse model injected with fluorescence contrast, the biodistribution of the fluorescent tracer is more accurately quantified and visualized. Another system under development is the FLARE™ system,25–27 which has incorporated color imaging with dual-band fluorescence imaging (Figs. 2A – 2C). This system utilizes an automated background subtraction algorithm in its operation, similar to the Zeiss OPMI Pentero® system, which isolates fluorescence emission from the excitation source, from other sources of background light in the operating room. Figure 2 contains representative images obtained using advanced FIGS systems.
Figure 2.
Example images obtained using advanced FIGS systems. (A–C) Sentinel lymph node (SLN) mapping using the FLARE™ system. (A) White-light image of SLNs. (B) 800 nm NIR fluorescence image following injection of 10 μM ICG:HSA (C) Pseudo-colored (lime green) overlay of the previous two (A,B) images.27 (D–F) Postmortem imaging of the surgically exposed mouse abdominal area, demonstrating light absorption correction. (D) White-light image. The double arrow indicates the inferior vena cava, while single-line arrows point to the lumbar lymph nodes. (E) Conventional fluorescence image showing poor contrast between lymph nodes and background, following injection of 8 μM Alexa Fluor 750. (F) Normalized/corrected image showing improved fluorescence contrast and quantification of signal from lymph nodes.24 (G–I) Quantitative fluorescence imaging (qFI) of a human GBM during surgery. Three hours prior to surgery, patient had received 20 mg/kg of ALA. (G) White-light image at the beginning of surgery. (H) Corresponding conventional fluorescence image. (I) Quantitative fluorescence image overlaid with the white-light view.61
FLUORESCENT CONTRAST AGENTS FOR NEUROSURGERY
Although several types of contrast agents are being developed for intraoperative surgical oncology, an extremely limited set of agents have been used in vivo during clinical studies. The overwhelming majority of agents have only been tested inpreclinical models, ranging from in vitro tissue cultures to ex vivo and in vivo animal models. Furthermore, certain examples provided below have only been tested on tumors outside of the brain and require further preclinical validation to assess their benefits for delineating gliomas. While a discussion of regulatory strategies is beyond the scope of this article, published case studies and reviews cover some of these issues, including the experiences of the PET and MRI communities.28,29
Fluorescent contrast agents can be categorized into three modes of tumor targeting: (1) passive, (2) metabolic, and (3) molecular. In practice, image contrast often results from a combination of these targeting mechanisms.
-
Passive targeting refers to the accumulation of contrast agents in various tissues due to diffusive processes commonly referred to as enhanced permeability and retention effects (EPR). Gadolinium is an example of an agent that largely relies upon disruption of the blood-brain-barrier to indicate brain tumor regions for MR imaging.
A small number of passively targeted optical contrast agents have been approved by the US FDA for clinical use, including sodium fluorescein, indocyanine green (ICG), and methylene blue. The use of these agents has not been approved for neurosurgery and their off-label use for brain-tumor resection should therefore be justified.30 Fluorescein and ICG are commonly used for retinal angiography and tend to remain within the circulatory network for several minutes after intravenous administration. Over time, these agents extravasate out of the vasculature and provide tissue and intercellular contrast, especially in tumor regions due to a compromised blood-brain barrier and leakiness in the tumor vasculature. A number of groups have investigated the use of these agents for both wide-field FIGS31–35 and high-resolution microscopy36–40 to guide brain tumor resection (Fig. 3A–C). Because of the passive nature of the delivery of these agents, as well as the non-specificity of their fluorescence, there is considerable ambiguity about the extent to which these contrast agents can correlate with histopathological diagnoses of tumor grade and tumor-cell density at the margins.
-
Metabolic targeting refers to a contrast agent that is activated or converted from a dark (eg. non-fluorescent) configuration into a signal-generating form due to a metabolic process that is highly active in the tissue of interest. Currently, the most widely investigated contrast agent for FIGS in the context of brain tumor resection is the metabolic agent 5-aminolevulinic acid (5-ALA or δ-ALA). In patients, 5-aminolevulinic acid (5-ALA) has been used as an orally administered prodrug that is intracellularly metabolized to form the fluorescent molecule protoporphyrin IX (PpIX).9,18,41–46 This heme-synthesis pathway substrate accumulates preferentially in tumor cells and epithelial tissues and emits red fluorescence (λ = 620–720 nm) when excited with violet light (λ = 400–410 nm). While disruption of the blood brain barrier (BBB) may play a role in the accumulation of 5-ALA in tumor tissues, studies have shown that 5-ALA-induced PpIX can also be generated strongly in brain tissues where the BBB is functional, suggesting that 5-ALA can cross the BBB.44 While additional studies are needed to fully elucidate the molecular pathways, cell-cell interactions, and cellular phenotypes that generate 5-ALA-induced PpIX, numerous studies have utilized this metabolic agent for both FIGS and photodynamic tumor therapy.
