Abstract
Epidemiological evidence indicates chronic environmental exposure to transition metals may play a role in chronic neurodegenerative conditions such as Parkinson’s disease (PD). Chronic inhalation exposure to welding fumes containing metal mixtures may be associated with development of PD. A significant amount of vanadium is present in welding fumes, as vanadium pentoxide (V2O5), and incorporation of vanadium in the production of high strength steel has become more common. Despite the increased vanadium use in recent years, the neurotoxicological effects of this metal are not well characterized. Recently, we demonstrated that V2O5 induces dopaminergic neurotoxicity via protein kinase C delta (PKCδ)-dependent oxidative signaling mechanisms in dopaminergic neuronal cells. Since anosmia (inability to perceive odors) and non-motor deficits are considered to be early symptoms of neurological diseases, in the present study, we examined the effect of V2O5 on the olfactory bulb in animal models. To mimic the inhalation exposure, we intranasally administered C57 black mice a low-dose of 182 µg of V2O5 three times a week for one month, and behavioral, neurochemical and biochemical studies were performed. Our results revealed a significant decrease in olfactory bulb weights, tyrosine hydroxylase (TH) levels, levels of dopamine (DA) and its metabolite, 3, 4-dihydroxyphenylacetic acid (DOPAC) and increases in astroglia of the glomerular layer of the olfactory bulb in the treatment groups relative to vehicle controls. Neurochemical changes were accompanied by impaired olfaction and locomotion. These findings suggest that nasal exposure to V2O5 adversely affects olfactory bulbs, resulting in neurobehavioral and neurochemical impairments. These results expand our understanding of vanadium neurotoxicity in environmentally-linked neurological conditions.
Keywords: vanadium, metals, olfactory system, neurotoxicity, non-motor symptoms, risk assessment, Parkinson’s disease
Introduction
Metal exposure has been considered a major chemical risk factor in the pathogenesis of chronic neurodegenerative conditions such as Parkinson’s disease (PD) (Dobson et al., 2004, Aschner et al., 2009, Furbee, 2011, Caudle et al., 2012, Kanthasamy et al., 2012). PD imposes an estimated economic burden of $23 billion per year in the United States alone (Weintraub et al., 2008). Multifactorial etiology is associated with progressive and substantive degeneration of nigral dopaminergic neurons and extra-nigral neurons underlying PD (Anglade et al., 1997, Braak and Braak, 2000, Braak et al., 2000, Allam et al., 2005, Przedborski, 2005, Przedborski and Ischiropoulos, 2005). The cause and mechanism of the disease’s progression are poorly understood and have not yet been exhaustively explored. Recently, many non-motor symptoms have been determined to precede the onset of motor symptoms and are considered hallmark in the early stages of PD. A non-motor, early stage symptom of PD is impaired olfactory function (Ansari and Johnson, 1975, Langston, 2006, Goldstein et al., 2010). However, the effect of environmental neurotoxic metals on the olfactory system has not been well characterized.
Case-control and epidemiological studies have linked metal exposure to the increased incidence of PD (Fleming et al., 1994, Schulte et al., 1996, Gorell et al., 1997, Liou et al., 1997, Marder et al., 1998, Smargiassi et al., 1998, Taylor et al., 1999, Priyadarshi et al., 2000, Ritz and Yu, 2000). Studies have shown that welders have an increased risk of developing PD (Racette et al., 2001, Park et al., 2005). Manganese (Mn), which is typically present in welding fumes mixed with other metals including vanadium, is the major metal that has been studied with respect to PD (Aschner et al., 2007, Aschner et al., 2009, Guilarte, 2010). Mn mainly targets the basal ganglia comprising the caudate nucleus, putamen, globus pallidus, substantia nigra, and subthalamic nucleus (Eriksson et al., 1992, Calne et al., 1994, Brenneman et al., 1999, Nagatomo et al., 1999, Aschner et al., 2009). Neurotoxicity resulting from excessive Mn exposure is distinct from sporadic PD in that the globus pallidus appears to be the most severely affected of all of the basal ganglia regions (Verity, 1999). However, studies have shown that dopamine (DA), which is the principal neurotransmitter in the striatum that is severely depleted in PD patients, is also decreased by Mn, with both in vivo (Parenti et al., 1986) and in vitro (Vescovi et al., 1991) exposure paradigms in animals. Dorman et al. reported the accumulation of MnSO4 in the olfactory bulb and striatum of inhalation-exposed rats relative to controls (Dorman et al., 2001).
The rapid growth and modernization of U.S. cities are dependent on ever-changing infrastructures. Central to the evolution of these structures is welding, one of the primary anthropogenic sources of environmental metals. Vanadium, typically present in welding fumes as vanadium pentoxide (V2O5), is emitted by welding rods commonly used in construction. Vanadium is also widely used in various steelmaking industrial applications, such as plane and ship building, in the production of temperature-resistant alloys and glass, and in pigment and paint manufacturing (McNeilly et al., 2004). Also, large quantities of vanadium compounds are released into the environment mainly through the burning of fossil fuels, with vanadium reported as the most abundant trace metal in petroleum samples (Amorim et al., 2007). Vanadium accumulates in soil, groundwater, and plants, and is consumed by animals and humans (Pyrzynska and Weirzbicki, 2004). The processing of vanadium slag (about 120 g/kg of vanadium pentoxide) generates dust, with vanadium concentrations ranging from 30 to 120 mg/m3 (IARC, 2006). Crude oil from Venezuela is believed to have the highest vanadium concentration, ranging up to 1400 mg/kg. Fifty percent vanadium pentoxide has been discovered in flue-gas deposits from oil-fired furnaces (IARC, 2006). Elevated levels of vanadium (4.7 mg/m3) have been found in the breathing air of steel industry workers (Kiviluoto et al., 1979). Vanadium exposure to humans has been shown to cause motor deficits (Done, 1979, WHO, 2000). Thus, the growing use of vanadium in a wide variety of applications warrants the full characterization of its neurotoxicological properties.
Chronic exposure to environmental toxicants, including herbicides, pesticides, solvents, and heavy metals, can alter the ability to smell (Doty and Hastings, 2001), with the best documented metal in this regard being cadmium, chromium, nickel, and manganese. Further, Avila-Costa et al. observed that inhaled V2O5 damages the nigrostriatal dopaminergic systems in rodent models (Avila-Costa et al., 2004). In a recent study, we showed that vanadium is neurotoxic to dopaminergic neurons in cell culture models (Afeseh Ngwa et al., 2009). In the present study, we further examine the neurotoxic properties of vanadium, specifically focusing on its effects on the olfactory bulb to determine whether subchronic nasal exposure impairs neurobehavioral and neurochemical processes associated with olfactory function.
Materials and Methods
Chemicals
Vanadium pentoxide (V2O5) salt, protease cocktail inhibitor, phosphatase inhibitors and anti-β-actin antibody were purchased from Sigma (St. Louis, MO). A Bradford protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA). Mouse monoclonal antibodies against tyrosine hydroxylase (TH) and GFAP were obtained from Millipore (Upstate, Billerica, MA, USA) and Cell Signaling Technology, Inc. (Danvers, MA), respectively. The anti-mouse and anti-rabbit secondary antibodies (Alexa Fluor 680 conjugated anti-mouse IgG and IRdye 800 conjugated anti-rabbit IgG) were purchased from Invitrogen and Rockland Inc., respectively.
Treatment paradigm
Six to eight week old male C57BL/6 mice were housed at room temperature under a 12 h light/dark cycle. The control and treatment animals were age-matched. Food and water were provided ad libitum and animal weights were monitored. Animals were cared for in accordance with institutional animal care guidelines. A previous study exposed mice to 5–20 mM V2O5 through inhalation route and examined neurotoxic effects of the metal (Avila-Costa et al., 2005, Fleming et al., 2008). In the present study, we used a low dose of 182 µg of V2O5 in 50 µL of de-ionized water and administered intranasally three times a week for period of one month. The vanadium pentoxide was administered to mice intranasally using micropipettes after briefly anaesthetizing the mice with isoflurane to prevent a gag reflex. The control animals received equal volumes of pH-adjusted deionized water (pH ~ 2.0 for V2O5 solution). Following the treatment, mice were subjected to behavioral and neurochemical tests one week following last dose of V2O5. Intranasal delivery was chosen because vanadium exposure mostly occurs through inhalation route. Intranasal delivery of chemicals takes advantage of an incomplete blood brain barrier in the olfactory epithelium (Graff and Pollack, 2005). The olfactory nerves bypass the blood brain barrier, therefore chemicals can be taken up by these neurons and transported directly into the brain (Graff and Pollack, 2005).
