Abstract
Neutrophil (PMN) transepithelial migration (TEM) and accumulation in luminal spaces is a hallmark of mucosal inflammation. TEM has been extensively modeled, however the functional consequences and molecular basis of PMN interactions with luminal epithelial ligands are not clear. Here we report that cytokine-induced expression of a PMN ligand, intercellular adhesion molecule-1 (ICAM-1), exclusively on the luminal (apical) membrane of the intestinal epithelium results in accumulation and enhanced motility of transmigrated PMN on the apical epithelial surface. Using complementary in-vitro and in-vivo approaches we demonstrate that ligation of epithelial ICAM-1 by PMN or with specific antibodies results in myosin light chain kinase (MLCK)-dependent increases in epithelial permeability that are associated with enhanced PMN TEM. Effects of ICAM-1 ligation on epithelial permeability and PMN migration in-vivo were blocked after intraluminal addition of peptides derived from the cytoplasmic domain of ICAM-1. These findings provide new evidence for functional interactions between PMN and epithelial cells after migration into the intestinal lumen. While such interactions may aid in clearance of invading microorganisms by promoting PMN recruitment, engagement of ICAM-1 under pathologic conditions would increase accumulation of epithelial-associated PMN, thus contributing to mucosal injury as observed in conditions including ulcerative colitis.
INTRODUCTION
During mucosal inflammation, neutrophil (PMN) infiltration of epithelial surfaces leads to injury and leaky mucosal barrier. Such barrier defects underlie the basis of a number of inflammatory disorders. For example, accumulation of PMN in the alveolar space and in epithelial intestinal crypts has been shown to directly correlate with the severity of diseases such as, acute lung injury (ALI)1, 2, cystic fibrosis (CF)3 and inflammatory bowel diseases, ulcerative colitis (UC) and Crohn's disease (CD)4, 5. PMN migration across epithelial layers and into luminal spaces is a sequential process, beginning with the extravasation of PMN from blood vessels6, migration through the interstitium, and terminating with transmigration across the epithelium in a basolateral to apical (luminal) direction. Interactions necessary for initial engagement of PMN with the basolateral surface of the intestinal epithelium are primarily mediated by the PMN β2-integrin CD11b/CD18 (Mac-1)7,8 and other yet unidentified ligands. These initial interactions have been shown to trigger intracellular signaling events leading to increased epithelial permeability, thus facilitating enhanced PMN transepithelial migration (TEM). Specifically, PMN contact with basolateral intestinal epithelial cell (IEC) ligands has been shown to activate protease-activated receptors-1 and 2 leading to enhanced phosphorylation of myosin light chain kinase (MLCK) and a subsequent increase in epithelial permeability9.
Following initial basolateral adhesion, PMN migrating across epithelial monolayers engage in adhesive interactions with adherens and tight junctional protein complexes10-12, and other epithelial ligands such as CD4713, before finally arriving at the luminal (apical) epithelial membrane. Here PMN remain in contact with the epithelial surface, and collections of apically-associated PMN in the intestinal crypts constitute a pathognomonic feature of the classic crypt abscess14.
PMN-epithelial cell interactions during the late stages of TEM have recently come into focus with the identification of several apically expressed epithelial PMN ligands. Specifically, expression of the PMN interacting proteins, CD5515, CD4416, and CD54 (ICAM-1)17 have been shown to be increased under inflammatory conditions. Importantly, CD44 and CD55 (decay accelerating factor, DAF) have been reported to play roles in facilitating PMN detachment from the apical surface after completion of TEM18,19, while ICAM-1 has been shown to mediate PMN adhesion through binding to Mac-120. In addition to mediating PMN-epithelial cell adhesion, ligands expressed on the apical epithelial surface have also been shown to modulate epithelial homeostasis through signaling events. In particular, CD44-associated signaling events have been implicated in regulating junctional composition and cell proliferation21.
ICAM-1 on endothelial cells has been previously shown to play a key role in regulating leukocyte trans-endothelial migration22,23. Moreover, ICAM-1 on endothelial cells has been shown to associate with cytoskeletal proteins and participate in cytoskeletal and junctional reorganization24,25. ICAM-1 is markedly upregulated in the epithelium of colonic biopsies from UC and CD patients26, as well as in intestinal epithelial cell lines, including T84 and Caco2 after stimulation with proinflammatory cytokines17,27. Although ICAM-1 binding to PMN Mac-1 can facilitate migration in the non-physiological apical-to-basolateral direction27, the role for ICAM-1 in PMN TEM in the physiological basolateral to apical direction is unknown as are the epithelial functional responses to such binding events.
In this study, we used in-vitro and in-vivo approaches to investigate the functional role of ICAM-1 during the final stages of PMN TEM. We show that ICAM-1 expression on the apical epithelial surface results in enhanced PMN adhesion after TEM. Furthermore, we show that apically associated PMN exhibit increased locomotion that is ICAM-1 and Mac-1 dependent. Importantly, we show that ligation of ICAM-1 at the apical epithelial membrane, leads to MLCK-dependent actomyosin contractility resulting in compromised barrier function, thereby facilitating enhanced PMN recruitment across the intestinal epithelium.
RESULTS
ICAM-1 promotes PMN adhesion and locomotion on the apical epithelial membrane under the conditions of inflammation
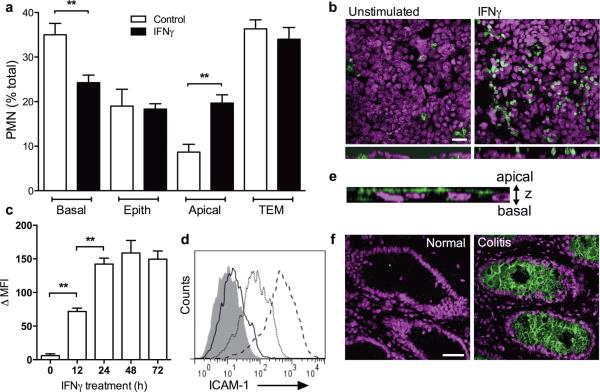
In crypt abscesses, as observed in active IBD, PMN infiltrating the intestinal crypts are often observed in intimate contact with the apical (luminal) epithelial surface14. To examine PMN-IEC interactions during the late stages of TEM, we modeled PMN migration across intestinal epithelium under inflammatory conditions using a previously described transwell setup19. PMN TEM in the physiological basolateral to apical direction across control or IFNγ treated T84 IECs was induced by the addition of a transepithelial fMLF gradient (100nM). Exposure of T84 IECs to IFNγ (100U/ml, 24h) had no significant effect on IEC barrier function or expression and subcellular localization of key junctional proteins JAM-A, Occludin and ZO-1 (Supplemental Figure 1). IFNγ treatment had no significant effect on the number of PMN that completed TEM, however it significantly increased the number of apically associated PMN (from 8.7±1.8%, untreated to 19.7±1.9%, IFNγ, apical, Fig. 1A). Consistent with this increase, the number of non-migrated PMN (PMN in the upper chamber; basal) was significantly reduced (~1.5-fold) following IFNγ treatment, suggesting an increased rate of migration (Fig. 1A). The number of PMN within the epithelial monolayer (epith) was not changed. Representative confocal immunofluorercence images (Fig. 1B, upper panels) and z-projections (bottom panels) show apically associated PMN after migration across IFNγ stimulated but not control T84 monolayers. Since IFNγ has been previously shown to induce ICAM-1 expression in inflamed epithelium27, we hypothesized that during inflammation, PMN migrating across epithelial monolayers remained adherent to the apical membrane through Mac-1 and ICAM-1 dependent interactions, and therefore would be capable of ICAM-1 dependent locomotion as has been previously observed in vascular endothelium24,28. Confirming previous findings, IFNγ treatment (100U/ml, 24h) induced a robust, time dependent increase in ICAM-1 expression (peaking 24 hours post-treatment, Fig. 1C,D) on the apical membrane of T84 IECs (Fig. 1E). This effect was specific to IFNγ, as exposure of T84 and SKCO15 IECs to TNFα (10ng/ml) failed to induce ICAM-1 expression (Supplemental Figure 2A,B). ICAM-1 upregulation was also observed in the crypt epithelium of colonic biopsies from patients with active UC compared to healthy mucosa (Fig. 1F). Further, confirming ICAM-1 and Mac-1 dependent PMN adhesion to apical IEC membranes, addition of either, anti-ICAM-1 or anti-Mac-1 function blocking Abs (20Lg/ml) reversed the IFNγ induced increase in PMN adhesion (Supplemental Figure 3A). Interestingly, inhibition of PMN Mac-1 resulted in a more potent reduction (>3-fold) in PMN adhesion, compared to inhibition of ICAM-1, suggesting that Mac-1 binds other epithelial surface ligands in addition to ICAM-1.
Figure 1. IFNγ-induced expression of ICAM-1 promotes PMN retention on the apical epithelial membrane.
