Abstract
Objectives
Recognizing population-level disparities for the treatment of patients with renal cell carcinoma (RCC) will inform clinical practice and health policy. Few studies, reporting conflicting results, have investigated race and sex disparities specifically among patients with small renal masses.
Methods and Materials
The Surveillance, Epidemiology and End Results-Medicare database (1995–2007) was queried for patients with localized T1a RCC undergoing radical nephrectomy, partial nephrectomy (PN), or deferred therapy (DT). Demographics, comorbidity, and treatment approach were assessed. Multivariable logistic regression models evaluated predictors of DT, and then PN among those receiving surgery. Cox proportional hazards evaluated survival differences for whites vs. blacks and women vs. men.
Results
A total of 6092 white and 617 black patients with T1a RCC met the inclusion criteria. Blacks were twice as likely to defer therapy compared to whites (odds ratio (OR) 1.95 (95% confidence interval (CI) 1.52–2.51)) and had worse overall survival (hazard ratio (HR) 1.36 (95%CI 1.19–1.56)). However, cancer-specific survival (CSS) was similar (p=0.429). The greatest discrepancy was among healthy (CCI≤1) blacks who had a much higher rate of DT compared to their white counterparts. Women were found to have decreased utilization of PN compared to men (OR 0.84 (95%CI 0.74–0.96)) and better CSS (HR 0.74 (95%CI 0.58–0.94)), but there were no differences by race.
Conclusions
The differential utilization of DT by race instead of purely by age and comorbidity is concerning but has not led to a significant difference in CSS. Women are less likely to undergo PN compared to men, but they also have a notably improved CSS.
Keywords: healthcare disparities, renal cell carcinoma, watchful waiting, comorbidity, SEER, Medicare
Introduction
Globally, the incidence and disability due to kidney cancer has steadily risen [1, 2]. Over 65000 new cases of renal cell carcinoma (RCC) and almost 14000 deaths are expected in the United States alone in 2013 [3]. Most patients present incidentally due to widespread use of cross-sectional imaging so that >50% of new diagnoses represent early-stage disease [4]. The standard of care for small renal masses (SRMs; ≤4cm) suspicious for RCC is extirpative surgery; research suggests no reduction in oncologic control but a potential benefit for renal function to reduce cardiovascular sequelae for partial nephrectomy (PN) over radical nephrectomy (RN) [5, 6]. At the same time, active surveillance has emerged as an alternative to surgery to reduce potential overtreatment [7].
As the utilization of PN for SRMs increases, there is a concern that health care disparities may exist in the treatment of early-stage kidney cancer [8, 9]. Women may be less likely than men to receive PN, and while 5-year survival has improved for whites with RCC, mortality rates among blacks have remained the same [8–10]. Most previous studies looking at survival by race and sex for cohorts of RCC patients found no significant differences in cancer survival but included patients with all stages of disease [11, 12]. Two studies have performed dedicated analyses on patients with T1a RCC to assess the impact of sociodemographics on rate of PN [13, 14]. However, these studies did not evaluate survival outcomes or include data on overall health status of each patient. Furthermore, no study has quantified disparities or predictors for patients who deferred cancer-directed therapy for T1a RCC while controlling for comorbidity. Patients who forgo surgery at the population-level, referred to as deferred therapy (DT), are not equivalent to patients monitored on active surveillance, so recognizing current disparities in this population could help providers and policy makers address potential concerns.
Therefore, the present study was designed to characterize associations of race and sex with surgical or deferred management of T1a RCC recognizing baseline comorbidity as a potential confounder. We also quantify other associated predictors of DT or PN and determine if differences in overall (OS) and cancer-specific survival (CSS) existed by race or sex.
Methods and Materials
Patient Population
Institutional Review Board approval was obtained. The Surveillance, Epidemiology, and End Results (SEER) cancer registry and linked Medicare claims data (1995–2007) were used to identify patients ≥65 years diagnosed with clinically localized, T1a (≤4cm) renal cortical tumors (AJCC TNM system,2009). Patients were included based on kidney cancer diagnosis codes ICD-0-2,C64.9 and 9th revision ICD-0-9,189.0 as well as self-identification of race as white or black according to SEER. Exclusion criteria included lacking Medicare A and/or B coverage, enrollment in managed care, regional disease, distant metastases, unknown stage, upper tract transitional cell carcinoma or ureteric, non-cortical renal tumors, multiple procedures, bilateral tumors, or ablative therapy.