Olfaction test
The ability of mice to detect pheromones from female bedding by sniffing was used as a measure of olfaction, as described in previous studies (Fleming et al., 2008, Kim et al., 2011). This test combines the principle behind the wooden block test, which relies on the ability of mice to discriminate between self and non-self odors (Fleming et al., 2008, Kim et al., 2011), and on the knowledge that female body odor and urine attract males (Lucas et al., 1982, Singer et al., 1988). For this test, the bedding from a mouse cage housing pregnant females was introduced in the cage of the male mice used in this study. The amount and location of bedding were kept constant each time. The total time spent sniffing the female bedding material was measured using a stop watch during a five minute testing session. Both vanadium-treated and control mice were subjected to the test at the same day. This test is an easy and effective measure of an animal’s olfactory capacity to detect a novel odor.
HPLC detection of dopamine and its metabolites in olfactory bulb
Following the completion of treatment, mice were sacrificed and the olfactory bulbs were dissected out at the junction between the olfactory bulbs and the rest of the brain for each mouse. The dissected olfactory bulbs were weighed. The differences in weight of olfactory bulb between control and vanadium exposed animals were determined. Levels of dopamine (DA) and its metabolite DOPAC in olfactory lobe tissues were determined by high-performance liquid chromatography (HPLC) with electrochemical detection. The samples were prepared as described previously (Zhang et al., 2007, Ghosh et al., 2013). Briefly, neurotransmitters were extracted from olfactory bulbs using an antioxidant extraction solution (0.1 M perchloric acid containing 0.05% Na2EDTA and 0.1% Na2S2O5). The extracts then were filtered in 0.22-µm spin tubes, and 100 µl of each sample were loaded for analysis at a 1:2 dilution in mobile phase buffer. DA and DOPAC were separated isocratically by a reversed-phase column with a flow rate of 0.7 mL/min. An HPLC system (ESA Inc., Bedford, MA) with an automatic sampler equipped with a refrigerated temperature control (model 542; ESA Inc.) was used for these experiments. The electrochemical detection system was composed of a Coulochem model 5100A with a microanalysis cell (model 5014A) and a guard cell (model 5020) (ESA Inc.). Standard stock solutions of catecholamines were prepared at 1 mg/ml in antioxidant solution, and then further diluted to a final working concentration of 50 pg/µL before injection. Data acquisition was performed using EZChrome HPLC Software (ESA Inc.) and analyzed using Microsoft Excel and Prism 4.0 software (GraphPad Software Inc., San Diego, CA). The DA and DOPAC levels were quantified as ng/mg of protein.
Western blot
Olfactory bulb lysates from control and treatment groups, containing equal amounts of protein, were loaded in each lane and separated on a 10 to 12% SDS-polyacrylamide electrophoresis gel, as described previously (Kanthasamy et al., 2006, Jin et al., 2011a, Jin et al., 2011b, Latchoumycandane et al., 2011). After this separation, the proteins were transferred to a nitrocellulose membrane, and nonspecific binding sites were blocked by treating with Odyssey blocking buffer (Licor Biosciences). The membranes with transferred proteins were then incubated with primary antibody directed against TH (mouse monoclonal; 1:1000). The primary antibody incubations were followed by incubation with either Alexa Fluor 680 conjugated anti-mouse or IRDye 800 conjugated anti-rabbit secondary antibody for 1 h at room temperature. To confirm equal protein loading in each lane, membranes were probed with β-actin antibody (1:5000 dilution). Western blot images were captured and analyzed with an Odyssey IR Imaging system (LI-COR). Densitometric analysis was performed on the TH bands.
Locomotor activity
Locomotor behavioral data were collected using VersaMax animal activity monitors (model RXYZCM-16; Accuscan Instruments Inc., Columbus, OH), as previously described by (Zhang et al., 2007, Ghosh et al., 2013). The clear Plexiglas chamber has dimensions of 40 × 40 × 30.5 cm, and is covered with a ventilated Plexiglas lid. Infrared monitoring sensors are located every 2.54 cm along the full perimeter of the square chamber (16 infrared beams along each side). On two opposite walls, sensors are 2.5 cm above the floor and on the other two opposite walls, sensors are located 8.0 cm above the floor. Data were collected and analyzed by a VersaMax analyzer (model CDA-8; Accuscan Instruments Inc.). Several parameters of locomotor activity are presented here. All raw data are expressed as percentage of the vehicle control group (mean ± S.E.M.; n = 5) and were obtained one week post-treatment.
Immunohistological analysis of olfactory bulb sections
Tyrosine hydroxylase immunolabeling was performed in olfactory bulb sections. Briefly, one week after the last dose of vanadium, mice were sacrificed and intracardiac perfusion was performed with 4% paraformaldehyde (PFA) and subsequently post-fixed with PFA and 30% sucrose. The fixed olfactory lobes of the perfused brains were then cut using a cryostat into 30-µm coronal sections and kept at −20 °C in a cryosolution of 30% sucrose-ethylene glycol. Sections were rinsed with PBS on the day of staining, and the free-floating sections blocked with 2% bovine serum albumin, 0.5 % Triton X-100 and 0.05% Tween-20 in PBS for 1 h at room temperature. Following blocking, the sections were incubated in either anti-GFAP or anti-TH primary antibody (Calbiochem mouse anti-rabbit, 1:1600) overnight at room temperature. The sections were washed in PBS and incubated for 90 min at room temperature with Alexa Fluor 488/568 anti-mouse secondary antibody. After washing the sections in PBS, they were incubated with 10 µg/ml Hoechst 33342 for 5 min at room temperature stain the nucleus. Sections were carefully mounted on Poly-L-lysine coated slides with the organic solvent DPX, then dehydrated by being kept for one minute each in water, 70% ethanol, 95% ethanol, 100% ethanol and Xylene in that order. The sections were viewed under an inverted fluorescence microscope (Nikon TE-2000U; NIKON, Tokyo, Japan). Images (2×, 30× and 60×) were captured with a SPOT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) using MetaMorph software, version 5.0 (Molecular Devices, Sunnyvale, CA).
Data analysis
Data analysis was performed using Prism 4.0 software (GraphPad Software Inc., San Diego, CA). Raw data were analyzed using a two-tailed unpaired student’s t-test. Statistically significant differences are indicated by asterisks as follows: * p<0.05, **p<0.01, and ***p<0.001.
Results
Intranasal vanadium exposure induces locomotor deficits
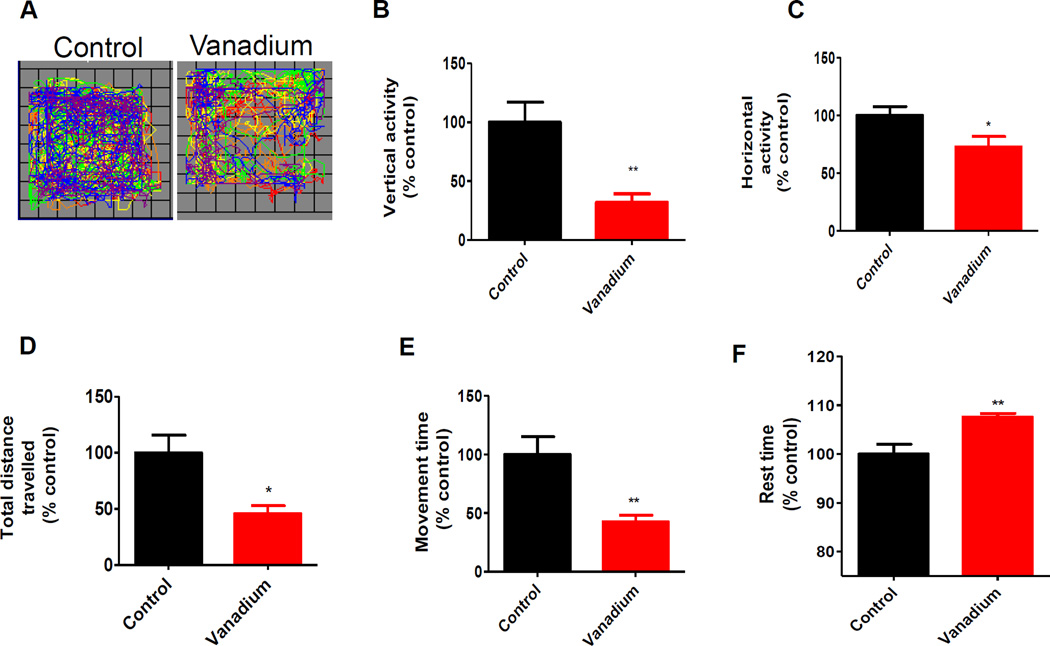
C57 black mice were administered V2O5 (182 µg) intranasally three times a week for one month. One month after initiating intranasal administration of vanadium, we evaluated the effect of the treatment on locomotor activity by measuring motor deficits using the VersaMax automated activity monitor. As shown in Fig. 1, significant decreases were observed in the various locomotor activities in the vanadium treatment group relative to the control. Fig. 1A shows the representative locomotor activity map of control and vanadium treated mice. Quantitative analysis indicated a decrease in motor activities in the vanadium treated mice relative to controls: 68% decrease in total vertical movement (Fig. 1B), 27% decrease in total horizontal movement (Fig. 1C), 57% decrease in total distance travelled (Fig. 1D), 54% decrease in total movement time (Fig. 1E), and a 7% increase in rest time (Fig. 1F).