(A) PMN were stimulated (100nM fMLF) to migrate across control or IFNγ treated T84 IECs. Non-migrated PMN (basal), PMN within the epithelial layer (epith), PMN associated with the apical IEC membrane after TEM (apical), and PMN that completed TEM (TEM) were quantified. (B) Following 1h of TEM, IEC monolayers were fixed and stained for cell nuclei (blue) and PMN Mac-1 (green). Representative images show enhanced apical attachment of PMN after migration across IFNγ treated T84 IECs. The bar is 20μm. Bottom panels are projections of images acquired in series in Z-direction. (C-F) Confluent T84 IECs were treated with IFNγ (100U/ml, 24h) to induce surface expression of ICAM-1. (C) Representative flow cytometry diagram and (D) quantification of ICAM-1 expression in response to IFNγ treatment. ICAM-1 expression was induced in a time dependent manner peaking at 24h. (E) T84 IECs were fixed in ethanol and immunofluorescently labeled for ICAM-1. Representative image (Z-projection of 7 confocal slices) demonstrates that ICAM-1 expression is restricted to apical epithelial surface. (F) Non-inflamed and inflamed tissue sections from biopsies of human patient with ulcerative colitis were stained for ICAM-1 (green) and nuclei (Topro-3, Blue). Representative immunofluorescence images show upregulation of ICAM-1 expression in inflamed tissue (right panel) but not in normal tissue (left panel). The bar is 50μm. N=5 independent experiments in triplicates, **(p<0.01), two-tailed Student's t-test (A), ANOVA with Newman-Keuls multiple comparison test (D).
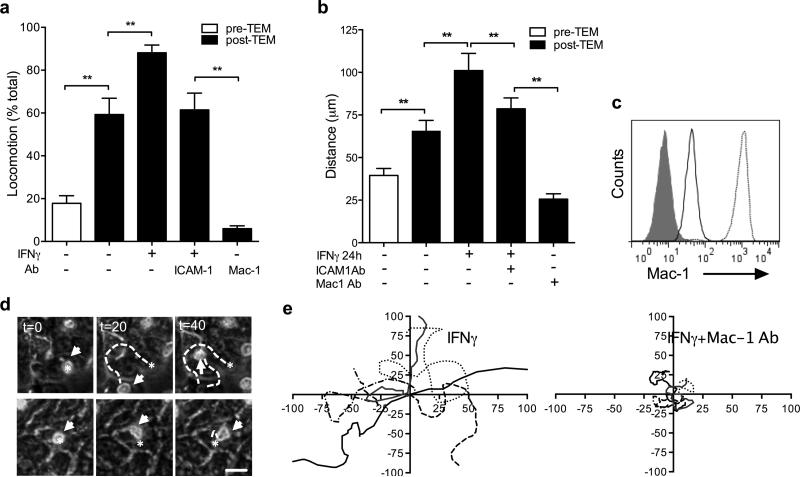
We next examined whether apically adhered PMN after crossing the epithelium, exhibited apical locomotion. Phase contrast time-lapse microscopy was used to track and quantify the behavior of individual PMN attached to the IEC apical membrane. PMN in the absence of exogenous stimulus exhibited little movement, however 59.3±7.6% of apically associated PMN after migration across IECs exhibited highly motile behavior (Fig. 2A) with average crawling distances of 65.6±6.2Lm (Fig. 2B). TEM induced a robust increase in Mac-1 expression on the PMN cell surface (Fig. 2C), consistent with its role in PMN-IEC apical interactions. Importantly, the number of PMN exhibiting apical locomotion was significantly increased when IECs were stimulated with IFNγ to upregulate ICAM-1 (88±3.6%, IFNγ vs 59.3±7.6%, untreated IEC). Under these conditions PMN traveled for significantly longer distances (101±10.0μm, IFNγ vs 65.6±6.2μm, untreated IECs, Fig. 2B) along the apical epithelial membrane. Confirming the role for ICAM-1 in mediating PMN locomotion, addition of a function blocking anti-ICAM-1 Ab reversed the IFNγ effects, reducing both the number and the distances traveled by PMN (61.4±7.8% and 73.6±6.1μm, respectively, Fig. 2A,B). Inhibition of Mac-1 abolished locomotion of the remaining adherent PMN (Fig. 2A and Supplemental movies 1,2), confirming an exclusive role for Mac-1 in this process. The effects of Mac-1 inhibition on PMN locomotion are further highlighted in time-lapse image sequences (Fig. 2D), and by displacement trajectories of 6 representative PMN (Fig. 2E). PMN crawling velocities ranged from 3-7 μm/min and were not significantly different on unstimulated versus IFNγ stimulated IECs (Supplemental Figure 3B).
Figure 2.
(A-B) Following TEM, PMN were allowed to apically adhere to IECs grown in the bottom chamber of transwells. The number of PMN exhibiting locomotion (A) and the traveled distances (B) were visualized and quantified in the presence or absence of the appropriate IgG control, or anti-ICAM-1 and Mac-1 function blocking Abs (20μg/ml), using phase contrast time lapse microscopy (Zeiss, Axiovert microscope) and ImageJ software. (C) PMN before (solid black line) and after TEM (dotted line) were analyzed for Mac-1 expression using flow cytometry. Representative flow diagram (n=4) shows robust upregulation of Mac-1 on PMN surface after TEM. The filled area represents control IgG staining. (D) Image sequence depicting representative PMN exhibiting locomotion on T84 IECs in the absence (upper panels) and in the presence of Mac-1 blocking antibody (bottom panels). Stars indicate initial PMN position, white arrows track PMN movement. The dashed lines highlight the path traveled by PMN over 40 minutes. The bar is 20μm. (E) Movement trajectories (in μm) of 6 representative PMN from 3 independent experiments over 40 minute time periods on the apical surface of IFNγ stimulated T84 IECs in the absence (upper panels) and in the presence of Mac-1 blocking antibody (bottom panels). N=4 independent experiments in duplicates, **(p<0.01), ANOVA with Newman-Keuls multiple comparison test (A,B).
PMN adhesion to the apical epithelial membrane compromises epithelial barrier function
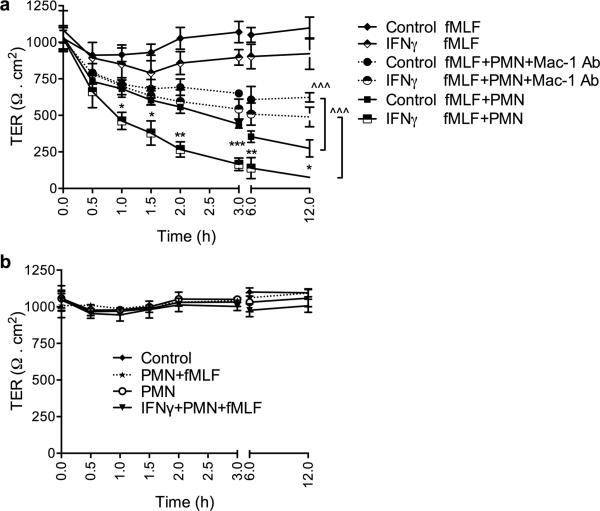
We next examined the effects of PMN interactions with apically expressed epithelial ligands on epithelial barrier function. In these experiments, PMN were added apically to confluent T84 IECs cultured on permeable supports (0.4μm pore size, too small to allow PMN TEM) and TER, as an index of epithelial barrier, was measured over 12h under the specified conditions. The 12h time period was selected to avoid the effects of apoptotic PMN on epithelial barrier29. fMLF (100nM) stimulated PMN adhesion to T84 IECs, triggered a time dependent, decrease in TER over a 12h time period (~60%, Fig. 3A). Importantly, addition of PMN to the apical membrane of T84 IECs that were pretreated with IFNγ, which we have shown leads to enhanced ICAM-1-dependent PMN adhesion (Fig. 1A), resulted in a further decrease in TER (~40% first 2h and ~90% over 12h). IFNγ treatment alone had no effect on TER. To test whether the observed increase in epithelial permeability was due to direct contact between epithelial cells and PMN, we used an anti-Mac-1 inhibitory Ab (CBRM1/29, 20μg/ml) to inhibit PMN adhesion to IFNγ stimulated IECs. Inhibition of Mac-1 significantly attenuated the PMN- induced decrease in TER after IFNγ stimulation (Fig. 3A). Importantly, in separate experiments we further confirmed that PMN-induced decrease in TER in both unstimulated and IFNγ treated epithelial monolayers was dependent on direct contact between PMN and the apical epithelial membrane, and not mediated through paracrine mechanisms. In such experiments PMN were added to the lower chamber of transwells containing inverted (apical side facing down) T84 monolayers, and stimulated with fMLF (100nM). Under these conditions, there was no effect of PMN on epithelial barrier as assessed by TER (Fig. 3B). Exposure of epithelial monolayers to 100nM fMLF for 12h had no significant effect on TER.
Figure 3. PMN interactions with the apical epithelial membrane compromise barrier function.