Recently, there has been increasing interest in developing a class of optical contrast agents that have been referred to as activity-based probes, fluorescence-quenched probes, or activatable probes (Fig. 3C).46–53 In general, these agents consist of a fluorophore that is prepared in a dark state by positioning a “quenching” molecule or object (i.e., a nanoparticle or nanotube) adjacent to the fluorophore. This quencher is typically designed to be removed or reconfigured through the action of an enzyme of interest, such as a protease. These strategies are promising for the imaging of aggressive tumors, which often generate large amounts of proteases to enable them to infiltrate their surroundings. However, in spite of numerous successful preclinical demonstrations, to our knowledge none have yet been approved for clinical studies. An example of a preclinical image obtained with an activity-based probe is shown in Fig. 3I, in which a probe is topically applied on normal brain (left) and tumor (right) in a mouse model causing an elevated fluorescence signal at the site of the tumor.49 In this case, the activity-based probe is unquenched by tumor-associated proteases (primarily cathepsin L), and also binds covalently to its protease target, which results in reduced washout of the activated probe.54
Molecular targeting and the field of molecular imaging has experienced explosive growth in recent years.51,55–59 Accessible cell-surface receptors such as EGFR and HER2 have been popular targets for imaging agents, just as they have been popular for the development of targeted pharmaceuticals. A recent article summarizes many of the biomarkers that have been popular targets for the development of imaging agents.59 A challenge with molecularly targeted contrast agents is distinguishing between specific binding of contrast agents with their antigen targets, versus nonspecific binding and accumulation of contrast agents. Regardless of whether molecular probes are delivered systemically or topically, uneven probe delivery and poor washout of unbound probes may create misleading sources of contrast. This is especially true considering the passive EPR effects mentioned previously, which affect the delivery of all exogenous agents. Ratiometric optical methods, utilizing dual-labeled probes, have been shown to greatly improve the ability to quantify the specific vs. nonspecific binding of exogenously applied fluorescent agents, as well as to quantify chemical binding potentials (see Figs. 3D–H).19–21 Recent efforts have also focused on the development of molecular probes to label normal brain structures, rather than tumors, in order to minimize iatrogenic injury to critical structures such as nerves.60
Figure 3.
Representative fluorescent images utilizing passive, metabolic and molecular contrast agents. (A–C) Example images obtained utilizing a passive agent, sodium fluorescein (Yellow 560). (A) Intraoperative white-light image of a metastatic brain tumor (B) Corresponding conventional fluorescence image. (C) Resection of lesion performed with fluorescence image guidance.33 (D–G) Fluorescence-guided, stepwise resection of a murine RCAS-PDGF glioblastoma, following injection of IRDye 800CW-conjugated RGD peptide (molecular imaging). The tumor (indicated by the black arrowheads) was exposed and a 2-step resection was conducted to remove the glioblastoma tissue (D) White-light image of exposed tumor. (E) Corresponding fluorescence image false-colored and overlaid with a white-light image. (F) Fluorescence image after first resection. (G) Fluorescence image following second resection.57 (H) Example image using a molecular contrast agent. Raw fluorescence image (false-colored) of a tumor margin stained with a VEGFR-1 probe (785-nm fluorescence excitation).105 (I) Example image of a metabolic contrast agent. Topical application of the quenched activity-based probe (qABP) GB119 reveals residual tumor in the right side of a mouse brain. The normal tissue on the left side of the brain does not generate strong fluorescence when GB119 is topically applied.49(J–M) Dual-reporter imaging of a human-neuronal glioblastoma (U251) tumor in a mouse at 1 hr post injection. (J) White-light image. The white arrows signal the location of the tumor. (K) Corresponding untargeted fluorescence uptake (IRdye 700DX), (L) Targeted fluorescence uptake (EGF-IRdye 800CW), and (M) dual-reporter image of the A431 tumor line. By utilizing a secondary, untargeted imaging reporter, this technique compensates for nonspecific uptake of the targeted fluorophore, hence achieving a more accurate image of molecular expression.20
With respect to the surgical workflow, contrast agents should ideally be administered intraoperatively or within 2 to 3 hours of incision. Passive agents such as fluorescein and ICG are generally intravenously injected during surgery immediately prior to imaging. Metabolic and molecular agents often require time to allow for metabolic activation or for binding to molecular targets, respectively. For example, 5-ALA is administered orally to patients 3 hours prior to the initiation of surgery since studies have shown that the production of PpIX in tumors peaks at approximately 6 hours after administration (ideally coinciding with the final stages of tumor resection)8. Topical application requires rapid activation or binding of contrast agents, preferably on a time scale of several minutes or less, in order to prevent surgical delays. For example, in Cutter et al. 54, an activity-based probe is activated by proteases within 5 to 20 min after topical application on brain tumor tissues. One potential challenge with topically applied probes is the limited penetration depth through tissue, such that multiple applications may be needed to guide the final stages of resection as tissues are progressively removed at the margins.
HIGH-RESOLUTION INTRAOPERATIVE MICROSCOPY
Rationale
In spite of the demonstrated clinical benefits of wide-field FIGS with 5-ALA contrast, a number of challenges remain. In particular, poor sensitivity and specificity of glioma detection with wide-field FIGS has been reported, especially for tumor subtypes that are less proliferative and accumulate less PpIX than high-grade gliomas (e.g. the low-grade glioma shown in Fig. 1B). 9,11–16 Some have advocated for the use of frozen-section pathology to confirm tissue status during glioma resection,8 which is time consuming and invasive. Others have suggested the need for quantitative measurements of PpIX fluorescence, such as with spectral measurement probes.9,12,61 These issues suggest that a potentially powerful complement to wide-field FIGS would be an intraoperative high-resolution optical-sectioning microscope that could be placed directly in contact with tissues to visualize and quantify the presence of labeled cells in real time. Wide-field imaging, in which each resolvable pixel represents an average signal from hundreds of cells, often lacks the sensitivity to detect the presence of sparse cell populations or microscopic punctate fluorescent structures, such as at the margins of all diffuse tumors or in LGGs. Interestingly, it has recently been reported that intraoperative high-resolution microscopy can visualize and quantify the presence of sparse and punctate PpIX generated in low-grade gliomas (Fig. 1D) even when wide-field imaging fails (Fig. 1B).13 The ability to resolve and detect sparse subpopulations of labeled cells, such as tumors cells at the diffuse margins of a glioma, could provide a standardized quantitative metric by which neurosurgeons may eventually be able to optimize their resections as well as to objectively quantify an unambiguous “extent-of-resection” for their surgeries.
