Abstract
Background
Increased mortality risk and its moderators is an important, but still under recognized, negative outcome of Late-Life Depression (LLD). Therefore, we aimed to evaluate whether LLD is a risk factor for all-cause mortality in a population-based study with over ten years of follow-up, and addressed the moderating effect of gender and symptom severity on mortality risk.
Methods
This analysis used data from the Bambuí Cohort Study of Aging. The study population comprised 1.508 (86.5%) of all eligible 1.742 elderly residents. Depressive symptoms were annually evaluated by the GHQ-12, with scores of 5 or higher indicating clinically significant depression. From 1997 to 2007, 441 participants died during 10,648 person-years of follow-up. We estimated the hazard ratio for mortality risk by Cox regression analyses.
Results
Depressive symptoms were a risk factor for all-cause mortality after adjusting for confounding lifestyle and clinical factors (adjusted HR=1.24 CI95% [1.00–1.55], p=0.05). Mortality risk was significantly elevated in men (adjusted HR=1.45 CI95% [1.01 – 2.07], p=0.04), but not in women (adjusted HR=1.13 CI95% [0.84 – 1.48], p=0.15). We observed a significant interaction between gender and depressive symptoms on mortality risk ((HR= 1.72 CI95% [1.18 – 2.49], p=0.004).
Conclusion
The present study provides evidence that LLD is a risk factor for all-cause mortality in the elderly, especially in men. The prevention and adequate treatment of LLD may help to reduce premature disability and death among elders with depressive symptoms.
Keywords: late-life depression, mortality, cohort study, risk factor
Introduction
Major depression is a common psychiatric disorder in older adults. Recent studies show one-year prevalence rates ranging from 4% to 12% in developed and developing countries1,2. Late-life depression (LLD) is linked to negative health outcomes, including a higher risk of cognitive impairment3, functional impairment4, and development of Alzheimer’s disease and vascular dementia5. LLD is also associated with a higher burden of medical illnesses and comorbid psychiatric disorders, independent of lifestyle factors6,7.
An important negative outcome of LLD may be the risk of death associated with this condition. Previous studies have shown an increased risk of all-cause mortality, including deaths due to suicide, in community-dwelling older adults with LLD, independent of comorbid medical conditions, lifestyle, health behavior, and cognitive decline8–10. Other studies, however, have not found an association between LLD and mortality and even found that mild depressive symptoms may protect against mortality in the elderly 11–13. Several reasons may help to explain these discrepant results. First, the studies used different instruments to evaluate depressive symptoms, and most did not carry out structured psychiatric interviews. Measurement variability could have led to differences in rates of depression caseness. Sample clinical (e.g. presence of medical comorbidities) and other socio-demographic characteristics differ among studies. In addition, varying follow-up periods may lead to differences in the estimation of mortality risk between studies. Finally, LLD is a heterogeneous disorder and may be related to distinct risk factors and neurobiological changes in different populations; therefore, subgroups of LLD subjects may be more prone to dying. Such differences in underlying neurobiology may not be captured in population-based studies.
Another reason for inconsistent findings of the association between depression and mortality in the elderly may be related to the presence of moderators of this association that have not been consistently evaluated in population-based studies. Gender may be an important moderator of mortality risk in LLD. Some studies suggest higher mortality risk in men compared to women14,15; though the opposite pattern has also been observed12. Severity of depressive episode may also moderate mortality risk. Community-based studies have shown that older adults with major depression or with more severe depressive episodes have higher mortality risk16,17. A recent meta-analysis including depressed and non-depressed participants across the lifespan showed that the mortality risk was significantly higher in individuals with subthreshold depression (RR=1.31) and with major depression (RR=1.58) compared to non-depressed individuals. Nonetheless, the relative risk of mortality between major and subthreshold depression was not different18. Why gender and depression severity may moderate the relationship between depression and mortality in the elderly is not clear. Nonetheless, differences in lifestyle, health behavior (including willingness to seek help for depression), and biological differences between gender, as well as the clinical and neurobiological correlates of depression severity may, in part, help to explain their potential moderating effects19–21. Age may also be a significant moderator of the relationship between LLD and mortality risk. Nonetheless, no study thus far has specifically addressed whether age is a significant moderator of mortality risk among individuals with LLD.
Despite a large number of studies linking depression and higher mortality risk in older adults, most of population-based studies were conducted in high-income countries and there are few population-based studies in low- and middle-income countries10,22,23. The latter studies found a marginal increase in the mortality risk in older adults with depression, but they did not evaluate the influence of moderators, like gender, age, and depression severity in LLD-related mortality risk. We expect an exponential rise of the elderly population in these countries in the coming decades, which will probably be accompanied by increased prevalence of age-related disorders as well as of depressive symptoms. Thus, given the paucity of evidence linking depression and mortality, and its moderators in older adults from low and middle-income countries22,23, this study aimed to assess whether LLD is a risk factor for all-cause mortality in a population-based study of elderly subjects with low socioeconomic status. We also addressed whether gender, age, and the intensity of depressive symptoms moderated the association with all-cause mortality risk in this population. Understanding whether depression is associated with increased mortality risk, and the potential moderators of this relationship in low and middle-income countries is important for the development of more specific interventions to prevent death in this population. In this study, we analyzed data from the Bambuí Cohort Study of Aging (BCSA), a large longitudinal population-based study, with over ten years of follow-up, which aimed to evaluate health outcomes in an elderly population with low socioeconomic status from Brazil24.
