Abstract
We examined event-related electroencephalography (EEG) oscillations, including event-related spectral perturbations (ERSP) and intertrial coherence (ITC), to compare feedback processing during a chance-based reward vs. non-reward task in groups of 10-12-year-old (n = 42), 13-14-year-old (n = 34) and 15-17-year-olds (n = 32). Because few, if any studies have applied these analytic methods to examine feedback processing in children or adolescents, we used a fine-grained approach that explored one half hertz by 16 ms increments during feedback (no win vs. win events) in the theta (4-8 Hz) frequency band. Complex wavelet frequency decomposition revealed that no win feedback was associated with enhanced theta power and phase coherence. We observed condition and age-based differences for both ERSP and ITC, with stronger effects for ITC. The transition from childhood to early adolescence (13-14 yrs.) was a point of increased differentiation of ITC favoring no win vs. wins feedback and also compared to children or older adolescents, a point of heightened ITC for no win feedback (quadratic effect).
Keywords: theta oscillations, reward, adolescence, event-related spectral analysis, inter-trial phase coherence
The adolescent developmental period paradoxically reflects a time of rapid increases in physical strength and decision-making capacity, yet is also a time of vulnerability related to increased risk-taking and novelty seeking behavior (Arnett, 1992; Dahl, 2004; DiClemente, Hansen, & Ponton, 1996). An influential neurobiological model of adolescent development suggests an imbalance between early developing emotion/reward-related brain regions and slower-to-mature cognitive control/decision-making regions (Casey, Getz, & Galvan, 2008; Yurgelun-Todd, 2007).
Contemporary decision making models propose that choices are guided by the respective value assigned to available options (Kahneman & Tversky, 1979), with the relative value computed in a system supported by the medial prefrontal cortex (mPFC) (Frank & Claus, 2006; O'Doherty, Dayan, Friston, Critchley, & Dolan, 2003; Pagnoni, Zink, Montague, & Berns, 2002; Pasupathy & Miller, 2005). As such, the interplay between expectancy, action and outcome-related feedback is central to reward learning models (Holroyd & Coles, 2002; Schultz & Dickinson, 2000). Such reinforcement learning relies on the use of both positive and negative performance feedback to adaptively guide behavior (Sutton & Barto, 1998). Phasic changes within the mesolimbic dopamine system are thought to encode reward prediction error signals that reflect the difference between actual and expected outcomes. Research has begun to specifically examine the role of prediction error signals in adolescent decision-making (Cohen et al., 2010). In particular, some recent work suggests increased functional connectivity of the ventral striatum and the mPFC from childhood through adolescence to adulthood, accompanied by a decrease in learning rate for negative prediction errors (van den Bos, Cohen, Kahnt, & Crone, 2012).
Electroencephalography (EEG) studies of reward processing and reinforcement learning mainly focus on the feedback related negativity (FRN), a mid-frontal event related potential (ERP) component peaking approximately 200-300ms post-stimulus. Holroyd and Coles’ (2002) reinforcement learning model postulates that the stimulus-locked FRN, and also the response locked error-related negativity (ERN), reflect activity emerging from a generic error processing system. According to a prevailing reinforcement learning model, FRN/ERN responses reflect a reward prediction error generated when transient dips in midbrain dopamine levels signal activation of disinhibitory neurons in the ACC (Holroyd & Coles, 2002). With regard to the FRN, both dipole and distributed source modeling studies indicate that the ACC and medial frontal cortical region are the main neural generators of the FRN (Gehring & Willoughby, 2002; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003).
Developmental EEG Studies of Reward Feedback
To date, the results of ERP studies on age-related feedback effects suggest that FRNs are generally greater in magnitude in younger groups (10-12 vs. 19-24 years, Eppinger, Mock, and Kray, 2009; 9-11 vs. 13-14, 30-40, 65-75 years, Hämmerer, Li, Müller, & Lindenberger, 2011; 10-12, 13-14 vs. 15-17 years, Crowley et al., in press; 14-17 vs. 22-26 years, Zottoli and Grose-Fifer, 2011). Some studies suggest greater differentiation of ERP responses for positive vs. negative outcomes across development (Hämmerer, Li, Müller, & Lindenberger, 2011; Zottoli and Grose-Fifer, 2011. There is also some evidence that FRN latency decreases from childhood through adolescence (Crowley et al., in press; Zottoli and Grose-Fifer, 2011). However, ERP studies have not documented any adolescent-specific reward processing changes at the level of the FRN.
An important issue that can affect the magnitude of the FRN across a task is whether or not the task involves learning. Müller and colleagues (Müller, Möller, Rodriguez-Fornells, & Münte, 2005) observed that as participants learn the mapping of choices and outcomes, they come to rely less on the external feedback and more on internal error awareness. In their age-based studies, Eppinger et al. (2009) and Hämmerer et al. (2011) speculate that in learning tasks, children show larger FRNs than do older groups due to a greater reliance on external feedback cues as opposed to internal representations of feedback emerging from learning. However, in a chance-based (non-learning) reward feedback task, in a large sample (n = 91), Crowley et al. (in press) observed reductions in FRN magnitude across 10 to 17 years. Thus, age differences in FRN amplitude, which do seem to be reliable, are not necessarily a function of learning. More recently, investigators have begun to look to EEG oscillations associated with reward feedback processing (Cavanagh, Frank, Klein, & Allen, 2010; Cavanagh, Zambrano-Vazquez, & Allen, 2012; Cohen, Elger, & Ranganath, 2007), but only in adult samples.
EEG Oscillatory Dynamics and Reward Processing
One important consideration regarding ERPs such as the FRN is that they are computed as the average signal across time-locked trials. Hence, ERPs inevitably only capture the stimulus- or response-driven partial phase alignment and power increases in the ongoing EEG brought about by the event (Le Van Quyen & Bragin, 2007; Sauseng et al., 2007). While this fixed-latency average amplitude approach has utility, it discards important information about task-relevant EEG oscillatory dynamics that may be important for interrogating the neurophysiology of reward processing (see Cohen, 2011). Using an approach broadly conceived as event-related brain dynamics (Makeig, Debener, Onton, & Delorme, 2004), advanced signal processing techniques such as short-time Fourier and wavelet transform can investigate the EEG signal in terms of frequency, power and phase. Importantly, characterizing oscillatory dynamics in this way probably more closely reflects the activity of underlying neuronal assemblies (Buzsáki, 2006).
Event time-locked frequency analyses of EEG allows for the measurement changes in EEG power and phase synchrony, across trials, on a millisecond time scale. In particular, event-related spectral perturbation (ERSP) is a temporally sensitive index of the relative change of mean EEG power from baseline associated with stimulus presentation or response execution. Unlike ERPs, ERSPs capture changes in spontaneous EEG activity that occurs across several frequency spectra and are sensitive to fluctuations that are temporally stable, but not coherent in phase angle (Makeig, 1993; Makeig et al., 2004). Although ERSPs are able to capture induced power changes, which are not revealed in typically averaged ERPs, they do not reveal details about the coherence in phase angle of the event-related EEG signals.