Fig. 1. Effects of intranasally administered vanadium on locomotor activity.
Male C57 black mice were intranasally administered 182 µg of V2O5 in 50 µL of de-ionized water three times a week for one month. The vehicle control animals were administered de-ionized water. Locomotor activity was measured using an automated VersaMax locomotor activity monitor before the termination of the study. A, Representative moving tracks of mice. B, Total vertical movement. C, Total horizontal movement. D, Total distance travelled. E, Total movement time. F, Total rest time. The vehicle-treated group served as the control. Data are in percent control and represent mean ± S.E.M. from at least five animals per group. Asterisks (*, p < 0.05; **, p<0.001) indicate significant differences between treatment and the control group.
Intranasal vanadium exposure induces olfaction deficits
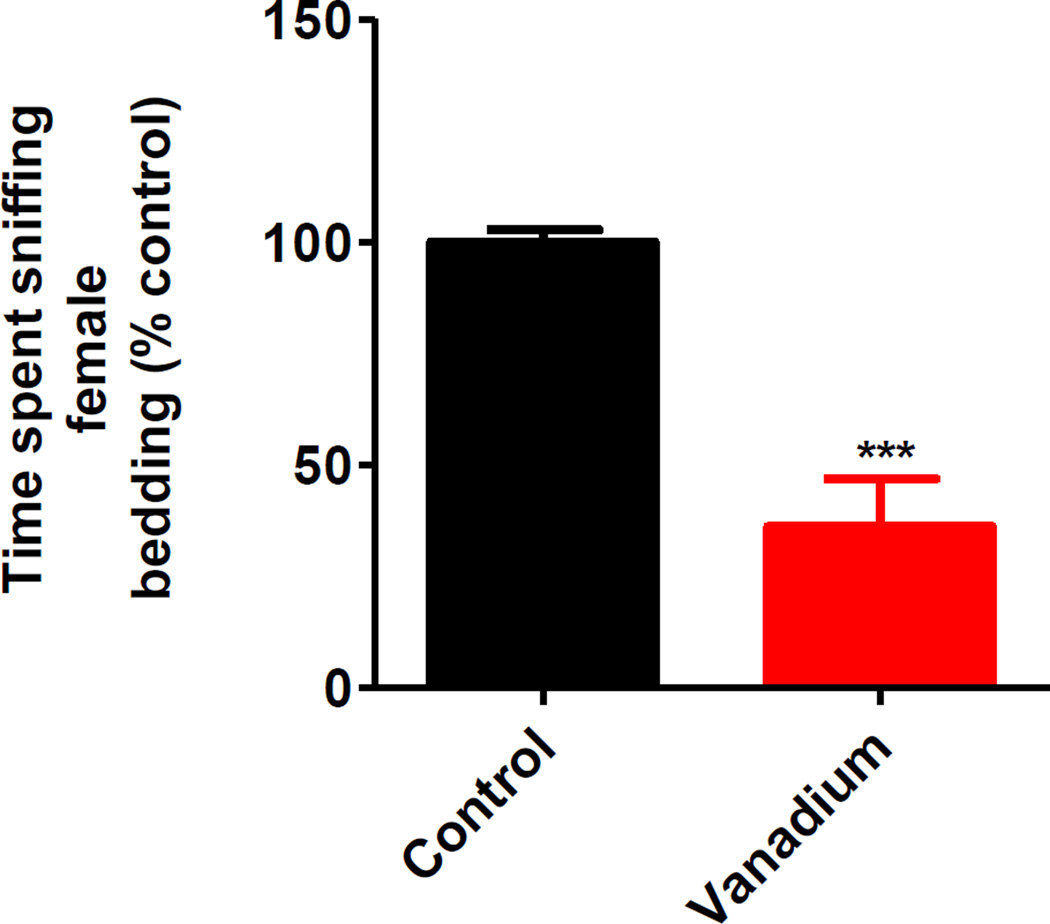
After measuring motor deficits, we determined the effect of vanadium on olfaction. We tested the ability of the male mice to detect female pheromones by measuring their ability to find and sniff bedding taken from pregnant female cages. As shown in Fig. 2, relative to controls, the vanadium treatment group spent 63% less time sniffing female bedding during a 5-minute testing session, indicating impaired olfaction following vanadium exposure.
Fig. 2. Effects of intranasally administered vanadium on pheromonal olfaction (sniffing ability).
Male C57 black mice (n ≥ 5 per group) were intranasally administered 182 µg of V2O5 in 50 µL of deionized water three times a week for one month. The vehicle control animals were administered deionized water. At the end of the study, animals were exposed to bedding from pregnant female cages, and the amount of time males spent sniffing the bedding during a five-minute time period was recorded. Asterisks (***, p < 0.001) indicate a significant difference between treatment and control group means ± S.E.M.
Intranasal vanadium exposure causes a decrease in the weight of olfactory bulbs
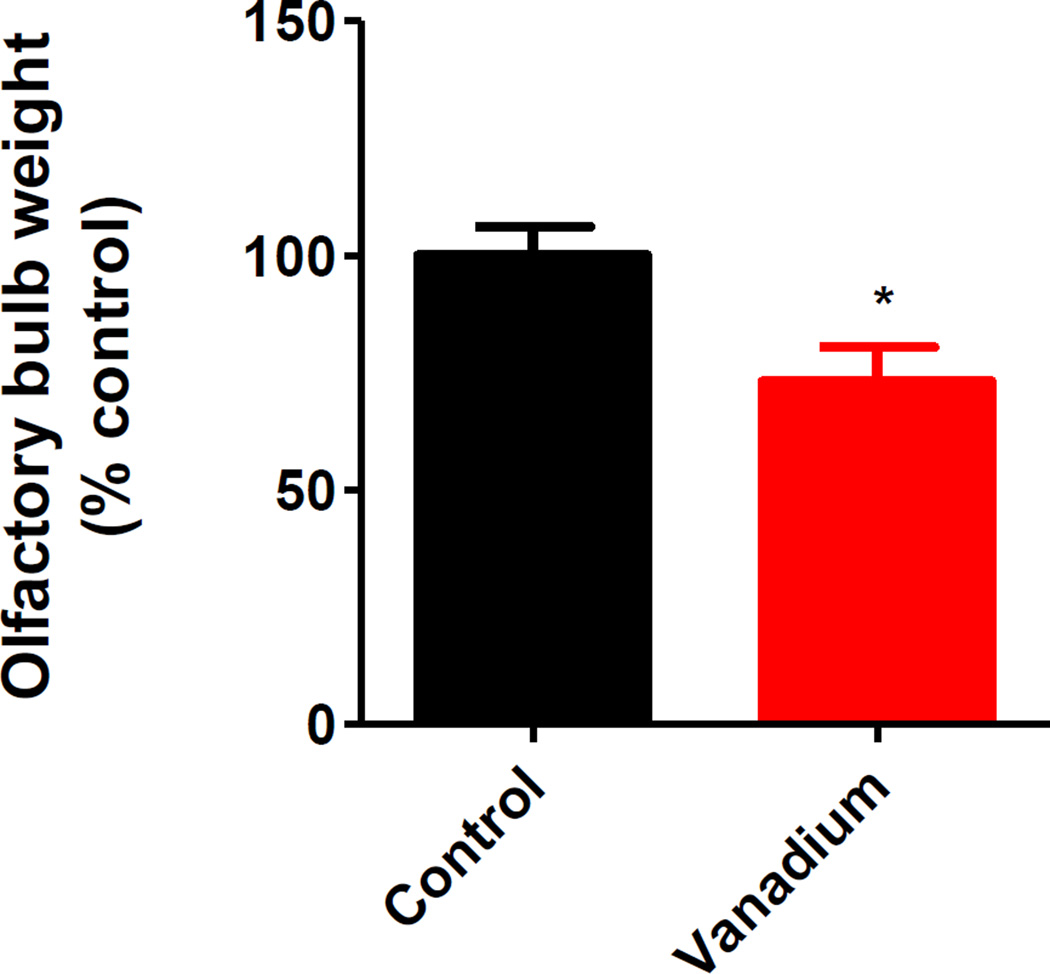
It has been observed that olfactory bulb volumes tend to decline with decreased smell function (Yousem et al., 1999, Rombaux et al., 2006). To determine the effect of intranasally administered vanadium on the volumes of olfactory bulbs, we dissected the olfactory bulbs and weighed each side. As shown in Fig. 3, we observed a 26% reduction in olfactory bulb weights in the vanadium treatment group relative to the control group.
Fig. 3. Effects of intranasally administered vanadium on the size of olfactory lobes.