(A) PMN (2.5×105 cells) were introduced to the apical surface of untreated (Control) or IFNγ (100U/ml, 24h) treated T84 IECs grown on permeable supports (0.4μm filter size, prevent PMN TEM), in the presence or absence of fMLF (100nM) and in the presence or absence of anti-Mac-1 inhibitory Ab (20μg/ml). The resulting changes in TER as an index of epithelial barrier function were measured at the indicated time points. PMN contact with apical epithelial surface resulted in time dependent decrease in TER, which was partially reversed with inhibition of PMN-epithelial cell interactions. N=4 independent experiments in triplicates, *(p<0.05), **(p<0.01) ***(p<0.001) significantly different from Control+fMLF+PMN, ^^^(p<0.001) significantly different, ANOVA with Newman-Keuls multiple comparison test. (B) PMN were added in the vicinity of the apical membrane of unstimulated (control) or IFNγ treated IECs (IFNγ+PMN+fMLF) without direct contact (lower chamber of transwell setup) in the presence or absence of fMLF stimulation (100nM). No changes in TER were observed, suggesting contact-dependent effects. N=4 independent experiments in triplicates.
Ligation of epithelial ICAM-1 triggers an MLCK-dependent increase in intestinal epithelial permeability
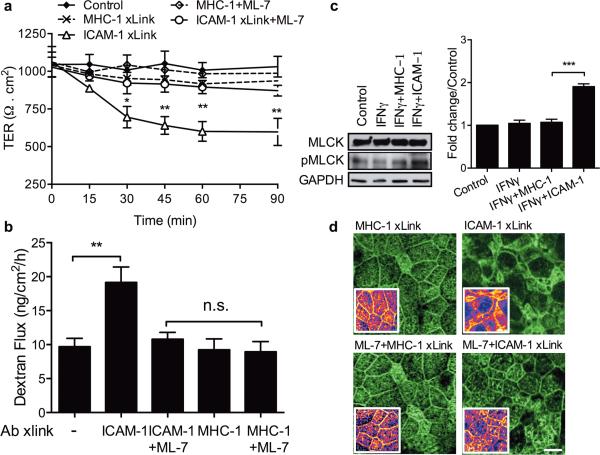
We observed that apical upregulation of ICAM-1 significantly potentiated the effects of Mac-1 dependent PMN adhesion on epithelial barrier (Fig. 3A). We thus hypothesized that the PMN induced increase in permeability of IFNγ treated epithelial monolayers was due to PMN contact-mediated ICAM-1 clustering, and an induction of ICAM-1 dependent signaling events, as previously described in vascular endothelium30,31. T84 IEC monolayers on permeable supports were treated with IFNγ to induce ICAM-1 expression, followed by addition of crosslinking Abs to ICAM-1. We confirmed that Ab-mediated crosslinking induced ICAM-1 clustering (Supplemental Figure 2C). As shown in Fig. 4A, Ab-mediated crosslinking of ICAM-1 resulted in a time dependent decrease in TER, which correlated with increased paracellular flux of FITC-dextran (3kd, Fig 4B). This effect was specific for ICAM-1, as crosslinking of other epithelial surface molecules, MHC-1 (Fig. 4) and the known PMN ligand, CD55 (Supplemental Figure 5) had no significant effect on permeability. Consistent with the absence of ICAM-1 expression on unstimulated T84 IECs, application of crosslinking Abs to the apical surface of these IECs in the absence of IFNγ stimulation had no effect on TER (Supplemental Figure 4A). Similarly, consistent with IFNγ-induced ICAM-1 localization on the apical epithelial membrane, application of crosslinking Abs to the basolateral aspect of epithelial monolayers had no significant effect on barrier function (Supplemental Figure 4B).
Figure 4. Ab-mediated crosslinking of ICAM-1 leads to an MLCK-dependent increase in epithelial permeability.
T84 IECs grown on permeable supports were stimulated with IFNγ (100U/ml, 24h) to induce ICAM-1 expression. ICAM-1 or control surface protein MHC-1 were crosslinked by incubation with primary Ab (20μg/ml, 60 min) followed by appropriate secondary antibodies (20μg/ml, 30 min). TER (A) and flux of FITC-dextran (3 kDa) (B) across T84 monolayers prior to and after ICAM-1 crosslinking were quantified at the indicated time points as an index of epithelial permeability. A pharmacological inhibitor of MLCK, ML-7 (20μM) was introduced an hour prior to initiation of the crosslinking protocols. ICAM-1 ligation resulted in decreased TER and increased flux of FITC-dextran, and was dependent on MLCK phosphorylation. (C) Representative western blots and densitometric analysis (using ImageJ) show increased MLCK phosphorylation after ICAM-1 crosslinking, which was reversed with the addition of ML-7. (D) Confocal microscopy and immunofluorescence labeling were used to examine actin remodeling after crosslinking of apically expressed control receptor MHC-1 and specifically ICAM-1, in the presence or absence of ML-7 (20μM). Ab-mediated crosslinking of ICAM-1 but not MHC-1 resulted in decreased apical brush border and junctional actin. This effect was reversed in the presence of ML-7. The bar is 50μm. N=4 independent experiments in triplicates (A,B), *(p<0.05), **(p<0.01) significantly different from control (A), **(p<0.01), ***(p<0.01), n.s. not significant, (B,C), ANOVA with Newman-Keuls multiple comparison test.
We have previously shown that early events in PMN TEM (at the level of the basolateral epithelial membrane) trigger MLCK mediated decreases in TER9. MLCK has been shown to play a key role in regulating epithelial permeability by regulating contraction of the perijunctional actomyosin ring through myosin II regulatory light chain phosphorylation32. We thus asked whether enhanced IEC permeability following ligation of apically expressed ICAM-1 was MLCK-dependent. Indeed, Ab-mediated crosslinking of ICAM-1 resulted in MLCK phosphorylation (Tyr 464), consistent with MLCK activation (Fig. 4C). Such activation was accompanied by decreased apical brush boarder and the perijunctional F-actin (Fig. 4D), indicative of actin cytoskeletal reorganization. Importantly, inhibition of ICAM-1 ligation-induced MLCK phosphorylation in T84 cells with ML-7 (20μM33, Supplemental Figure 4C) prevented ICAM-1 induced F-actin reorganization (Fig 4D), the decrease in TER (Fig. 4A), and the increase in paracellular dextran flux (Fig. 4B). ML-7 treatment (20μM) alone had no effect on TER. Together, these data suggest that under inflammatory conditions, PMN contact with the apical surface of crypt epithelial cells triggers ICAM-1 dependent signaling events resulting in enhanced permeability.
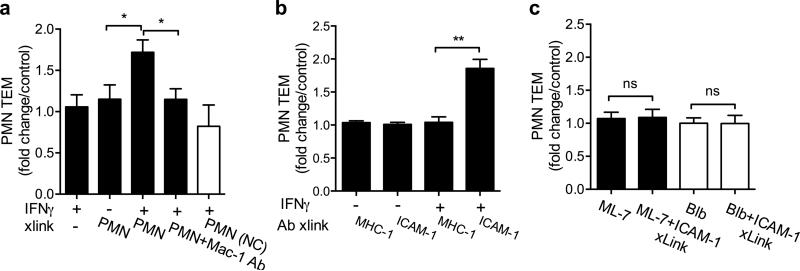
Engagement of ICAM-1 on the apical epithelial membrane facilitates enhanced PMN TEM
Since ligation of apically expressed ICAM-1 increased epithelial permeability, we performed experiments to examine whether ICAM-1 dependent alterations in epithelial barrier function would result in enhanced PMN TEM. We first investigated whether PMN engagement of ICAM-1 on the apical IEC membrane affected PMN TEM in the physiologically relevant basolateral-to-apical direction34. In these experiments, PMN were stimulated to migrate across epithelial monolayers and the transmigrated cells were collected and re-applied for 1h to the apical surface of new epithelial monolayers (2.5×105 PMN/monolayer) with or without IFNγ pretreatment. After 1h of PMN-epithelial contact, the monolayers were washed free of adherent PMN and used for subsequent PMN TEM assays in the basolateral-to-apical direction. Apical introduction of PMN to IFNγ-treated, but not to untreated epithelial monolayers triggered a significant increase in PMN TEM (1.7-fold, Fig. 5A). IFNγ treatment alone or PMN presence near the apical surface, but without direct contact with monolayers had no effect on PMN TEM (Fig. 5A). In parallel experiments, PMN TEM was examined following specific, Ab-mediated crosslinking of ICAM-1. Consistent with the effect of ICAM-1 crosslinking on IEC barrier function, Ab-mediated crosslinking of ICAM-1 on IFNγ treated T84 IECs significantly increased PMN TEM (1.9±fold, Fig. 5B) compared to crosslinking of an apically expressed control molecule, MHC-1. As expected, application of ICAM-1 crosslinking protocols to untreated IEC monolayers had no significant effect on PMN TEM. Together, these findings suggest that PMN adherent to the apical (luminal) IEC membrane through engagement of ICAM-1 may trigger alterations in the epithelial barrier function and contribute to the regulation of PMN recruitment.
Figure 5. Engagement of apically expressed ICAM-1 induces MLCK-dependent increase in PMN TEM.