Inter-trial coherence (ITC) can be used to assess the extent to which EEG oscillations become phase aligned following feedback. Thus, ITC reflects the extent to which a specific task event (e.g., stimulus or response) generates changes in phase synchrony (or induces phase re-setting) of ongoing oscillations across frequency spectra. Analogous to a correlation coefficient, ITC values refer to the degree of association across trials, ranging from zero to one. ITC allows for the assessment of millisecond-to-millisecond fluctuations in partial phase synchrony induced by experimental events, independent of changes in EEG power (Makeig et al., 2004). ITC is assessed at a single location or region and thus reflects “temporal coherence,” to be distinguished from “spatial coherence” assessed across brain regions.
Converging evidence suggests that performance monitoring processes associated with activation of the medial frontal cortex are reflected in a common oscillatory substrate in the theta rhythm (4-8 Hz)(Cavanagh et al., 2010; Cavanagh, Zambrano-Vazquez, et al., 2012; Cohen et al., 2007). For instance, several groups have now documented greater theta power and phase coherence for loss feedback compared to gain feedback (Cavanagh, Zambrano-Vazquez, et al., 2012; Cohen, Elger, & Fell, 2009; Cohen et al., 2007; Marco-Pallares et al., 2008). Despite a growing body of work on the family of frontal oscillations, reward/feedback processing studies have only focused on adult samples.
The present study
Here we examine age differences in theta oscillations for reward versus non-reward feedback from middle childhood through adolescence across three groups of children, 10-12 years, 13-14 years, and 15-17 years. To our knowledge there are no published studies examining oscillatory aspects of reward feedback processing in children or adolescents. In reward feedback studies with adults, researchers typically average across the 4-8 Hz frequency range relying on a preselected temporal window reflecting visual inspection of a time/frequency plot or the peak amplitude of the ERP. Importantly, these decisions regarding a preselected frequency range and time window in averaged data may obscure the actual frequencies, timing and potential markers of subcomponent processes that reflect age differences in reward processing. We address this issue directly with a fine-grained examination of the theta band, moment by moment over the course of reward feedback processing. To this end we examined EEG oscillatory activity at 0.5 Hz increments and 16 ms time windows, relying on false discovery rate (FDR) methods to control for multiple comparisons. We draw on work in the field of functional magnetic resonance imaging (fMRI), where high-dimensional data create the potential for many statistical comparisons to be made and investigators have increasingly relied on FDR procedures (Benjamini & Hochberg, 1995; Benjamini, Krieger, & Yekutieli, 2006; Benjamini & Yekutieli, 2001) to control type I error rates. These approaches are just beginning to take hold in EEG research (Crowley, Wu, McCreary, Miller, & Mayes, 2012; Groppe, Urbach, & Kutas, 2011; Lage-Castellanos, Martinez-Montes, Hernandez-Cabrera, & Galan, 2010).
We hypothesized that complex wavelet frequency decomposition would show that EEG responses to non-rewards versus rewards would be associated with enhanced power and phase coherence in the theta frequency band. Given that a large portion of the feedback related negativity is attributable to activity in the theta band (Cavanagh, Eisenberg, Guitart-Masip, Huys, & Frank, 2013; Cavanagh et al., 2010; Cohen et al., 2007) and several age comparison studies have found that the FRN (positive and negative outcome) is generally larger in children, decreasing in amplitude across adolescence, we also expected to see a decrease in post-feedback theta power across the age groups in our sample. However, we also acknowledge that recent neuroimaging work suggests increased sensitivity to rewards for some adolescents (Cohen et al., 2010; Galvan, Hare, Voss, Glover, & Casey, 2007). It is surprising that the published studies of the FRN comparing adolescence to other developmental periods have failed to identify reward-related EEG neural correlates unique to adolescence. One possibility is that the signal averaging approach obscures important aspects of feedback processing that may emerge with an event-related oscillatory approach. Thus, we remain open to the possibility that oscillatory patterns do not follow a linear pattern across development.
Material and Methods
Participants
The original sample from which our participants were drawn consisted of 144 children (72 female), recruited via mass mailing list provided by the credit mailing company Experian, targeting the towns surrounding New Haven, Connecticut, USA. Among the total sample, 109 children (52 female) provided sufficient artifact-free EEG data for this report. All children were assessed between 4:30 and 5:30 p.m. Children were fluent in English with no evidence of serious mental illness (e.g., psychosis) as assessed via a parental telephone screen. The mean age of the children was 13.67 (SD = 2.11). The ethnic backgrounds of the children were 8.3 % African American, 6.4 % Hispanic, 6.4 % Asian, 1.8 % Native American, 76.2 % Caucasian, and 0.9 % other ethnic background. Children were grouped by age into three groups: 10-12 years (n=43); 13-14 years (n=34); and 15-17 years (n=32) (see Table 1 for age and sex breakdowns). The sample participants were mainly from stable middleclass families. Ninety-five percent of children were living with their biological mother. Mothers reported 84.4% of the time that they were either married or in a committed relationship, with 8.3% indicating they were separated or divorced. A majority of families (67%) had an income greater than 60,000 dollars, 11% had incomes between 35,000 and 59,999 dollars, 7.3% had and income less than 35,000 dollars and 14.7% chose not to report their family income.
Table 1.
Sample Characteristics: Age and Sex by Group
| Group | 10-12 yrs. | 13-14 yrs. | 15-17 yrs. |
|---|---|---|---|
| Age M (SD) | 11.52 (.79) | 13.95 (.61) | 16.09 (1.27) |
| Males / Females | 20 / 22 | 20 / 14 | 17 / 16 |
| Total N | 42 | 34 | 33 |
ERP Reward-Feedback Task
A reward task modeled after Holroyd and colleagues (Holroyd, Nieuwenhuis, Yeung, & Cohen, 2003) presented the participant with four different colored balloon images (red, green, orange, blue). Balloons randomly appeared in serial positions along a row centered on the screen. Although there were four options (i.e., different colored balloons) on a given trial, feedback was rigged to have the probability of 50% win (reward 10 cents) and 50% no win (non-reward) outcomes across the task. Feedback was random and there was no pattern for certain balloons predicting specific outcomes. To keep the children engaged in the task, they were led to believe that some people “can figure out a pattern some of the time”. Participants selected balloons with a four-button response pad, using the middle and index fingers of both hands, and were reminded to look at the screen and not their hands, as they would in video game play. Subsequent to balloon selection on each trial, feedback was delayed variably between 1000 and 1200ms. This was done to make the task feel less monotonous and to mitigate against the possibility of a contingent negative variation (CNV) in the EEG signal. Feedback lasted 1000 ms followed by a 1000-1200ms crosshair, and a 100ms blank screen before the balloons reappeared. Participants' selection of a balloon was self-paced on each trial.