Limitations
An important trade-off for high-resolution microscopy is its small field of view – typically on the order of 0.5 mm in diameter. Additional challenges include the need to visualize microscopic images on a separate monitor located near the patient, as well as the surgeon’s need to hold a portable imaging device with sufficient stability and dexterity to produce high-quality images. Nevertheless, recent reports on the use of intraoperative handheld microscopy13,36,37,62 and portable spectroscopy9,10,61 have suggested the clinical utility of performing a real-time “optical biopsy” of select regions of the resection cavity considered to be at high risk for residual tumor. For example, in eloquent territories of the brain, a real-time high-resolution “optical biopsy” would provide an accurate measurement that, in combination with intraoperative neuronavigation and other surgical cues, could better inform the surgeon’s intraoperative decisions regarding extent of resection vs. functional preservation. While current neurosurgical tools are limited to a precision of no better than ~0.5 cm, a high-resolution microscope image can provide a point measurement (a real-time, noninvasive alternative to biopsy) that facilitates a rapid risk-benefit assessment of further resection. Real-time image-processing algorithms would ultimately need to be developed to quantify fluorescence expression and to display this information to surgeons.
Intraoperative microscope technology
In recent years, miniature high-resolution microscopes have played a critical role in the rapidly developing field of in vivo microendoscopy and point-of-care pathology for both clinical applications and biological investigations. Microscope designs have utilized various approaches towards achieving optical sectioning such as multiphoton excitation,63 single-axis confocal microscopy,64,65 dual-axis confocal (DAC) microscopy66 and structured illumination.67–69 Figure 4 provides an example of two such microscope designs and representative images. Designs have also differed in their scanning mechanisms, including proximally scanned coherent fiber bundles,70–79 distally scanned fiber tips,80–84 and MEMS scanners.66,83,85–91
Figure 4.

(A) A handheld confocal microscope (Zeiss Optiscan®) used during neurosurgery.37 (B) Zeiss Optiscan® image revealing localized subcellular 5-ALA-induced tumor fluorescence in a low-grade glioma.13 (C) Intraoperative confocal image of tumor microvasculature following injection of sodium fluorescein.37 (D) Miniature dual-axis confocal microscope prototype.66 (E) Image of GFP-expressing tumor cells in a spontaneous mouse model of medulloblastoma. (F) Fluorescence image of mouse brain vasculature (depth 55 μm)98 following retro-orbital injection of fluorescein-dextran.
To date, the only high-resolution optical-sectioning microscope that has been used in vivo for brain tumor resection in humans is the Zeiss Optiscan® system.13,36,37 As a result of the unique resonant-scanning mechanism used for imaging, this microscope can be limited by a slow frame rate (0.8 frames/sec) that leads to motion artifacts and makes the clinical use of the device less effective. In addition, current devices have a fixed illumination wavelength and optics that are optimized at 488 nm, which is not ideal for imaging PpIX fluorescence (405-nm illumination wavelength, 625-nm emission). Figs. 4B–C show example images obtained using this system. Additional surgical microscope technologies for brain tumor resection are under various stages of laboratory or preclinical development, and may provide certain advantages such as faster frame rates.
A number of groups and at least one company (Mauna Kea Technologies, Paris, France) have developed miniature microscopes based on coherent fiber-bundle technologies.71,72,74–76,78,92 These confocal microscopes treat each fiber within the bundle as a unique confocal pinhole for spatial filtering of out-of-focus and scattered light for high-contrast imaging of tissues at modest depths (0–200 microns). Proximal scanning allows the distal tip of these devices to be extremely small (0.5 to 3 mm) and flexible. One disadvantage of these technologies is that they often do not allow for axial adjustment of the focal plane since the mechanisms for doing so would significantly increase the size of the distal tip of these devices. While the ability to image deeply is not a fundamental necessity for intraoperative determinations of tissue status, there are practical advantages for being able to adjust the focal plane of an optical-sectioning device during surgery. For example, adjustment of the axial imaging depth allows the surgeon to search for an optimal imaging plane in which the tissues show minimal signs of surgical disruption, and at which signal levels and contrast are optimal. Another limitation to fiber-bundle-based approaches is that current fiber-bundle manufacturers utilize ion-doped glass fibers that create large autofluorescence backgrounds when excited at 405 nm.93 This is less of an issue at other excitation wavelengths, such as at 488 nm, but the autofluorescence background limits the ability of these technologies to be utilized for imaging 5-ALA-induced PpIX, in which the optimal absorption peak is at 405 nm.
