Abstract
Goals
Investigate the role of self-efficacy during HCV treatment.
Background
Adherence to chronic hepatitis C virus (HCV) treatment is critical. Self-efficacy (SE) is an important predictor of medication adherence in a number of chronic disease populations and medication regimens, but its role during HCV treatment remains unknown.
Study
Data from the prospective Virahep-C study was analyzed to examine relationships between SE and patient-driven deviations (i.e., missed doses measured using electronic pill caps, and nonpersistence) from adherence to HCV antiviral treatment. SE was measured using the 17-item HCV Treatment Self-Efficacy scale. This measure provides a global estimate of a patient’s confidence to undergo and adhere to HCV treatment, and can estimate SE in four underlying domains: communication SE (i.e., confidence to communicate with healthcare provider), physical coping SE (i.e., confidence to cope with physical side effects), psychological coping SE (i.e., confidence to cope with psychiatric side effects), and treatment adherence SE (i.e., confidence to take all medication as prescribed and attend doctor visits). Generalized estimating equations and Cox proportional hazards models were used to assess associations between SE and missed doses and nonpersistence, respectively.
Results
SE was associated with being in a relationship, educated, privately insured, and less depressed. Higher communication SE at TW24 reduced the risk of missed doses between TW24 and 48. Higher baseline treatment adherence SE reduced the likelihood of nonpersistence between baseline and TW24.
Conclusions
Self-efficacy’s relationship to HCV treatment adherence has promising clinical and research implications.
Keywords: medication adherence, compliance, self-efficacy, Hepatitis C, antiviral therapy
Introduction
Chronic hepatitis C viral (HCV) infection is estimated to affect 2% of the United States adult population and is responsible for over 10,000 deaths annually 1, 2. While the incidence of acute HCV infections in the U.S. has decreased dramatically, the individual and public health burdens of chronic HCV are expected to rise over the next 20–30 years 3. Fortunately, antiviral medical regimens are available which can cure HCV and reduce mortality and morbidity 4. Historically, the backbone of these regimens was pegylated interferon (IFN) and ribavirin (RBV), which induced several severe treatment-related side effects (e.g., flu-like, fatigue, anemia, insomnia, nausea, depression, and irritability), making adherence to the medical regimen a significant challenge. New developments in antiviral therapy, beginning with protease inhibitors (i.e., “triple therapy”) have become the standard of care, yet introduced more complex dosing schedules, additional side effects, and the potential for viral resistance in the presence of sub-optimal medication adherence 4, 5. Given the treatment-related side effects and complex dosing regimen of the current antiviral treatments for HCV, patients’ capacities to take multiple medications as prescribed, as well as their abilities to persist to the end of the treatment course, is paramount to treatment success.
Previous research has demonstrated that HCV patients need to be maintained on at least 80% of IFN and 80% of ribavirin, for at least 80% of the treatment duration (24 or 48 weeks) to maximize their chance of achieving a sustained virological response (SVR) (i.e., “cure”) 6. However, medically-necessary deviations, such as dose reductions and premature treatment discontinuations due to dangerous side effects, and patient-initiated deviations, such as missing doses or stopping treatment early due to unpleasant, but not life-threatening side effects, can interfere with achievement of the 80/80/80 standard and attenuate the chance of cure. Of the patient-driven deviations, how well patients take their medications as prescribed (referred to as “medication adherence” or “execution of dosing” in the broader health behavior literature) can affect treatment success 4, 7–9. In addition, persistence, or the total duration of time a patient takes their medication from first to last dose, can be significantly shortened. Patients may discontinue treatment earlier than recommended due to factors, such as intolerance to unpleasant side effects, noncompliance with treatment protocols, dropout, and patient preference 4, 7, 8.
The extent to which patients miss doses (i.e., medication nonadherence) of IFN/RBV during HCV treatment has been examined in only a handful of studies using self-report, pharmacy refill data, pill counts, and electronic monitoring caps 6, 10–16. Collectively, these studies show that (a) patients miss doses, particularly the twice daily oral RBV tablets; (b) the proportion of missed doses increases over time; and (c) missed doses are associated with worse virological response to treatment. Less is known about nonpersistence on IFN/RBV treatment, defined as patient-driven premature treatment discontinuations. A recent study identified several unmodifiable sociodemographic characteristics (e.g., age, race, education level, insurance status, marital status) associated with missing doses or nonpersistence during HCV treatment, highlighting specific patient cohorts that may benefit from additional support 16. However, it is important to identify additional predictors of missed doses and nonpersistence which may be modifiable through behavioral or psychological interventions.
Identifying predictors to explain how and why patients missed doses and do not persist on medication has relatively unstudied in the HCV population 4. Internal attitudes, such as self-efficacy or confidence in one’s ability to mobilize internal and/or external resources to engage in specific goal-directed behaviors, have been the focus of empirical and theoretical exploration across myriad health behaviors and medical conditions17, 18. However, self-efficacy’s relationship to missed doses and nonpersistence in the context of HCV treatment has yet to be explored 19. While self-efficacy’s relationship to missed doses and nonpersistence is largely unknown in the HCV population, its role in adherence to medications for other chronic disease treatment, including the HIV population, has been explored. From the HIV literature, higher levels of self-efficacy lead to fewer missed doses, lower depressive symptoms, increased problem-solving abilities, and improved patient-provider interactions during HIV treatment 20–22. Therefore, it is plausible that HCV patients who have higher levels of self-efficacy during HCV treatment will miss fewer doses than those with low self-efficacy. While no published studies have examined self-efficacy as a predictor of nonpersistence to HCV regimens, one might expect that higher levels of self-efficacy serve to enable individuals to persevere through an arduous and unpleasant treatment with multiple side effects, consistent with the self-efficacy construct and evidence found in the broader health behavior literature 23–26.