SEER and Medicare data have a high concordance to identify patients who do not undergo cancer-directed surgery [15]. SEER data also has a high agreement (97%) with Medicare claims data for classifying patients receiving PN versus RN [16]. Patients were divided into those receiving surgical management and those who did not. While previous studies have named this group non-surgical management, they are classified as DT in this study to recognize a small percentage (<4%) eventually do undergo intervention [17, 18]. Patients receiving surgery were classified as undergoing PN based on CPT codes (50240,50280,50290,50543) and ICD-9-CM codes (55.31,55.39,55.4) or RN based on CPT codes (50220,50225,50230,50545,50546) and ICD-9-CM codes (55.51,55.52,55.54).
Demographic, Comorbidity, and Outcomes Data
SEER data were used to ascertain patient demographic data including age, sex, race, marital status, urban-rural location, tumor size, and year of intervention. Besides residence, socioeconomic status was estimated using census tract information on percent of tract residents living below the poverty level. However, only 41% of patients had complete agreement between SEER, Medicare, and census tract so this information was only included in subset analyses for comparison. Comorbidity data were collected from the Medicare Provider Analysis and Review file to calculate Charlson comorbidity index (CCI) [19]. Outcomes were OS through May 31, 2010 (Medicare follow-up) and CSS through December 31, 2007 (SEER follow-up for cause of death).
Statistical Analysis
Chi-square (χ2) tests were used to evaluate differences in baseline demographics, comorbidity, and treatment by race as well as treatment utilization by comorbidity status. A multivariable logistic regression model was developed to determine predictors of patients who receive DT over surgery. A second multivariable logistic regression model assessed predictors of PN over RN. The cohort was then stratified by CCI to compare the exact prevalence rates of whites and blacks receiving either DT or PN across strata of CCI.
A survival analysis was conducted using the Kaplan-Meier method to calculate survival functions for OS and CSS by race and sex. Cox proportional hazards regression was used to obtain adjusted hazard ratios (HR) by controlling for potential confounders influencing survival. Fine and Grey competing risks regression was employed for CSS treating other-cause mortality as a competing risk of death with kidney cancer. All statistical analyses were performed using STATA v.12.0 (STATA Corp, College Station, TX, 2011).
Results
Baseline Characteristics