Current earnings were displayed numerically on the screen, centered just below the middle two balloons. There were four blocks, each containing approximately 30 trials (1-3 extra win trials were added to two blocks to provide variable winnings from block to block). A coin jar was presented after each block, into which coins (dimes) appeared, one by one, each followed by a coin sound. The coin jar was introduced using three practice trials at the outset of the game. A total of 120 trials (60 per condition) were administered for the purpose of computing ERPs. Three additional trials were added such that the total winnings were $6.30 for each participant. Participants received this payment as part of a larger compensation ($60), for a study on stress and decision-making.
Procedures
After obtaining IRB-approved parental permission and child assent, each child was seated 24 inches in front of a 19-inch computer LCD monitor. The E-prime v.2.0 (PST, Inc.) software package controlled the stimulus presentation. The task lasted approximately 10 minutes and each child's electroencephalogram (EEG) and behavior were continuously monitored across the session so that stimulus presentation occurred only when the child was sitting still and looking at the monitor. The location of coordinate Cz was marked as the juncture of the halfway point between nasion to inion and left and right preauricular notches. Recordings were done using a Hydrocel high-density electroencephalogram net of 128 Ag/AgCl electrodes (Geodesic Sensor Net, EGI Inc.). The filter was set at 100 Hz low pass. Brain wave data were recorded through the Netstation v.4.4 software package (EGI, Inc.) and EGI high impedance amplifiers, sampling at 250 Hz (EGI, Inc. Series 300 amplifier). All electrodes were referenced to Cz for recording and then re-referenced offline for data analysis. All impedances were assessed at or under 40 kohms before recording.
Offline, the EEG data were first processed through a 0.1 Hz first order high-pass filter and a 30 Hz low-pass filter. Continuous data were segmented into 2-second epochs containing a 1000ms pre-stimulus baseline and a 1000ms post-stimulus interval. Eye channels were inspected visually. Those likely reflecting a poor connection to the scalp, either flat (no discernable EEG signal) or reflecting white noise, were manually marked as contaminated and interpolated by surrounding channels. Automatic artifact rejection removed any segments containing extreme voltage fluctuations (threshold 200 μV) or muscle activity association with saccades and eye blinks (threshold 150 μV). Epochs with any eye blink or eye movement (threshold 150 μV) were rejected. Epochs with more than 10 bad channels (40% or more segments marked bad) were rejected as well. Then the remaining bad channels were replaced by surrounding channels. The single trial data were re-referenced from the vertex (Cz) to an average reference of all electrodes because the latter is thought to be a better representation of a true zero (Junghofer, Elbert, Tucker, & Braun, 1999). The data were baseline corrected to the 1000 ms pre-stimulus interval.
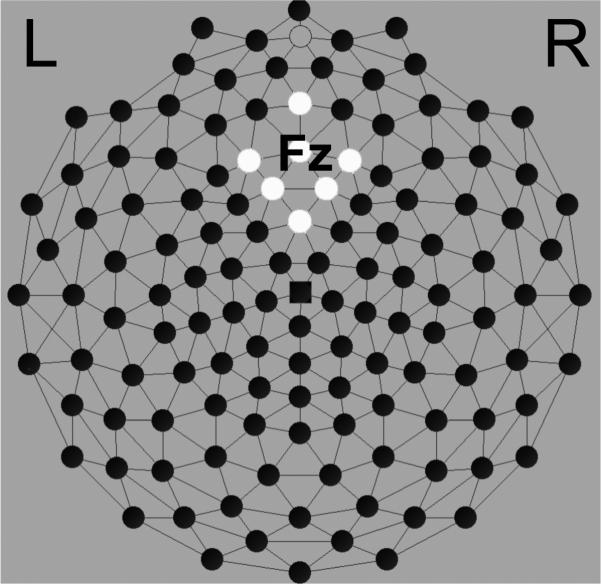
Data for participants with fewer than twenty-seven artifact free trials per condition received additional preprocessing with statistical eye-blink removal (blink threshold 14 μV/ms) (Gratton, Coles, & Donchin, 1983). Participants whose data yielded 27 good trials per condition with this additional approach (n = 84) were then included in the overall statistical analysis (n = 109). This report builds on a previous report (Crowley et al. in press), which examined the feedback related negativity (FRN) in a sample of 94 participants who were a subset of the participants included in the present report. The current report focuses specifically on ERSP and ITC in the larger sample. Past work on the feedback negativity has localized the FRN to the medial frontal region along the midline at site Fz (10-10 system). We relied on the average signal across seven electrodes extending over the frontal midline and regions, specifically electrode numbers 11 (Fz), 16, 19, 12, 5, 4, and 6 (Electrical Geodesics Incorporated, Hydrocel net, see Figure 1).
Figure 1.
Selected medial frontal channels surrounding site Fz from a high density Hydrocel EEG array (Electrical Geodesics Inc.).
EEG Signal Analysis
ERSP and ITC measures were examined with EEGLab version 11.0.4.3b, MATLab version R2011a, with statistical analyses performed in R1.15.1. Our data processing pipeline involved computing ERSP and ITC values in EEGLab, using algorithms for implementing a time × frequency spectrogram (Delorme & Makeig, 2004). Specifically, we relied on the EEGLab function “newtimef” to calculate the average ERSP and ITC across the medial frontal channel cluster. ERSP and ITC calculations relied on both fast Fourier Transform (at the lowest frequency) and wavelet decomposition (at the highest frequency). Using the standard setting with EEGLab (cycles were set as [3,0.5]), cycles increase linearly with frequency from 0 for FFT (same window width at all frequencies) to 1 for wavelet (same number of cycles at all frequencies). Specifically, the software uses 3 cycles at lowest frequency to 11.25 cycles at highest. The time-frequency decomposition yields a time × frequency transform with a complex number for every time point, frequency, and trial. ERSP and ITC data were obtained at 53 linear-spaced frequencies, 4.0-30.0 Hz, and 290 linear-spaced time points from -582 to 578 ms (i.e., 836 ms sliding window) relative to the stimulus onset1.
ERSP and ITC data were collected every 4 ms and every 0.5 Hz. The ERSP and ITC values were further averaged every 16 ms. Consequently, the ERSP and ITC values ranged temporally from 0 to 576 ms, with 16 ms temporal resolution (36 values) and spectrally from 4 to 30 Hz, with 0.5 Hz spectral precision. Based on previous work for feedback processing, only 4 to 8 Hz (8 values) were brought into the R statistical package further analysis. Thus for each participant, the total number of dependent measures were 36 × 8 = 288.