Male C57 black mice (n ≥ 5 per group) were intranasally administered 182 µg of V2O5 in 50 µL of deionized water three times a week for one month. The vehicle control animals were administered deionized water. At the end of treatment, olfactory lobes were dissected out and weighed. Asterisks (*, p < 0.05) indicate a significant difference between treatment and control group means ± S.E.M.
Intranasal vanadium exposure causes a decrease in tyrosine hydroxylase (TH) levels in the olfactory bulb
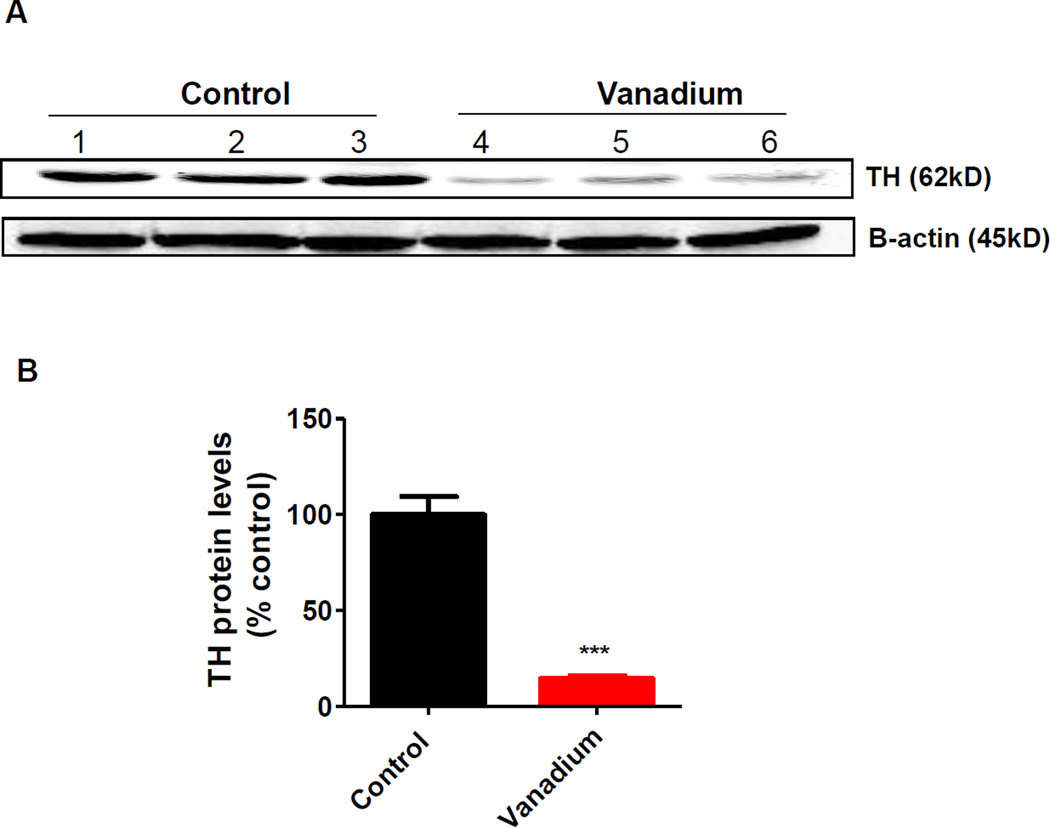
The glomerular layer of the olfactory bulb has been reported to have an abundance of dopaminergic neurons (Halasz et al., 1981, Davila et al., 2003). Dopamine is believed to play an important role in olfaction (Hsia et al., 1999). Tyrosine hydroxylase (TH) is the rate-limiting enzyme responsible for synthesizing dopamine, and therefore, TH is used as a marker of dopaminergic neuronal integrity. First, we used Western blots to measure changes in TH levels in the dissected olfactory bulb, following intranasally administered vanadium. As depicted in Fig. 4A, we observed a dramatic reduction in TH levels in the vanadium-treated olfactory bulbs. The changes in TH levels corresponded to an 85% decrease in the vanadium-treated olfactory bulbs compared with the control group, as shown by densitometric analysis of the Western blot (Fig. 4B). Beta-actin was used as a loading control for Western blot studies.
Fig. 4. Effect of intranasally administered vanadium on TH expression levels in olfactory lobes.
Male C57 black mice (n ≥ 3 per group) were intranasally administered 182 µg of V2O5 in 50 µL of deionized water three times a week for one month. The vehicle control animals were administered deionized water. At the end of the treatment animals were sacrificed and TH expression was detected in the olfactory lobe by Western blot using mouse monoclonal antibody against TH. (A) A representative Western blot analysis of TH expression in control and vanadium treated mice. β-actin immunoblot was used to confirm equal protein loading in each lane. (B) The bands were quantified for densitometric analysis and data are expressed as a percentage of vehicle-treated bands. Asterisks (***, p < 0.001) indicate a significant difference between treatment and control group means ± S.E.M.
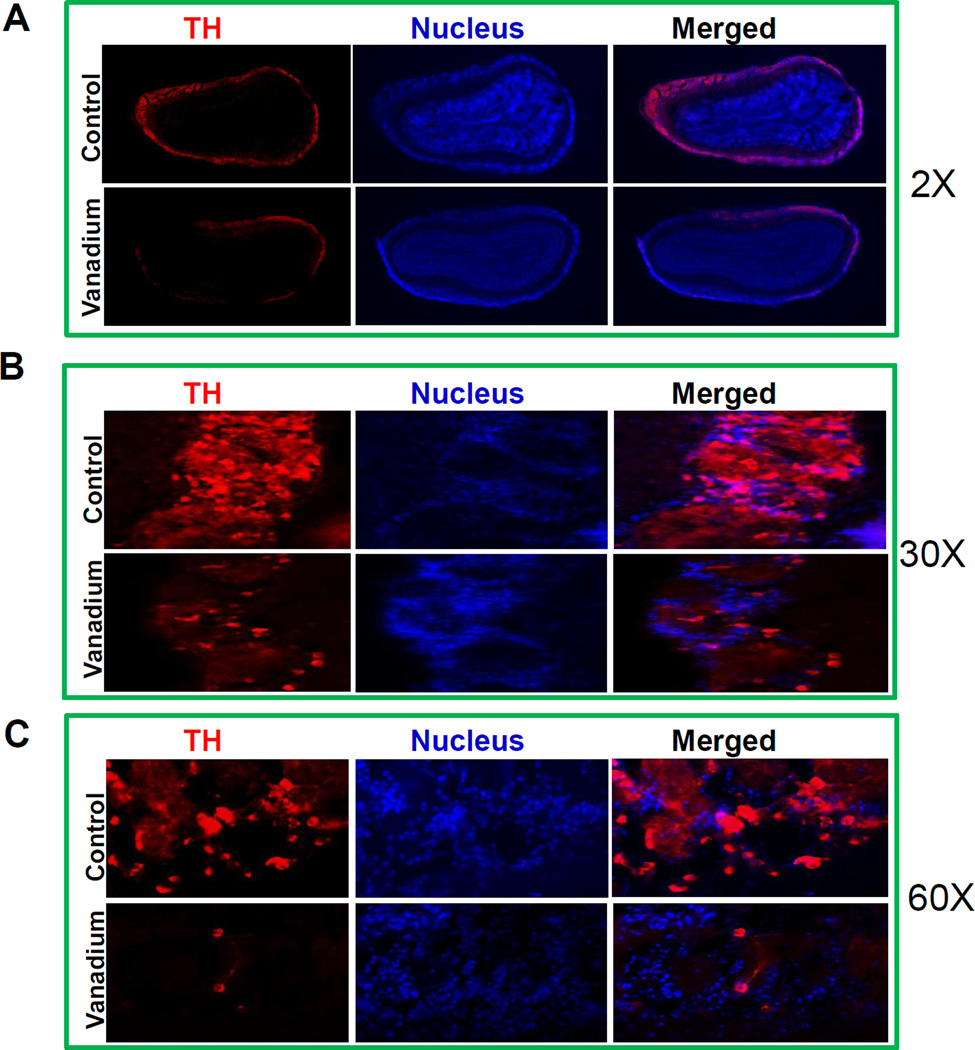
Next we examined the vanadium exposure on the integrity of dopaminergic neurons in the glomerular layer of olfactory bulb. As shown in Fig. 5A, a dense distribution of TH positive neurons was noted throughout the glomerular layer in control mice. However, a dramatic loss of TH positive neurons was observed in the glomerular layer of vanadium treated mice. Higher magnifications (Fig. 5B: 30×, Fig. 5C: 60×) clearly revealed the extent of dopaminergic neuronal loss in vanadium treated olfactory bulb compared to control olfactory bulb. Collectively, both Western blot and immunohistochemical studies indicated that vanadium exposure dramatically affects dopaminergic neurons in olfactory bulb.