PMN TEM in the basolateral-to-apical direction across untreated (control) or IFNγ treated (to induce ICAM-1 expression) T84 monolayers, was induced by a gradient of fMLF (100nM). (A) Transmigrated PMN were collected and introduced to the apical side of new T84 monolayers in the presence of fMLF (100nM), or added to bottom chambers of transwells, facing the apical membrane, but without direct contact with the monolayers (2.5×105 PMN/well, 1h). After washing off the apically adhered PMN, subsequent PMN TEM was quantified. PMN apical interactions specifically with IFNγ treated T84 IECs significantly increased PMN TEM. This effect was reversed in the presence of anti-Mac-1 inhibitory Ab (20μg/ml). For all conditions the number of PMN that remained adherent to the apical epithelial membrane after washes was determined and subtracted from the total number of transmigrated PMN. (B) PMN TEM after Ab-mediated crosslinking of ICAM-1 or control protein (MHC-1) was quantified. Crosslinking of ICAM-1 but not MHC-1 on IFNγ treated T84 cells significantly increased PMN TEM. (C) Control and IFNγ treated T84 monolayers were preincubated with ML- 7 (MLCK inhibitor, 20SM) or Blebbistatin (myosin motor II inhibitor, 10μm) alone or followed by ICAM-1 crosslinking. The effects of these inhibitors on ICAM-1 crosslinking induced increases in PMN TEM were quantified. Both inhibitors prevented ICAM-1 crosslinking induced increases in PMN TEM. For all panels the data presented as fold increase above PMN TEM across unstimulated T84 IECs. N=4 independent experiments in triplicates, *(p<0.05), **(p<0.01), n.s. not significant, ANOVA with Newman-Keuls multiple comparison test.
ICAM-1 crosslinking resulted in MLCK activation suggesting actin-myosin contraction (Fig. 4). Thus we next examined the effects of MLCK inhibition and inhibition of F-actin contractile forces on PMN TEM following ICAM-1 crosslinking. Increased PMN TEM induced by ICAM-1 crosslinking, was reversed when IECs were pretreated with either MLCK inhibitor ML-7 (20μM, 1h33) or the myosin motor II inhibitor, Blebbistatin (10μM, 1hr35, Fig. 5C). These findings suggest a role for actomyosin contraction downstream of ICAM-1 in regulating PMN TEM. Treatment with ML-7 or Blebbistatin alone had no effect on PMN TEM (Fig. 5C).
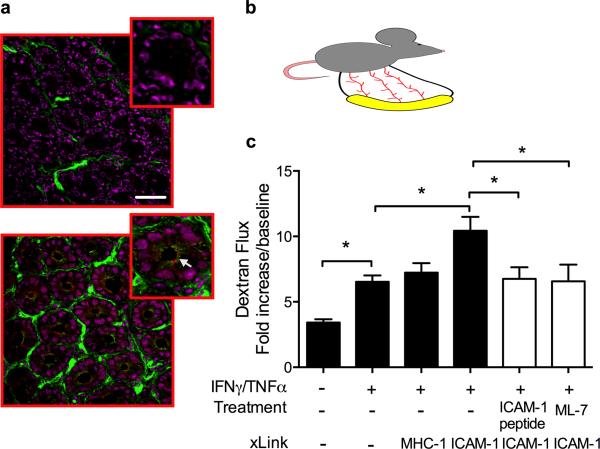
Ab-mediated ligation of ICAM-1 in murine intestinal lumen leads to MLCK-dependent increase in epithelial permeability and enhanced PMN recruitment
We next performed in-vivo experiments to examine the effect of ICAM-1 ligation on intestinal epithelial barrier function and PMN recruitment, using a mouse intestinal loop model (Fig. 6B). In this model, permeability of intact, blood perfused segments of small intestine was assessed after introduction of FITC-dextran to the intestinal lumen. As shown in figure 6A, while ICAM-1 was not detected in unstimulated epithelium (upper panel), intraperitoneal administration of IFNγ and TNFα (500ng, 24h) resulted in a robust induction of ICAM-1 expression. In particular, induced ICAM-1 localized to apical regions of murine crypt IECs above Claudin-2 (Fig. 6A, bottom panel). In parallel we observed that cytokine treatment resulted in ~2-fold increase in permeability of 3kDa FITC-dextran (Fig. 6C).
Figure 6. Engagement of ICAM-1 in murine intestine in-vivo leads to MLCK-dependent increase in intestinal permeability.
To induce ICAM-1 expression, mice were injected with a mixture of IFNγ and TNFα (500ng each, 24h, IP). (A) OCT-frozen segments of mouse intestine were sectioned (7μm-width) and immunofluorescently stained for ICAM-1 (green) and the tight junction protein, Claudin-2 (red). Representative confocal images show apical induction of ICAM-1 expression (white arrow) after IFNγ/TNFα treatment (bottom panel) compared to non-activated tissue (upper panel). The bar is 50μm. (B) Cartoon depicts the mouse intestinal loop model as described Methods. (C) In-vivo intestinal epithelial permeability was measured under the specified conditions as described in Methods. ICAM-1 tail peptide (100μm/ml) was introduced luminally 30 minutes prior to ICAM-1 crosslinking protocols. MLCK inhibitor ML-7 (20μM) was introduced by intraperitoneal injection 2.5 hours prior to ICAM-1 crosslinking. Increased FITC dextran flux after Ab-crosslinking of ICAM-1 was prevented with both the addition of ICAM-1 tail peptide and pretreatment with ML-7. N=4 mice/condition, *(p<0.05), ANOVA with Newman-Keuls multiple comparison test.
Importantly, introduction of ICAM crosslinking Abs, but not Abs to the control protein, MHC-1 into the lumen of cytokine stimulated intestinal loops induced a further ~1.5-fold increase in permeability to dextran when compared to cytokine treatment alone (Fig. 6C). To confirm that increases in intestinal epithelial permeability were mediated specifically by epithelial ICAM-1-induced signaling events, we used peptides derived from the cytoplasmic domain of ICAM-1 (ICAM-1 peptide) to inhibit the ability of ICAM-1 to mediate downstream signaling events. It has been previously shown in vascular endothelium that ICAM-1, but not control peptide inhibited interactions between ICAM-1 and target proteins, thereby preventing ICAM-1 dependent signaling without affecting binding to extracellular leukocyte ligands22, 24. Addition of ICAM-1, but not the control peptide (100μg/ml, 30 min) inhibited ICAM-1 ligation-induced increases in intestinal permeability (Fig. 6C). Abs introduced intraluminally into intestinal loops (unstimulated and following IFNγ/TNFα activation) were confirmed to not cross the epithelium, thus ruling out the potential indirect contribution of lamina propria cells to the observed effects (Supplemental Figure 6).
In further support of the role of MLCK in ICAM-1 mediated signaling, inhibition of MLCK activation, using ML-7 (1mg/kg, IP36) prior to ICAM-1 crosslinking prevented the increase in permeability (Fig. 6C). These data demonstrate that increased intestinal epithelial permeability in-vivo following ICAM-1 ligation is MLCK-dependent.
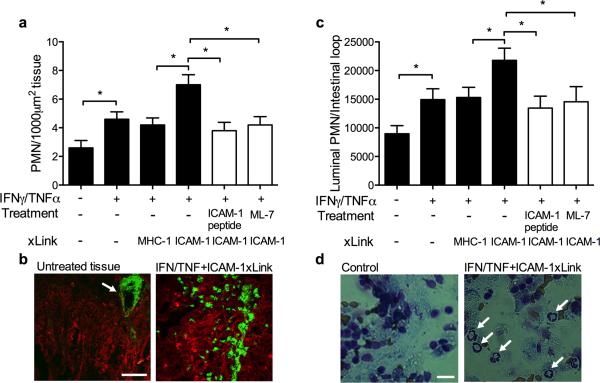
Since increased epithelial permeability is associated with enhanced PMN migration, we asked whether ligation of ICAM-1 would lead to enhanced PMN recruitment in-vivo. Using the murine intestinal loop model, PMN infiltration into the intestinal mucosa and migration into the lumen was induced by luminal administration of chemoattractant CXCL1 (1μM in 200μl HBSS+), and quantified by immunofluorescence labeling/confocal microscopy and analysis of lavaged fluid prepared on cytospins and stained with Diff-Quik, respectively. In the absence of chemoattractant, PMN were not detected in the intestinal epithelium or in the intestinal lumen (not shown), however, luminal administration of CXCL1 triggered significant PMN migration into the epithelial layer (Fig. 7A) and accumulation in the intestinal lumen (Fig. 7C). Consistent with the cytokine-induced increase in permeability, IFNγ/TNFα treatment increased by ~2.2-fold the number of PMN in the intestinal epithelium, and ~1.7-fold in the lumen. Importantly, addition of ICAM-1 crosslinking Abs into the lumen of cytokine treated intestinal loops further increased (over cytokine treatment alone) the number of PMN both in the epithelium (~1.6-fold, Fig. 7A,B) and in the lumen (~1.4-fold, Fig. 7C,D). PMN (green) infiltrating the intestinal epithelium (red) are shown in representative images of tissue sections from cytokine activated intestinal loops after ICAM-1 ligation (Fig. 7B). Similarly, PMN that migrated into the lumen after ICAM-1 ligation are shown in representative images of lavage fluids from intestinal loops (Fig. 7D).