Results
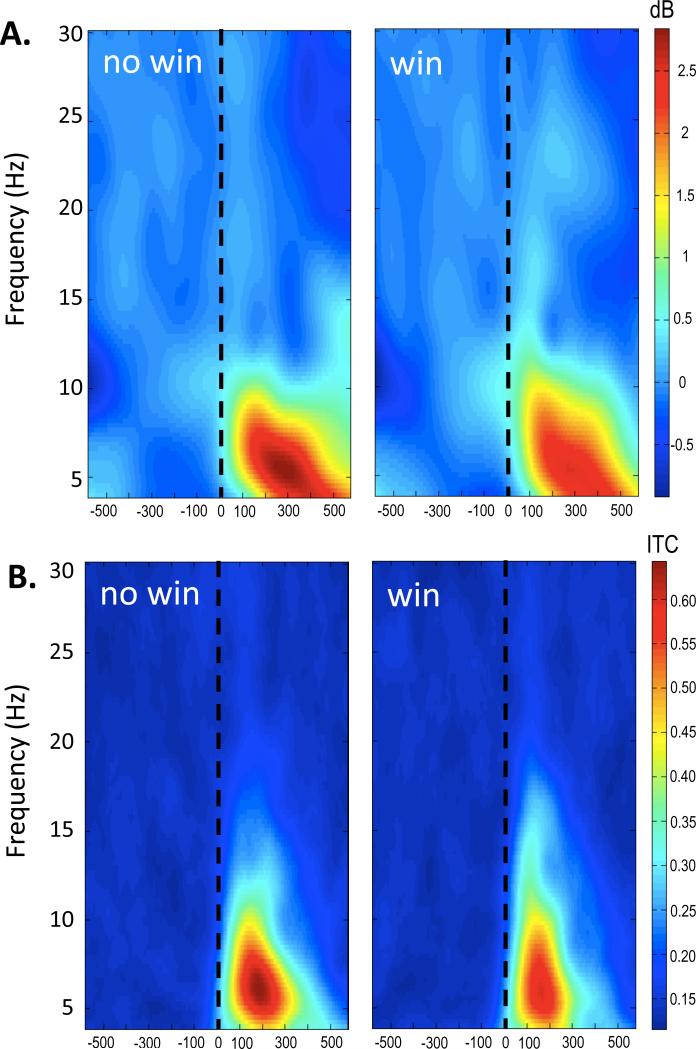
Sample characteristics including age and gender breakdown by age group are presented in Table 1. The mean number of trials available for the no win condition and win condition were 43.81 (SD = 8.45) and 45.07 (SD = 7.8), respectively. For descriptive purposes, we display ERSP and ITC plots for the range from 4 to 30 Hz. Figure 2A displays ERSP values for no win and win conditions. Figure 2B displays analogous plots for ITC. As can be seen in Figure 2, the theta band (4-8 Hz) captured a large portion of the oscillatory activity for both ERSP (Figure 2A) and ITC (Figure 2B).
Figure 2.
ERSP (panel A) plot and ITC (panel B) for no win and win conditions at 4 to 30 Hz. The dashed line indicates the onset of feedback. The theta band clearly predominates across conditions and plot types.
Analyses consisted of a condition (2: no win vs. win) × age (3: 10-12 years, 13-14 years, 15-17 years) × sex (2: male, female) repeated measures analysis of variance. Because sex did not account for significant variance in any model at the level of individual ANOVAs, this factor was dropped from subsequent analyses. Type III repeated measures ANOVAs were conducted on each of the 288 dependent measures within the R statistical package (R version 2.15.2), which were then subjected to false discovery rate correction (Benjamini & Hochberg, 1995). ERSP values were directly exported from EEGLAB as dependent measures in the ANOVAs. However, because ITC values reflect a correlation-like measure, bounded by 0 and 1, these values were Fisher r-to-z transformed prior to statistical analysis.
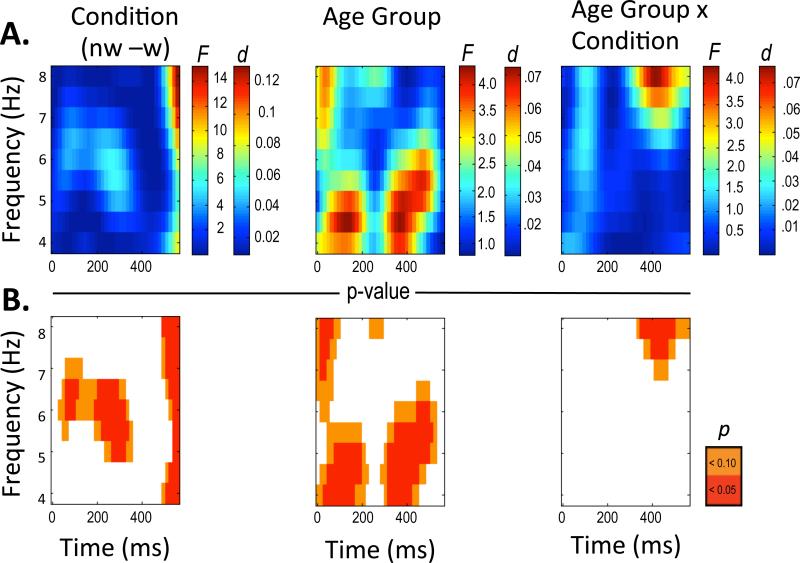
F-values, effect sizes (d) and p-values were calculated for each ANOVA. These are displayed graphically for ERSP and ITC analyses in Figures 3 and 4, respectively. Following standard procedures, p-values were submitted to FDR, to maintain the overall false discovery rate at p < .05 for ERSP and ITC analyses, respectively. The large numbers of tests preclude a full narrative of the significant effects for each omnibus test. Thus, we present summary effects. By “summary” we mean effects representing the average for selected frequency and time windows based on visual inspection of clusters of statistically significant effects. These are presented in tabular form in Table 3 for ERSP and Table 5 for ITC with repeated measures ANOVAs conducted on each effect. Consequently, in some cases, an interaction emerged at the aggregate level of analysis that was not present in the fine-grained analysis. We acknowledge that we have imposed rectangular shapes on significant effective ranges displayed (Tables 2-5 and Figure 3 and Figure 5). Means and standard errors for summary effects are presented in Table 2 for ERSP and Table 4 for ITC.
Figure 3.
Plotted ERSP statistical results from .5 Hz by 16 ms repeated measures ANOVAs with F-values and effect size for Condition, Age and Condition by Age Interaction (panel A). Plotted uncorrected p-values for Condition, Age and Condition by Age Interaction at p < .05 (red) and p< .10 (orange) (panel B).