A total of 6092 white and 617 black patients with stage T1aN0M0 RCC met inclusion criteria with 605(9.9%), 1554(25.5%), and 3933(64.5%) undergoing DT, PN, and RN, respectively. Statistically significant differences existed for baseline characteristics by race (Table 1). Compared to whites, a greater proportion of black patients received DT, lived in large metro areas, were unmarried, and had higher CCI (all p<0.01). There was no difference in CCI by race among only DT patients (p=0.984). Black patients were also somewhat younger (71.7 vs. 73.4 years, p<0.01). Proportions by tumor size and year of diagnosis did not differ.
Table 1.
Demographics, treatment approach, and Charlson comorbidity index by race for patients ≥65 years of age with T1a renal cell carcinoma, SEER-Medicare 1995–2007.
| White | (%) | Black | (%) | p-valuea | ||
|---|---|---|---|---|---|---|
| N | 6092 | (90.8) | 617 | (9.2) | ||
| Age | <0.01 | |||||
| 65–69 | 1536 | (25.2) | 213 | (34.5) | ||
| 70–74 | 1722 | (28.3) | 199 | (32.3) | ||
| 75–79 | 1591 | (26.1) | 131 | (21.2) | ||
| 80–84 | 873 | (14.3) | 48 | (7.8) | ||
| >85 | 370 | (6.1) | 26 | (4.2) | ||
| Managementb | <0.01 | |||||
| RN | 3933 | (64.5) | 352 | (57.1) | ||
| PN | 1554 | (25.5) | 162 | (26.3) | ||
| DT | 605 | (9.9) | 103 | (16.7) | ||
| Sex | <0.01 | |||||
| Male | 3389 | (55.6) | 306 | (49.6) | ||
| Female | 2703 | (44.4) | 311 | (50.4) | ||
| Residencec | <0.01 | |||||
| Large Metro | 3515 | (57.7) | 479 | (77.6) | ||
| Metro | 1637 | (26.9) | 107 | (17.3) | ||
| Urban | 368 | (6.0) | 18 | (2.9) | ||
| Less Urban | 477 | (7.8) | - | - | ||
| Rural | 95 | (1.6) | - | - | ||
| Marital Status | <0.01 | |||||
| Married | 3879 | (65.9) | 289 | (48.9) | ||
| Not Married | 2010 | (34.1) | 302 | (51.1) | ||
| CCId | <0.01 | |||||
| 0 | 3224 | (52.9) | 246 | (39.9) | ||
| 1 | 1694 | (27.8) | 188 | (30.5) | ||
| 2 | 690 | (11.3) | 89 | (14.4) | ||
| 3 | 250 | (4.1) | 49 | (7.9) | ||
| 4+ | 234 | (3.8) | 45 | (7.3) | ||
| Tumor Size | 0.833 | |||||
| <2 cm | 945 | (15.6) | 90 | (14.7) | ||
| 2–<3cm | 2077 | (34.2) | 214 | (34.9) | ||
| 3–4 cm | 3057 | (50.3) | 310 | (50.5) | ||
| Year of Diagnosis | 0.58 | |||||
| 1995–1999 | 890 | (14.6) | 98 | (15.9) | ||
| 2000–2003 | 1985 | (32.6) | 191 | (31.0) | ||
| 2004–2007 | 3217 | (52.8) | 328 | (53.2) | ||
Chi-square (χ2) test
RN: Radical nephrectomy; PN: Partial nephrectomy; DT: Deferred therapy
values suppressed according to SEER-Medicare regulations for cell sizes <11
Charlson comorbidity index
Predictors of Deferred Therapy vs. Surgical Intervention
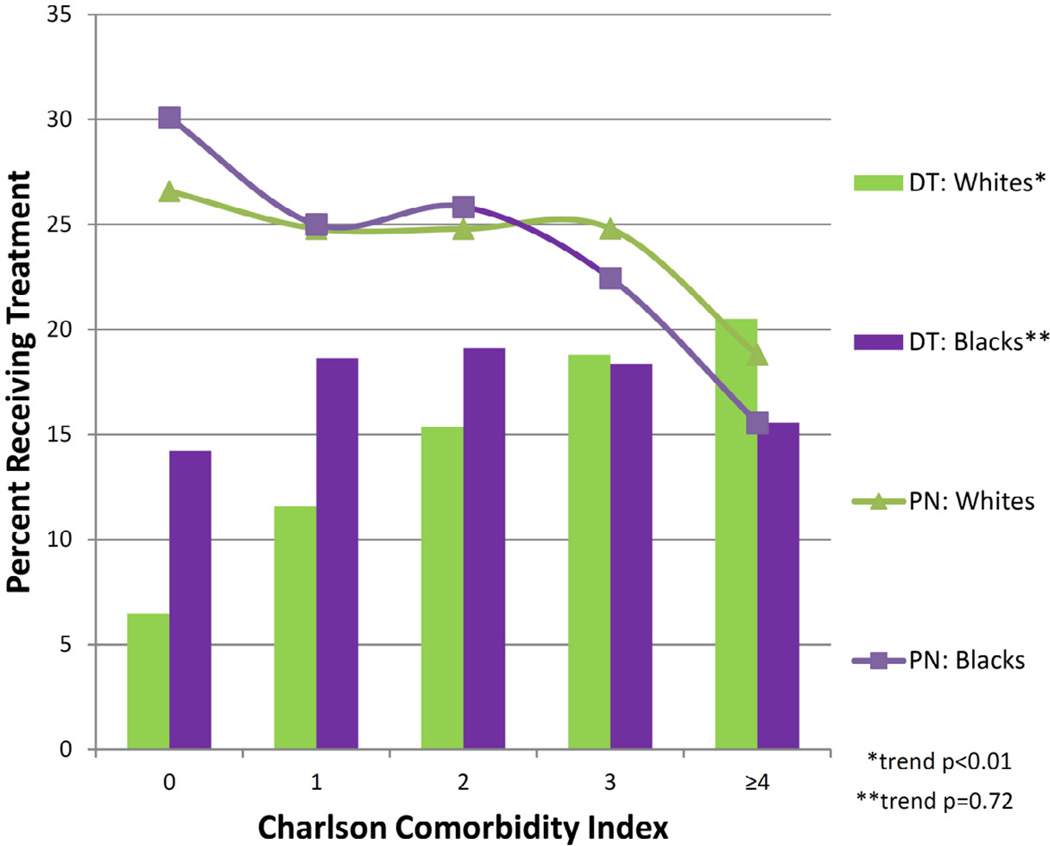
Adjusted multivariable model showed blacks were twice as likely to defer therapy compared to whites (odds ratio (OR) 1.95 (95% confidence interval (CI) 1.52–2.51)) (Table 2). The rate of DT stratified by CCI is shown in Figure 1. A greater proportion of whites received DT as they get sicker (higher CCI), which is a statistically significant trend (p<0.01) with a range of 6.4% to 20.5%. The proportion of blacks who received DT, on the other hand, stayed similar across strata of CCI (p=0.72) with a range of 14.2% to 19.1%.