Study Aims
The present study is the first empirical analysis of self-efficacy and adherence to HCV antiviral therapy. This study’s aims were two-fold. First, we sought to identify relationships between patient characteristics and self-efficacy before and during HCV treatment. We were interested specifically in self-efficacy’s relationship with other sociodemographic characteristics found previously in the literature to predict missed doses and nonpersistence 16. We were also interested in potential interactions between depression and self-efficacy, since this relationship exists in the literature in other chronic conditions and patients with HCV commonly suffer from premorbid or treatment-induced depression 27, 28. Second, we sought to determine whether self-efficacy predicted missed doses or nonpersistence during HCV treatment. To further expand on this second aim, we examined interactions between self-efficacy and age, gender, and depressive symptomatology, as evidence in the HIV literature suggests that relationships among these factors may affect self-management behaviors necessary to adhere to treatment regimens 29–31.
Materials and Methods
Design and Participants
The present study is a secondary data analysis of data from the NIH-funded Viral Resistance to Antiviral Therapy of Chronic Hepatitis C (Virahep-C) study. Virahep-C was a multicenter, prospective, longitudinal study designed to evaluate factors that may explain racial disparities in SVR rates 11. The study enrolled African American (n=196) and Caucasian (n=205) patients with HCV genotype 1 across eight U.S. medical centers. All participants were new to HCV treatment. Virahep-C had many exclusion criteria, but included: (a) severe psychiatric disorders within the past 6 months including severe depression, schizophrenia, bipolar illness, obsessive-compulsive disorder, severe anxiety, or personality disorder; (b) psychiatric hospitalization or suicide attempt within last 5 years; and (c) evidence of substance abuse (drugs or alcohol) in past 6 months. Participants engaged in a protocol-based treatment that consisted of weekly self-injections of IFN and twice daily dosed RBV tablets. All patients were treated for at least 24 weeks. Per study protocol, participants with undetectable HCV viral load at week 24 of treatment (“TW24 Responders”) were continued on treatment for an additional 24 weeks, while those with detectable viral load at week 24 were discontinued from treatment per the study protocol (“Nonresponders”). Specific guidelines regarding discontinuation of treatment are detailed in the Virahep-C protocol on file at the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository: https://www.niddkrepository.org/static/studies/virahep-c/protocol/VirahepC_Protocol.pdf. Since the goal of Virahep-C was to investigate factors associated with treatment efficacy, maximizing adherence to the treatment protocol was essential. Therefore, an extensive Patient Education and Adherence Program was administered throughout the study to encourage medication adherence and side effect management for all participants. This program used structured interactions with study personnel to help participants enhance self-management for the duration of the study protocol. Virahep-C participants provided written informed consent and the study was approved by the respective Institutional Review Boards of participating research sites. Complete details about the Virahep-C study can be found at https://www.niddkrepository.org/studies/virahep-c/.
Measures
Baseline Sociodemographic Characteristics
In the present study, sociodemographic variables of interest included age, gender, race, marital status, education level, employment status, and health insurance status.
HCV Treatment Self-efficacy Survey
The original self-efficacy instrument used in Virahep-C was a 24-item measure developed for the Adult AIDS Clinical Trials Group (AACTG), which was modified for use with individuals undergoing HCV treatment. A recent factor analysis of the instrument led to refining the measure into a 17-item scale that yielded a Global Self-Efficacy scale (GSE) and four self-efficacy subscales: (a) communication SE (i.e., confidence to communicate with healthcare provider); (b) physical coping SE (i.e., confidence to cope with physical side effects of treatment); (c) psychological coping SE (i.e., confidence to cope with emotional side effects of treatment); and (d) treatment adherence SE (i.e., confidence to take all medication as prescribed and attend doctor visits) (See Table 1)19. The revised HCV Treatment Self-Efficacy Survey demonstrated good reliability for the GSE and the four subscales (Cronbach’s α range = .85 to .96) and good discriminant validity with other psychosocial measures 19. Higher scores on each scale indicate higher levels of self-efficacy. In the Virahep-C protocol, self-efficacy was measured only at baseline and treatment week 24.
Table 1.
The Hepatitis C Treatment Self-Efficacy Survey
| Question Stem: How confident are you that you can… |
|---|
Domain 1: Communication Self-Efficacy
|
Domain 2: Physical Coping Self-Efficacy
|
Domain 3: Psychological Coping Self-Efficacy
|
Domain 4: Treatment Adherence Self-Efficacy
|
Note: Responses range from 0 (Cannot do at all) to 10 (Certain to Do). Global Self-Efficacy and sub-scale scores are calculated by averaging the items. Higher scores indicate higher levels of self-efficacy. Reprinted with permission from Bonner, JE, Esserman, D., and Evon, DM. Reliability and validity of a self-efficacy instrument for hepatitis C antiviral treatment regimens. J Viral Hepat 2012 May; 19(5):316-26.
Depression
Depression was measured using the Center for Epidemiologic Studies–Depression (CES-D) scale, a 20-item self-report measure 32. Items range from 0 (never) to 3 (almost always), with total scores ranging from 0 to 60. Higher scores indicate more depressive symptomatology. In the present study, accepted cutoffs of <16 (no depressive symptomatology, referred to as “No Depression” or “None”), 16–22 (possible depression, referred to as “Mild-to-Moderate”), and ≥ 23 (probable depression, referred to as “Severe”) were used for categorical comparisons and analyses 28, 32. The CES-D was measured at multiple time points during the Virahep-C study, but for these analyses, we focused on measurement conducted at baseline and treatment week 24, concurrent with measurement of self-efficacy.