Methods
The Bambuí Cohort Study of Aging (BCSA)
Study area and population
This analysis used data from a population-based prospective cohort study of aging, which was carried out in Bambuí city (approximately 15,000 inhabitants), situated in southeastern Brazil. The local Human Development Index was 0.70, which implies moderate development. The original goal of the BCSA was to investigate the incidence and predictors of health outcomes in an elderly population with low social economic level24.
The BCSA recruitment and procedures have been described in detail elsewhere25. Briefly, the baseline cohort population comprised all residents aged 60 and over on January 1st, 1997, who were identified by a population census carried out in November and December of 1996, by the BCSA team. From a total of 1,742 older residents in Bambui, 1,606 (92.2%) participated in the baseline survey. Baseline data collection was carried out from February to May 1997. All interviews were carried out in participants’ homes. Neither interviewers nor respondents were aware of clinical results at the time of the interview.
A standardized survey questionnaire was administered to all participants and included information on: (1) social and demographic characteristics; (2) self-perceived health conditions and history of selected diseases; (3) medication use; (4) health service utilization and source of medical care; (5) lifestyle (physical activities, smoking, drinking and eating habits); (6) psychosocial aspects (personality traits, social support and life events); (7) reproductive history; (8) physical functioning and (9) mental health (depressive symptoms, cognitive functioning and sleeping habits), including the administration of the General Health Questionnaire-12, Mini-Mental State Examination. A laboratory work-up was also done in all participants and included the following blood tests: (1) biochemical assays (glucose, creatinine, urea, total protein, albumin, uric acid, calcium, phosphorus, magnesium, total cholesterol, HDL cholesterol, LDL cholesterol, VLDL cholesterol, and triglycerides); (2) haematological assays (red blood cell count, haemoglobin, hematocrit, red blood cell indices, white blood cell count, and platelet count); and (3) Chagas’ disease serological tests.
Participants (and their family members, if available) underwent annual follow-up evaluation and responded to the same survey questionnaire administered at the baseline assessment.
Mortality data source
Deaths occurring up to June 30th 2007 were included in this analysis. Deaths were reported by next of kin during the annual follow-up interview and verified through the Brazilian System of Information on Mortality, available with the permission of the Ministry of Health. Death certificates were obtained for 98.9% of individuals. The endpoint in this analysis was all-cause death, including deaths due to suicide. Due to lack of standardised report of specific death causes in death certificates, we did not evaluate specific causes of death in this study.
Depressive symptoms assessment
Symptoms of depression were assessed by the General Health Questionnaire-12 (GHQ-12). Originally the GHQ was designed for the assessment of common mental disorders in the general population26. Shorter versions of this scale were developed, and the GHQ-12 version has been shown to have similar accuracy as the Geriatric Depression Scale (GDS) for the identification of depression in the elderly27. We used a cut-off score of 5 or more points in the GHQ-12 to define major depression (“LLD group”), based on a previous validation study in this population27. Otherwise, participants were included in the “non-depressed group”.
Covariates
Information on socio-demographic variables was obtained in the baseline BHAS interview. Monthly income was determined according to the Brazilian Minimum Wage at the time of assessment. Subjects were classified according to size of monthly minimum wage between 1 and 1.99 times monthly minimum wage; between 2 and 3.99 times monthly minimum wage; between 4 to 5.99 times monthly minimum wage; between 6 and 9.99 monthly minimum wage; between 10 to 19.99 monthly minimum wage; and more than 20 times monthly minimum wage. Body mass index (weight (kg) divided by height (m)2) was calculated from height and weight measurements. Activities of daily living were ascertained by the Katz Index of Activities of Daily Living28. Participants were questioned about the following lifestyle characteristics: alcohol consumption (drinking at least one glass of alcohol per week in the previous 12 months), smoking habits, and regular physical activity, and use of psychotropic medication in the past 90 days. Smoking habits were defined as never smoked, past smoker, and current active smoker. Regular physical activity was defined as any exercise during leisure time for at least 20–30 minutes at least three times a week in the previous 90 days. Diabetes mellitus was defined as fasting blood glucose equal to or greater than 126 mg/dl and/or current treatment for diabetes29. Blood hypertension was defined as systolic blood pressure equal to or greater than 140 mmHg, and/or diastolic blood pressure equal to or greater than 90 mmHg and/or the use of antihypertensive drugs30. Myocardial infarct was ascertained by self-report and defined as physician diagnosis of myocardial infarct. Infection with T. cruzi was defined by seropositivity in both indirect hemagglutination assay and enzyme-linked immunoabsorbent assay (Biolab-Mérieux and Abbott, respectively). The scores on the MMSE were corrected for educational levels, according to previously published Brazilian norms31. The BCSA was approved by the Ethics Committee of the Oswaldo Cruz Foundation. All participants were informed about the objectives and procedures of the project and gave full-informed written consent.
Statistical analysis
Baseline differences between depression groups were tested by t-tests for continuous and Chi-square tests for categorical variables. We carried out Kaplan-Meyer survival analysis with log-rank tests to ascertain differences in survival between depressed and non-depressed elderly at baseline on annual follow-up assessments. The association between depression and risk of all-cause mortality was tested with Cox proportional hazard regressions, yielding hazard ratios (HR) and the corresponding 95% confidence interval (CI95%). All analysis were adjusted for gender, baseline age, baseline monthly income, marital status, use of psychotropic medication, retirement, educational level, activities of daily living, baseline MMSE scores, drinking and smoking habits, hypertension, myocardial infarction, Chagas disease, physical activity, BMI, diabetes mellitus 2. The proportionality assumption for the Cox regression models was assessed by checking the Kaplan-Meyer survival curves. All analyses were carried out for the whole sample and then stratified according to gender, age (below or equal 70 years-old or greater than 70 years-old), and intensity of depressive symptoms.