Figure 7. Engagement of ICAM-1 in murine intestine in-vivo leads to MLCK-dependent increase in PMN recruitment.
PMN TEM in the murine intestinal loop model was induced by luminal introduction of CXCL1 (1μM in 200μl HBSS+). (A) PMN in the mucosal epithelia were quantified from cryosections of the intestinal loop immunofluorescently labeled for PMN (green) and F-actin (red). (B) Representative images show robust PMN infiltration after ICAM-1 crosslinking (right panel) compared to intestinal tissue without introduction of CXCL1, where PMN are localized inside blood vessels (white arrow). The bar is 50μm. (C) PMN that migrated into the lumen were isolated by lavage and quantified from cytospins stained with Diff-Quik. Data presented as percent of all cells in the lavage fluid. (D) Representative images of the cytospins depict transmigrated PMN in the intestinal luminal lavage after ICAM-1 crosslinking (right panel, PMN are indicated by white arrows). PMN are not detected in the intestinal lumen without the introduction of the chemoattractant (left panel). The bar is 10μm. N=4 mice/condition, *(p<0.05), ANOVA with Newman-Keuls multiple comparison test.
Consistent with ICAM-1 ligation-induced increases in epithelial permeability, enhanced PMN migration into the intestinal lumen was dependent on ICAM-1 mediated signaling events and MLCK activation. Both, intraluminal addition of ICAM-1 peptide, but not the control peptide (not shown) (100μg/ml, 30 min), as well as inhibition of MLCK (ML-7, 1mg/kg, IP) prior to Ab-mediated crosslinking of ICAM-1 completely inhibited ICAM-1 ligation-induced increases in PMN migration (Fig. 7A,C). These findings suggest that engagement of ICAM-1 on the apical intestinal epithelial membrane under conditions of inflammation compromises the barrier function, thus facilitating enhanced PMN recruitment in-vivo.
DISCUSSION
ICAM-1 expressed on the luminal aspect of vascular endothelium under inflammatory conditions37 mediates leukocyte migration across the endothelium in a luminal (apical) to basolateral direction. Engagement of ICAM-1 during early stages of the extravasation cascade, and ICAM-1 clustering around migrating leukocytes23, is essential for efficient leukocyte transendothelial migration. Furthermore, PMN-mediated ligation of endothelial ICAM-1 triggers signaling events, resulting in cytoskeletal and junctional reorganization, thus priming the endothelium for leukocyte passage24. In mucosal organs, it is now appreciated that ICAM-1 expression is induced under inflammatory conditions in bronchial38, alveolar39 and intestinal epithelial cells17. However the localization of epithelial ICAM-1 is not in a position to mediate early adhesive interactions with migrating PMN. In fact, epithelial-expressed ICAM-1 localizes to the apical or luminal membrane so that migrating PMN that are recruited into the intestine would only encounter epithelial ICAM-1 after crossing the epithelium. Given these observations, the functional role for ICAM-1 in epithelial cells has not been clearly defined.
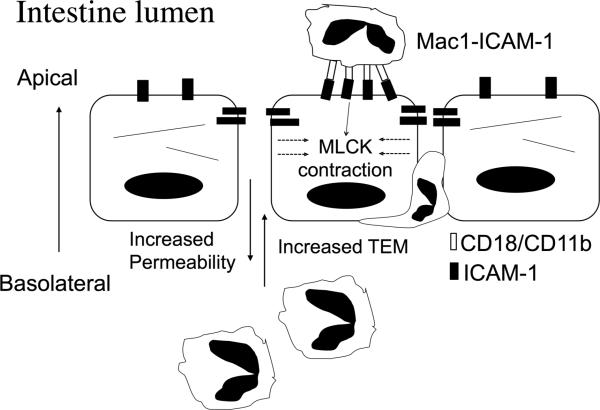
In the current work, we used complementary in-vitro and in-vivo approaches to show that engagement of ICAM-1 on the apical epithelial membrane by PMN or by antibody-mediated crosslinking resulted in MLCK-mediated actin reorganization, increased epithelial permeability, and enhanced PMN TEM. Thus far, regulation of PMN migration across epithelial layers has been largely attributed to PMN interactions with the basolateral aspects of epithelia9, and to PMN secreted protease-induced reorganization of epithelial junctional complexes during TEM40. Our findings introduce a new mechanism suggesting that under inflammatory conditions the presence of post-migrated PMN on the luminal epithelial surface and interactions with apically expressed epithelial ligands have regulatory effects on epithelial permeability and recruitment of PMN. Furthermore, under pathologic conditions characterized by persistence of large numbers of infiltrated PMN, such as observed in active ulcerative colitis, sustained engagement of ICAM-1 could enhance PMN TEM, thus contributing to PMN-associated epithelial injury. However, such mechanisms may also be beneficial in providing a robust pro-inflammatory response that aids in clearance of invading microorganisms by promoting PMN recruitment. These findings are summarized in a model depicted in Figure 8.
Figure 8. Hypothetical model depicting PMN engagement of ICAM-1 on the apical epithelial surface, and alteration of epithelial barrier function thereby facilitating enhanced PMN TEM.
PMN adhered to apically expressed ICAM-1 after completing TEM exhibit luminal locomotion and ligation of ICAM-1. Engagement of ICAM-1 triggers MLCK activation, reorganization of the actin cytoskeleton, and cell contraction leading to increased paracellular permeability. As a result of the compromised barrier function the number of PMN migrating across epithelial monolayers is significantly enhanced.
Exposure of T84 IECs to IFNγ induced ICAM-1 expression, resulting in enhanced PMN retention on the apical epithelial membrane after TEM. Intriguingly, apically associated PMN after completing TEM were observed to exhibit ICAM-1 and Mac-1-dependent apical locomotion, as has been previously described for vascular endothelium24. In blood vessels, luminal locomotion of PMN is an essential mechanism used by adherent PMN to locate the specific endothelial regions that can support PMN transmigration. In contrast, in the intestine or the lung PMN luminal locomotion would occur post-transepithelial migration, thus clearly serving a different purpose. While it has been suggested that PMN can migrate through mucus41, we speculate that PMN migrating along the luminal IEC surface would be more efficient in reaching the specific sites of inflammation. Moreover, PMN exhibiting locomotion may lead to clustering of apically expressed ligands, which in the case of ICAM-1 results in initiation of signaling events that alter epithelial barrier function, thereby facilitating enhanced PMN recruitment during acute inflammatory responses.
In support of this, we found that PMN interactions with the luminal epithelial surface following stimulation with IFNγ resulted in increased epithelial permeability. These changes were PMN-IEC contact dependent, as they were reversed upon inhibition of PMN adhesion/locomotion by anti-Mac-1 function-blocking Abs.
Several other apical epithelial ligands have been identified as being important for PMN-IEC interactions. For example, CD55 has been reported to promote PMN detachment18, thus potentially competing with ICAM-1 for neutrophil interactions. CD44 is another apical epithelial ligand that is markedly upregulated during inflammation19,42. While it has been implicated in promoting PMN detachment19, similar to ICAM-1, CD44 has also been shown to act as a signaling receptor through interactions with the actin cytoskeleton, thus contributing to tumor metastasis43. While we established that ligation of CD55 had no effect on epithelial permeability, whether this is true for other apically expressed PMN ligands should be explored in future studies.
We also demonstrate the importance of actomyosin dynamics in regulation of PMN TEM. Specifically, ICAM-1 ligation induced MLCK activation, and MLCK-dependent reorganization of the actin cytoskeleton, resulting in increased epithelial permeability and PMN TEM. These effects were likely due to increased actomyosin contractility, as MLCK has been previously shown to mediate cell contraction through actin cytoskeleton remodeling and activation of actomyosin contraction33,44. Inhibition of both MLCK activation and myosin motor II mediated actomyosin contraction, prevented ICAM-1 ligation-induced increases in permeability and PMN TEM (Figs 4A,B and 5C). It was previously shown that ICAM-1 associates with the actin cytoskeleton through interactions of its cytosolic domain (ICAM-1 tail) with adapter proteins45. Inhibition of these interactions in-vivo, using cell permeable peptides constituting the cytoplasmic domain of ICAM-1 also prevented ICAM-1 ligation induced increases in permeability and in PMN TEM, confirming the specificity of the observed effects to ICAM-1 mediated signaling. Interestingly, we have also shown that stimulus-dependent PMN contact with the basolateral epithelial surface, which was induced by a chemoattractant gradient, resulted in MLCK-depended alterations in the epithelial barrier function9. These effects were induced downstream of basolaterally expressed protease-activated receptors-1 and -2. Together with current observations these findings suggest that similar cellular events, such as MLCK activation, that are capable of modulating epithelial function, can be induced through engagement of ligands localized to either basolateral or apical epithelial surfaces during both early and late stages of PMN TEM.
In summary, these findings highlight an important role for post-migrated PMN on the luminal epithelial surface, and their interactions with apical epithelial ligands in the regulation of epithelial barrier function during inflammation. Impaired epithelial barrier and increased numbers of infiltrating PMN are ultimately linked to mucosal injury, as often observed in inflammatory disorders of the mucosal surfaces.