Table 2.
Multivariable logistic regression models evaluating predictors of deferred therapy among the entire cohort and predictors of partial nephrectomy among patients receiving surgery for patients ≥65 years of age with T1a renal cell carcinoma, SEER-Medicare 1995–2007.
| Predictors of Deferred Therapy |
Predictors of Partial Nephrectomy |
||||||
|---|---|---|---|---|---|---|---|
| Variable | ORa | (95% CI) | p-value | ORa | (95% CI) | p-value | |
| Race | White | Ref | - | Ref | - | ||
| Black | 1.95 | (1.52–2.51) | <0.01 | 1.15 | (0.93–1.42) | 0.203 | |
| Sex | Male | Ref | - | Ref | - | ||
| Female | 0.87 | (0.73–1.04) | 0.135 | 0.84 | (0.74–0.96) | <0.01 | |
| Age | 65–69 | Ref | - | Ref | - | ||
| 70–74 | 1.21 | (0.92–1.57) | 0.169 | 0.88 | (0.75–1.02) | 0.093 | |
| 75–79 | 1.55 | (1.19–2.02) | <0.01 | 0.77 | (0.66–0.91) | <0.01 | |
| 80–84 | 2.76 | (2.09–3.64) | <0.01 | 0.64 | (0.52–0.79) | <0.01 | |
| >85 | 10.36 | (7.70–13.93) | <0.01 | 0.32 | (0.22–0.48) | <0.01 | |
| Residence | Large Metro | Ref | - | Ref | - | ||
| Metro | 0.85 | (0.69–1.04) | 0.124 | 0.87 | (0.76–1.00) | 0.052 | |
| Urban | 0.84 | (0.57–1.23) | 0.361 | 0.78 | (0.59–1.02) | 0.068 | |
| Less Urban | 1.17 | (0.85–1.61) | 0.327 | 0.8 | (0.63–1.02) | 0.074 | |
| Rural | 1.38 | (0.71–2.67) | 0.341 | 0.78 | (0.46–1.33) | 0.367 | |
| Marital Status | Married | Ref | - | Ref | - | ||
| Not Married | 1.43 | (1.19–1.72) | <0.01 | 0.95 | (0.83–1.09) | 0.45 | |
| CCIb | 0 | Ref | - | Ref | - | ||
| 1 | 1.83 | (1.49–2.23) | <0.01 | 0.94 | (0.82–1.08) | 0.382 | |
| 2 | 2.23 | (1.73–2.86) | <0.01 | 1.03 | (0.85–1.26) | 0.757 | |
| 3 | 2.53 | (1.78–3.62) | <0.01 | 1.13 | (0.83–1.54) | 0.453 | |
| 4+ | 3.29 | (2.33–4.63) | <0.01 | 0.69 | (0.49–0.98) | 0.036 | |
| Tumor Size | <2 cm | Ref | - | Ref | - | ||
| 2–<3cm | 0.84 | (0.66–1.08) | 0.181 | 0.67 | (0.57–0.79) | <0.01 | |
| 3–≤4 cm | 0.72 | (0.57–0.91) | <0.01 | 0.29 | (0.24–0.34) | <0.01 | |
| Year | Since 1995 | 1.03 | (1.00–1.06) | 0.024 | 1.15 | (1.12–1.17) | <0.01 |
Logistic regression adjusted for age, sex, race, residence, marital status, Charlson comorbidity index, tumor size, and year of diagnosis
CCI: Charlson comorbidity index
Figure 1.
Rates of partial nephrectomy and deferred therapy by Charlson comorbidity index comparing blacks and whites. DT = deferred therapy, PN = partial nephrectomy.
Notably, there was no statistically significant difference in the receipt of surgery by sex (OR 0.87 (95%CI 0.73–1.04)). Older age, higher CCI, larger tumor size, being unmarried, and being diagnosed in a more recent year were all associated with greater odds of DT. Urban-rural residence did not appear to be a significant predictor.