Fig. 5. Loss of glomerular TH neurons in olfactory lobes following intranasally administered vanadium.
Male C57 black mice were intranasally administered 182 µg of V2O5 in 50 µL of de-ionized water three times a week as described in methods. The vehicle control animals were administered de-ionized water. At the end of treatment, animals were intracardially perfused and TH neurons were detected in the glomerular layer of the olfactory lobe by immunohistochemistry. (A) Representative 2× pictures of TH neurons in the glomerular layer of the olfactory lobe of control and vanadium-treated mice. (B) Representative 30× pictures of TH neurons in the glomerular layer of the olfactory lobe of control and vanadium-treated mice. (C) Representative 60× pictures of TH neurons in the glomerular layer of the olfactory lobe of control and vanadium-treated mice.
Intranasal vanadium exposure induces dopamine depletion in the olfactory bulb
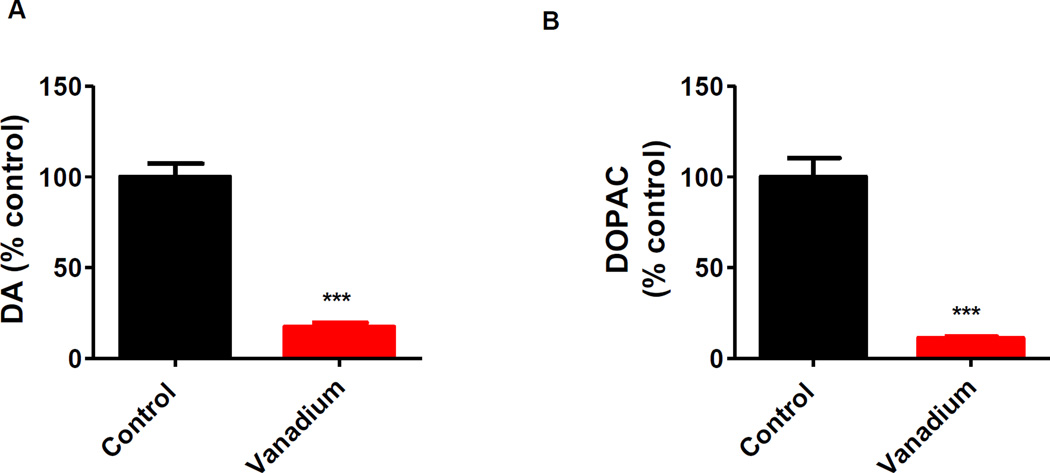
Since we found significant reductions in TH in the olfactory bulb following intranasally administered vanadium, we proceeded to measure the neurochemical changes in the dissected olfactory bulbs using HPLC. We observed that the level of DA was reduced by 82% (Fig. 6A), and that of its metabolite DOPAC by 88% (Fig. 6B), relative to control group. Together, these results show that vanadium induces a significant dopaminergic neurochemical deficit in the olfactory bulb.
Fig. 6. Effects of intranasally administered vanadium on olfactory bulb dopamine and DOPAC levels.
Male C57 black mice (n ≥ 5 per group) were intranasally administered 182 µg of V2O5 in 50 µL of deionized water three times a week for one month. The vehicle control animals were administered deionized water. Animals were sacrificed following the last treatment, and the neurochemical analysis (A, dopamine; B, DOPAC) was performed in olfactory lobe tissues using HPLC. Asterisks (***, p < 0.001) indicate a significant difference between treatment and control group means ± S.E.M.
Subchronic intranasal exposure to vanadium induces migration and accumulation of astrocytes in the glomerular layer of the olfactory bulb
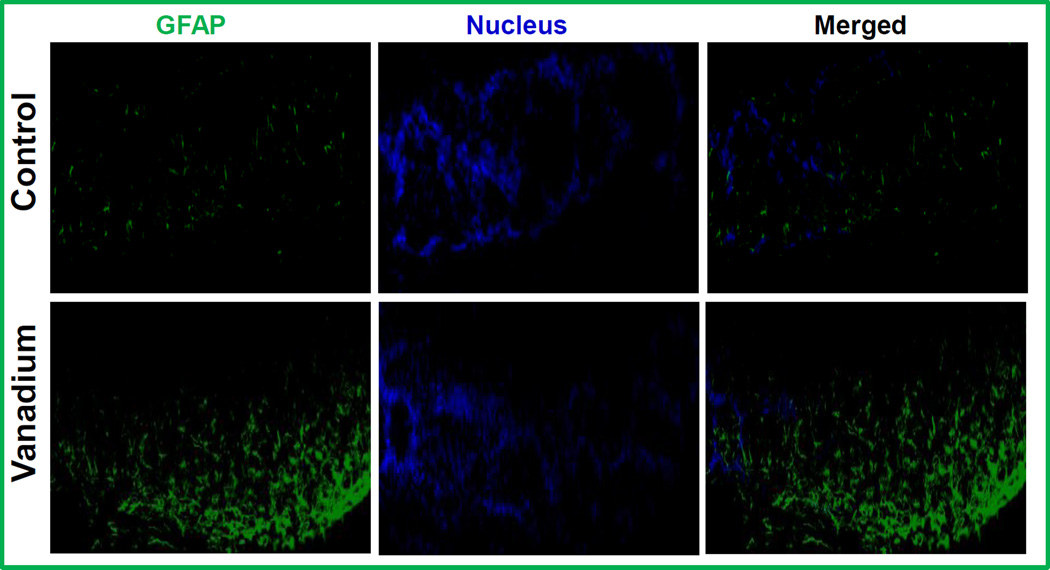
Having shown the degeneration of olfactory bulb dopaminergic neurons in the glomerular layer, we probed for increased presence of astroglia in the same layer, as a marker and indicator of neurodegeneration and neuroinflammation. As shown in Fig. 7, GFAP immunostaining revealed a dramatic increase in astroglial proliferation in the glomerular layer of vanadium exposed olfactory bulb. No GFAP staining was detected in control animals. These results further confirmed that vanadium exposure induced degeneration processes in olfactory bulb.
Fig. 7. Astrocyte Proliferation in the olfactory lobe glomerular layer following intranasally administered vanadium.
Male C57 black mice were intranasally administered 50 µl (182 ug) of vanadium as described in the methods. The vehicle control group was administered de-ionized water. These treated animals were intracardially perfused and the astrocytes in the olfactory lobe were stained for GFAP by immunohistochemistry. A representative 20× pictures of GFAP astroglia in the olfactory lobe of the control and vanadium treated mice is shown. The data is representative of 3 mice per treatment group.
Discussion
The effects of neurotoxic metals on the olfactory system are not well characterized. In light of recently observed correlations between olfactory dysfunction and environmentally linked neurodegenerative disorders, identification of the effects of neurotoxic metals on the olfactory bulb was warranted. The main findings of our study are that intranasal administration of a low dose of vanadium caused a significant reduction in a) tyrosine hydroxylase protein levels, b) dopamine levels and c) weight of olfactory bulbs. These effects were accompanied by decreased ability of animals to detect pheromones in pregnant female bedding. In addition, intranasal vanadium exposure produced significant locomotor deficits. To our knowledge, this is the first report demonstrating adverse effects of vanadium exposure on the olfactory system.
Vanadium is one of the light metals with very high strength, and this unique property makes the metal desirable for high strength steel alloy industrial applications, including ship and aircraft manufacturing (Korchynsky, 2001). Elevated levels of vanadium in the air (4.7 mg/m3) were reported in the breathing zones of steel industry workers (Kiviluoto et al., 1979) as well as in welding fumes (IARC, 2006). Vanadium is also used in the production of temperature-resistant alloys and glass, and in pigment and paint manufacturing. Through the Vanadium Technology Partnership, the United States Army is partnering with the vanadium industry to explore the potential applications of high-strength, light-weight and low-cost vanadium micro-alloyed steel in creating a stronger and lighter military. The program demonstrates that military support structures like vehicles, trailers, barriers and buildings can be improved in terms of protection and greater mobility through vanadium-alloying and hot-rolled steel technology. Another major source of environmental vanadium exposure is through use of fossil fuels. It has been reported that 12000 – 24000 tons of vanadium per year are generated from the burning of fossil fuels (Bertine and Goldberg, 1971), and given that fossil fuel demand has risen considerably since then (Huntington, 2010), the emission rate attributable to fossil fuel burning has likely risen dramatically. Also, vanadium concentrations have been reported in cigarette smoke to range from 0.49 to 5.33 µg/g (Adachi et al., 1998), and smoking has been linked to olfactory dysfunction (Frye et al., 1990, Martin et al., 2009).