METHODS
Cells
Human T84 IECs were grown in DMEM-F12 50:50 (Sigma) with supplements as previously described34. For PMN isolation, human blood was drawn and handled according to protocols for the protection of human subjects, as approved by the Emory University Hospital Institutional Review Board. PMN were isolated by density gradient centrifugation34,46, and were used in experiments within 2 hours of isolation.
Antibodies and Reagents
Function blocking anti-human ICAM-1 (15.2) from Serotec (Raleigh, NC), anti-mouse ICAM-1mAb (YN1/1.7.4) and anti-Ly-6G conjugated to Alexa 488 from eBioscience (San Diego, CA), anti-CD11b/CD18 mAb (CBRM1/29) has been described47, anti-Mouse-Alexa488 and anti-rabbit-Alexa555 from Invitrogen (Carlsbad, CA), anti-MHC Class-I (1.B.548) from Abcam (Cambridge, MA), Isotype control IgG1 from BD Biosciences (San Jose, CA), rabbit polyclonal anti-MLCK from ProteinTech (Chicago IL) and anti-phospho MLCK from Santa Cruz (Santa Cruz, CA). HRP-conjugated anti-mouse and anti-rabbit IgGs from Jackson Immunoresearch (West Grove, PA). ABTS (2,2'-azinobis- 3-ethylbenzothiazoline-6-sulfonic acid), HBSS with Ca2+ and Mg2+ (HBSS+) and HBSS without Ca2+ and Mg2+ (HBSS-), DMEM including media supplements as well as chemotactic peptide fMLF, mouse IFNγ and TNFα from Sigma (St Louis, MO). Fluorescein-labeled dextran (3kDa) from Molecular Probes (Eugene, OR)
Western Blot
Epithelial cells were prepared for western blot analysis using standard techniques, as previously described19.
Flow cytometry
Adherent epithelial cell monolayers were trypsinized (Trypsin-EDTA, Sigma), washed and stained for ICAM-1 (15.2 FITC, 5 μg/ml). PMN before and after TEM were stained for Mac-1 (ICRF44, 20 μl/test). Cell samples were analysed using FACS Calibur and FlowJo software.
Cell adhesion assay
PMN were incubated with confluent monolayers in 24-well tissue culture plates (2.5×105 cell/well in H+, 1h, 37°C). Following three consecutive washes, the remaining adherent PMN were quantified in 10 randomly selected fields of view, in triplicate, for each experimental condition.
Immunofluorescence microscopy
IEC monolayers were fixed/permeabilized (95% ethanol), and incubated with appropriate primary (10μg/ml, over night at 4°C) followed by fluorescently labeled secondary Abs (1μg/ml, 1h at RT). Nuclei were stained with ToPro-3 iodide (Invitrogen). For mouse tissue, segments of mouse intestine were snap frozen in OCT, 7μm thick sections were cut on a freezing microtome and mounted on slides for immunofluorescence labeling. All images were acquired on a LSM510 confocal microscope (Carl Zeiss, Thornwood, NY) with Plan-Neofluor 60x and 40x objectives.
Mouse intestinal loop model
Male C57BL6J mice (Jackson Laboratories, age 11-15 weeks) were maintained under specific pathogen-free conditions at Emory Division of Animal Resources facilities. When indicated, inflammation was induced by intraperitoneal injection of mouse recombinant TNFα and IFNγ (0.5μg each) in 0.25ml saline 24 hours prior to the start of the surgical preparation. Animals were anesthetized by subcutaneous intramascular injection of ketamine and xylazine mixture at doses of 100 and 5 mg/kg, respectively. A midline abdominal incision was made and a 4-cm loop of small intestine was exteriorized and clipped at proximal and distal ends (Fig. 6B). After luminal administration of the desired treatment the excised loops were reinserted into the peritoneal cavity for the duration of the experimental procedures. At the end of all experimental procedures animals were euthanized via rapid cervical dislocation. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Emory University.
PMN TEM assays
For in-vivo PMN migration experiments, exteriorized intestinal loops were injected with 1μM PMN chemoattractant CXCL1 (KC) in 200μl HBSS+. 90 minutes later intestinal loops were isolated and lavaged twice with 200μl HBSS+. PMN in the lavaged fluid (PMN that migrated into the intestinal lumen) were quantified on 100μL cytospins stained with Diff-Quik (Dade Behring, Newark, DE). In-vitro PMN TEM assays were performed in the physiologically relevant, basolateral-to-apical direction and quantified by assaying for the PMN azurophilic granule protein MPO, as previously described34. Apically associated PMN were collected by centrifugation (500 RPM, 3min) and assayed for MPO. When indicated IECs were pretreated with IFNγ (100U/ml) for 24 hours to induce ICAM-1 expression.
PMN locomotion on epithelial cells
Following migration across T84 IEC monolayers PMN adhered to the apical membrane of confluent T84 IEC monolayers grown in the bottom chamber of the transwell setup, were visualized using phase contrast timelapse microscopy (Zeiss Axiovert microscope). PMN locomotion (% locomoting cells, distances, and velocity) was quantified using ImageJ software.
Epithelial permeability assays
For in-vivo dextran flux assay, exteriorized intestinal loops were injected with FITC-dextran (3kDa, 1mg/ml in 200μl saline) and reinserted into the peritoneal cavity. 60 minutes later, fluorescence intensity in whole blood (obtained through cardiac puncture) was analyzed on a fluorescence plate reader (Fluostar Galaxy, BMG LabTech, Germany), using excitation/emission wavelengths of 480/520nm as previously described48. For in-vitro dextran flux assay, 10μg/ml fluorescein-labeled dextran (3kDa) was added apically to IEC monolayers grown on permeable supports. Samples were taken from the bottom chambers at the indicated time points and fluorescence intensity in the samples was measured.
ICAM-1 crosslinking
In-vitro, primary anti-ICAM-1 (clone 15.2, 20μg/ml, 1h) followed by secondary crosslinking Abs (20μg/ml, 30min) were added apically to control/IFNγ pre-exposed epithelial monolayers. Where specified IECs were preincubated with ML-7 (20μM) and blebbistatin (10μM) for 1h at 37°C. In-vivo, prior to introduction of FITC dextran or CXCL1, isolated intestinal loops were cannulated at proximal and distal ends with 0.76-mm internal diameter polyethylene tubing, filled with ICAM-1 (YN1/1.7.4) or MHC-1 (1.B.548) Ab solutions (50μg/ml in 200μl saline warmed to 37°C, 1h), flushed with saline and refilled with secondary crosslinking Abs (50μg/ml in 200μl saline) for an additional 30 minutes. Where specified, ICAM-1 cytoplasmic domain peptide (13 C-terminal amino acids of mouse ICAM-1 (QRKIRIYKLQQAQ) attached to penetratin (RQIKIWFQNRRMKWKK)24, 100μg/ml) or control peptide (an irrelevant sequence from rat rodopsin, CKPMSNFRFGENH) was introduced intraluminally 30 minutes prior to ICAM-1 crosslinking. The MLCK inhibitor ML-7 (1mg/kg) was introduced by intraperitoneal injection 2.5 hours prior to initiation of surgical protocols.
Statistics
Statistical significance was assessed by Student t-test or by one-way ANOVA with Newman-Keuls Multiple Comparison Test using Graphpad Prism (V4.0). Statistical significance was set at P<0.05. For all experiments the data shown as ± SEM.
Supplementary Material
Supplemental movie 1: Timelapse series (36 minutes, acquired at 1 frame per minute) shows highly motile behavior of PMN adherent to the apical epithelial membrane after completing TEM. PMN were induced to migrate across epithelial monolayers by the addition of a transepithelial fMLF gradient (100nM). The locomotion of PMN adhered to the apical membrane of epithelial cells grown in the bottom chamber of transwells after exposure to IFNγ (100U/ml, 24h, to induce ICAM-1 expression) was visualized using phase contrast time-lapse microscopy.
Supplemental movie 2: Timelapse series (36 minutes, acquired at 1 frame per minute) shows that the addition of function blocking anti-Mac-1 Ab inhibits the motile behavior of post-migrated PMN on apical epithelial membrane. PMN were induced to migrate across epithelial monolayers by the addition of a transepithelial fMLF gradient (100nM). Locomotion of PMN adhered to the apical membrane of epithelial cells that were pre-exposed to IFNγ (100U/ml, 24h) after Mac-1 inhibition was visualized using phase contrast time-lapse microscopy. Addition of an anti Mac-1 inhibitory Ab (Cbrm 1/29, 20μg/ml) significantly inhibited PMN apical locomotion.