We then estimated the population attributable risk (PAR) of dying due to depression according to the following formula:
Where p is the prevalence of the condition in the population, HR is the adjusted hazard ratio for all-cause mortality due to LLD. The PAR was calculated only in case the HR for mortality was statistically significant.
Statistical significance was set at α ≤ 5%. All analyses were conducted with the Statistical Package for Social Science (SPSS), v18 for Windows.
Results
At baseline assessment, 1606 older adults were evaluated. Ninety-eight were excluded due to absent information or incomplete data for depressive symptoms at baseline. Thus, the final baseline sample included in the analysis was 1508 subjects (922 women and 586 men). At baseline 581 subjects (38.5%) had GHQ-12 scores equal to or greater than 5 and were considered to be in a major depressive episode (“LLD group”). Table 1 shows the baseline socio-demographic and clinical characteristics according to depression status. The median follow-up period was 8.9 years, interquartile range [6.3 years – 9.2 years]. Four hundred and forty one deaths occurred during 10,648 person-years of follow-up. Men showed a significant higher proportion of death compared to women (women, 27.1% vs. men, 32.4%, X2=5.02, df=1, p=0.02).
Table 1.
Baseline socio-demographic and clinical characteristics of the sample.
| Controls (N=927) | LLD (N=581) | Statistics | df | p | |
|---|---|---|---|---|---|
| Baseline age (years) | 68.6 ± 6.8 | 69.6 ± 7.6 | t= 2.85 | 1506 | 0.005 |
| Gender | |||||
| Females | 55.9% | 69.5% | Χ2=28.03 | 1 | <0.001 |
| Males | 44.1% | 30.5% | |||
| Marital status | |||||
| Married | 54% | 42% | Χ2=18.86 | 1 | <0.001 |
| Not married* | 46% | 58% | |||
| Retirement | |||||
| No | 41% | 35% | Χ2=6.88 | 1 | 0.009 |
| Yes | 59% | 65% | |||
| Schooling | |||||
| ≥4 years | 39.5% | 30.1% | Χ2=13.6 | 1 | <0.001 |
| < 3 years | 60.5% | 69.9% | |||
| Physical exercise | |||||
| No | 15.7% | 9.1% | Χ2=13.7 | 1 | <0.001 |
| Yes | 84.3% | 90.9% | |||
| BMI | 25.1 ± 4.8 | 25.3 ± 5.3 | t=−0.703 | 1506 | 0.5 |
| Hypertension | |||||
| No | 38.3% | 37.5% | Χ2=0.1 | 1 | 0.7 |
| Yes | 61.7% | 62.5% | |||
| Diabetes | |||||
| No | 84.7% | 85.3% | Χ2=0.1 | 1 | 0.7 |
| Yes | 15.3% | 14.7% | |||
| Chagas disease | |||||
| No | 67.2% | 54.4% | Χ2=23.3 | 1 | <0.001 |
| Yes | 32.8% | 45.6% | |||
| Previous MI | |||||
| No | 96.3% | 93.3% | Χ2=6.78 | 1 | 0.009 |
| Yes | 3.7% | 6.7% | |||
| Drinking habits | |||||
| No | 79% | 85% | Χ2=9.64 | 1 | 0.002 |
| Yes | 21% | 15% | |||
| Smoking | |||||
| Never | 58.8% | 58.7% | Χ2=0.56 | 2 | 0.7 |
| Past smoking | 22.4% | 23.8% | |||
| Current smoking | 18.8% | 17.6% | |||
| Use of psychoactive drugs | |||||
| No | 84% | 30% | Χ2=41.24 | 1 | <0.001 |
| Yes | 16% | 70% | |||
| Katz ADL | 0.07 ± 0.008 | 0.18 ± 0.16 | T=5.82 | 1506 | <0.001 |
| Baseline MMSE | 25.3 ± 3.9 | 23.7 ± 4.6 | t=6.94 | 1506 | <0.001 |
| Baseline GHQ-12 | 1.5 ± 1.4 | 7.7 ± 2.1 | t=68.11 | 1506 | <0.001 |
| Follow-up (months) | 91.0 ± 28.3 | 85.1 ± 31.7 | t=3.76 | 1506 | <0.001 |
| Death** | |||||
| No | 75.1% | 63.9% | Log-rank χ2 =27.28 | 1 | <0.001 |
| Yes | 24,.9% | 36.1% | |||
BMI: body mass index; MI: myocardial infarction; MMSE: Mini-mental State Examination; GHQ-12: General Health Questionnaire-12;
Not married: single, divorced, widowed
Kaplan-Meyer survival analysis.