In addition to miniature optical-sectioning devices that utilize conventional single-axis confocal approaches, recent efforts have been made to develop intraoperative microscopes using an alternative confocal architecture called a dual-axis confocal microscope or a divided-pupil confocal microscope.66,94–101 In the dual-axis confocal architecture, the illumination and collection beam paths are spatially separated, as opposed to the common-path configuration of typical microscopes. Simulations and experiments have shown that the dual-axis confocal configuration provides certain benefits in terms of optical-sectioning contrast in tissues, including the ability to image at deeper depths compared to conventional single-axis confocal microscopes.96,99,102,103 In addition, dual-axis designs, which utilize low-NA (weakly focusing) beams as opposed to the high-NA beams preferred for conventional confocal microscopy, have been shown to be scalable in portable devices with diameters ranging from 5 – 10 mm.98,101,104 These devices have utilized miniature microelectromechanical systems (MEMS) scanning mirrors to scan an image within tissues (Fig. 4D–F) at a high frame rate (up to 30 Hz),101 which is beneficial in clinical settings to reduce motion artifacts (image blur) during handheld use.
CONCLUSION
Fluorescence image-guided surgery is emerging as a valuable technology to improve glioma resections. Numerous contrast agents are in various stages of preclinical and clinical development. 5-ALA-induced PpIX, in particular, has been shown to be a relatively reliable biomarker for gliomas. However, there are still a number of limitations inherent to all wide-field (low-resolution) imaging techniques (i.e., FIGS and MRI), such as limited sensitivity to detect glioma infiltration at the margins and ambiguous image contrast. Recent works suggest that intraoperative high-resolution microscopy, a real-time alternative to invasive biopsy and histopathology, has the potential to better quantify tumor burden at the final stages of surgery and ultimately to improve patient outcomes when combined with wide-field imaging approaches. Additional studies are needed to further elucidate the clinical benefits of these new technologies for glioma patients. In particular, the desire to increase the extent of resection must be balanced by neurological morbidity. As imaging technologies improve in their ability to rapidly quantify tumor burden over large fields of view, clinical studies will eventually determine precise resection thresholds based upon cellular tumor burden for patients with gliomas.
Acknowledgments
FINANCIAL SUPPORT: We would like to acknowledge funding support from the National Institute for Biomedical Imaging and Bioengineering: R00 EB008557 (Liu), the National Institute of Dental and Craniofacial Research: R01 DE023497 (Liu), the National Institute of Neurological Diseases and Stroke: R01 NS082745 (Sanai), and the National Cancer Institute: R01 CA175391 (Liu and Sanai).
Footnotes
DISCLOSURE: The authors report no conflict of interest concerning the surgical methods reviewed in this paper.
References
- 1.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 2.Hardesty DA, Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol. 2012;3:140. doi: 10.3389/fneur.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffau H, Mandonnet E. The “onco-functional balance” in surgery for diffuse low-grade glioma: integrating the extent of resection with quality of life. Acta Neurochir (Wien ) 2013;155:951–957. doi: 10.1007/s00701-013-1653-9. [DOI] [PubMed] [Google Scholar]
- 4.Duffau H. A new concept of diffuse (low-grade) glioma surgery. Adv Tech Stand Neurosurg. 2012;38:3–27. doi: 10.1007/978-3-7091-0676-1_1. [DOI] [PubMed] [Google Scholar]
- 5.Liao H, Noguchi M, Maruyama T, et al. An integrated diagnosis and therapeutic system using intra-operative 5-aminolevulinic-acid-induced fluorescence guided robotic laser ablation for precision neurosurgery. Med Image Anal. 2012;16:754–766. doi: 10.1016/j.media.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Nabavi A, Thurm H, Zountsas B, et al. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery. 2009;65:1070–1076. doi: 10.1227/01.NEU.0000360128.03597.C7. [DOI] [PubMed] [Google Scholar]
- 7.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 8.Tonn JC, Stummer W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: practical use, risks, and pitfalls. Clin Neurosurg. 2008;55:20–26. [PubMed] [Google Scholar]
- 9.Valdes PA, Leblond F, Kim A, et al. Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg. 2011;115:11–17. doi: 10.3171/2011.2.JNS101451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdes PA, Kim A, Brantsch M, et al. delta-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: the need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro Oncol. 2011;13:846–856. doi: 10.1093/neuonc/nor086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floeth FW, Sabel M, Ewelt C, et al. Comparison of (18)F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur J Nucl Med Mol Imaging. 2011;38:731–741. doi: 10.1007/s00259-010-1690-z. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara R, Katayama Y, Watanabe T, Yoshino A, Fukushima T, Sakatani K. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol Med Chir (Tokyo) 2007;47:53–57. doi: 10.2176/nmc.47.53. [DOI] [PubMed] [Google Scholar]