Adherence
The present study focused on two patient-driven deviations from the prescribed HCV treatment protocol: missed doses and treatment nonpersistence.
Missed Doses
While HCV treatment includes weekly injections of IFN and daily oral RBV, we chose to focus only on RBV in the present study. Previous research indicates that (a) patients miss a greater proportion of daily RBV compared to weekly IFN injections; (b) HCV treatment regimens currently in development are all-oral, IFN-free that includes combinations with RBV; and (c) decrements in RBV exposure, rather than IFN, may play a more critical role in nonresponse to HCV treatment in individuals naïve to treatment 5, 10, 12, 33. Missed doses of RBV were measured using Medication Event Management System (MEMS) caps (AARDEX Group Ltd., Switzerland). MEMS caps use a computer chip in the cap of a medication vial to record the precise date and time the vial was opened and presumably, when the medication was taken. When used properly, MEMS has been shown to be a valid, sensitive, specific representation of dose-taking behavior 16. MEMs data were downloaded at each study visit by study coordinators. Patients were fully trained in the correct use of the MEMS caps at baseline and throughout the study. Missed doses were defined as deviations from the number of RBV doses prescribed on a daily basis (0,1, or 2 doses taken by the participant out of a total number of doses prescribed [up to 2 doses of RBV per day]) based on MEMS cap openings 16. If a participant was instructed by a provider to discontinue RBV for a period of time (or change the number of doses), and they did not open the MEMS cap during the appropriate periods of time, they were considered adherent to the study protocol (i.e., it was not counted as a missed dose). Time 0 (first day of treatment) was excluded for all participants. In the present study, we analyzed missed RBV doses during two time periods: baseline to week 24 (n=384) and week 24 to 48 in TW24 Responders only (n=170).
Nonpersistence
Nonpersistence was defined as the time to patient-driven premature study or medication discontinuation at any time during the study, excluding virological nonresponse to treatment 16. Reasons for patient-driven discontinuation of study or medications included: intolerance to side effects; patient preference; nonadherence to study protocol; drop out (withdrawal); or refusal 16. Medically (rather than patient)-driven treatment discontinuations due to life-threatening lab abnormalities or medical conditions were censored from these analyses. In the present study, we analyzed nonpersistence events during two time periods: baseline to week 24 (n=384) and week 24 to 48 in TW24 Responders only (n=170).
Statistical Analysis
Descriptive statistics included the median and interquartile range (IQR) for the baseline and TW24 measures of self-efficacy. Differences in the distribution of GSE score across categorical patient characteristics were assessed using a Wilcoxon rank sum (2 categories) or Kruskal-Wallis (3 categories) test, as appropriate. Post-hoc comparisons of Kruskal-Wallis tests were adjusted using the Bonferroni method. Spearman’s rank correlation was used to examine relationships between continuous patient characteristics (e.g., CES-D) and GSE. Non-parametric methods were used due to the non-normal distribution of GSE scores (i.e., GSE scores tended to be negatively skewed).
The primary exposure of interest for both measures of adherence was the GSE score. We were interested in both the main effect on missed doses and nonpersistence, as well the possible effect of the interactions of GSE and age, gender, and CES-D score. We used baseline GSE (n=384) to predict missed doses and nonpersistence from baseline to week 24, and TW24-GSE to predict missed doses and nonpersistence among TW24 Responders (n=170) from weeks 24 to 48. For reasons unknown, 5 participants had missing baseline self-efficacy measures, and 71 participants who were TW24 Responders had missing TW24 self-efficacy data. These participants were not included in this study’s TW24 analyses. We compared baseline patient characteristics and self-efficacy scores between those with (n=165) and without (n=71) TW24 self-efficacy to determine if any differences existed. Patients who were missing TW24 self-efficacy data had higher baseline GSE scores, higher physical coping and psychological coping self-efficacy scores, and lower baseline CES-D scores. However, these CES-D scores (for both those with and without TW24 GSE data) were mostly within the no depression (CES-D < 16) range.
Predicting Missed Doses
Generalized estimating equations (GEE) with a multinomial distribution and cumlogit link function were used to estimate odds ratios (OR) and 95% confidence intervals (CI) of missing more RBV doses (i.e., 2 doses missed vs. 1 and 0 doses missed, and 1 and 2 doses missed vs. 0 doses missed). Both unadjusted models and models adjusted for age, race, gender, insurance status, marital status, employment status, and CES-D (baseline CES-D for baseline to week 24, and week 24 CES-D for weeks 24 to 48) were run for the baseline sample and the TW24 Responders. These covariates were selected a priori based on the empirical literature of factors related to missed doses/nonpersistence in HCV 16, as well as factors that predict or confound the relationship with medication adherence to HIV treatment 29–31, 34, 35.
Predicting Nonpersistence
A Cox proportional hazards model was used to estimate hazard ratios (HR) for time to nonpersistence. Due to the small number of nonpersistence events from treatment weeks 24 to 48 (n=13), we were only able to analyze the baseline to week 24 data. Models were adjusted for the same a priori covariates as selected for missed doses analyses.
Statistical significance was established at 0.05 for main effects and 0.10 for interaction effects. In addition, using the same methodology, we analyzed the four self-efficacy subscales as predictors of missed doses and nonpersistence. No formal corrections were made for multiple testing of these subscales, as these were only exploratory in nature; however, we were conservative in our interpretation of these findings. All analyses were carried out using SAS version 9.2 (Cary, NC).