Baseline depressive symptoms and mortality risk
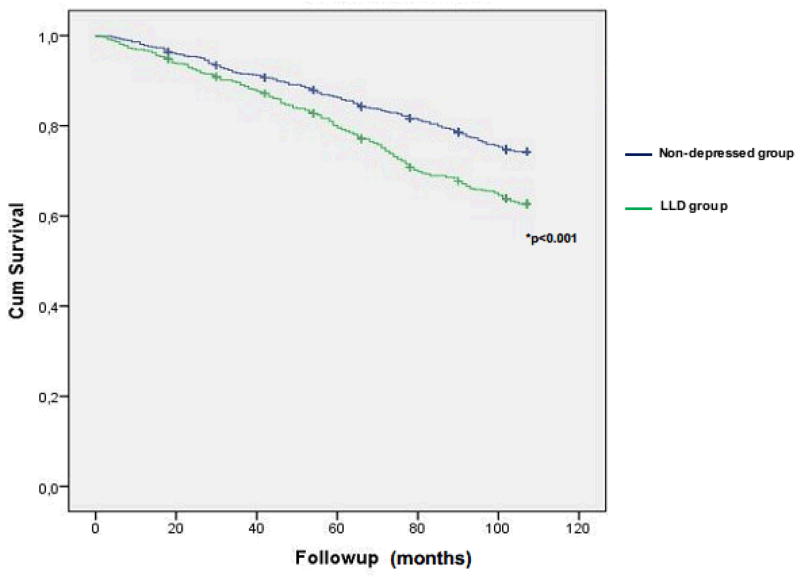
Kaplan-Meyer survival analysis showed shorter survival in subjects in the LLD group (Log-rank Chi2 = 27.28, df=1, p<0.001). Cox regression analysis showed a significant association between GHQ-12 scores (as a continuous measure) and mortality risk (adjusted HR = 1.05 CI95% [1.02 – 1.08], p=0.003) (table 2). We dichotomized the sample according to the GHQ-12 score and those participants in the LLD group (GHQ-12 scores ≥ 5) at baseline showed a higher mortality risk (adjusted HR = 1.24 CI95% [1.00 – 1.54], p=0.05) (table 2).
Table 2.
Hazard ratio for mortality risk.
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Comparisons | HR | 95% CI | p | HR | 95% CI | p |
| GHQ-12 scores (continuous measure) | 1.07 | 1.05 – 1.10 | <0.001 | 1.05 | 1.02 – 1.08 | 0.003 |
| LLD group vs. non-depressed group | 1.56 | 1.30 – 1.88 | <0.001 | 1.24 | 1.00 – 1.55 | 0.05 |
| Depression severity | ||||||
| “low depressive symptoms” group vs. non-depressed group | 1.41 | 1.14 – 1.75 | 0.002 | 1.03 | 0.81 – 1.33 | 0.77 |
| “high depressive symptoms” groups vs. non-depressed group | 1.87 | 1.46 – 2.41 | <0.001 | 1.44 | 1.07 – 1.93 | 0.01 |
gender, baseline age, baseline monthly income, marital status, use of psychoactive drugs, retirement, educational level, activities of daily living, MMSE scores, drinking and smoking habits, hypertension, myocardial infarct, Chagas disease, physical activity, BMI, diabetes mellitus 2
The PAR of death due to LLD was 8.5% (CI95%: 0% – 17.2%).
We conducted additional analyses to assess whether the higher depressive symptoms was associated with differential risk of mortality. Although the cut point of 5 of the GHQ-12 is based on previous validation, there is no cut point available in the literature to distinguish among lower vs. higher levels of depressive symptoms based on the GHQ-12 scores. We therefore used the quartiles of the distribution of GHQ-12 scores among LLD participants in the sample to form two groups: “higher depressive symptoms” group (participants with scores above the third quartile (i.e. scores ≥ 9), and “lower depressive symptoms” (defined as scores below the third quartile (i.e. scores between 5 and 8).
Cox regression analysis showed that participants in the “higher depressive symptoms” group showed an increased mortality risk compared to the non-depressed group (i.e. those with GHQ-12 scores below 5) (adjusted HR=1.44 CI95% [1.07 – 1.93], p=0.01) (table 2). Participants in the “lower depressive symptoms” group did not show a higher mortality risk relative to the non-depressed group after adjustment for confounding variables (table 2). Mortality was higher in the “higher depressive symptoms” group compared to the “lower depressive symptoms” group (adjusted HR 1.39 CI95% [1.01 – 1.91], p=0.04).
The PAR of due in the “higher depressive symptoms” group was 14.5% (CI95% 2.6% – 26.4%).
The moderating effect of gender and age on LLD-related mortality
Gender may be an important moderator in the association between LLD and mortality risk. Therefore, we stratified all analysis according to gender. Kaplan-Meyer survival analysis showed that men and women with LLD differed with respect to survival. In the Cox regression analysis, participants with LLD showed a higher mortality risk in men (adjusted HR=1.45 CI95% [1.01 – 2.07], p=0.04), but not in women (adjusted HR=1.13 CI95% [0.84 – 1.48], p=0.15) (table 3).
Table 3.
Effect of gender on mortality risk.
| Men | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Comparisons | Unadjusted | Adjusted* | ||||
|
| ||||||
| HR | 95% CI | p | HR | 95% CI | P | |
| GHQ-12 scores (continuous measure) | 1.05 | 1.02 – 1.09 | 0.004 | 1.09 | 1.04 – 1.14 | 0.001 |
| LLD group vs. non-depressed group | 1.90 | 1.43 – 2.54 | <0.001 | 1.45 | 1.01 – 2.07 | 0.04 |
| Depression severity | ||||||
| “low depressive symptoms” group vs. non-depressed group | 1.60 | 1.13 – 2.26 | 0.008 | 1.23 | 0.80 – 1.87 | 0.34 |
| “high depressive symptoms” groups vs. non-depressed group | 2.56 | 1.74 – 3.76 | <0.001 | 1.90 | 1.19 – 3.04 | 0.007 |
| Women
| ||||||
|---|---|---|---|---|---|---|
| Comparisons | Unadjusted | Adjusted* | ||||
|
| ||||||
| HR | 95% CI | p | HR | 95% CI | P | |
| GHQ-12 scores (continuous measure) | 1.12 | 1.08 – 1.16 | <0.001 | 1.01 | 0.97 – 1.06 | 0.5 |
| LLD group vs. non-depressed group | 1.48 | 1.16 – 1.90 | 0.002 | 1.13 | 0.84 – 1.48 | 0.45 |
| Depression severity | ||||||
| “low depressive symptoms” group vs. non-depressed group | 1.40 | 1.06 – 1.85 | 0.02 | 1.01 | 0.73 – 1.39 | 0.9 |
| “high depressive symptoms” groups vs. non-depressed group | 1.66 | 1.18 – 2.32 | 0.003 | 1.38 | 0.93 – 2.06 | 0.11 |
baseline age, baseline monthly income, use of psychoactive drugs, marital status, retirement, educational level, activities of daily living, MMSE scores, drinking and smoking habits, hypertension, myocardial infarct, Chagas disease, physical activity, BMI, diabetes mellitus 2
The PAR of dying due to depression in men was 12% (CI95% 0.3% – 24.4%).