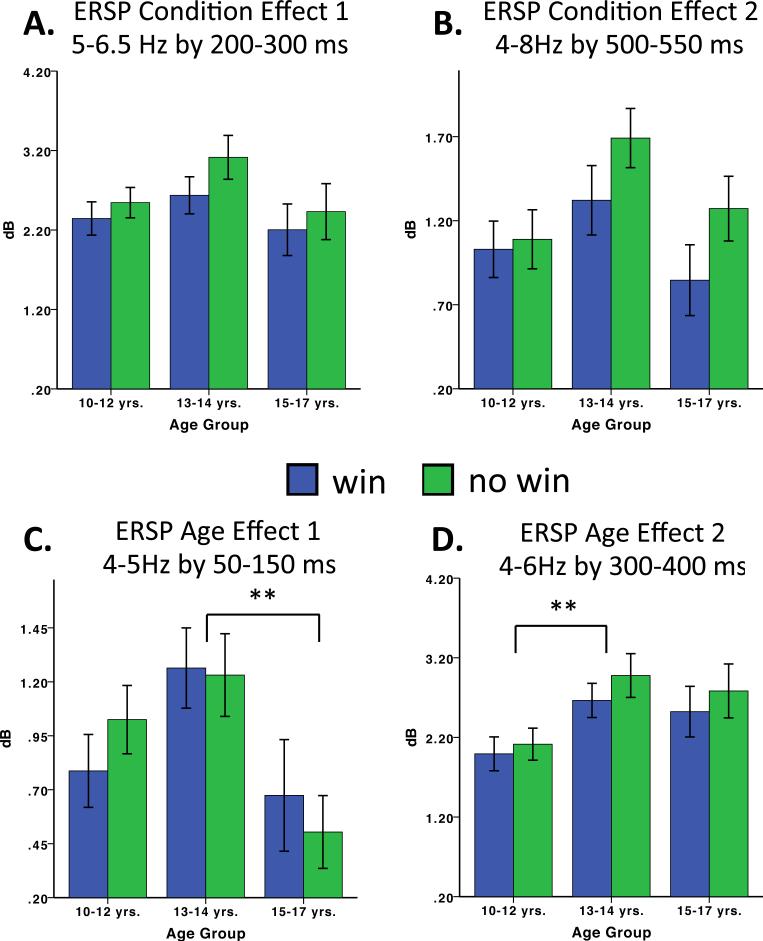
Figure 4.
ERSP summary effects graphed for selected frequency and time windows based on clusters of statistically significant effects (uncorrected). Bar graphs A-D were derived from effective ranges of uncorrected significant effects in Figure 3.
Table 3.
Event-related Spectral Perturbation Prototypical Effects
| Frequency by Time | Condition Effect 1 5-6.5 Hz by 200-300 ms | Condition Effect 2 4-8 Hz by 500-550 ms | Age Effect 1 4-5 Hz by 50-150 ms | Age Effect 2 4-6 Hz by 300-400 ms |
|---|---|---|---|---|
| ANOVA | ||||
| Condition | F(1, 106) = 4.792, p = .031* | F(1, 106) = 6.230, p = .014* | F(1, 106) = .010, p = .920 | F(1, 106) = 2.492, p = .117 |
| Age | F(2, 106) = 1.483, p = .232 | F(2, 106) = 2.532, p = .084 | F(2, 106) = 3.969, p = .022* | F(2, 106) =3.419, p = .036* |
| Interaction | F(2, 106) =.406, p = .667 | F(2, 106) = 1.071, p = .346 | F(2, 106) = 1.098, p = .337 | F(2, 106) = .163, p = .849 |
| Post Hoc | ||||
| Condition | no win > win, t(108) = 2.164, p = .033* | no win > win, t(108) = 2.353, p = .020* | - | - |
| Age | - | - | Y=M, t(74) = -1.71, p = .091 Y=O, t(73) = -1.383, p = .171 M>O, t(65) = 2.638, p = .010* |
Y<M, t(74) = −2.852, p = .006** Y=O, t(73) = −1.808, p = .075 M=O, t(65) = .465, p = .643 |
Y=10-12 yrs., M=13-14 yrs., O=15-17 yrs.
Table 5.
Intertrial Coherence Prototypical Effects
| Frequency by Time | Condition Effect 4.5-8 Hz × 200-400 ms | Age Effect 1 4-6 Hz × 150-250 ms | Age Effect 2 4-5 Hz × 350-450 ms |
|---|---|---|---|
| ANOVA | |||
| Condition | F(1,106) = 25.47, p < .001** | F(1,106) = 2.77, ns | F(1,106) = 12.72, p < .001*** |
| Age | F(2, 106) = 2.32, p = .103 | F(2, 106) = 6.62, p = .002** | F(2,106) = 6.14, p = .003** |
| Interaction | F(2,106) = 3.88, p = .024* | F(2, 106) = 2.17, ns | F(2,106) = 3.66, p = .03* |
| Post Hoc | |||
| Condition | - | - | - |
| Age | - | Y= M, t(74) = .429, p = .669 Y > O, t(73) = 3.458, p = .001*** M > O, t(65) = 2.876, p = .006** |
M/O, no win>win; Y, no win= win. Diff. of no win vs. win: M>O>Y |
| Interaction |
no win>win; Y, t(41) = .902, p = .372 M, t(34) = 4.066, p = .000, O, t(32) = 3.438, p = .002 |
- |
no win>win; Y, t(41) = .101, p = .920 M, t(33) = 2.690, p = .011, O, t(32) = 2.455, p = .020 |
Y=10-12 yrs., M=13-14 yrs., O=15-17 yrs.
Table 2.
Means and Standard Deviations for Event Related Spectral Perturbation Prototypical Effects
| Frequency by Time | 10-12 yrs. | 13-14 yrs. | 15-17 yrs. |
|---|---|---|---|
| 5-6.5 Hz by 200-300 ms | |||
| Win | 2.3 45 (1.359) | 2.6 37 (1.360) | 2.2 03 (1.864) |
| No win | 2.5 45 (1.236) | 3.1 15 (1.611) | 2.4 31 (2.020) |
| Ave. Feedback | 2.4447 (1.111) | 2.8756 (1.261) | 2.3174 (1.819) |
| 4-8 Hz by 500-550 ms | |||
| Win | 1.0 30 (1.088) | 1.3 21 (1.205) | 0.8 46 (1.210) |
| No win | 1.0 89 (1.205) | 1.6 92 (1.027) | 1.2 72 (1.105) |
| Ave. Feedback | 1.0 60 (0.973) | 1.5 07 (0.922) | 1.0 59 (0.986) |
| 4-5 Hz by 50-150 ms | |||
| Win | 0.7 87 (1.094) | 1.2 64 (1.084) | 0.6 74 (1.485) |
| No win | 1.0 25 (1.025) | 1.2 31 (1.117) | 0.5 04 (0.969) |
| Ave. Feedback | 0.9061 (0.845) | 1.2476 (0.891) | 0.5 89 (1.142) |
| 4-6 Hz by 300-400 ms | |||
| Win | 1.9 94 (1.379) | 2.6 64 (1.253) | 2.5 24 (1.825) |
| No win | 2.1 15 (1.305) | 2.9 77 (1.601) | 2.7 84 (1.947) |
| Ave. Feedback | 2.0 54 (1.148) | 2.8 21 (1.186) | 2.6 54 (1.716) |
Figure 5.