Results
Patient Characteristics Associated with Self-Efficacy
Patient characteristics of the full Virahep-C (n=401) study cohort are described elsewhere 11. The overall sample’s baseline median GSE score was 8.7 out of 10 (IQR: 7.8–9.5) and TW24 median GSE was 8.3 (IQR: 7.0–9.3). The distribution of GSE scores across patient characteristics for baseline and TW24 are summarized in Table 2. Three-way post-hoc comparisons revealed that participants with higher baseline self-efficacy scores were in a relationship and privately insured (p’s<0.001). Among TW24 Responders, GSE at TW24 was higher among those married/partnered compared to divorced/widowed/separated (p<0.001) and privately insured compared to publicly insured individuals (p=0.004).
Table 2.
Patient Characteristics by Global Self-Efficacy Score at Baseline and Treatment Week 24.
| Characteristic | Baseline Global Self-Efficacy Score (N=384) | Week 24 Global Self-Efficacy Score (N=170) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | % | Median (IQR) | p-value | n | % | Median (IQR) | p-value | |
| Gender | 0.079 | 0.316 | ||||||
| Male | 248 | 66 | 8.8 (7.7–9.6) | 101 | 59 | 8.4 (7.1–9.4) | ||
| Female | 136 | 34 | 8.5 (7.8–9.3) | 69 | 41 | 8.1 (6.7–9.2) | ||
| Race | 0.768 | 0.393 | ||||||
| AA | 183 | 48 | 8.7 (7.8–9.5) | 65 | 38 | 8.4 (7.2–9.4) | ||
| CA | 201 | 52 | 8.7 (7.7–9.4) | 105 | 62 | 8.2 (6.7–9.2) | ||
| Marital Status* | <0.001 | 0.003 | ||||||
| Married/Relationship | 186 | 48 | 9.0 (8.1–9.7) | 80 | 48 | 8.4 (7.7–9.5) | ||
| Never Married | 71 | 19 | 8.2 (7.5–9.2) | 29 | 18 | 8.2 (7.1–9.1) | ||
| Divorced/Widow/Separated | 127 | 33 | 8.6 (7.5–9.2) | 57 | 34 | 7.5 (6.5–9.1) | ||
| Education Level* | 0.043 | 0.461 | ||||||
| < High School | 69 | 18 | 8.4 (7.4–9.4) | 30 | 18 | 7.7 (6.6–9.4) | ||
| ≥ High School | 314 | 82 | 8.8 (7.9–9.5) | 135 | 82 | 8.3 (7.0–9.2) | ||
| Employment Status* | <0.001 | <0.001 | ||||||
| Employed | 245 | 64 | 8.9 (8.2–9.6) | 104 | 63 | 8.5 (7.4–9.4) | ||
| Unemployed | 139 | 36 | 8.3 (7.6–9.3) | 62 | 37 | 7.5 (6.5–8.5) | ||
| Insurance* | <0.001 | 0.028 | ||||||
| Private | 230 | 60 | 8.9 (8.2–9.6) | 90 | 55 | 8.5 (7.4–9.4) | ||
| Public | 85 | 22 | 8.2 (7.5–9.2) | 34 | 21 | 7.4 (6.5–8.4) | ||
| Uninsured | 67 | 18 | 8.4 (7.3–9.1) | 40 | 24 | 8.1 (6.6–9.0) | ||
Note: IQR: Interquartile Range. Self-efficacy scores range from 0–10, with higher scores indicative of higher self-efficacy.
Sample sizes may not sum to overall N. Three level post-hoc pairwise comparisons analyzed using Kruskal-Wallis test with a Bonferroni correction for multiple comparisons set at 0.05/3=0.0167.
Self-Efficacy and Depressive Symptomatology
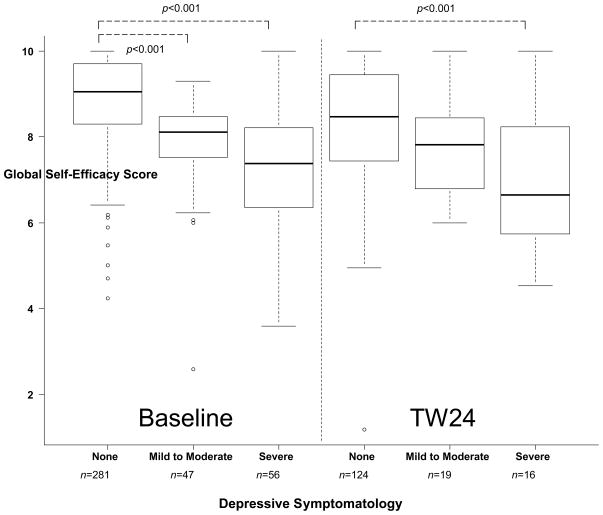
GSE at baseline was higher among those with no depressive symptoms compared to those with mild-to-moderate (p<0.001) or severe (p<0.001) symptoms (Figure 1). The difference in baseline GSE between mild-to-moderate and severe depressive symptoms at baseline did not reach statistical significance (p=0.0168) due to Bonferonni correction for multiple comparisons (α=0.0167). Likewise, differences at TW24 between no depressive symptoms and mild-to-moderate (p=0.057), and mild-to-moderate to severe (p=0.052) did not reach statistical significance after Bonferonni correction, although TW24 GSE was higher in those with no depressive symptoms at TW24 compared to those with severe depressive symptoms (p<0.001). Correlational analyses revealed a negative relationship between baseline depressive symptoms (continuous CES-D score) and the baseline GSE score (rs= − 0.62, p<0.001, n=384). This relationship was similar between TW24 CES-D and TW24 GSE (rs= − 0.50, p<0.001, n=165).