We observed an increase in the mortality risk among men with baseline GHQ scores of 9 or higher (HR=1.90 CI95% [1.19 – 3.04], p=0.007), but not in women (HR=1.38 CI95% [0.93 – 2.06], p=0.15) (table 3). Low depressive symptoms were not associated with increased risk of mortality in either men or women after adjustment for confounding variables (table 3).
The PAR of dying due to depression in men with severe depressive symptoms was 21.4% (CI95% 5.4% – 38.1%).
We conducted additional analyses to further address the moderating effect of gender and age on mortality risk due to depression. We tested the interaction between gender, age, and depressive symptoms in the risk of death in this cohort. We found a significant interaction between gender and clinically significant depressive symptoms (HR= 1.72 CI95% [1.18 – 2.49], p=0.004). We did not find a significant interaction between age and clinically significant depressive symptoms (HR= 1.16 CI95% [0.75 – 1.79], p=0.5), or and interaction between gender, age and clinically significant depressive symptoms (HR=1.03 CI95% [0.59 – 1.82), p=0.9).
Discussion
In this population-based prospective cohort study, GHQ-12 scores of 5 or greater constituted a risk factor for mortality in older adults. The attributable risk of dying due to LLD was high (8.5%). Additional analyses revealed that the mortality risk was more pronounced in older adults with GHQ-12 depression scores of 9 or higher (indicating a higher level of depressive symptoms). We found a significant gender and depression interaction on mortality risk and the relationship between mortality risk and depression is considerably stronger in men compared to women after controlling for confounding variables. Thus, the present study provides additional evidence that depression is an important risk factor for mortality in older adults with low socioeconomic status and illustrates the moderating role of gender and intensity of depressive symptoms in depression-related mortality risk.
Late-life depression is usually associated with negative health behaviors (e.g. smoking, low physical activity level), worse physical health (e.g. obesity, hypertension, cardiovascular disease, diabetes), as well as adverse socioeconomic condition7,32–34. These are well-known risk factors for death in older adults and may explain in part the relationship between depression and mortality in these subjects. Nonetheless, we found that the mortality risk persisted after adjustment for lifestyle factors; therefore, other systemic biological factors may be related to the increased mortality risk in older subjects.
Abnormalities in inflammatory control, with persistent low-grade pro-inflammatory activity, is a common feature of several medical illness, such as diabetes, dyslipidemia, hypertension, obesity and is associated with higher risk of death in these patients35–36. Several lines of evidence suggest that depression in older adults is also associated with low-grade pro-inflammatory status with a similar pattern observed in the latter disorders37–39. Therefore, inflammatory abnormalities may help to explain the independent, higher risk of mortality in LLD.
Other mechanisms may also play an important mediating role in depression-related mortality in the elderly. Depressed older adults, especially those with more severe depressive episodes, show significant HPA axis dysfunction40, increased oxidative stress41, reduced neurotrophic support42, increased GSK-3β19, and reduced telomere length and increased telomerase activity43. These abnormalities may result in higher systemic allostatic load, leading to accelerating normal aging processes and, finally, to premature death in these individuals44,45. In addition, recent studies showed that cardiovascular, endocrine-metabolic and depressive disorders share biological, genetic, and environmental susceptibility factors that may increase the risk of death in these conditions46,47. Therefore, studies are needed to better understand the neurobiologic and systemic mediators of higher risk of mortality in older adults with depression.
It is widely accepted that gender modulates the expression of affective disorders. The prevalence and incidence of major depression and distress is higher in women than in men; men tend not to report milder symptoms or seek treatment until depressive symptom are more severe20,48. In addition, men have higher prevalence and risk of death due to cardiovascular disease and endocrine-metabolic disorders; though these differences attenuate with aging21,49. These factors may explain in part the higher risk of death in men compared to women in the present study. Biological differences between men and women, in particular hormonal differences can also contribute to the differences in the mortality risk between men and women. It is worth noting that a previous study found that older women with subthreshold depressive symptoms had a significant lower risk of dying compared to men13. The authors hypothesized that subthreshold depression may be an adaptive state in women that protects against death. Methodological differences as well as differences in the socio-demographic characteristics of the samples between these studies may help to explain the observed differences in mortality risk between men and women. Nonetheless, no study thus far has specifically addressed depression-related biological mechanisms that confer a higher mortality risk in men compared to women. A better understanding of the mechanisms by which gender moderates the mortality risk in LLD is of utmost importance to develop personalized preventive and therapeutic interventions aiming the reduction of mortality in these subjects.