Supplemental Figure 1: IFNγ treatment induces time dependent expression of ICAM-1 on the apical epithelial surface. (A-B) Confluent T84 (A) and SKCO15 (B) IECs were treated with TNFα (10ng/ml, 24h), and the expression of ICAM-1 was examined using flow cytometry. TNFα treatment failed to induce ICAM-1 expression in both cell lines as shown in representative flow diagrams. (C) To confirm that crosslinking protocols indeed result in clustering of ICAM-1, IFNγ stimulated IEC monolayers were labeled for ICAM-1 using either an anti-ICAM-1 Ab primary conjugated to Alexa 488 (20μg/ml, 60 min, left panel) or an anti-ICAM-1 Ab (20μg/ml, 60 min) followed by secondary crosslinking Ab conjugated to Alexa 488 (20μg/ml, 30 min, right panel). Punctate distribution of ICAM-1 (indicative of ICAM-1 clustering) can be seen after the addition of secondary crosslinking Ab. The bar is 20μm. N=3
Supplemental Figure 2: T84 IEC treatment with IFNγ for 24 hours has no effect barrier function. T84 IECs cultured on permeable supports were stimulated with IFNγ (100u/ml) for 24h. (A) TER and (B) Dextran flux (data presented as the rate of flux) were measured before (control) and after IFNγ treatment. (C) Protein levels of ICAM-1 and key junctional proteins that regulate IEC barrier function, JAM-A, Occludin and ZO-1 were assayed using western blot analysis. No changes were observed. (D) Localization of the junctional proteins was examined using confocal microscopy and immunofluorescence labeling, and was found not altered after 24h IFNγ treatment. N=4, n.s. not significant, two-tailed Student's t-test.
Supplemental Figure 3: IFNγ induced ICAM-1 expression leads to increased PMN adhesion and locomotion on the apical T84 IECs membrane. PMN after TEM were collected and incubated with the apical membrane of untreated/IFNγ treated T84 IECs in the presence or absence of control IgG or function inhibitory Abs against ICAM-1 and Mac-1 (20μg/ml). (A) Adhesion, (B) Locomotion velocity were quantified. N=4 independent experiments in duplicates, *(p<0.05), **(p<0.01), ***(p<0.001), ANOVA with Newman-Keuls multiple comparison test.
Supplemental Figure 4: Ligation of ICAM-1 at the luminal IEC surface alters epithelial barrier function. T84 IECs were cultured in an inverted orientation in a transwell setup. (A) Ab crosslinking protocols were applied to the luminal surface of T84 IECs that were not treated with IFNγ (no ICAM-1 expression). No significant effects on TER were observed. (B) Ab crosslinking protocols were applied to the basal surface of IFNγ treated T84 IECs. Similarly, no significant effects on TER were observed, confirming that IFNγ induced expression of ICAM-1 on IECs is restricted to the luminal surface. (C) Inhibition of MLCK activation with ML-7. T84 IECs were treated IFNγ (100U/ml, 24h) to induce ICAM-1 expression. Prior to application of crosslinking protocols IECs were preincubated with ML- 7 (20μM, 1h). As shown by representative western blot (left panel) and quantified using densitometry analysis (right panel), ML-7 treatment prevented the ICAM-1 crosslinking induced, significant increase in MLCK phosphorylation. N=4, ***(p<0.001), ANOVA with Newman-Keuls multiple comparison test.
Supplemental Figure 5: Ab-mediated crosslinking of CD55 has no effect on epithelial permeability. T84 IECs grown on permeable supports were stimulated with IFNγ (100U/ml, 24h) and CD55 was crosslinked by incubation with primary Ab (20μg/ml, 60 min) followed by appropriate secondary antibodies (20μg/ml, 30 min). TER (A) and flux of FITC-dextran (3 kDa, data presented as rate of flux) (B) across T84 monolayer without (control) or with CD55 crosslinking were quantified at the indicated time points. N=3 independent experiments in triplicates, n.s. not significant, two-tailed Student's t-test.
Supplemental Figure 6: Luminally introduced anti-ICAM-1 Ab in the intestinal loop preferentially labels only luminal epithelial surface. To confirm that luminally introduced Abs in the intestinal loop model under the conditions of inflammation remain in the lumen and do not cross the epithelium, the binding of rat anti-ICAM-1 Ab (YN1/1.7.4, introduced intraluminally, 50μg/ml in 200μl saline warmed to 37°C, 2h) was assessed in the intestinal mucosa from mice that were stimulated with IFNγ/TNFα (500ng each, 24h, IP), using immunofluorescence labeling and confocal microscopy. OCT-frozen segments of the intestinal loop after anti-ICAM-1 Ab treatment were sectioned (7Sm-width) and immunofluorescently stained with an Alexa 488 conjugated anti-rat Ab secondary alone (left panel). In separate experiments frozen sections of the mucosa from cytokine treated intestines were stained using standard protocols of anti-ICAM-1 Ab (YN1/1.7.4) followed by an Alexa 488 conjugated secondary anti-rat Ab (right panel). Intraluminally introduced anti-ICAM-1 Ab exclusively labeled ICAM-1 on the luminal surface of the crypt epithelia without any staining of sub-epithelial structures. In contrast imuunofluorescence staining of the mucosa using standard protocols demonstrate robust staining of sub-epithelial blood vessels (white arrows) in addition to the staining of the apical epithelial membrane. The bar is 50μm.
AKNOWLEGMENTS
The authors thank Emory DDRDC core facility for culturing intestinal epithelial cell lines (supported NIH DK064399). We thank Dr. Ingrid H Sarelius for kindly providing the ICAM-1 and control peptides. This work was supported in part by grants from the NIH (DK072564, DK061379, DK079392 to CP, and DK055679, DK059888 to AN) and CDA from the Crohn's and Colitis Foundation of America to RS.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;295:L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Lisle RC, Mueller R, Boyd M. Impaired mucosal barrier function in the small intestine of the cystic fibrosis mouse. J. Pediatr. Gastroenterol. Nutr. 2011;53:371–379. doi: 10.1097/MPG.0b013e318219c397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz H, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 5.Ma TY. Intestinal epithelial barrier dysfunction in Crohn's disease. Proc. Soc. Exp. Biol. Med. 1997;214:318–327. doi: 10.3181/00379727-214-44099. [DOI] [PubMed] [Google Scholar]
- 6.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 7.Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J. Immunol. 2002;169:5270–5278. doi: 10.4049/jimmunol.169.9.5270. [DOI] [PubMed] [Google Scholar]
- 8.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J. Clin. invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J. Immunol. 2008;181:5702–5710. doi: 10.4049/jimmunol.181.8.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am. J. Respir. Cell. Mol. Biol. 2009;40:519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mumy KL, McCormick BA. The role of neutrophils in the event of intestinal inflammation. Curr. Opin. Pharmacol. 2009;9:697–701. doi: 10.1016/j.coph.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, et al. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J. Biol. Chem. 2002;277:10028–10036. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- 14.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 15.Louis NA, Hamilton KE, Kong T, Colgan SP. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. FASEB J. 2005;19:950–959. doi: 10.1096/fj.04-3251com. [DOI] [PubMed] [Google Scholar]
- 16.Leir SH, Holgate ST, Lackie PM. Inflammatory cytokines can enhance CD44-mediated airway epithelial cell adhesion independently of CD44 expression. Am. J. Physiol. Lung. Cell Mol. Physiol. 2003;285:L1305–1311. doi: 10.1152/ajplung.00255.2002. [DOI] [PubMed] [Google Scholar]
- 17.Kaiserlian D, Rigal D, Abello J, Revillard JP. Expression, function and regulation of the intercellular adhesion molecule-1 (ICAM-1) on human intestinal epithelial cell lines. Eur. J. Immunol. 1991;21:2415–2421. doi: 10.1002/eji.1830211018. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence DW, et al. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J. Exp. Med. 2003;198:999–1010. doi: 10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brazil JC, et al. Neutrophil migration across intestinal epithelium: evidence for a role of CD44 in regulating detachment of migrating cells from the luminal surface. J. Immunol. 2010;185:7026–7036. doi: 10.4049/jimmunol.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang GT, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM)-1) expression and neutrophil adhesion. J. Clin. Invest. 1996;98:572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, et al. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumagin R, Sarelius IH. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J. Immunol. 2010;184:5242–5252. doi: 10.4049/jimmunol.0903319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 26.Vainer B, Nielsen OH, Horn T. Comparative studies of the colonic in situ expression of intercellular adhesion molecules (ICAM-1, -2, and -3), beta2 integrins (LFA-1, Mac-1, and p150,95), and PECAM-1 in ulcerative colitis and Crohn's disease. Am. J. Surg. Pathol. 2000;24:1115–1124. doi: 10.1097/00000478-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Parkos CA, et al. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol. Med. 1996;2:489–505. [PMC free article] [PubMed] [Google Scholar]