- 13.Sanai N, Snyder LA, Honea NJ, et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011;115:740–748. doi: 10.3171/2011.6.JNS11252. [DOI] [PubMed] [Google Scholar]
- 14.Stockhammer F, Misch M, Horn P, Koch A, Fonyuy N, Plotkin M. Association of F18-fluoro-ethyl-tyrosin uptake and 5-aminolevulinic acid-induced fluorescence in gliomas. Acta Neurochir (Wien ) 2009;151:1377–1383. doi: 10.1007/s00701-009-0462-7. [DOI] [PubMed] [Google Scholar]
- 15.Valdes PA, Kim A, Leblond F, et al. Combined fluorescence and reflectance spectroscopy for in vivo quantification of cancer biomarkers in low- and high-grade glioma surgery. J Biomed Opt. 2011;16:116007. doi: 10.1117/1.3646916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widhalm G, Wolfsberger S, Minchev G, et al. 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with nonsignificant contrast enhancement. Cancer. 2010;116:1545–1552. doi: 10.1002/cncr.24903. [DOI] [PubMed] [Google Scholar]
- 17.Widhalm G, Kiesel B, Woehrer A, et al. 5-aminolevulinic Acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS One. 2013;8:e76988. doi: 10.1371/journal.pone.0076988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stummer W, Stepp H, Moller G, Ehrhardt A, Leonhard M, Reulen HJ. Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir (Wien ) 1998;140:995–1000. doi: 10.1007/s007010050206. [DOI] [PubMed] [Google Scholar]
- 19.Liu JT, Helms MW, Mandella MJ, Crawford JM, Kino GS, Contag CH. Quantifying cell-surface biomarker expression in thick tissues with ratiometric three-dimensional microscopy. Biophys J. 2009;96:2405–2414. doi: 10.1016/j.bpj.2008.12.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tichauer KM, Samkoe KS, Sexton KJ, Gunn JR, Hasan T, Pogue BW. Improved tumor contrast achieved by single time point dual-reporter fluorescence imaging. J Biomed Opt. 2012;17:066001. doi: 10.1117/1.JBO.17.6.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tichauer KM, Samkoe KS, Sexton KJ, et al. In vivo quantification of tumor receptor binding potential with dual-reporter molecular imaging. Mol Imaging Biol. 2012;14:584–592. doi: 10.1007/s11307-011-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogaards A, Sterenborg HJ, Trachtenberg J, Wilson BC, Lilge L. In vivo quantification of fluorescent molecular markers in real-time by ratio imaging for diagnostic screening and image-guided surgery. Lasers Surg Med. 2007;39:605–613. doi: 10.1002/lsm.20525. [DOI] [PubMed] [Google Scholar]
- 23.van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 24.Themelis G, Yoo JS, Soh KS, Schulz R, Ntziachristos V. Real-time intraoperative fluorescence imaging system using light-absorption correction. J Biomed Opt. 2009;14:064012. doi: 10.1117/1.3259362. [DOI] [PubMed] [Google Scholar]
- 25.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–255. [PMC free article] [PubMed] [Google Scholar]
- 26.Keereweer S, Kerrebijn JD, van Driel PB, et al. Optical image-guided surgery--where do we stand? Mol Imaging Biol. 2011;13:199–207. doi: 10.1007/s11307-010-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrio JR, Marcus CS, Hung JC, Keppler JS. A rational regulatory approach for positron emission tomography imaging probes: from “first in man” to NDA approval and reimbursement. Mol Imaging Biol. 2004;6:361–367. doi: 10.1016/j.mibio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman JM, Gambhir SS, Kelloff GJ. Regulatory and reimbursement challenges for molecular imaging. Radiology. 2007;245:645–660. doi: 10.1148/radiol.2453060737. [DOI] [PubMed] [Google Scholar]
- 30.Reimer P, Vosshenrich R. Off-label use of contrast agents. Eur Radiol. 2008;18:1096–1101. doi: 10.1007/s00330-008-0886-0. [DOI] [PubMed] [Google Scholar]
- 31.Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuda T, Yoshioka H, Kato A. Fluorescence-guided surgery for glioblastoma multiforme using high-dose fluorescein sodium with excitation and barrier filters. J Clin Neurosci. 2012;19:1719–1722. doi: 10.1016/j.jocn.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 33.Rey-Dios R, Cohen-Gadol AA. Technical principles and neurosurgical applications of fluorescein fluorescence using a microscope-integrated fluorescence module. Acta Neurochir (Wien ) 2013;155:701–706. doi: 10.1007/s00701-013-1635-y. [DOI] [PubMed] [Google Scholar]
- 34.Schebesch KM, Proescholdt M, Hohne J, et al. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery--a feasibility study. Acta Neurochir (Wien ) 2013;155:693–699. doi: 10.1007/s00701-013-1643-y. [DOI] [PubMed] [Google Scholar]
- 35.Kuroiwa T, Kajimoto Y, Ohta T. Development of a fluorescein operative microscope for use during malignant glioma surgery: a technical note and preliminary report. Surg Neurol. 1998;50:41–48. doi: 10.1016/s0090-3019(98)00055-x. [DOI] [PubMed] [Google Scholar]
- 36.Eschbacher J, Martirosyan NL, Nakaji P, et al. In vivo intraoperative confocal microscopy for real-time histopathological imaging of brain tumors. J Neurosurg. 2012;116:854–860. doi: 10.3171/2011.12.JNS11696. [DOI] [PubMed] [Google Scholar]