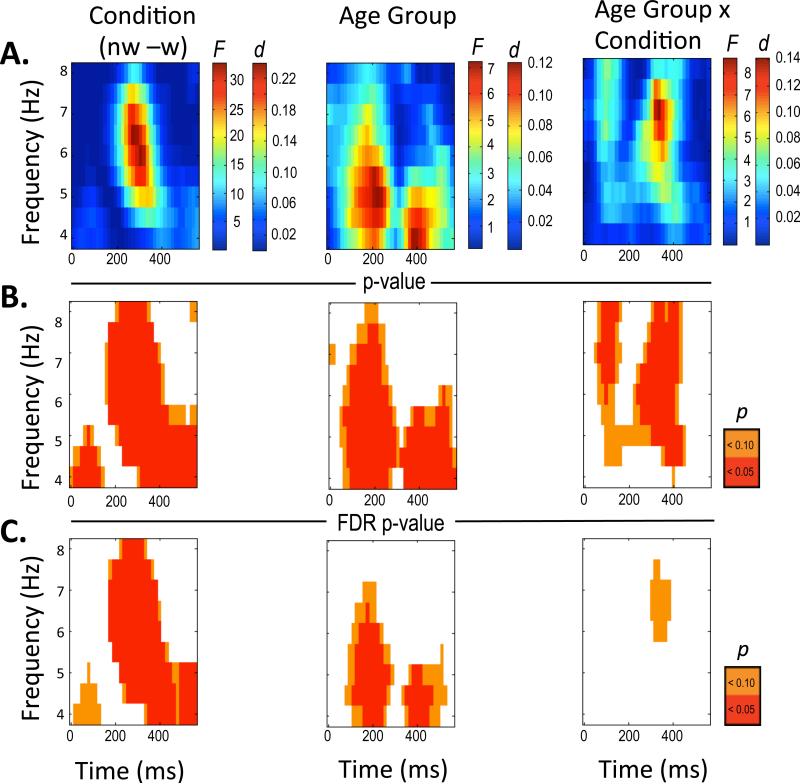
Plotted ITC statistical results from .5 Hz by 16 ms repeated measures ANOVAs with F-values and effect size for Condition, Age and Condition by Age Interaction (panel A). Plotted uncorrected p-values for Condition, Age and Condition by Age Interaction at p < .05 (red) and p< .10 (orange) (panel B). Plotted FDR corrected p-values Condition, Age and Condition by Age Interaction at p < .05 (red) and p < .10 (orange) (panel C).
Table 4.
Means and Standard Deviations for Intertrial Coherence Prototypical Effects
| Frequency by Time 4.5-8 Hz × 200-400 ms | 10-12 yrs. | 13-14 yrs. | 15-17 yrs. |
|---|---|---|---|
| Win | 0.407 (0.144) | 0.370 (0.128) | 0.323 (0.135) |
| No win | 0.429 (0.143) | 0.490 (0.149) | 0.414 (0.184) |
| Ave. Feedback | 0.4178 (0.121) | 0.4301 (0.110) | 0.3685 (0.142) |
| 4-6 Hz × 150 to 250 ms | |||
| Win | 0.5846 (0.206) | 0.5388 (0.213) | 0.4559 (0.213) |
| No win | 0.6114 (0.226) | 0.6195 (0.215) | 0.44 (0.187) |
| Ave. Feedback | 0.598 (0.190) | 0.5792 (0.191) | 0.448 (0.182) |
| 4-5 Hz × 350-450 ms | |||
| Win | 0.2595 (0.098) | 0.3229 (0.142) | 0.2813 (0.192) |
| No win | 0.2614 (0.113) | 0.4373 (0.255) | 0.3546 (0.219) |
| Ave. Feedback | 0.2605 (0.086) | 0.3801 (0.165) | 0.3179 (0.188) |
Event Related Spectral Perturbation
In terms of ERSP, age, condition and age × condition interaction effects are presented in Figure 3. None of the effects remained significant after FDR was applied. The use of 16 ms by .5 Hz data values may have been overly ambitious, yielding too many omnibus tests and excessive loss of statistical power. Nevertheless, having already examined the data in this way, we felt returning to broader units of frequency and time would have been statistically unsound. We also could have let the previous adult studies of feedback processing guide our window in terms of frequency and time. Previous studies in adults have tended to sum across the theta band (4-7 or 4-8 Hz), allowing visual inspection to guide the time window capturing the theta band for feedback. This would have been at odds with a major goal of this paper, which was to employ an “age sensitive” approach. Thus, here for descriptive purposes we use our uncorrected omnibus tests to guide selection of relevant frequencies and time windows. We acknowledge these analyses are exploratory. Thus, we report effects collapsed across individually significant time and frequency ranges so that these ranges might guide future developmental studies (See Table 2 for means and standard deviations and Table 3 for post hoc tests). Main effects emerged for ERSP in four main clusters, two condition effects and two age effects (see Table 3 and Figure 4).
In terms of condition, one effect overlapped with the time window commonly reflecting the feedback negativity ERP, ~200-300 ms and 5-6.5 Hz (ERSP Condition Effect 1, p = .031, see Figure 4A). Note that the Condition panel spectral plot in Figure 3A has a different range of effect size (larger), which appears less intense than the age group plot in Figure 3A because of the broader effective range for the Condition plot. A second condition effect appeared at the end of our analysis windo ~500-550 ms and 4-8 Hz (ERSP Condition Effect 2, p = .014, see Figure 4B). Both Condition Effect 1 and 2 reflected greater spectral power for the no win condition (Table 3, post hoc). In terms of age group (average feedback collapsed across condition) one main effect (ERSP Age Effect 1, p = .022, Figure 4C, bottom left panel) emerged early ~50-150 ms and 4-5 Hz. Pairwise comparisons suggest this effect mainly reflected greater spectral power for the 13-14-year-old group compared to the 15-17 year-old group (p = .010, Figure 4C) and the 10-12-year-old group (trend, p = .091). The second effect (ERSP Age Effect 2, p = .036, Figure 4D, bottom right panel).) appeared in the window of 4-6 Hz and 300-400 ms. This effect mainly reflected that the 13-14-year-old group had more spectral power in the 4-6 Hz range than the 10-12-year-old group, p = .006 (Figure 4D). The 15-17-year old group had less power in this range than the 13-14-year old group (trend, p = .075).
In summary, our ERSP data suggest that a smaller frequency window (5-6.5) Hz may better characterize “theta” for greater response to no win vs. win feedback in our child and adolescent sample (ERSP Condition Effect 1, Figure 4A), with the full range of theta (4-8 Hz) being engaged differentially only later in stimulus processing (Condition Effect 2, 500-550 ms, Figure 3 (Age Group) and Figure 4B). Age effects suggest that the 13-14-year-old group produces greater theta activity than the older group for early feedback stimulus processing at 50-150 ms and 4-5 Hz (Figure 4C) and greater theta activity than the younger group at 300-400 ms and 4-6 Hz (Figure 4D).