- 28.Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J. Immunol. 2010;185:7057–7066. doi: 10.4049/jimmunol.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross EA, et al. Interaction between integrin alpha9beta1 and vascular cell adhesion molecule-1 (VCAM-1) inhibits neutrophil apoptosis. Blood. 2006;107:1178–1183. doi: 10.1182/blood-2005-07-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am. J. Physiol. Heart. Circ. Physiol. 2008;295:H969–H977. doi: 10.1152/ajpheart.00400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1. Am. J. Physiol. Cell. Physiol. 2011;301:C804–813. doi: 10.1152/ajpcell.00135.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham KE, Turner JR. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Annals of the New York Academy of Sciences. 2012;1258:34–42. doi: 10.1111/j.1749-6632.2012.06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner JR, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 1997;273:C1378–1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 34.Parkos CA, Colgan SP, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J. Cell Biol. 1992;117:757–764. doi: 10.1083/jcb.117.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol. Biol. Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CC, Lu YZ, Wu LL, Yu LC. Role of myosin light chain kinase in intestinal epithelial barrier defects in a rat model of bowel obstruction. BMC Gastroenterol. 2010;10:39. doi: 10.1186/1471-230X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumagin R, Sarelius IH. TNF-alpha activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2116–2125. doi: 10.1152/ajpheart.00248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan SC, et al. Upregulation of ICAM-1 expression in bronchial epithelial cells by airway secretions in bronchiectasis. Respir. Med. 2008;102:287–298. doi: 10.1016/j.rmed.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Madjdpour C, et al. Lipopolysaccharide induces functional ICAM-1 expression in rat alveolar epithelial cells in vitro. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278:L572–579. doi: 10.1152/ajplung.2000.278.3.L572. [DOI] [PubMed] [Google Scholar]
- 40.Ginzberg HH, et al. Neutrophil-mediated epithelial injury during transmigration: role of elastase. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G705–717. doi: 10.1152/ajpgi.2001.281.3.G705. [DOI] [PubMed] [Google Scholar]
- 41.Parkhurst MR, Saltzman WM. Leukocytes migrate through three-dimensional gels of midcycle cervical mucus. Cellular immunology. 1994;156:77–94. doi: 10.1006/cimm.1994.1154. [DOI] [PubMed] [Google Scholar]
- 42.Mackay CR, et al. Expression and modulation of CD44 variant isoforms in humans. J. Cell Biol. 1994;124:71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathew J, et al. CD44 is expressed in hepatocellular carcinomas showing vascular invasion. J. Pathol. 1996;179:74–79. doi: 10.1002/(SICI)1096-9896(199605)179:1<74::AID-PATH531>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 44.Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology. 2002;123:1179–1190. doi: 10.1053/gast.2002.36002. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, et al. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J. Immunol. 2006;177:6440–6449. doi: 10.4049/jimmunol.177.9.6440. [DOI] [PubMed] [Google Scholar]
- 46.Chin AC, et al. CD47 and TLR-2 cross-talk regulates neutrophil transmigration. J. Immunol. 2009;183:5957–5963. doi: 10.4049/jimmunol.0900789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balsam LB, Liang TW, Parkos CA. Functional mapping of CD11b/CD18 epitopes important in neutrophil-epithelial interactions: a central role of the I domain. J. Immunol. 1998;160:5058–5065. [PubMed] [Google Scholar]
- 48.Laukoetter MG, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental movie 1: Timelapse series (36 minutes, acquired at 1 frame per minute) shows highly motile behavior of PMN adherent to the apical epithelial membrane after completing TEM. PMN were induced to migrate across epithelial monolayers by the addition of a transepithelial fMLF gradient (100nM). The locomotion of PMN adhered to the apical membrane of epithelial cells grown in the bottom chamber of transwells after exposure to IFNγ (100U/ml, 24h, to induce ICAM-1 expression) was visualized using phase contrast time-lapse microscopy.
Supplemental movie 2: Timelapse series (36 minutes, acquired at 1 frame per minute) shows that the addition of function blocking anti-Mac-1 Ab inhibits the motile behavior of post-migrated PMN on apical epithelial membrane. PMN were induced to migrate across epithelial monolayers by the addition of a transepithelial fMLF gradient (100nM). Locomotion of PMN adhered to the apical membrane of epithelial cells that were pre-exposed to IFNγ (100U/ml, 24h) after Mac-1 inhibition was visualized using phase contrast time-lapse microscopy. Addition of an anti Mac-1 inhibitory Ab (Cbrm 1/29, 20μg/ml) significantly inhibited PMN apical locomotion.
Supplemental Figure 1: IFNγ treatment induces time dependent expression of ICAM-1 on the apical epithelial surface. (A-B) Confluent T84 (A) and SKCO15 (B) IECs were treated with TNFα (10ng/ml, 24h), and the expression of ICAM-1 was examined using flow cytometry. TNFα treatment failed to induce ICAM-1 expression in both cell lines as shown in representative flow diagrams. (C) To confirm that crosslinking protocols indeed result in clustering of ICAM-1, IFNγ stimulated IEC monolayers were labeled for ICAM-1 using either an anti-ICAM-1 Ab primary conjugated to Alexa 488 (20μg/ml, 60 min, left panel) or an anti-ICAM-1 Ab (20μg/ml, 60 min) followed by secondary crosslinking Ab conjugated to Alexa 488 (20μg/ml, 30 min, right panel). Punctate distribution of ICAM-1 (indicative of ICAM-1 clustering) can be seen after the addition of secondary crosslinking Ab. The bar is 20μm. N=3
Supplemental Figure 2: T84 IEC treatment with IFNγ for 24 hours has no effect barrier function. T84 IECs cultured on permeable supports were stimulated with IFNγ (100u/ml) for 24h. (A) TER and (B) Dextran flux (data presented as the rate of flux) were measured before (control) and after IFNγ treatment. (C) Protein levels of ICAM-1 and key junctional proteins that regulate IEC barrier function, JAM-A, Occludin and ZO-1 were assayed using western blot analysis. No changes were observed. (D) Localization of the junctional proteins was examined using confocal microscopy and immunofluorescence labeling, and was found not altered after 24h IFNγ treatment. N=4, n.s. not significant, two-tailed Student's t-test.
Supplemental Figure 3: IFNγ induced ICAM-1 expression leads to increased PMN adhesion and locomotion on the apical T84 IECs membrane. PMN after TEM were collected and incubated with the apical membrane of untreated/IFNγ treated T84 IECs in the presence or absence of control IgG or function inhibitory Abs against ICAM-1 and Mac-1 (20μg/ml). (A) Adhesion, (B) Locomotion velocity were quantified. N=4 independent experiments in duplicates, *(p<0.05), **(p<0.01), ***(p<0.001), ANOVA with Newman-Keuls multiple comparison test.
Supplemental Figure 4: Ligation of ICAM-1 at the luminal IEC surface alters epithelial barrier function. T84 IECs were cultured in an inverted orientation in a transwell setup. (A) Ab crosslinking protocols were applied to the luminal surface of T84 IECs that were not treated with IFNγ (no ICAM-1 expression). No significant effects on TER were observed. (B) Ab crosslinking protocols were applied to the basal surface of IFNγ treated T84 IECs. Similarly, no significant effects on TER were observed, confirming that IFNγ induced expression of ICAM-1 on IECs is restricted to the luminal surface. (C) Inhibition of MLCK activation with ML-7. T84 IECs were treated IFNγ (100U/ml, 24h) to induce ICAM-1 expression. Prior to application of crosslinking protocols IECs were preincubated with ML- 7 (20μM, 1h). As shown by representative western blot (left panel) and quantified using densitometry analysis (right panel), ML-7 treatment prevented the ICAM-1 crosslinking induced, significant increase in MLCK phosphorylation. N=4, ***(p<0.001), ANOVA with Newman-Keuls multiple comparison test.
Supplemental Figure 5: Ab-mediated crosslinking of CD55 has no effect on epithelial permeability. T84 IECs grown on permeable supports were stimulated with IFNγ (100U/ml, 24h) and CD55 was crosslinked by incubation with primary Ab (20μg/ml, 60 min) followed by appropriate secondary antibodies (20μg/ml, 30 min). TER (A) and flux of FITC-dextran (3 kDa, data presented as rate of flux) (B) across T84 monolayer without (control) or with CD55 crosslinking were quantified at the indicated time points. N=3 independent experiments in triplicates, n.s. not significant, two-tailed Student's t-test.
Supplemental Figure 6: Luminally introduced anti-ICAM-1 Ab in the intestinal loop preferentially labels only luminal epithelial surface. To confirm that luminally introduced Abs in the intestinal loop model under the conditions of inflammation remain in the lumen and do not cross the epithelium, the binding of rat anti-ICAM-1 Ab (YN1/1.7.4, introduced intraluminally, 50μg/ml in 200μl saline warmed to 37°C, 2h) was assessed in the intestinal mucosa from mice that were stimulated with IFNγ/TNFα (500ng each, 24h, IP), using immunofluorescence labeling and confocal microscopy. OCT-frozen segments of the intestinal loop after anti-ICAM-1 Ab treatment were sectioned (7Sm-width) and immunofluorescently stained with an Alexa 488 conjugated anti-rat Ab secondary alone (left panel). In separate experiments frozen sections of the mucosa from cytokine treated intestines were stained using standard protocols of anti-ICAM-1 Ab (YN1/1.7.4) followed by an Alexa 488 conjugated secondary anti-rat Ab (right panel). Intraluminally introduced anti-ICAM-1 Ab exclusively labeled ICAM-1 on the luminal surface of the crypt epithelia without any staining of sub-epithelial structures. In contrast imuunofluorescence staining of the mucosa using standard protocols demonstrate robust staining of sub-epithelial blood vessels (white arrows) in addition to the staining of the apical epithelial membrane. The bar is 50μm.