- 37.Sanai N, Eschbacher J, Hattendorf G, et al. Intraoperative confocal microscopy for brain tumors: a feasibility analysis in humans. Neurosurgery. 2011;68:282–290. doi: 10.1227/NEU.0b013e318212464e. [DOI] [PubMed] [Google Scholar]
- 38.Schlosser HG, Suess O, Vajkoczy P, van Landeghem FK, Zeitz M, Bojarski C. Confocal neurolasermicroscopy in human brain - perspectives for neurosurgery on a cellular level (including additional comments to this article) Cent Eur Neurosurg. 2010;71:13–19. doi: 10.1055/s-0029-1237735. [DOI] [PubMed] [Google Scholar]
- 39.Snuderl M, Wirth D, Sheth SA, et al. Dye-enhanced multimodal confocal imaging as a novel approach to intraoperative diagnosis of brain tumors. Brain Pathol. 2013;23:73–81. doi: 10.1111/j.1750-3639.2012.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wirth D, Snuderl M, Sheth S, et al. Identifying brain neoplasms using dye-enhanced multimodal confocal imaging. J Biomed Opt. 2012;17:026012. doi: 10.1117/1.JBO.17.2.026012. [DOI] [PubMed] [Google Scholar]
- 41.Collaud S, Juzeniene A, Moan J, Lange N. On the selectivity of 5-aminolevulinic acid-induced protoporphyrin IX formation. Curr Med Chem Anticancer Agents. 2004;4:301–316. doi: 10.2174/1568011043352984. [DOI] [PubMed] [Google Scholar]
- 42.Duffner F, Ritz R, Freudenstein D, Weller M, Dietz K, Wessels J. Specific intensity imaging for glioblastoma and neural cell cultures with 5-aminolevulinic acid-derived protoporphyrin IX. J Neurooncol. 2005;71:107–111. doi: 10.1007/s11060-004-9603-2. [DOI] [PubMed] [Google Scholar]
- 43.Gibbs SL, Chen B, O’Hara JA, Hoopes PJ, Hasan T, Pogue BW. Protoporphyrin IX level correlates with number of mitochondria, but increase in production correlates with tumor cell size. Photochem Photobiol. 2006;82:1334–1341. doi: 10.1562/2006-03-11-RA-843. [DOI] [PubMed] [Google Scholar]
- 44.Olivo M, Wilson BC. Mapping ALA-induced PPIX fluorescence in normal brain and brain tumour using confocal fluorescence microscopy. Int J Oncol. 2004;25:37–45. [PubMed] [Google Scholar]
- 45.Roberts DW, Valdes PA, Harris BT, et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg. 2011;114:595–603. doi: 10.3171/2010.2.JNS091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tung CH, Mahmood U, Bredow S, Weissleder R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000;60:4953–4958. [PubMed] [Google Scholar]
- 47.Alencar H, Funovics MA, Figueiredo J, Sawaya H, Weissleder R, Mahmood U. Colonic adenocarcinomas: near-infrared microcatheter imaging of smart probes for early detection--study in mice. Radiology. 2007;244:232–238. doi: 10.1148/radiol.2441052114. [DOI] [PubMed] [Google Scholar]
- 48.Blum G, von DG, Merchant MJ, Blau HM, Bogyo M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat Chem Biol. 2007;3:668–677. doi: 10.1038/nchembio.2007.26. [DOI] [PubMed] [Google Scholar]
- 49.Cutter JL, Cohen NT, Wang J, et al. Topical application of activity-based probes for visualization of brain tumor tissue. PLoS One. 2012;7:e33060. doi: 10.1371/journal.pone.0033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgington LE, Berger AB, Blum G, et al. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat Med. 2009;15:967–973. doi: 10.1038/nm.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hilderbrand SA, Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14:71–79. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi H, Choyke PL. Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc Chem Res. 2011;44:83–90. doi: 10.1021/ar1000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu TW, Chen J, Zheng G. Peptide-based molecular beacons for cancer imaging and therapy. Amino Acids. 2011;41:1123–1134. doi: 10.1007/s00726-010-0499-1. [DOI] [PubMed] [Google Scholar]
- 54.Blum G, Mullins SR, Keren K, et al. Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat Chem Biol. 2005;1:203–209. doi: 10.1038/nchembio728. [DOI] [PubMed] [Google Scholar]
- 55.Achilefu S. Lighting up tumors with receptor-specific optical molecular probes. Technol Cancer Res Treat. 2004;3:393–409. doi: 10.1177/153303460400300410. [DOI] [PubMed] [Google Scholar]
- 56.Alford R, Ogawa M, Choyke PL, Kobayashi H. Molecular probes for the in vivo imaging of cancer. Mol Biosyst. 2009;5:1279–1291. doi: 10.1039/b911307j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang R, Vider J, Kovar JL, et al. Integrin alphavbeta3-targeted IRDye 800CW near-infrared imaging of glioblastoma. Clin Cancer Res. 2012;18:5731–5740. doi: 10.1158/1078-0432.CCR-12-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hellebust A, Richards-Kortum R. Advances in molecular imaging: targeted optical contrast agents for cancer diagnostics. Nanomedicine (Lond) 2012;7:429–445. doi: 10.2217/nnm.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation--a new cutting edge. Nat Rev Cancer. 2013;13:653–662. doi: 10.1038/nrc3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitney MA, Crisp JL, Nguyen LT, et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotechnol. 2011;29:352–356. doi: 10.1038/nbt.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valdes PA, Leblond F, Jacobs VL, Wilson BC, Paulsen KD, Roberts DW. Quantitative, spectrally-resolved intraoperative fluorescence imaging. Sci Rep. 2012;2:798. doi: 10.1038/srep00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sankar T, Delaney PM, Ryan RW, et al. Miniaturized handheld confocal microscopy for neurosurgery: results in an experimental glioblastoma model. Neurosurgery. 2010;66:410–417. doi: 10.1227/01.NEU.0000365772.66324.6F. [DOI] [PubMed] [Google Scholar]