Predictors of Partial Nephrectomy vs. Radical Nephrectomy
Among patients who underwent surgery, utilization of PN over RN did not appear to differ between whites and blacks (OR 1.15 (95%CI 0.93–1.42)) (Table 2). Rate of PN stratified by CCI was similar by race (Figure 1). In other words, for patients who underwent surgery, similar proportions of white and black patients received PN across all strata of CCI.
However, women had decreased utilization of PN compared to men (OR 0.84 (95%CI (0.74–0.96)). At the same time, older age and larger tumor size were associated with decreased use of PN. Only the highest category of CCI (4+) was associated with decreased PN use (OR 0.69 (95%CI (0.49–0.98)) while there was a 15% increase in PN use each year since 1995. Marital status and residence did not exert significant adjusted associations.
Survival Outcomes
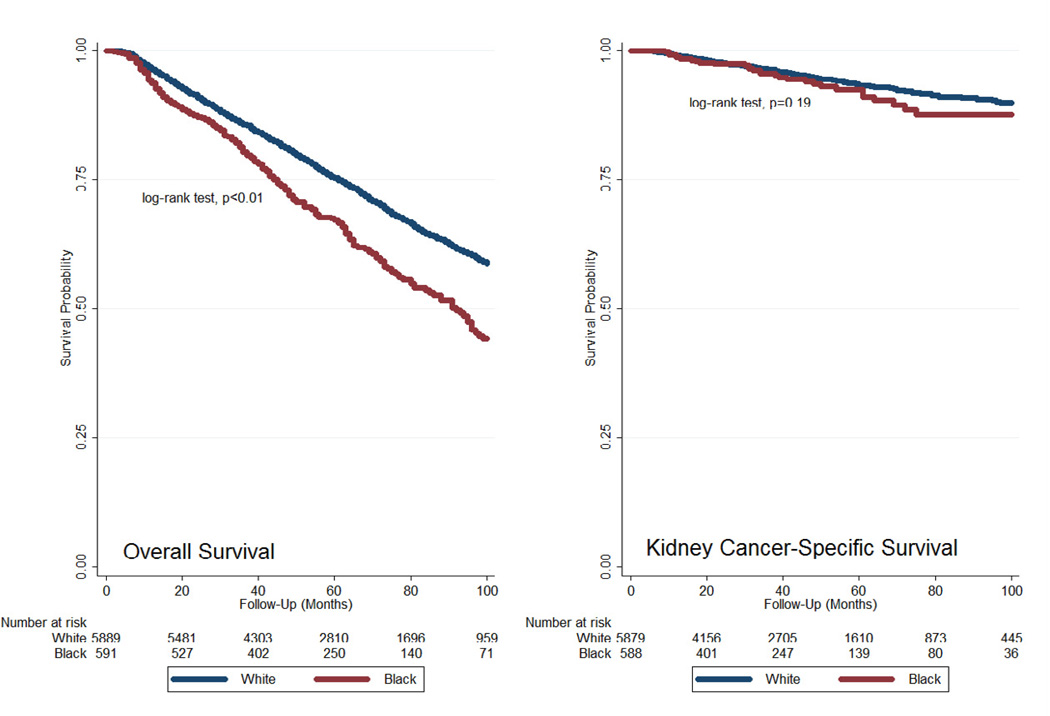
The median follow-up for OS was 57 months (interquartile range 38–84 months). Unadjusted Kaplan-Meier curves showed decreased OS for blacks compared to whites (log-rank test, p<0.01) but similar CSS (log-rank test, p=0.19) (Figure 2). Adjusted Cox proportional hazards survival analysis demonstrated similar findings with decreased OS for blacks compared to whites but comparable CSS (Table 3). Survival analysis by sex showed improved OS (hazard ratio (HR) 0.79 (95%CI 0.72–0.86)) as well as improved CSS (HR 0.74 (95%CI 0.58–0.94)) for women compared to men. The results were similar in the subset of patients (N=2480) with complete data on percent of census tract residents living below the poverty line.
Figure 2.
Kaplan-Meier survival curves for overall survival and cancer-specific survival comparing blacks and whites.
Table 3.