Figure 1.
Comparison of Global Self-Efficacy (GSE) Scores by Level of Depressive Symptomatology at Baseline (left panel, N=384) and Treatment Week 24 (right panel, N=165). Significance bars represent pairwise comparisons using Kruskal-Wallis test with a Bonferroni correction for multiple comparisons set at 0.05/3=0.0167. Among baseline participants, GSE was highest among those with no depressive symptomatology at baseline when compared to participants with mild-to-moderate (p<0.001) and severe (p<0.001) depressive symptoms, respectively. Among participants who continued on treatment from week 24 to 48, GSE at treatment week 24 was higher among those with no depressive symptoms when compared with those with severe depressive symptoms (p<0.001).
Self-Efficacy and Missed Doses
Missed doses of RBV increased over time: on average, participants missed 15% of doses by TW24 and 25% of doses by treatment week 48 (TW48). As noted in Table 3, there was no association between GSE and missed doses of RBV, either from baseline to TW24 or among TW24 Responders from week 24 to 48. In exploratory analyses of the four subscales, we only found statistically significant interactions for TW24 communication SE among Responders from TW24 to TW48. Specifically, interactions between TW24 communication SE and gender (p=0.001) and CES-D (p=0.024) were associated with missing doses of RBV, even after adjusting for age, race, insurance status, marital status, and employment status. That is, after controlling for all other covariates, as communication SE increased, the odds of missing doses increased at a higher rate for women (OR=45.4; 95% CI: 4.11, 501.6) compared to men (OR=1.41; 95% CI: 0.92, 2.16). Due to the lack of precision in this finding (e.g., CI width of almost 500 for women), we give little weight to these point estimates. Similarly, the association between higher communication SE and missing fewer doses was greater in severely depressed individuals (e.g., CES-D≥23; OR=0.13; 95% CI: 0.01, 1.16) compared to mild to moderately depressed individuals (e.g. CES-D=16–22; OR=0.27; 95% CI: 0.05, 1.32).
Table 3.
Self-Efficacy at Baseline and Treatment Week 24 as a Predictor of Ribavirin Missed Doses
| Baseline to Treatment Week 24 (N=384) | Treatment Week 24 to Treatment Week 48 (N=165) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusteda | Adjustedb | Unadjustedc | Adjustedd | ||||||||||||
|
| |||||||||||||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||||||||
| Global Self-Efficacy | 0.98 (0.86, 1.11) | 0.749 | 1.01 (0.86, 1.17) | 0.926 | 0.95 (0.86, 1.12) | 0.799 | 1.00 (0.80, 1.26) | 0.999 | |||||||
| Communication Self-Efficacy† | 0.95 (0.86, 1.10) | 0.788 | 0.99 (0.88, 1.11) | 0.856 | 0.95 (0.84, 1.07 | 0.400 | |||||||||
| Male | 1.41 (0.92, 2.16 | 1.11 | |||||||||||||
| Female | 45.4 (4.11, 501.6) | 0.002 | |||||||||||||
| CESD=16 | 0.27 (0.05, 1.32) | 0.105 | |||||||||||||
| CESD=23 | 0.13 (0.01, 1.16) | 0.067 | |||||||||||||
| Physical Coping Self-Efficacy | 1.00 (0.93, 1.08) | 0.952 | 1.01 (0.93, 1.10) | 0.744 | 1.01 (0.93, 1.10) | 0.744 | 1.02 (0.90, 1.16) | 0.754 | |||||||
| Psychological Coping Self-Efficacy | 1.02 (0.93, 1.11) | 0.744 | 1.05 (0.94, 1.18) | 0.420 | 1.00 (0.91, 1.11) | 0.936 | 1.04 (0.88, 1.22) | 0.659 | |||||||
| Adherence Self-Efficacy | 0.90 (0.79, 1.03) | 0.170 | 0.92 (0.81, 1.05) | 0.255 | 0.91 (0.77, 1.07) | 0.275 | 0.94 (0.79, 1.11) | 0.452 | |||||||
Note: OR: Odds Ratio; CI: Confidence Interval.
includes Baseline measures only;
adjusted for age, race, gender, insurance status, marital status, employment status, and baseline depression (CESD) score;
Treatment Week 24 Responders only;
Treatment Week 24 measures;
adjusted for age, race gender, insurance status, marital status, employment status, treatment week 24 CESD score;
Treatment Week 24 (Adjusted) interactions between Communication Self-Efficacy and (1) gender, and (2) CESD (Nondepressed versus high) were significant at p<.10 level.
Self-Efficacy and Nonpersistence
Although it appeared that GSE and three of the four subscales were associated with nonpersistence from baseline to week 24, only the relationship between nonpersistence and the treatment adherence SE subscale remained statistically significant (p=0.013) after adjusting for covariates (Table 4). This finding suggested that risk of nonpersistence was lower among participants who had higher levels of adherence self-efficacy at baseline. Although not statistically significant after adjusting for covariates, the relationship between baseline GSE and nonpersistence approached statistical significance (p=0.078).
Table 4.