- 63.Grewe BF, Langer D, Kasper H, Kampa BM, Helmchen F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nat Methods. 2010;7:399–405. doi: 10.1038/nmeth.1453. [DOI] [PubMed] [Google Scholar]
- 64.Maitland KC, Gillenwater AM, Williams MD, El-Naggar AK, Descour MR, Richards-Kortum RR. In vivo imaging of oral neoplasia using a miniaturized fiber optic confocal reflectance microscope. Oral Oncol. 2008;44:1059–1066. doi: 10.1016/j.oraloncology.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanbakuchi AA, Rouse AR, Udovich JA, Hatch KD, Gmitro AF. Clinical confocal microlaparoscope for real-time in vivo optical biopsies. J Biomed Opt. 2009;14:044030. doi: 10.1117/1.3207139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu JT, Mandella MJ, Loewke NO, et al. Micromirror-scanned dual-axis confocal microscope utilizing a gradient-index relay lens for image guidance during brain surgery. J Biomed Opt. 2010;15:026029. doi: 10.1117/1.3386055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bozinovic N, Ventalon C, Ford T, Mertz J. Fluorescence endomicroscopy with structured illumination. Opt Express. 2008;16:8016–8025. doi: 10.1364/oe.16.008016. [DOI] [PubMed] [Google Scholar]
- 68.Lim D, Ford TN, Chu KK, Mertz J. Optically sectioned in vivo imaging with speckle illumination HiLo microscopy. J Biomed Opt. 2011;16:016014. doi: 10.1117/1.3528656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neil MA, Juskaitis R, Wilson T. Method of obtaining optical sectioning by using structured light in a conventional microscope. Opt Lett. 1997;22:1905–1907. doi: 10.1364/ol.22.001905. [DOI] [PubMed] [Google Scholar]
- 70.Carlson K, Chidley M, Sung KB, et al. In vivo fiber-optic confocal reflectance microscope with an injection-molded plastic miniature objective lens. Appl Opt. 2005;44:1792–1797. doi: 10.1364/ao.44.001792. [DOI] [PubMed] [Google Scholar]
- 71.Jean F, Bourg-Heckly G, Viellerobe B. Fibered confocal spectroscopy and multicolor imaging system for in vivo fluorescence analysis. Opt Express. 2007;15:4008–4017. doi: 10.1364/oe.15.004008. [DOI] [PubMed] [Google Scholar]
- 72.Laemmel E, Genet M, Le GG, Perchant A, Le Gargasson JF, Vicaut E. Fibered confocal fluorescence microscopy (Cell-viZio) facilitates extended imaging in the field of microcirculation. A comparison with intravital microscopy. J Vasc Res. 2004;41:400–411. doi: 10.1159/000081209. [DOI] [PubMed] [Google Scholar]
- 73.Liang C, Sung KB, Richards-Kortum RR, Descour MR. Design of a high-numerical-aperture miniature microscope objective for an endoscopic fiber confocal reflectance microscope. Appl Opt. 2002;41:4603–4610. doi: 10.1364/ao.41.004603. [DOI] [PubMed] [Google Scholar]
- 74.Makhlouf H, Gmitro AF, Tanbakuchi AA, Udovich JA, Rouse AR. Multispectral confocal microendoscope for in vivo and in situ imaging. J Biomed Opt. 2008;13:044016. doi: 10.1117/1.2950313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muldoon TJ, Pierce MC, Nida DL, Williams MD, Gillenwater A, Richards-Kortum R. Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy. Opt Express. 2007;15:16413–16423. doi: 10.1364/oe.15.016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sabharwal YS, Rouse AR, Donaldson L, Hopkins MF, Gmitro AF. Slit-scanning confocal microendoscope for high-resolution in vivo imaging. Appl Opt. 1999;38:7133–7144. doi: 10.1364/ao.38.007133. [DOI] [PubMed] [Google Scholar]
- 77.Sun Y, Phipps J, Elson DS, et al. Fluorescence lifetime imaging microscopy: in vivo application to diagnosis of oral carcinoma. Opt Lett. 2009;34:2081–2083. doi: 10.1364/ol.34.002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sung KB, Liang C, Descour M, et al. Near real time in vivo fibre optic confocal microscopy: sub-cellular structure resolved. J Microsc. 2002;207:137–145. doi: 10.1046/j.1365-2818.2002.01049.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang TD, Friedland S, Sahbaie P, et al. Functional imaging of colonic mucosa with a fibered confocal microscope for real-time in vivo pathology. Clin Gastroenterol Hepatol. 2007;5:1300–1305. doi: 10.1016/j.cgh.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flusberg BA, Jung JC, Cocker ED, Anderson EP, Schnitzer MJ. In vivo brain imaging using a portable 3.9 gram two-photon fluorescence microendoscope. Opt Lett. 2005;30:2272–2274. doi: 10.1364/ol.30.002272. [DOI] [PubMed] [Google Scholar]
- 81.Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope. high-resolution brain imaging in freely moving animals. Neuron. 2001;31:903–912. doi: 10.1016/s0896-6273(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 82.Lee CM, Engelbrecht CJ, Soper TD, Helmchen F, Seibel EJ. Scanning fiber endoscopy with highly flexible, 1 mm catheterscopes for wide-field, full-color imaging. J Biophotonics. 2010;3:385–407. doi: 10.1002/jbio.200900087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan Y, Xie H, Fedder GK. Endoscopic optical coherence tomography based on a microelectromechanical mirror. Opt Lett. 2001;26:1966–1968. doi: 10.1364/ol.26.001966. [DOI] [PubMed] [Google Scholar]