Unadjusted and adjusted survival analysis comparing blacks to whites and women to men among patients ≥65 years of age with T1a renal cell carcinoma, SEER-Medicare 1995–2007.
| Unadjusted | Adjusteda | ||||||
|---|---|---|---|---|---|---|---|
| Survivalb | Variable | HR | (95% CI) | p-value | HRa | (95% CI) | p-value |
| White | Ref | Ref | - | ||||
| Black | 1.45 | (1.27–1.65) | <0.01 | 1.36 | (1.19–1.56) | <0.01 | |
| OS | |||||||
| Male | Ref | - | Ref | - | |||
| Female | 0.86 | (0.79–0.93) | <0.01 | 0.79 | (0.72–0.86) | <0.01 | |
| White | Ref | - | Ref | - | |||
| Black | 1.32 | (0.92–1.88) | 0.131 | 1.17 | (0.80–1.71) | 0.429 | |
| CSS | |||||||
| Male | Ref | - | Ref | - | |||
| Female | 0.81 | (0.65–1.01) | 0.058 | 0.74 | (0.58–0.94) | 0.013 | |
Cox regression adjusted for age, sex, race, treatment strategy, residence, marital status, Charlson comorbdity index, tumor size, and year of diagnosis; Fine and Gray competing risks regression used to obtain subhazard ratios for CSS
OS: Overall survival; CSS: Kidney cancer-specific survival
Discussion
Race and sex disparities in the treatment of SRMs have important clinical implications for patient management as well as health policy implications for patients’ ability to access care. The present study shows blacks were twice as likely to not receive surgery compared to whites in the United States even after controlling for differences in baseline comorbidity. Notably, race was not a significant factor determining surgical management among those who did receive an intervention. In contrast, patient sex did not affect whether a patient received surgery, but women were less likely to undergo PN compared to men when they did undergo intervention. The second question was whether these associations translated into differences in cancer survival for patients with T1a RCC. Our survival analysis showed no difference in CSS for blacks compared to whites but a significantly better CSS among women compared to men.
Racial Disparities
We expected that the rate of DT should not differ by race, after controlling for age and comorbidity, if patients were being appropriately selected and monitored. Strikingly, even relatively healthy blacks (CCI≤1) were not receiving surgery, and the expected pattern of a greater proportion of patients receiving DT with increasing CCI was notably absent (p=0.72). It is not clear from these data why exactly healthy black patients are forgoing extirpative surgery, but it does not appear to be fully explained by socioeconomic status or proximity to urban medical centers [14]. Well selected patients on active surveillance with SRMs have experienced excellent short-term oncologic outcomes [7, 20]. However, a racial disparity leading to inappropriate population-based practice where healthy individuals cannot access care could undermine widespread implementation of active surveillance. As all patients in the cohort were insured through Medicare, the findings may be even more relevant or pronounced in uninsured populations. While the study was not specifically focused on SRMs, Berndt et al reported that blacks were significantly less likely to undergo nephrectomy compared to whites in a cohort of SEER-Medicare patients with all stages of RCC [12]. Compared to their significant 7% difference, we found blacks were almost half as likely to undergo surgery for T1a RCC. Becker et al reported a 23% difference but was not able to control for comorbidity [14].
Among surgical patients, several groups have reported on trends or associations with PN vs. RN use with conflicting results. Kates et al reported an increased rate of RN among blacks, but sex seemed to be a more significant factor with a 24% and 47% increased rate of RN among white and black women, respectively [13]. Hollenbeck et al, however, reported an association of black race with a greater likelihood of receiving PN [21]. Our results indicate no racial disparity in use of PN vs. RN, which was also recently noted in a cohort in Maryland [9].
Berndt et al also reported that blacks had worse OS compared to whites [12]. However, this was largely due to comorbid conditions and lower rates of surgical treatment, which resulted in a nonsignificant difference after adjustment for CCI and treatment approach. In our analysis, blacks continued to have worse OS after adjustment for comorbidity, treatment strategy, and demographics. The differences may be due to a more pronounced disparity among patients with early-stage cancer compared with later stage disease or represent a widening trend over time as the prior study only included patients through 1999. An increase in racial disparities has been noted for ovarian cancer treatment, which may be paralleled in the SRM population [22]. The difference in OS may also be related to other-cause mortality with being less likely to get surgery serving as a marker for general health care. While the comparable CSS by race is encouraging, this is likely because the majority received intervention. Previous studies in similar SEER-Medicare cohorts have shown forgoing intervention is associated with lower CSS [17, 18]. Another consideration of note is biologic differences in RCC between blacks and whites, which might not be apparent in CSS of a cohort of early-stage RCC patients.