Baseline Self-Efficacy as a Predictor of Ribavirin Nonpersistence
| Baseline to Treatment Week 24 | ||||
|---|---|---|---|---|
|
| ||||
| Unadjusted (N=384) | Adjusteda (N=384) | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Global Self-Efficacy | 0.73 (0.59, 0.90) | 0.004 | 0.77 (0.58, 1.03) | 0.078 |
| Communication Self-Efficacy | 0.83 (0.70, 0.98) | 0.026 | 0.87 (0.73, 1.04) | 0.125 |
| Physical Coping Self-Efficacy | 0.86 (0.75. 0.98) | 0.026 | 0.93 (0.79, 1.09) | 0.380 |
| Psychological Coping Self-Efficacy | 0.88 (0.75, 1.03) | 0.107 | 0.97 (0.79, 1.18) | 0.745 |
| Adherence Self-Efficacy | 0.77 (0.65, 0.91) | 0.002 | 0.78 (0.63, 0.95) | 0.013 |
Note: HR: Hazard Ratio, calculated using Cox Proportional Hazards Model. CI: Confidence Interval.
Model adjusted for age, race, gender, employment status, marital status and baseline depressive symptomatology. Interpretation: when HR < 1, higher self-efficacy was associated with a lower risk of ribavirin nonpersistence.
Discussion
When patients deviate from the prescribed treatment regimens for HCV, such as missing doses of medication or failing to persist to the end of the treatment course, treatment response and cure rates are comprised 4, 6, 10, 36. Research into understanding which patient factors may be related to and possibly influence missed doses and nonpersistence during HCV treatment is in its infancy 4, 16. The present study sought to address this gap by exploring the relationship between the well-known construct of self-efficacy, and missed doses (i.e., “medication adherence”) and nonpersistence, which while investigated in other medical populations and treatments, had not been applied in the setting of HCV treatment.
In this large prospective study sample, participants’ overall confidence in their ability to undergo HCV treatment was fairly high, however, higher self-efficacy ratings were found among certain subgroups, such as those who were in a relationship, better educated, employed, or privately insured. Considerable differences were noted between level of depressive symptoms and global self-efficacy. Participants with greater depressive symptoms at baseline tended to be less confident in their ability to engage in HCV treatment. This was true at baseline and for TW24 Responders. This relationship is clinically relevant, first because depressive symptoms are common among patients diagnosed with HCV, are induced by IFN treatment, and can be related to worse treatment outcomes 27, 28, 37. Second, among patients with HIV, depressive symptoms predict both lower adherence self-efficacy and self-reported medication adherence, and the prediction of self-reported adherence by depressive symptoms has been shown to be at least partially mediated by adherence self-efficacy beliefs. 31, 38. In the parent study, researchers were blinded to both CES-D and GSE scores throughout the study, and so could not have acted upon these differences. However, these findings suggest that the relationship between self-efficacy, depression, and adherence in HCV may be a critical target for future clinical and research investigation to help in the clinical management of patients’ medication adherence and ability to persevere to the end of treatment.
Contrary to our expectations, neither baseline nor TW24 GSE predicted missed RBV doses. There are several possible reasons for the lack of association between GSE and missed doses, but perhaps the most salient is the purpose and study design of the parent study. The Patient Education and Adherence intervention was delivered to all participants from baseline to the end of treatment and may have bolstered patients’ confidence to ensure optimal adherence. Also, patients across the board had high levels of GSE at baseline and week 24, suggesting the possibility of a ceiling effect. Perhaps the high self-efficacy scores were due to fairly rigorous exclusion criteria and patient selection for those who were judged most likely to adhere to the treatment protocol. Finally, it may simply be that confidence in one’s ability to undergo HCV treatment is unrelated to missing doses in HCV, although this would be inconsistent with studies in HIV and other chronic illnesses which have found a stable, prospective relationship between self-efficacy and medication adherence 22, 31, 38. Interestingly, among TW24 Responders, higher confidence in one’s ability to communicate, discuss issues, and work out difficulties with clinicians served as a protective factor against missing RBV doses in those individuals who experienced severe depressive symptoms at TW24. This finding suggests that even among individuals experiencing significant depressive symptoms, greater confidence in one’s ability to communicate with a medical provider could buffer patients from poorer outcomes during HCV treatment.
Only baseline treatment adherence self-efficacy predicted nonpersistence between baseline and treatment week 24. Individuals who reported greater confidence in taking their medications exactly as prescribed, attending follow-up clinic visits, and doing so despite feeling fatigued or depressed, were less likely to drop out of treatment early 4. These findings suggest that clinicians should consider monitoring adherence self-efficacy and intervening when appropriate to bolster confidence levels to help patients persevere through a difficult treatment course.
Strengths of the present study include a large sample size, rigorous prospective study design, measurement of medication adherence using objective electronic monitoring technology, and use of an psychometrically sound measure of HCV treatment self-efficacy 19. However, a few limitations are noted. First, the nature of the Virahep-C study, including its stringent exclusion criteria, may have excluded individuals with lower baseline levels of self-efficacy (e.g., those with severe psychiatric comorbidities, alcohol or drug dependence, social instability, etc.). A second limitation was the missing TW24 self-efficacy data among Responders which may have affected TW24 analyses of missed doses and nonpersistence between TW24 and TW48. As these Responders had higher levels of baseline GSE, it is possible that their TW24 scores could have affected the TW24 analyses. Third, the lack of power due to the low number of nonpersistence events that occurred during the study (n=39) may have precluded detecting statistically significant associations between self-efficacy and nonpersistence. Moreover, we were unable to examine nonpersistence from weeks 24 to 48 due to too few events. The Patient Education and Adherence intervention that was delivered to all participants before and during treatment may have improved medication adherence and persistence during the study in all patients, limiting the number of missed doses and nonpersistence events and perhaps reducing potential variability in self-efficacy scores. Unfortunately, the study was not designed with a comparison condition to evaluate the benefits of such an intervention on patient self-efficacy, dose-taking behavior, or persistence.