Vanadium is known to be present in the earth’s crust in average concentrations of about 150 µg/g, with soil concentrations ranging from 3 to 310 µg/g (Waters, 1977). In fact, concentrations as high as 400 µg/g have been documented in fly ash polluted areas (Bengtsson and Tyler, 1976). Urban areas have annual airborne vanadium concentration averaging from 0.05 to 0.18 µg/m3, with maxima as high as 2 µg/m3 occurring on the coldest winter nights in the most densely populated areas. These airborne vanadium concentrations have been on the rise in Europe, due mostly to the increased combustion of crude oil residues in community-heating systems and power plants (WHO, 2000).
Vanadium concentrations in drinking-water range from 0.2 to 100 µg/L (Vouk, 1979), with the estimated mean dietary intake of being 20 µg/day (Myron et al., 1977). Using the exposure commitment method of analysis, Davies and Bennett reported that a person living in a rural environment with an assumed airborne concentration of 8 ng/m3 vanadium will have a body-burden of about 100 µg of vanadium (Davies and Bennett, 1983), over 80% of which is derived from the diet. Vanadium concentrations recorded and reported in urban areas range from 50 to 200 ng/m3, which translate to a body burden of about 142–570 µg (Davies and Bennett, 1983). In this case, the inhalation route contributes more than half of the total body burden. The use of high-vanadium fuel-oil for heating during winter escalated concentrations to about 2000 ng/m3 in bigger cities, hence an estimated body burden of over 5700 µg of vanadium following exposure. Vanadium concentrations observed in workplace air (0.01–60 mg/m3) far exceed those reported for the general environment. Considering more than half of the total environmental vanadium exposure is contributed through inhalation route (Davies and Bennett, 1983), we used intranasal exposure in our experiment. Thus, the dose of 182 µg vanadium used in our study is within the environmentally relevant range.
Our results show that vanadium affects the dopaminergic neurotransmitter system in the olfactory bulb. Olfactory system is important route of various neurotoxicant exposures. Our findings are consistent with results from other studies showing that the classic catecholaminergic neurotoxicants methamphetamine and amphetamine cause dopamine depletion in the olfactory bulb (Deng et al., 2007, Atianjoh et al., 2008). Another study showed both intranasal irrigation in mice with either ZnSO4 or Triton X-100 and surgical deafferentation or axotomy in rats are associated with decreased DA, DOPAC, TH enzyme activity, and olfactory bulb weights (Baker et al., 1983). These authors also found a clear correlation between reduction in TH, DA and DOPAC levels and reduced bulb weights (Baker et al., 1983). Agents like viruses, nanoparticles and prions are thought to enter the brain through the olfactory mucosa by damaging the olfactory epithelium. Olfactory receptor neurons have been shown in animal studies to take up and transport cadmium, gold, and manganese ions toward the olfactory bulbs at rates ranging from 2.5 to 3 mm/hour (Gottofrey and Tjalve, 1991, Tjalve et al., 1995, Tjalve et al., 1996, Doty, 2009). Antunes et al. carried out a study measuring olfactory function in welders employed in the construction of the San Francisco-Oakland Bay Bridge for durations ranging from 6 to 28 months. They concluded that professional welders may be at risk of losing their ability to smell properly (Antunes et al., 2007).
The olfactory bulb contains five layers, namely, the subependymal, combined mitral and granule cells, external plexiform and the glomerular layers (Lledo et al., 2006); the glomerular layer abundantly expresses dopaminergic neurons (Halasz et al., 1981, Davila et al., 2003). In the present study we show that vanadium exposure can decrease both TH and dopamine levels in the olfactory bulb. Dopamine plays an important role in olfaction (Doty and Risser, 1989, Wilson and Sullivan, 1995, Duchamp-Viret et al., 1997, Hsia et al., 1999, Koster et al., 1999), and changes in dopamine levels affect olfaction. Hsia et al. reported that dopamine regulates transmission between the olfactory bulb epithelium and the olfactory bulb glomeruli to mediate entry of olfactory information into the brain (Hsia et al., 1999). Consistent with this notion, our results indicate that the depletion of dopamine is accompanied by significantly decreased locomotion and decreased time spent sniffing pregnant female bedding. Thus, dopamine depletion in the olfactory bulb following intranasally administered vanadium may impair olfaction (odor detection) by disinhibition of neural transmission in olfactory glomeruli, leading to impaired olfactory processing. This observation may be relevant to clinical observations that patients who suffer from Parkinson’s disease experience hyposmia, which typically precedes motor deficits (Hawkes, 2003). The clinical assessment of olfactory deficits in PD patients is conducted by testing for odor identification, odor discrimination, threshold detection and odor recognition memory experiments (Mesholam et al., 1998). Parkinson’s disease patients have demonstrated impairments in odor detection, differentiation and identification (Ward et al., 1983, Doty et al., 1992, Tissingh et al., 1998). Most occupationally related environmental neurotoxicant exposures occur through the nostrils, and Avila-Costa and coworkers have shown that vanadium enters the brain parenchyma following inhalation exposure (Avila-Costa et al., 2005). Furthermore, prolonged inhalation exposure to vanadium can damage the nigral dopaminergic system (Avila-Costa et al., 2004). Our study shows that depletion of dopamine in the olfactory bulb may contribute to the locomotor deficits that are characteristic of PD, suggesting that vanadium may have important effects in the nigrostriatal dopaminergic system. Although we did not find any significant change in either striatal dopamine or nigral TH+ neurons, the nigra did exhibit moderately increased oxidative damage, as measured by 4-hydroxy noneneal (4HNE), as well as slightly increased alpha-synuclein protein levels (data not shown). Future studies will address whether the nigrostriatal dopaminergic pathway is also affected following prolonged intranasal exposure to vanadium.
Behavioral and neurochemical results of our study correlated well with histological analysis of olfactory bulb. TH immunohistochemical analysis revealed a severe loss of dopaminergic neurons in granular layer of olfactory bulb. In addition to dopaminergic neuronal loss, we observed the migration and accumulation of astroglia in the glomerular layer of the olfactory bulb as measured by GFAP immunostaining. The GFAP staining and TH-positive dopaminergic neuronal loss was limited to the glomerular layer, indicating that dopaminergic neurons in glomerular layer are preferentially affected by intranasal exposure to vanadium. An increased number of astroglia has been observed in many neurodegenerative conditions including PD (Forno et al., 1992, Maragakis and Rothstein, 2006). Since glial cells play a role in neuroinflammatory processes (Tansey et al., 2008), it is possible that the observed glomerular dopaminergic neurodegeneration is accompanied by neuroinflammation. Further detailed studies examining microglia activation and other proinflammatory processes are required to implicate inflammatory mechanisms in vanadium neurotoxicity.
In summary, we observed that subchronic exposure to a low dose of vanadium via the intranasal route induces olfactory dysfunction which is characterized by decreased olfactory bulb volume and the loss of dopaminergic neurotransmission to the olfactory bulb. Our study reveals the neurotoxicity of vanadium to the olfactory system, and may be useful in future risk assessments and regulation of vanadium.
Highlights.
Low dose intranasal vanadium exposure induces locomotor impairment
Intranasal vanadium exposure also causes profound olfactory deficits
Intranasal vanadium exposure decreases the levels of TH protein and dopamine in the olfactory bulb
Intranasal vanadium exposure also increases astroglial proliferation in the olfactory bulb
Acknowledgements
The writing of this chapter was supported by the National Institutes of Health RO1 grants, ES10586, ES19267, and NS074443 to AGK and NS65167 to AK. The W. Eugene and Linda Lloyd Endowed Chair to AGK is also acknowledged. The authors acknowledge Mr. Gary Zenitsky for his assistance in the preparation of this manuscript.