Sex Disparities
The underutilization of PN for women is well established and consistently reported by several studies, however a definitive explanation is still lacking [9, 13, 23]. Potential contributors include perceived differences in baseline renal function or surgical complexity compared to males making RN a more attractive choice. Women may be perceived to have better cardiovascular or renal health compared to men, and hence less likely to experience consequences of chronic kidney disease from loss of a single renal unit. Finally, as supported by the data in this analysis, women are less likely to die of their renal malignancy. Although our regression models attempt to control for surgical approach and it did not achieve significance, the higher rate of radical surgery may confer a survival benefit as one randomized trial has suggested [24].
It is also interesting to compare OS and CSS between female and male patients. Prior studies on patients with all stages of RCC have noted a worse OS for men compared to women [25–27]. However, much of the difference could be due to an inherently longer life expectancy among women compared to men with no relevance to the underlying RCC. Two recent studies have suggested better CSS for women with RCC compared to men. A Korean cohort of 1616 patients with 73 months mean follow-up suggested histology might contribute to better CSS in women [27]. An international cohort of 5654 patients posited that the CSS advantage for women is not due to difference in pathologic features but inherent hormonal effects on RCC development and progression [28]. Other studies assessing the impact of sex on CSS have found no statistically significant difference, but most hinted at some association of male sex with worse CSS [23, 26, 29]. Our results, in a cohort of T1a RCC patients in the United States, adds to the evidence of improved CSS for women and is the first to suggest a survival advantage among patients with early-stage disease.
Limitations
Potential limitations deserve mention. Patients were identified retrospectively, and it is unclear if conclusions extend to patients <65 years old where about half of RCCs are diagnosed [30]. Evaluation of other races was limited without sufficient power to evaluate potential disparities among Hispanics or Asian-Americans. Socioeconomic status based on poverty level could only be assessed in a subset (41%) with complete data, and although results were very similar in subanalysis limited to these patients, further exploration of socioeconomic status by multiple categorizations should be pursued [14]. Residual confounding persists as we cannot control for unmeasured comorbidity. Case ascertainment is also a limitation as only 51.3% of DT patients had microscopic confirmation of disease, and upstaged SRMs on nephrectomy would be excluded from the surgical cohorts. Lastly, DT patients reflect current practice patterns in the United States and do not to represent a true active surveillance cohort. Notwithstanding the limitations, the study population is a nationally representative cohort of older adults with T1a RCC providing important insights into contemporary race and sex disparities.
Conclusions
A greater proportion of relatively healthy black patients are not receiving surgery for T1a RCC compared to whites. OS was worse for blacks, which might indicate a lack of access to health care in general. The differential utilization of DT by race instead of purely by age and comorbidity is concerning but has not led to a significant difference in CSS. Women were less likely to undergo PN compared to men, but they also had a notably improved CSS possibly due to inherent protective biological or hormonal effects on RCC progression, which deserves further study.
Acknowledgement
This project was supported, in part, by the Predoctoral Clinical Research Training Program and the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by Grant Number UL1 TR 000424-06 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH.
Abbreviations and Acronyms
- CCI
Charlson comorbidity index
- CSS
cancer-specific survival
- DT
deferred therapy
- HR
hazard ratio
- OR
odds ratio
- OS
overall survival
- PN
partial nephrectomy
- RCC
renal cell carcinoma
- RN
radical nephrectomy
- SEER
Surveillance, Epidemiology and End Results
- SRM
small renal mass
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no disclosures.