Despite these limitations, the present study is the first empirical investigation of self-efficacy during HCV antiviral treatment. In light of the present study’s findings that aspects of self-efficacy likely influence aspects of HCV treatment adherence, particularly among certain subgroups, there appears to be a fertile ground for future clinical and research endeavors to elucidate self-efficacy’s role during HCV treatment. Because self-efficacy is a behavioral construct that research has shown can be enhanced through interventions, and can improve health behaviors when enhanced, it is a particularly useful target for improving clinical outcomes. Future empirical investigations should be more inclusive of “real world” HCV patients, many of whom have mental health and/or substance abuse issues which likely impacts one’s level of self-efficacy 4, 39. These studies, within both rigorous trial settings and real world clinical settings, need to investigate whether: (a) self-efficacy predicts missed doses and nonpersistence, and (b) whether tailored interventions can bolster self-efficacy or reduce depression and in turn improve medication adherence and health outcomes, as shown in HIV populations 31. Future investigations should consider examining self-efficacy within the framework of an overarching health behavior model, to advance the understanding of how intrapersonal factors, including self-efficacy, may enable patient’s to adhere to a difficult treatment regimen17, 40. Lastly, future research should examine the role of self-efficacy within the context of the newer, more complex HCV treatment regimens which require greater precision in dosing and introduce the possibility of viral resistance, which could occur if patient’s drop out of treatment too soon or fail to take their medication appropriately.
Conclusions
Personal, highly self-efficacious beliefs have been well-demonstrated in the broader health behavior literature as playing an important role in how patient’s pursue goal-oriented health behaviors. While the importance of self-efficacy to taking medications has been demonstrated in other patient populations, self-efficacy’s role in taking HCV medications and persisting on difficult antiviral treatment had not been investigated. The findings of this study provide researchers and clinicians initial insights into self-efficacy’s relationship with missed doses and nonpersistence during HCV treatment. This study highlights that patient selection and access to adherence-enhancing strategies may influence study results, and that, at least for HCV treatment, self-efficacy may not be as important to dose-taking behaviors, as it is to persistence on treatment. These initial findings lay the groundwork for further investigations into the relationship between self-efficacy and adherence to HCV treatment.
Acknowledgments
Financial Support: This study was supported, in part, by the UNC Clinical and Translational Science Award (UL1RR025747; Bonner & Esserman); a Center for Aids Research grant (P30-AI50410; Golin); and by the National Institutes of Health Award (K23DK089004-02; Evon).
The VIRAHEP-C study was conducted by multiple investigators and supported by the National Institute of Digestive and Kidney Diseases (NIDDK). This manuscript was not prepared in collaboration with the investigators of Virahep-C and does not necessarily reflect the opinions or views of the Virahep-C study or the NIDDK. The authors would like to thank the Virahep-C investigators, coordinators, and study participants who contributed to Virahep-C.
Footnotes
Conflict of Interest Statement: Donna M. Evon received research grant support from Roche, and served as an ad hoc consultant to Vertex in the past 12 months. All other authors have nothing to disclose.
References
- 1.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006 May 16;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011 Sep;31(8):1090–101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 3.Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010 Feb;138(2):513–21. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 4.Evon DM, Golin CE, Fried MW, et al. Chronic Hepatitis C and Antiviral Treatment Regimens: Where Can Psychology Contribute? J Consult Clin Psychol. 2012 Jun 25; doi: 10.1037/a0029030. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsch C, Jesudian A, Zeuzem S, et al. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut. 2012 May;61( Suppl 1):i36–i46. doi: 10.1136/gutjnl-2012-302144. [DOI] [PubMed] [Google Scholar]
- 6.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002 Oct;123(4):1061–9. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 7.Urquhart J, Vrijens B. New findings about patient adherence to prescribed drug dosing regimens: An introduction to pharmionics. The European Journal of Hospital Pharmacy Science. 2005;11(5):103–106. [Google Scholar]
- 8.Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008 May 17;336(7653):1114–7. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008 Jan;11(1):44–7. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 10.Alam I, Stainbrook T, Cecil B, et al. Enhanced adherence to HCV therapy with higher dose ribavirin formulation: final analyses from the ADHERE registry. Aliment Pharmacol Ther. 2010 Aug;32(4):535–42. doi: 10.1111/j.1365-2036.2010.04381.x. [DOI] [PubMed] [Google Scholar]
- 11.Conjeevaram HS, Fried MW, Jeffers LJ, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006 Aug;131(2):470–7. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Lo RV, III, Amorosa VK, Localio AR, et al. Adherence to hepatitis C virus therapy and early virologic outcomes. Clin Infect Dis. 2009 Jan 15;48(2):186–93. doi: 10.1086/595685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcellin P, Chousterman M, Fontanges T, et al. Adherence to treatment and quality of life during hepatitis C therapy: a prospective, real-life, observational study. Liver Int. 2011 Apr;31(4):516–24. doi: 10.1111/j.1478-3231.2011.02461.x. [DOI] [PubMed] [Google Scholar]