Abbreviations
- V2O5
vanadium pentoxide
- Mn
manganese
- PD
Parkinson’s disease
- TH
tyrosine hydroxylase
- OB
olfactory bulb
- DA
dopamine
- DOPAC
3, 4-dihydroxyphenylacetic acid
- HPLC
high-performance liquid chromatography
- PFA
paraformaldehyde
- PKCδ
protein kinase C delta
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Adachi A, Asai K, Koyama Y, Matsumoto Y, Kobayashi T. Vanadium content of cigarettes. Bull Environ Contam Toxicol. 1998;61:276–280. doi: 10.1007/s001289900759. [DOI] [PubMed] [Google Scholar]
- Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson's disease. Toxicol Appl Pharmacol. 2009;240:273–285. doi: 10.1016/j.taap.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allam MF, Del Castillo AS, Navajas RF. Parkinson's disease risk factors: genetic, environmental, or both? Neurol Res. 2005;27:206–208. doi: 10.1179/016164105X22057. [DOI] [PubMed] [Google Scholar]
- Amorim FA, Welz B, Costa AC, Lepri FG, Vale MG, Ferreira SL. Determination of vanadium in petroleum and petroleum products using atomic spectrometric techniques. Talanta. 2007;72:349–359. doi: 10.1016/j.talanta.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Anglade P, Vyas S, Hirsch EC, Agid Y. Apoptosis in dopaminergic neurons of the human substantia nigra during normal aging. Histol Histopathol. 1997;12:603–610. [PubMed] [Google Scholar]
- Ansari KA, Johnson A. Olfactory function in patients with Parkinson's disease. J Chronic Dis. 1975;28:493–497. doi: 10.1016/0021-9681(75)90058-2. [DOI] [PubMed] [Google Scholar]
- Antunes MB, Bowler R, Doty RL. San Francisco/Oakland Bay Bridge Welder Study: olfactory function. Neurology. 2007;69:1278–1284. doi: 10.1212/01.wnl.0000276988.50742.5e. [DOI] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson's disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–266. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atianjoh FE, Ladenheim B, Krasnova IN, Cadet JL. Amphetamine causes dopamine depletion and cell death in the mouse olfactory bulb. Eur J Pharmacol. 2008;589:94–97. doi: 10.1016/j.ejphar.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Costa MR, Colin-Barenque L, Zepeda-Rodriguez A, Antuna SB, Saldivar OL, Espejel-Maya G, Mussali-Galante P, del Carmen Avila-Casado M, Reyes-Olivera A, Anaya-Martinez V, Fortoul TI. Ependymal epithelium disruption after vanadium pentoxide inhalation. A mice experimental model. Neurosci Lett. 2005;381:21–25. doi: 10.1016/j.neulet.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Avila-Costa MR, Montiel Flores E, Colin-Barenque L, Ordonez JL, Gutierrez AL, Nino-Cabrera HG, Mussali-Galante P, Fortoul TI. Nigrostriatal modifications after vanadium inhalation: an immunocytochemical and cytological approach. Neurochem Res. 2004;29:1365–1369. doi: 10.1023/b:nere.0000026398.86113.7d. [DOI] [PubMed] [Google Scholar]
- Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson S, Tyler G. Vanadium in the environment. London: University of London Monitoring and Assessment Research Centre; 1976. vol. (MARC Report No. 2) [Google Scholar]
- Bertine KK, Goldberg ED. Fossil fuel combustion and the major sedimentary cycle. Science. 1971;173:233–235. doi: 10.1126/science.173.3993.233. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol. 2000;247(Suppl 2):II3–I10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Sandmann-Keil D, Gai WP, de Vos RA, Jansen Steur EN, Arai K, Braak E. Parkinson's disease: affection of brain stem nuclei controlling premotor and motor neurons of the somatomotor system. Acta Neuropathol (Berl) 2000;99:489–495. doi: 10.1007/s004010051150. [DOI] [PubMed] [Google Scholar]
- Brenneman KA, Cattley RC, Ali SF, Dorman DC. Manganese-induced developmental neurotoxicity in the CD rat: is oxidative damage a mechanism of action? Neurotoxicology. 1999;20:477–487. [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Guillot TS, Lazo CR, Miller GW. Industrial toxicants and Parkinson's disease. Neurotoxicology. 2012;33:178–188. doi: 10.1016/j.neuro.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DJA, Bennett BG. Exposure commitment assessments of environmental pollutants. London: University of London Monitoring Assessment and Research Centre; 1983. vol. (MARC Report No. 30) [Google Scholar]
- Davila NG, Blakemore LJ, Trombley PQ. Dopamine modulates synaptic transmission between rat olfactory bulb neurons in culture. J Neurophysiol. 2003;90:395–404. doi: 10.1152/jn.01058.2002. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Jayanthi S, Cadet JL. Methamphetamine administration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol Psychiatry. 2007;61:1235–1243. doi: 10.1016/j.biopsych.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Done AK. Of metals and chelation. Emer Med. 1979;11:186–218. [Google Scholar]
- Dorman DC, Struve MF, James RA, Marshall MW, Parkinson CU, Wong BA. Influence of particle solubility on the delivery of inhaled manganese to the rat brain: manganese sulfate and manganese tetroxide pharmacokinetics following repeated (14-day) exposure. Toxicol Appl Pharmacol. 2001;170:79–87. doi: 10.1006/taap.2000.9088. [DOI] [PubMed] [Google Scholar]
- Doty RL. The olfactory system and its disorders. Semin Neurol. 2009;29:74–81. doi: 10.1055/s-0028-1124025. [DOI] [PubMed] [Google Scholar]
- Doty RL, Hastings L. Neurotoxic exposure and olfactory impairment. Clin Occupat Environ Med. 2001;1:547–575. [Google Scholar]
- Doty RL, Risser JM. Influence of the D-2 dopamine receptor agonist quinpirole on the odor detection performance of rats before and after spiperone administration. Psychopharmacology (Berl) 1989;98:310–315. doi: 10.1007/BF00451680. [DOI] [PubMed] [Google Scholar]
- Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1992;55:138–142. doi: 10.1136/jnnp.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp-Viret P, Coronas V, Delaleu JC, Moyse E, Duchamp A. Dopaminergic modulation of mitral cell activity in the frog olfactory bulb: a combined radioligand binding-electrophysiological study. Neuroscience. 1997;79:203–216. doi: 10.1016/s0306-4522(96)00646-x. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Tedroff J, Thuomas KA, Aquilonius SM, Hartvig P, Fasth KJ, Bjurling P, Langstrom B, Hedstrom KG, Heilbronn E. Manganese induced brain lesions in Macaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch Toxicol. 1992;66:403–407. doi: 10.1007/BF02035130. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson's disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF. Olfactory deficits in mice overexpressing human wildtype alpha-synuclein. Eur J Neurosci. 2008;28:247–256. doi: 10.1111/j.1460-9568.2008.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Di Monte D, Langston JW. Astrocytes and Parkinson's disease. Prog Brain Res. 1992;94:429–436. doi: 10.1016/s0079-6123(08)61770-7. [DOI] [PubMed] [Google Scholar]
- Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990;263:1233–1236. [PubMed] [Google Scholar]
- Furbee B. Welding and parkinsonism. Neurologic clinics. 2011;29:623–640. doi: 10.1016/j.ncl.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Saminathan H, Kanthasamy A, Anantharam V, Jin H, Sondarva G, Harischandra DS, Qian Z, Rana A, Kanthasamy AG. The peptidyl-prolyl isomerase Pin1 up-regulation and proapoptotic function in dopaminergic neurons: relevance to the pathogenesis of Parkinson disease. The Journal of biological chemistry. 2013;288:21955–21971. doi: 10.1074/jbc.M112.444224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sewell L, Holmes C. Association of anosmia with autonomic failure in Parkinson disease. Neurology. 2010;74:245–251. doi: 10.1212/WNL.0b013e3181ca014c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology. 1997;48:650–658. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- Gottofrey J, Tjalve H. Axonal transport of cadmium in the olfactory nerve of the pike. Pharmacol Toxicol. 1991;69:242–252. doi: 10.1111/bcpt.1991.69.4.242. [DOI] [PubMed] [Google Scholar]
- Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005;94:1187–1195. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson's disease: a critical review and new findings. Environ Health Perspect. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz N, Johansson O, Hokfelt T, Ljungdahl A, Goldstein M. Immunohistochemical identification of two types of dopamine neuron in the rat olfactory bulb as seen by serial sectioning. J Neurocytol. 1981;10:251–259. doi: 10.1007/BF01257970. [DOI] [PubMed] [Google Scholar]
- Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord. 2003;18:364–372. doi: 10.1002/mds.10379. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol. 1999;82:1082–1085. doi: 10.1152/jn.1999.82.2.1082. [DOI] [PubMed] [Google Scholar]
- Huntington HG. Oil demand and technical progress. Applied Economics Letters. 2010;17:1747–1751. [Google Scholar]