- 84.Seibel EJ, Smithwick QY. Unique features of optical scanning, single fiber endoscopy. Lasers Surg Med. 2002;30:177–183. doi: 10.1002/lsm.10029. [DOI] [PubMed] [Google Scholar]
- 85.Dickensheets DL, Kino GS. Micromachined scanning confocal optical microscope. Opt Lett. 1996;21:764–766. doi: 10.1364/ol.21.000764. [DOI] [PubMed] [Google Scholar]
- 86.Fu L, Jain A, Cranfield C, Xie H, Gu M. Three-dimensional nonlinear optical endoscopy. J Biomed Opt. 2007;12:040501. doi: 10.1117/1.2756102. [DOI] [PubMed] [Google Scholar]
- 87.Kumar K, Avritscher R, Wang Y, et al. Handheld histology-equivalent sectioning laser-scanning confocal optical microscope for interventional imaging. Biomed Microdevices. 2010;12:223–233. doi: 10.1007/s10544-009-9377-6. [DOI] [PubMed] [Google Scholar]
- 88.Piyawattanametha W, Barretto RP, Ko TH, et al. Fast-scanning two-photon fluorescence imaging based on a microelectromechanical systems two-dimensional scanning mirror. Opt Lett. 2006;31:2018–2020. doi: 10.1364/ol.31.002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ra H, Piyawattanametha W, Mandella MJ, et al. Three-dimensional in vivo imaging by a handheld dual-axes confocal microscope. Opt Express. 2008;16:7224–7232. doi: 10.1364/oe.16.007224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ren H, Waltzer WC, Bhalla R, et al. Diagnosis of bladder cancer with microelectromechanical systems-based cystoscopic optical coherence tomography. Urology. 2009;74:1351–1357. doi: 10.1016/j.urology.2009.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin HJ, Pierce MC, Lee D, Ra H, Solgaard O, Richards-Kortum R. Fiber-optic confocal microscope using a MEMS scanner and miniature objective lens. Opt Express. 2007;15:9113–9122. doi: 10.1364/oe.15.009113. [DOI] [PubMed] [Google Scholar]
- 92.Rouse AR, Gmitro AF. Multispectral imaging with a confocal microendoscope. Opt Lett. 2000;25:1708–1710. doi: 10.1364/ol.25.001708. [DOI] [PubMed] [Google Scholar]
- 93.Udovich JA, Kirkpatrick ND, Kano A, Tanbakuchi A, Utzinger U, Gmitro AF. Spectral background and transmission characteristics of fiber optic imaging bundles. Appl Opt. 2008;47:4560–4568. doi: 10.1364/ao.47.004560. [DOI] [PubMed] [Google Scholar]
- 94.Dwyer PJ, DiMarzio CA, Rajadhyaksha M. Confocal theta line-scanning microscope for imaging human tissues. Appl Opt. 2007;46:1843–1851. doi: 10.1364/ao.46.001843. [DOI] [PubMed] [Google Scholar]
- 95.Dwyer PJ, DiMarzio CA, Zavislan JM, Fox WJ, Rajadhyaksha M. Confocal reflectance theta line scanning microscope for imaging human skin in vivo. Opt Lett. 2006;31:942–944. doi: 10.1364/ol.31.000942. [DOI] [PubMed] [Google Scholar]
- 96.Gareau DS, Abeytunge S, Rajadhyaksha M. Line-scanning reflectance confocal microscopy of human skin: comparison of full-pupil and divided-pupil configurations. Opt Lett. 2009;34:3235–3237. doi: 10.1364/OL.34.003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koester CJ, Khanna SM, Rosskothen HD, Tackaberry RB, Ulfendahl M. Confocal slit divided-aperture microscope: applications in ear research. Appl Opt. 1994;33:702–708. doi: 10.1364/AO.33.000702. [DOI] [PubMed] [Google Scholar]
- 98.Leigh SY, Liu JT. Multi-color miniature dual-axis confocal microscope for point-of-care pathology. Opt Lett. 2012;37:2430–2432. doi: 10.1364/OL.37.002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu JT, Mandella MJ, Crawford JM, Contag CH, Wang TD, Kino GS. Efficient rejection of scattered light enables deep optical sectioning in turbid media with low-numerical-aperture optics in a dual-axis confocal architecture. J Biomed Opt. 2008;13:034020. doi: 10.1117/1.2939428. [DOI] [PubMed] [Google Scholar]
- 100.Liu JT, Mandella MJ, Friedland S, et al. Dual-axes confocal reflectance microscope for distinguishing colonic neoplasia. J Biomed Opt. 2006;11:054019. doi: 10.1117/1.2363363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu JT, Mandella MJ, Ra H, et al. Miniature near-infrared dual-axes confocal microscope utilizing a two-dimensional microelectromechanical systems scanner. Opt Lett. 2007;32:256–258. doi: 10.1364/ol.32.000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Y, Wang D, Liu JT. Assessing the tissue-imaging performance of confocal microscope architectures via Monte Carlo simulations. Opt Lett. 2012;37:4495–4497. doi: 10.1364/OL.37.004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wong LK, Mandella MJ, Kino GS, Wang TD. Improved rejection of multiply scattered photons in confocal microscopy using dual-axes architecture. Opt Lett. 2007;32:1674–1676. doi: 10.1364/ol.32.001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piyawattanametha W, Ra H, Qiu Z, et al. In vivo near-infrared dual-axis confocal microendoscopy in the human lower gastrointestinal tract. J Biomed Opt. 2012;17:021102. doi: 10.1117/1.JBO.17.2.021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang D, Chen Y, Leigh SY, Haeberle H, Contag CH, Liu JT. Microscopic Delineation of Medulloblastoma Margins in a Transgenic Mouse Model Using a Topically Applied VEGFR-1 Probe. Transl Oncol. 2012;5:408–414. doi: 10.1593/tlo.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]