- 14.Weiss JJ, Bhatti L, Dieterich DT, et al. Hepatitis C patients’ self-reported adherence to treatment with pegylated interferon and ribavirin. Aliment Pharmacol Ther. 2008 Aug 1;28(3):289–93. doi: 10.1111/j.1365-2036.2008.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SR, Wahed AS, Kelley SS, et al. Assessing the validity of self-reported medication adherence in hepatitis C treatment. Ann Pharmacother. 2007 Jul;41(7):1116–23. doi: 10.1345/aph.1K024. [DOI] [PubMed] [Google Scholar]
- 16.Evon DM, Esserman DA, Bonner JE, et al. Adherence to PEG/ribavirin treatment for chronic hepatitis C: prevalence, patterns, and predictors of missed doses and nonpersistence. Journal of Viral Hepatitis. 2013 Aug;20:536–549. doi: 10.1111/jvh.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarzer R, Lippke S, Luszczynska A. Mechanisms of health behavior change in persons with chronic illness or disability: The health action process approach (HAPA) Rehabil Psychol. 2011 Aug;56(3):161–70. doi: 10.1037/a0024509. [DOI] [PubMed] [Google Scholar]
- 18.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004 Apr;31(2):143–64. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 19.Bonner JE, Esserman D, Evon DM. Reliability and validity of a self-efficacy instrument for hepatitis C antiviral treatment regimens. J Viral Hepat. 2012 May;19(5):316–26. doi: 10.1111/j.1365-2893.2011.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds NR, Testa MA, Marc LG, et al. Factors influencing medication adherence beliefs and self-efficacy in persons naive to antiretroviral therapy: a multicenter, cross-sectional study. AIDS Behav. 2004 Jun;8(2):141–50. doi: 10.1023/B:AIBE.0000030245.52406.bb. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MO, Chesney MA, Goldstein RB, et al. Positive provider interactions, adherence self-efficacy, and adherence to antiretroviral medications among HIV-infected adults: A mediation model. AIDS Patient Care STDS. 2006 Apr;20(4):258–68. doi: 10.1089/apc.2006.20.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson MO, Neilands TB, Dilworth SE, et al. The role of self-efficacy in HIV treatment adherence: validation of the HIV Treatment Adherence Self-Efficacy Scale (HIV-ASES) J Behav Med. 2007 Oct;30(5):359–70. doi: 10.1007/s10865-007-9118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandura A. Self-Efficacy: The Exercise of Control. New York: W. H. Freeman and Company; 1997. [Google Scholar]
- 24.Schwarzer R. Self-Efficacy in the Adoption and Maintenance of Health Behaviors: Theoretical Approaches and a New Model. In: Schwarzer R, editor. Self-Efficacy: Thought Control of Action. London, England: Hemisphere Publishing Corporation; 1992. [Google Scholar]
- 25.Schwarzer R. Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. Applied Psychology: An International Review. 2008;57:1–29. [Google Scholar]
- 26.Conner M. Initiation and maintenance of health behaviors. Applied Psychology: An International Review. 2008;57:42–50. [Google Scholar]
- 27.Evon DM, Simpson KM, Esserman D, et al. Barriers to accessing care in patients with chronic hepatitis C: the impact of depression. Aliment Pharmacol Ther. 2010 Nov;32(9):1163–73. doi: 10.1111/j.1365-2036.2010.04460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evon DM, Ramcharran D, Belle SH, et al. Prospective Analysis of Depression During Peginterferon and Ribavirin Therapy of Chronic Hepatitis C: Results of the VIRAHEP-C Study. American Journal of Gastroenterology. 2009 Sep 29;104:2949–58. doi: 10.1038/ajg.2009.528. [DOI] [PubMed] [Google Scholar]
- 29.Berg KM, Demas PA, Howard AA, et al. Gender differences in factors associated with adherence to antiretroviral therapy. J Gen Intern Med. 2004 Nov;19(11):1111–7. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barclay TR, Hinkin CH, Castellon SA, et al. Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology. 2007 Jan;26(1):40–9. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cha E, Erlen JA, Kim KH, et al. Mediating roles of medication-taking self-efficacy and depressive symptoms on self-reported medication adherence in persons with HIV: a questionnaire survey. Int J Nurs Stud. 2008 Aug;45(8):1175–84. doi: 10.1016/j.ijnurstu.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 33.van Vlerken LG, Huisman EJ, van SH, et al. Ribavirin rather than PEG-interferon pharmacodynamics predict nonresponse to antiviral therapy in naive chronic hepatitis C patients. J Viral Hepat. 2012 Jan;19(1):39–46. doi: 10.1111/j.1365-2893.2010.01399.x. [DOI] [PubMed] [Google Scholar]
- 34.Golin CE, Liu H, Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002 Oct;17(10):756–65. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bottonari KA, Tripathi SP, Fortney JC, et al. Correlates of antiretroviral and antidepressant adherence among depressed HIV-infected patients. AIDS Patient Care STDS. 2012 May;26(5):265–73. doi: 10.1089/apc.2011.0218. [DOI] [PubMed] [Google Scholar]
- 36.Lo RV, III, Teal V, Localio AR, et al. Relationship between adherence to hepatitis C virus therapy and virologic outcomes: a cohort study. Ann Intern Med. 2011 Sep 20;155(6):353–60. doi: 10.1059/0003-4819-155-6-201109200-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evon DM, Verma A, Dougherty KA, et al. High deferral rates and poorer treatment outcomes for HCV patients with psychiatric and substance use comorbidities. Dig Dis Sci. 2007 Nov;52(11):3251–8. doi: 10.1007/s10620-006-9669-0. [DOI] [PubMed] [Google Scholar]
- 38.Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychol. 2006 Jan;25(1):74–81. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonner JE, Barritt AS, Fried MW, et al. Time to rethink antiviral treatment for hepatitis C in patients with coexisting mental health/substance abuse issues. Dig Dis Sci. 2012 Jun;57(6):1469–74. doi: 10.1007/s10620-012-2141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lippke S, Ziegelmann JP. Theory-based health behavior change: Developing, testing, and applying theories for evidence-based interventions. Applied Psychology: An International Review. 2008;57(698):716. [Google Scholar]