- IARC. Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide, Indium Phosphide and Vanadium Pentoxide, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 86. Lyon, France: International Agency for Research on Cancer; 2006. pp. 227–292. [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Anantharam V, Rana A, Kanthasamy AG. Transcriptional regulation of pro-apoptotic protein kinase Cdelta: implications for oxidative stress-induced neuronal cell death. The Journal of biological chemistry. 2011a;286:19840–19859. doi: 10.1074/jbc.M110.203687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Ghosh A, Yang Y, Anantharam V, Kanthasamy AG. alpha-Synuclein negatively regulates protein kinase Cdelta expression to suppress apoptosis in dopaminergic neurons by reducing p300 histone acetyltransferase activity. J Neurosci. 2011b;31:2035–2051. doi: 10.1523/JNEUROSCI.5634-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthasamy AG, Anantharam V, Zhang D, Latchoumycandane C, Jin H, Kaul S, Kanthasamy A. A novel peptide inhibitor targeted to caspase-3 cleavage site of a proapoptotic kinase protein kinase C delta (PKCdelta) protects against dopaminergic neuronal degeneration in Parkinson's disease models. Free radical biology & medicine. 2006;41:1578–1589. doi: 10.1016/j.freeradbiomed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Choi C, Jin H, Harischandra DS, Anantharam V, Kanthasamy A. Effect of divalent metals on the neuronal proteasomal system, prion protein ubiquitination and aggregation. Toxicology letters. 2012;214:288–295. doi: 10.1016/j.toxlet.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Lussier S, Rane A, Choi SW, Andersen JK. Inducible dopaminergic glutathione depletion in an alpha-synuclein transgenic mouse model results in age-related olfactory dysfunction. Neuroscience. 2011;172:379–386. doi: 10.1016/j.neuroscience.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviluoto M, Pyy L, Pakarinen A. Serum and urinary vanadium of vanadium-exposed workers. Scand J Work Environ Health. 1979;5:362–367. doi: 10.5271/sjweh.2645. [DOI] [PubMed] [Google Scholar]
- Korchynsky M. A New Role for Microalloyed Steels: Adding Economic Value. Infacon 9 Quebec City, Canada: 2001. [Google Scholar]
- Koster NL, Norman AB, Richtand NM, Nickell WT, Puche AC, Pixley SK, Shipley MT. Olfactory receptor neurons express D2 dopamine receptors. J Comp Neurol. 1999;411:666–673. doi: 10.1002/(sici)1096-9861(19990906)411:4<666::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Langston JW. The Parkinson's complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Jin H, Kanthasamy A. Dopaminergic neurotoxicant 6-OHDA induces oxidative damage through proteolytic activation of PKCdelta in cell culture and animal models of Parkinson's disease. Toxicology and applied pharmacology. 2011;256:314–323. doi: 10.1016/j.taap.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson's disease: a case-control study in Taiwan. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lucas PD, Donohoe SM, Thody AJ. The role of estrogen and progesterone in the control of preputial gland sex attractant odors in the female rat. Physiol Behav. 1982;28:601–607. doi: 10.1016/0031-9384(82)90037-3. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Marder K, Logroscino G, Alfaro B, Mejia H, Halim A, Louis E, Cote L, Mayeux R. Environmental risk factors for Parkinson's disease in an urban multiethnic community. Neurology. 1998;50:279–281. doi: 10.1212/wnl.50.1.279. [DOI] [PubMed] [Google Scholar]
- Martin GE, Junque C, Juncadella M, Gabarros A, de Miquel MA, Rubio F. Olfactory dysfunction after subarachnoid hemorrhage caused by ruptured aneurysms of the anterior communicating artery. Clinical article. J Neurosurg. 2009;111:958–962. doi: 10.3171/2008.11.JNS08827. [DOI] [PubMed] [Google Scholar]
- McNeilly JD, Heal MR, Beverland IJ, Howe A, Gibson MD, Hibbs LR, MacNee W, Donaldson K. Soluble transition metals cause the pro-inflammatory effects of welding fumes in vitro. Toxicol Appl Pharmacol. 2004;196:95–107. doi: 10.1016/j.taap.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- Myron DR, Givand SH, Nielsen FH. Vanadium content of selected foods as determined by flameless atomic absorption spectroscopy. J Agric Food Chem. 1977;25:297–300. doi: 10.1021/jf60210a036. [DOI] [PubMed] [Google Scholar]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, Osame M. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J Neurol Sci. 1999;162:102–105. doi: 10.1016/s0022-510x(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Parenti M, Flauto C, Parati E, Vescovi A, Groppetti A. Manganese neurotoxicity: effects of L-DOPA and pargyline treatments. Brain Res. 1986;367:8–13. doi: 10.1016/0006-8993(86)91571-4. [DOI] [PubMed] [Google Scholar]
- Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005;48:63–77. doi: 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology. 2000;21:435–440. [PubMed] [Google Scholar]
- Przedborski S. Pathogenesis of nigral cell death in Parkinson's disease. Parkinsonism Relat Disord. 2005;11(Suppl 1):S3–S7. doi: 10.1016/j.parkreldis.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Ischiropoulos H. Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson's disease. Antioxid Redox Signal. 2005;7:685–693. doi: 10.1089/ars.2005.7.685. [DOI] [PubMed] [Google Scholar]
- Pyrzynska K, Weirzbicki T. Determination of vanadium species in environmental samples. Talanta. 2004;64:823–829. doi: 10.1016/j.talanta.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Ritz B, Yu F. Parkinson's disease mortality and pesticide exposure in California 1984– 1994. Int J Epidemiol. 2000;29:323–329. doi: 10.1093/ije/29.2.323. [DOI] [PubMed] [Google Scholar]
- Rombaux P, Mouraux A, Bertrand B, Nicolas G, Duprez T, Hummel T. Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope. 2006;116:436–439. doi: 10.1097/01.MLG.0000195291.36641.1E. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Burnett CA, Boeniger MF, Johnson J. Neurodegenerative diseases: occupational occurrence and potential risk factors, 1982 through 1991. Am J Public Health. 1996;86:1281–1288. doi: 10.2105/ajph.86.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AG, Clancy AN, Macrides F, Agosta WC, Bronson FH. Chemical properties of a female mouse pheromone that stimulates gonadotropin secretion in males. Biol Reprod. 1988;38:193–199. doi: 10.1095/biolreprod38.1.193. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Mutti A, De Rosa A, De Palma G, Negrotti A, Calzetti S. A case-control study of occupational and environmental risk factors for Parkinson's disease in the Emilia-Romagna region of Italy. Neurotoxicology. 1998;19:709–712. [PubMed] [Google Scholar]
- Tansey MG, Frank-Cannon TC, McCoy MK, Lee JK, Martinez TN, McAlpine FE, Ruhn KA, Tran TA. Neuroinflammation in Parkinson's disease: is there sufficient evidence for mechanism-based interventional therapy? Front Biosci. 2008;13:709–717. doi: 10.2741/2713. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Saint-Hilaire MH, Cupples LA, Thomas CA, Burchard AE, Feldman RG, Myers RH. Environmental, medical, and family history risk factors for Parkinson's disease: a New England-based case control study. Am J Med Genet. 1999;88:742–749. [PubMed] [Google Scholar]
- Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen AG, van Royen EA, Stoof JC, Wolters EC. Iodine-123-N-omega-fluoropropyl-2beta-carbomethoxy-3beta-(4-iod ophenyl)tropane SPECT in healthy controls and early-stage, drug-naive Parkinson's disease. J Nucl Med. 1998;39:1143–1148. [PubMed] [Google Scholar]
- Tjalve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol Toxicol. 1996;79:347–356. doi: 10.1111/j.1600-0773.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Tjalve H, Mejare C, Borg-Neczak K. Uptake and transport of manganese in primary and secondary olfactory neurones in pike. Pharmacol Toxicol. 1995;77:23–31. doi: 10.1111/j.1600-0773.1995.tb01909.x. [DOI] [PubMed] [Google Scholar]
- Verity MA. Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology. 1999;20:489–497. [PubMed] [Google Scholar]
- Vescovi A, Facheris L, Zaffaroni A, Malanca G, Parati EA. Dopamine metabolism alterations in a manganese-treated pheochromocytoma cell line (PC12) Toxicology. 1991;67:129–142. doi: 10.1016/0300-483x(91)90137-p. [DOI] [PubMed] [Google Scholar]
- Vouk V. Vanadium. In: Handbook on the toxicology of metals. Friberg L, et al., editors. Amsterdam: Elsevier-North Holland Biomedical Press; 1979. pp. 659–674. [Google Scholar]
- Ward CD, Hess WA, Calne DB. Olfactory impairment in Parkinson's disease. Neurology. 1983;33:943–946. doi: 10.1212/wnl.33.7.943. [DOI] [PubMed] [Google Scholar]
- Waters MD. Toxicology of vanadium. In: Goyer RA, Mehlman MA, editors. Advances in modern toxicology Vol 2 Toxicology of trace elements. New York: Wiley; 1977. pp. 147–189. [Google Scholar]
- Weintraub D, Comella CL, Horn S. Parkinson's disease--Part 1: Pathophysiology, symptoms, burden, diagnosis, and assessment. The American journal of managed care. 2008;14:S40–S48. [PubMed] [Google Scholar]
- WHO Vanadium. Chapter 6.12. Denmark: Copenhagen; 2000. Air Quality Guidelines for Europe - Second Edition: WHO Regional Office for Europe. [Google Scholar]
- Wilson DA, Sullivan RM. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J Neurosci. 1995;15:5574–5581. doi: 10.1523/JNEUROSCI.15-08-05574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousem DM, Geckle RJ, Bilker WB, Kroger H, Doty RL. Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad Radiol. 1999;6:264–272. doi: 10.1016/s1076-6332(99)80449-8. [DOI] [PubMed] [Google Scholar]
- Zhang D, Anantharam V, Kanthasamy A, Kanthasamy AG. Neuroprotective effect of protein kinase C delta inhibitor rottlerin in cell culture and animal models of Parkinson's disease. J Pharmacol Exp Ther. 2007;322:913–922. doi: 10.1124/jpet.107.124669. [DOI] [PubMed] [Google Scholar]