Late-life depression is a treatable and potentially preventable medical condition50,51, and may be a modifiable risk factor for preventing premature death in older adults. A recent analysis of long-term outcomes of a clinical trial for LLD treatment showed that the risk of death, in particular due to cancer, was reduced by approximately 24% in older adults in full remission after antidepressant treatment52. This result suggests that the adequate and long-term treatment of depression and maintenance of remission of depressive episode can reduce mortality risk in LLD. However, no clinical trial to date has evaluated whether the prevention of depression in the elderly can also reduce the mortality associated with this disorder. Given the high attributable risk of dying due to LLD, and the expected increase in LLD cases due to the increasingly older population, it is necessary to conduct novel clinical trials to investigate the potential impact of prevention and treatment of depression on risk of death in elderly people. Finally, given the moderating effect of gender on mortality risk, these interventions should be tailored to gender to maximize their effectiveness.
The present results should be viewed in light of some study limitations. Diagnosis of LLD and of depression severity was based on pre-defined cut-off scores of the GHQ-12, and no structured psychiatric interview was carried out with cohort members. Therefore, some participants with milder symptoms might have been misclassified as non-depressed. On the other hand, this cut-off value yielded a very high prevalence of clinically significant depressive symptoms in this population (38.5%). Therefore, some non-depressed participants may have been misclassified as presenting clinically significantly depressive symptoms, yielding a very high prevalence of depression caseness (38.5%)27,53. The assessment of depressive symptoms at baseline may reflect either a temporary or acute state, or may reflect the presence of more chronic depressive symptoms. Therefore, we cannot determine with the current data whether the mortality risk is due to the effect of acute and/or persistent depressive symptoms. Given this limitation, we have tried to evaluate the relationship between late-life depression through different perspectives, in order to show how approaches to measurement and ascertainment of caseness can influence estimates of mortality risk, and the results were robust across several analyses. We did not have information on past psychiatric history and on whether participants were under treatment for depression. Thus, we were not able to evaluate the impact of either variable on mortality risk. Finally, this study was conducted in one small city in Brazil and we did not address the relationship between depressive symptoms and specific causes of death, including deaths due to suicide or trauma. These factors may limit the generalizability of these results and should be replicated in other population-based studies.
In conclusion, in the present study we found that LLD is associated with increased risk for all-cause mortality in the elderly, in particular in those with more severe depressive symptoms. In addition, gender may moderate mortality risk, being elevated in men more so than in women. Therefore, it is necessary to carry out long-term clinical trials to investigate the potential impact of treatment and prevention of depression on the risk of all-cause mortality in this population.
Figure 1.
Survival curve for mortality for participants with clinically significant depressive symptoms (LLD group) vs. non-depressed group.
Acknowledgments
This study was sponsored by the Supporting Agency of Studies and Projects (FINEP) and by the Oswaldo Cruz Foundation, Brazil.
The Brazilian National Research Council (CNPq) provided Dr. M. F. Lima-Costa and Dr. JOA Firmo’s scholarships. E Castro-Costa is supported by the Programa Nacional de Pós-doutorado em Saúde-PNDS. Breno Satler Diniz receives research support from John A. Hartford Foundation and from Intramural Grant from the Federal University of Minas Gerais. Charles F. Reynolds III receives pharmaceutical support for NIH-sponsored research studies from Bristol-Myers Squibb, Forest, Pfizer, and Lilly; receiving grants from the National Institute of Mental Health, National Institute on Aging, National Center for Minority Health Disparities, National Heart Lung and Blood Institute, Center for Medicare and Medicaid Services (CMS), Patient Centered Outcomes Research Institute (PCORI), the Commonwealth of Pennsylvania, the John A Hartford Foundation, Clinical and Translational Science Institute (CTSI), and the American Foundation for Suicide Prevention; and serving on the American Association for Geriatric Psychiatry editorial review board. He is the co-inventor (Licensed Intellectual Property) of Psychometric analysis of the Pittsburgh Sleep Quality Index (PSQI) PRO10050447 (PI: Buysse). Meryl A. Butters: receives support from the National Institute of Mental Health (NIMH), the National Institute on Aging (NIA) and has received remuneration for neuropsychological services from GlaxoSmithKline.
Footnotes
The authors do not have any conflict of interest regarding the elaboration of this manuscript.