Intertrial Coherence
Because ITC values reflect a measure of association akin to correlation, bounded from zero to one, ITC values were converted to z-scores prior to analyses, with actual ITC values reflected in Figures 2 and 5. Effects for ITC were robust after applying the FDR procedure. Following the same repeated measures analysis as with ERSP, ITC effects emerged for condition and age (Figure 5). Figure 5A shows the respective F-tests and effect sizes (d) for condition, age, and the condition × age interaction. Figure 5B, middle panel, shows the p-values for uncorrected omnibus tests and Figure 5C, bottom panel, shows FDR corrected omnibus tests. After FDR, a condition effect remained from ~200-400 ms by ~4.5 Hz to 8 Hz, largely consistent with the time window capturing the FRN. Two other age-related effects emerged, one at ~150-250 ms and from 4 Hz to 6 Hz and a second at ~350-450 ms and from 4 Hz to 5 Hz. Thus we report the three frequency by time windows reflecting these summary effects (see Table 4 for means and standard deviations and Table 5 for tests of summary effects).
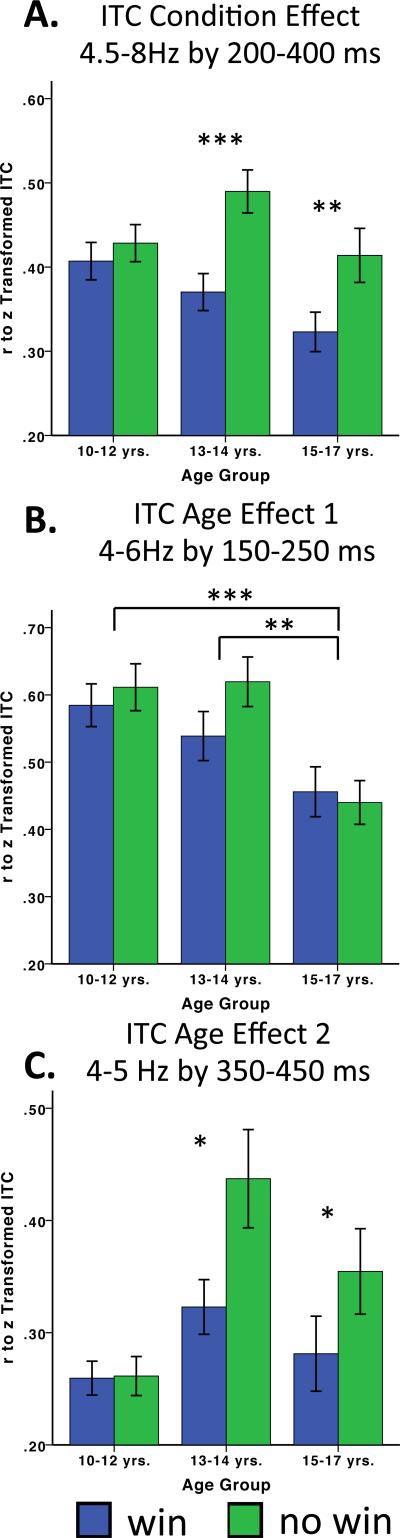
The ITC Condition Effect (Table 5) was qualified by an age × condition interaction, p = .024. This interaction was largely accounted for by a lack of a no win vs. win condition difference for the 10-12-year-old group, p = .372, but a significant difference between no win and win for both the 13-14-year-old group and the 15-17-year old group, ps < .001 and .01, respectively (see Figure 6A).
Figure 6.
ITC summary effects graphed for selected frequency and time windows based on clusters of statistically significant effects (FDR corrected). Bar graphs A-C were derived from effective ranges of uncorrected significant effects in Figure 3.
Because our data also suggest differential trends across age group, we conducted a secondary a post-hoc trend analysis comparing a linear trend to a quadratic trend separately for the ITC no win and win conditions. For the no win condition, the linear contrast was not significant, t(106) = −.397, p = .692, but the quadratic contrast was reliable, t(106) = 2.094, p = .039. Conversely, for the win condition, there was a linear effect, t(106) = −2.650, p = .009, whereas the quadratic contrast was not significant, t(106) = .186, p = .853.
Age effects emerged in two clusters (Table 5). Age Effect 1 occurred between ~150-250 ms in the 4 Hz to 6 Hz range, but did not reveal a Condition effect or Condition × Age interaction. Post hoc comparisons indicated that the 10-12-year-old group and the 13-14-year old group had significantly greater ITC, irrespective of condition, compared to the 15-17-year-old group, p < .001 and p < .01, respectively (see Figure 6B).
Age Effect 2 (Table 5) occurred between from ~350-450 ms in the 4 Hz to 5 Hz range (. This window included a significant condition effect, p < .001, age effect, p < .01 and Age × Condition interaction, p < .05. Similar to the ITC Condition Effect, post hoc comparisons indicated that the 10-12-year-old group did not have significantly different ITC across no win vs. win conditions, p = .920, whereas ITC was greater for no-win compared to win feedback in the 13-14-year-old group and the 15-17-year-old group, p = .011 and p = .020, respectively (see Figure 6C). Given the plot of means for ITC Age Effect 2 resembled the ITC Condition Effect (Figure 6C) we repeated the trend analysis. For the no win condition, the linear contrast was significant, t(106) = 2.012, p = .047, but the quadratic contrast was a better fit for the data, t(106) = 3.133, p = .002. For the win condition, neither the linear, t(106) = .644, p = .521, nor the quadratic contrasts, t(106) = 1.745, p = .084, were statistically reliable.
In summary, our ITC data suggest that the typical window of 4-8 Hz for theta largely captures the synchronous feedback activity coincident with the feedback negativity (we observed 4.5-8 Hz). In turn, the greater phase locking of theta for no win vs. win feedback (ITC Condition Effect 1), was absent for the 10-12-year-olds, but emerged for the older groups. A later effect (~350-450 ms × 4-5 Hz; ITC Age Effect 2) similarly suggested a lack of differentiation for no- win vs. win feedback among the younger group, which emerges across the middle and older adolescent groups. Moreover, differences across the age groups (Condition Effect, Age Effect 2) indicate a quadratic pattern for no win theta ITC, reduced at 10-12 years, increased at 13-14 years and then decreased again for the 15-17-year-old group. A second, early aspect of feedback processing (~150-250 ms × 4-6 Hz; ITC Age Effect 1), reflected greater trial-to-trial coherence, irrespective of condition, for the younger groups, which was markedly reduced for the older group.
Discussion
This study was designed to provide a fine-grained look at child and adolescent age trends in reward processing from the perspective of event related theta oscillations. In a relatively large sample we explored win and no win feedback among children and adolescents 10-17, in three groups (10-12 years, 13-14 years, 15-17 years). We took a relatively ambitious approach to examining fine-grained developmental effects (.5 Hz by 16 ms). Generally, the no win feedback led to greater theta activity than the win feedback, for both spectral power and phase coherence. Phase coherence yielded more statistically robust effects than did spectral power (ERSP), which did not survive FDR error correction.