References
- 1.Mathew A, Devesa SS, Fraumeni JF, Jr, Chow WH. Global increases in kidney cancer incidence, 1973 – 1992. Eur J Cancer Prev. 2002;11:171–178. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Patel HD, Kushner AL, Allaf ME. Waiting for Global Access to Urologic Care. Eur Urol. 2013;64:344–345. doi: 10.1016/j.eururo.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Cancer facts and figures: 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 4.Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 5.Pettus JA, Jang TL, Thompson RH, et al. Effect of baseline glomerular filtration rate on survival in patients undergoing partial or radical nephrectomy for renal cortical tumors. Mayo Clin Proc. 2008;83:1101–1106. doi: 10.4065/83.10.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors- Is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierorazio PM, Hyams ES, Mullins JK, Allaf ME. Active surveillance for small renal masses. Rev Urol. 2012;14:13–19. [PMC free article] [PubMed] [Google Scholar]
- 8.Sun M, Abdollah F, Bianchi M, et al. Treatment management of small renal masses in the 21st century: a paradigm shift. Ann Surg Oncol. 2012;19:2380–2387. doi: 10.1245/s10434-012-2247-0. [DOI] [PubMed] [Google Scholar]
- 9.Patel HD, Mullins JK, Pierorazio PM, et al. Trends in renal surgery: robotic technology is associated with increased use of partial nephrectomy. J Urol. 2013;189:1229–1235. doi: 10.1016/j.juro.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 11.Aron M, Nguyen MM, Stein RJ, Gill IS. Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur Urol. 2008;54:133–140. doi: 10.1016/j.eururo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Berndt SI, Carter HB, Schoenberg MP, Newschaffer CJ. Disparities in treatment and outcome for renal cell cancer among older black and white patients. J Clin Oncol. 2007;25:3589–3595. doi: 10.1200/JCO.2006.10.0156. [DOI] [PubMed] [Google Scholar]
- 13.Kates M, Whalen MJ, Badalato GM, McKiernan JM. The effect of race and gender on the surgical management of the small renal mass. Urol Oncol. 2013;31:1794–1799. doi: 10.1016/j.urolonc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Becker A, Roghmann F, Trinh QD, et al. Sociodemographic disparities in the treatment of small renal masses. BJU Int. 2013;111:E274–E282. doi: 10.1111/bju.12111. [DOI] [PubMed] [Google Scholar]
- 15.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40:IV-43-8. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 16.Miller DC, Saigal CS, Warren JL, et al. External validation of a claims-based algorithm for classifying kidney-cancer surgeries. BMC Health Serv Res. 2009;9:92. doi: 10.1186/1472-6963-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun M, Becker A, Tian Z, et al. Management of Localized Kidney Cancer: Calculating Cancer-specific Mortality and Competing Risks of Death for Surgery and Nonsurgical Management. Eur Urol. 2014;65:235–241. doi: 10.1016/j.eururo.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Patel HD, Kates M, Pierorazio PM, et al. Survival after Diagnosis of Localized T1a Kidney Cancer: Current Population-based Practice of Surgery and Nonsurgical Management. Urology. 2014;83:126–133. doi: 10.1016/j.urology.2013.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118:997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254–259. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Terplan M, Schluterman N, McNamara EJ, Tracy JK, Temkin SM. Have racial disparities in ovarian cancer increased over time? An analysis of SEER data. Gynecol Oncol. 2012;125:19–24. doi: 10.1016/j.ygyno.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Dulabon LM, Lowrance WT, Russo P, Huang WC. Trends in renal tumor surgery delivery within the United States. Cancer. 2010;116:2316–2321. doi: 10.1002/cncr.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Aron M, Nguyen MM, Stein RJ, Gill IS. Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur Urol. 2008;54:133–140. doi: 10.1016/j.eururo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Schrader AJ, Sevinc S, Olbert PJ, Hegele A, Varga Z, Hofmann R. Gender-specific characteristics and survival of renal cell carcinoma. Urologe A. 2008;47(1182):1184–1186. doi: 10.1007/s00120-008-1832-0. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Jeon HG, Kwak C, et al. Gender-specific clinicopathological features and survival in patients with renal cell carcinoma (RCC) BJU Int. 2012;110:E28–E33. doi: 10.1111/j.1464-410X.2011.10667.x. [DOI] [PubMed] [Google Scholar]
- 28.Rampersaud EN, Klatte T, Bass G, et al. The effect of gender and age on kidney cancer survival: Younger age is an independent prognostic factor in women with renal cell carcinoma. Urol Oncol. 2013 doi: 10.1016/j.urolonc.2012.10.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Woldrich JM, Mallin K, Ritchey J, Carroll PR, Kane CJ. Sex differences in renal cell cancer presentation and survival: an analysis of the National Cancer Database, 1993–2004. J Urol. 2008;179:1709–1713. doi: 10.1016/j.juro.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Nepple KG, Yang L, Grubb RL, 3rd, Strope SA. Population based analysis of the increasing incidence of kidney cancer in the United States: evaluation of age specific trends from 1975 to 2006. J Urol. 2012;187:32–38. doi: 10.1016/j.juro.2011.09.028. [DOI] [PubMed] [Google Scholar]