References
- 1.Viana MC, Andrade LH. Lifetime Prevalence, age and gender distribution and age-of-onset of psychiatric disorders in the Sao Paulo Metropolitan Area, Brazil: results from the Sao Paulo Megacity Mental Health Survey. Rev Bras Psiquiatr. 2012;34:249–60. doi: 10.1016/j.rbp.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML. High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry. 2010;67(5):489–96. doi: 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–95. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 4.Yen YC, Rebok GW, Gallo JJ, Jones RN, Tennstedt SL. Depressive symptoms impair everyday problem-solving ability through cognitive abilities in late life. Am J Geriatr Psychiatry. 2011;19(2):142–50. doi: 10.1097/JGP.0b013e3181e89894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–35. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallegos-Carrillo K, Garcia-Pena C, Mudgal J, Romero X, Duran-Arenas L, Salmeron J. Role of depressive symptoms and comorbid chronic disease on health-related quality of life among community-dwelling older adults. J Psychosom Res. 2009;66(2):127–35. doi: 10.1016/j.jpsychores.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Lyness JM, Niculescu A, Tu X, Reynolds CF, 3rd, Caine ED. The relationship of medical comorbidity and depression in older, primary care patients. Psychosomatics. 2006;47(5):435–9. doi: 10.1176/appi.psy.47.5.435. [DOI] [PubMed] [Google Scholar]
- 8.Almeida OP, Alfonso H, Hankey GJ, Flicker L. Depression, antidepressant use and mortality in later life: the Health In Men Study. PLoS One. 2010;5(6):e11266. doi: 10.1371/journal.pone.0011266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler S, Verhey F, Weyerer S, et al. Depression, non-fatal stroke and all-cause mortality in old age: A prospective cohort study of primary care patients. J Affect Disord. doi: 10.1016/j.jad.2013.02.020. Epub ahead of print Mar 7 2013. [DOI] [PubMed] [Google Scholar]
- 10.Sun W, Schooling CM, Chan WM, Ho KS, Lam TH. The association between depressive symptoms and mortality among Chinese elderly: a Hong Kong cohort study. J Gerontol A Biol Sci Med Sci. 2011 Apr;66(4):459–66. doi: 10.1093/gerona/glq206. [DOI] [PubMed] [Google Scholar]
- 11.Fredman L, Magaziner J, Hebel JR, Hawkes W, Zimmerman SI. Depressive symptoms and 6-year mortality among elderly community-dwelling women. Epidemiology. 1999;10(1):54–9. [PubMed] [Google Scholar]
- 12.Penninx BW, Geerlings SW, Deeg DJ, van Eijk JT, van Tilburg W, Beekman AT. Minor and major depression and the risk of death in older persons. Arch Gen Psychiatry. 1999;56(10):889–95. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- 13.Hybels CF, Pieper CF, Blazer DG. Sex differences in the relationship between subthreshold depression and mortality in a community sample of older adults. Am J Geriatr Psychiatry. 2002;10(3):283–91. [PubMed] [Google Scholar]
- 14.Fuhrer R, Dufouil C, Antonucci TC, Shipley MJ, Helmer C, Dartigues JF. Psychological disorder and mortality in French older adults: do social relations modify the association? Am J Epidemiol. 1999;149(2):116–26. doi: 10.1093/oxfordjournals.aje.a009776. [DOI] [PubMed] [Google Scholar]
- 15.Schoevers RA, Geerlings MI, Beekman AT, et al. Association of depression and gender with mortality in old age. Results from the Amsterdam Study of the Elderly (AMSTEL) Br J Psychiatry. 2000;177:336–42. doi: 10.1192/bjp.177.4.336. [DOI] [PubMed] [Google Scholar]
- 16.Jeong HG, Lee JJ, Lee SB, et al. Role of severity and gender in the association between late-life depression and all-cause mortality. Int Psychogeriatr. 2013;25(4):677–84. doi: 10.1017/S1041610212002190. [DOI] [PubMed] [Google Scholar]
- 17.Ryan J, Carriere I, Ritchie K, et al. Late-life depression and mortality: influence of gender and antidepressant use. Br J Psychiatry. 2008;192(1):12–8. doi: 10.1192/bjp.bp.107.039164. [DOI] [PubMed] [Google Scholar]
- 18.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry. 2013;202(1):22–7. doi: 10.1192/bjp.bp.112.112169. [DOI] [PubMed] [Google Scholar]
- 19.Diniz BS, Talib LL, Joaquim HP, de Paula VR, Gattaz WF, Forlenza OV. Platelet GSK3B activity in patients with late-life depression: marker of depressive episode severity and cognitive impairment? World J Biol Psychiatry. 2011;12(3):216–22. doi: 10.3109/15622975.2010.551408. [DOI] [PubMed] [Google Scholar]
- 20.Gorman JM. Gender differences in depression and response to psychotropic medication. Gender Medicine. 2006;3(2):93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- 21.Vaccarino V, Badimon L, Corti R, et al. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovascular Res. 2011;90(1):9–17. doi: 10.1093/cvr/cvq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu CC, Lee YM, Chen JD. Association between depressive symptoms and twelve-year mortality among elderly in a rural community in Taiwan. J Formos Med Assoc. 2003;102(4):234–9. [PubMed] [Google Scholar]
- 23.Jotheeswaran AT, Williams JD, Prince MJ. Predictors of mortality among elderly people living in a south Indian urban community; a 10/66 Dementia Research Group prospective population-based cohort study. BMC Public Health. 2010;10:366. doi: 10.1186/1471-2458-10-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima-Costa MF, Firmo JO, Uchoa E. Cohort profile: the Bambui (Brazil) Cohort Study of Ageing. Int J Epidemiol. 2011;40(4):862–7. doi: 10.1093/ije/dyq143. [DOI] [PubMed] [Google Scholar]
- 25.Costa MF, Uchoa E, Guerra HL, Firmo JO, Vidigal PG, Barreto SM. The Bambui health and ageing study (BHAS): methodological approach and preliminary results of a population-based cohort study of the elderly in Brazil. Rev Saude Publica. 2000;34(2):126–35. doi: 10.1590/s0034-89102000000200005. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg DP, Blackwell B. Psychiatric illness in general practice. A detailed study using a new method of case identification. BMJ. 1970;1(5707):439–43. doi: 10.1136/bmj.2.5707.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa E, Barreto SM, Uchoa E, Firmo JO, Lima-Costa MF, Prince M. Is the GDS-30 better than the GHQ-12 for screening depression in elderly people in the community? The Bambui Health Aging Study (BHAS) Int Psychogeriatr. 2006;18(3):493–503. doi: 10.1017/S1041610205002954. [DOI] [PubMed] [Google Scholar]
- 28.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 29.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 31.Castro-Costa E, Fuzikawa C, Uchoa E, Firmo JO, Lima-Costa MF. Norms for the mini-mental state examination: adjustment of the cut-off point in population-based studies (evidences from the Bambuí health aging study) Arq Neuropsiquiatr. 2008;66(3A):524–8. doi: 10.1590/s0004-282x2008000400016. [DOI] [PubMed] [Google Scholar]
- 32.Li N, Pang L, Chen G, Song X, Zhang J, Zheng X. Risk factors for depression in older adults in Beijing. Can J Psychiatry. 2011;56(8):466–73. doi: 10.1177/070674371105600804. [DOI] [PubMed] [Google Scholar]
- 33.van Gool CH, Kempen GI, Penninx BW, Deeg DJ, Beekman AT, van Eijk JT. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Age Ageing. 2003;32(1):81–7. doi: 10.1093/ageing/32.1.81. [DOI] [PubMed] [Google Scholar]
- 34.Wong SY, Mercer SW, Woo J, Leung J. The influence of multi-morbidity and self-reported socio-economic standing on the prevalence of depression in an elderly Hong Kong population. BMC Public Health. 2008;8:119. doi: 10.1186/1471-2458-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Candore G, Caruso C, Jirillo E, Magrone T, Vasto S. Low grade inflammation as a common pathogenetic denominator in age-related diseases: novel drug targets for anti-ageing strategies and successful ageing achievement. Curr Pharm Des. 2010;16(6):584–96. doi: 10.2174/138161210790883868. [DOI] [PubMed] [Google Scholar]
- 36.Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H. Low grade inflammation as measured by levels of YKL-40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol. 2010;143(1):35–42. doi: 10.1016/j.ijcard.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 37.Diniz BS, Teixeira AL, Talib LL, Mendonca VA, Gattaz WF, Forlenza OV. Increased soluble TNF receptor 2 in antidepressant-free patients with late-life depression. J Psychiatr Res. 2010;44(14):917–20. doi: 10.1016/j.jpsychires.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Diniz BS, Teixeira AL, Campos AC, et al. Reduced serum levels of adiponectin in elderly patients with major depression. J Psychiatr Res. 2012;46(8):1081–5. doi: 10.1016/j.jpsychires.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 39.Wium-Andersen MK, Orsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry. 2013;70(2):176–84. doi: 10.1001/2013.jamapsychiatry.102. [DOI] [PubMed] [Google Scholar]
- 40.Penninx BW, Beekman AT, Bandinelli S, et al. Late-life depressive symptoms are associated with both hyperactivity and hypoactivity of the hypothalamo-pituitary-adrenal axis. Am J Geriatr Psychiatry. 2007;15(6):522–9. doi: 10.1097/JGP.0b013e318033ed80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimopoulos N, Piperi C, Psarra V, Lea RW, Kalofoutis A. Increased plasma levels of 8-iso-PGF2alpha and IL-6 in an elderly population with depression. Psychiatry Res. 2008;161(1):59–66. doi: 10.1016/j.psychres.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Diniz BS, Teixeira AL, Machado-Vieira R, Talib LL, Gattaz WF, Forlenza OV. Reduced serum nerve growth factor in patients with late-life depression. Am J Geriatr Psychiatry. 2013;21(5):493–6. doi: 10.1016/j.jagp.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Wolkowitz OM, Mellon SH, Epel ES, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One. 2011;6(3):e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 45.McKinney BC, Oh H, Sibille E. Age-by-disease biological interactions: implications for late-life depression. Front Genet. 2012;3:237. doi: 10.3389/fgene.2012.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hidalgo CA, Blumm N, Barabasi AL, Christakis NA. A dynamic network approach for the study of human phenotypes. PLoS Comput Biol. 2009;5(4):e1000353. doi: 10.1371/journal.pcbi.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCaffery JM, Frasure-Smith N, Dube MP, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68(2):187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- 48.Alexandrino-Silva C, Wang YP, Carmen Viana M, Bulhoes RS, Martins SS, Andrade LH. Gender differences in symptomatic profiles of depression: results from the Sao Paulo Megacity Mental Health Survey. J Affect Disord May. 2013;147(1–3):355–364. doi: 10.1016/j.jad.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 49.Mikkola TS, Gissler M, Merikukka M, Tuomikoski P, Ylikorkala O. Sex differences in age-related cardiovascular mortality. PLoS One. 2013;8(5):e63347. doi: 10.1371/journal.pone.0063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reynolds CF, 3rd, Cuijpers P, Patel V, et al. Early intervention to reduce the global health and economic burden of major depression in older adults. Annu Rev Public Health. 2012;33:123–35. doi: 10.1146/annurev-publhealth-031811-124544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuijpers P, Beekman AT, Reynolds CF., 3rd Preventing depression: a global priority. JAMA. 2012;307(10):1033–4. doi: 10.1001/jama.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallo JJ, Morales KH, Bogner HR, et al. Long term effect of depression care management on mortality in older adults: follow-up of cluster randomized clinical trial in primary care. BMJ. 2013;346:f2570. doi: 10.1136/bmj.f2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castro-Costa E, Lima-Costa MF, Carvalhais S, Firmo JO, Uchoa E. Factors associated with depressive symptoms measured by the 12-item General Health Questionnaire in community-dwelling older adults (The Bambuí Health Aging Study) Rev Bras Psiquiatr. 2008;30(2):104–9. doi: 10.1590/s1516-44462008005000007. [DOI] [PubMed] [Google Scholar]