We did observe uncorrected significant effects for ERSP. Owing to the developmental thrust of this report, that the fine-grained decomposition of the data was quite restrictive in terms of statistical power, and that our analyses might guide future work by other investigators, we proceeded to exploratory analysis of our ERSP omnibus effects. ERSP analysis suggested that rather than the 4-8 Hz theta band typically applied in adult studies, a smaller frequency range (5-6.5 Hz) may capture the aspect of feedback processing corresponding temporally to the FRN. Consistent with other studies (Cavanagh, Figueroa, Cohen, & Frank, 2012; Cavanagh, Zambrano-Vazquez, et al., 2012) we observed a burst of theta activity coinciding with the time window of the FRN (200-300 ms). However, we also observed a second theta burst overlapping with the full theta range (4-8 Hz) at the end of the frequency range we examined. This process was observed up to the end of our temporal window (500-550 ms). Future studies might sample further into the post feedback period. We chose not to because of movement artifact present in the larger temporal window.
Two ERSP age-related oscillatory trends emerged from our feedback data, independent of feedback type. Early in feedback processing (50-150 ms), our 13-14-year-old adolescent group showed greater theta power than 15-17-year-olds and a trend in the same direction compared to 10-12-year-olds (see Figure 4C). Although we focused on a cluster of channels over the medial frontal cortex, the effects in these early temporal windows (i.e., ~0 to 200 ms post-stimulus) likely reflect the activation of different generators in the medial frontal cortex than those commonly attributed to the FRN. There is appreciable involvement of other sensory and association cortices during the early stages of stimulus processing, and future studies should consider, for example, differences in early occipital activation as a function of reward type, developmental stage, or their interactions. On the other hand, our 13-14-year-old adolescent group had larger burst of theta than the 10-12-year-oldsfor later processing of reward feedback (4-6 Hz, 300-400 ms), but not the 15-17-year-olds. This increase in mean evoked theta power likely reflects the engagement of generators in the medial frontal cortex and anterior cingulate that account for the FRN (Asada, Fukuda, Tsunoda, Yamaguchi, & Tonoike, 1999).
Based on previous work (Crowley et al., in press, 2013; Eppinger, Mock, & Kray, 2009; Hämmerer, Li, Müller, & Lindenberger, 2011; Zottoli & Grose-Fifer, 2012), we expected to see increased theta spectral power for children compared to our younger and older adolescent groups. Although we did not present averaged ERP data here, our previous study found a decrease in average FRN amplitude with age (Crowley et al., in press) among children (n=91) who were a subset of those presented here. The lack of finding here for ERSP suggests that the reduction in FRN feedback amplitudes across age reflects a reduction in phase locking rather than a reduction in theta power across childhood and adolescence.
More generally, these effects point to a developmental model of reward function that recognizes feedback processing as unfolding and dynamic, having both early (50-150 ms) and later processing stages (300-400 ms) which could reflect the transient activation of multiple cortical sources that support reward processing. In our sample, increased entrainment for reward related feedback was observed in the 13-14-year-old group. Future work will need to determine whether or not aged-related differences in oscillatory activity reflect maturation of subcomponents of reward processing, and whether not these effects are reliable even in the face of conservative or targeted hypothesis testing procedures.
In terms of phase coherence, our ITC analysis yielded stronger effects, surviving the FDR procedure. We observed an approximate 200-400 ms by4.5 Hz to 8 Hz condition effect, with greater ITC for no win vs. win, consistent with the typical time window of the FRN. This effect was qualified by a Condition by Age interaction, partly driven by the 10-12-year-old group, who showed comparable ITC for the win and no win conditions. Both the 13-14 year-olds and the 15-17-year olds displayed greater ITC for no win vs. win. Two ITC age effects emerged irrespective of condition, before aggregating for summary effects. The first reflected a reduction in overall phase coherence in the 15-17-year-old group compared to the younger groups from 150-250 ms and 4-6 Hz. The second effect, when examined in aggregate, resembled the ITC condition effect. Specifically we observed an age by condition interaction from 350-450 ms in the 4-5 Hz range, again reflecting a lack of ITC difference for the 10-12-year-old group, but significantly greater ITC for no win compared to win for the older groups. These findings suggest that the transition from childhood to adolescence is reflected by greater phase consistency of the theta oscillations following non-rewards.
As a second approach to interpreting the condition by age group interactions for ITC we conducted trend analyses (200-400 ms by 4.5 Hz to 8 H and 350-450 ms in the 4-5 Hz ranges). These ranges consistently reflected a quadratic pattern for no win theta trial to trial phase synchrony (ITC), which was reduced in the 10-12-year-old group, increased in the13-14-year-old group and then decreased again for the 15-17-year-old group. Future work will need to link developmental patterns of theta oscillation to learning (Cavanagh et al. 2010), decision making (Cohen et al., 2009) and risk behavior inside and outside the laboratory (Lejuez, Aklin, Zvolensky, & Pedulla, 2003).
We have shown that evoked mean spectral power (ERSP) and ITC vary by developmental period vis-à-vis reward vs. non-reward (no win) processing. The meaning of these age related correlates still requires further study. For instance, as developmental trends, they may relate to greater efficiency of processing feedback, but they could also be associated with aspects of greater reward sensitivity or risk taking proclivity (Galvan et al., 2006). As noted by Casey et al. (2008), there is a relative paucity of research focused on the role of the frontal cortex and, more specifically, the functional interaction it has with subcortical reward circuitry with respect to risky behavior. Future studies should consider these functional relationships and how they vary across individuals, such as adolescents showing high levels of sensation-seeking (Mackiewicz Seghete, Cservenka, Herting, & Nagel, 2013).
In addition, in the pursuit of endophenotypes, oscillatory measures of basic mechanisms such as reward feedback processing may hold particular promise because they allow for examination of specific frequencies, their timing and extent of phase locking (ITC), which may be more precisely tied to the postsynaptic potentials and physiology that underlies the EEG. Thus, whereas ERPs can be limited by assumptions about which factors influence the emergence of distinct event-related brain potentials (Makeig et al., 2004), considering the oscillatory nature can better inform our understanding of brain-behavior relationships as this approach retains a richer representation of EEG signals across time-frequency domains.
Limitations
We specifically focused on the theta band for feedback processing of no win and win conditions because the body of adult data suggested the relevance of this frequency range for feedback processing. Our overall spectral plots (Figure 2) are consistent with this choice. However, we could have considered other frequency ranges and other cortical regions that may vary with reward feedback processes. Other work suggests that reward feedback may elicit midfrontal beta band activity(Marco-Pallares et al., 2008; van de Vijver, Ridderinkhof, & Cohen, 2011). Similar to Cavanagh et al. (2010) we saw little if any evidence for elevated beta activity in the midfrontal region for either ERSP or ITC (see our Figure 2). Because both chance-based (e.g., this study) and learning tasks (e.g., Cavanagh et al. 2010) have been employed, the absence of a beta effect for reward in our study is probably not due to this factor.
We focused on the contrast of rewards versus their absence. Thus, we can only generalize to these two types of feedback. All the other published work on oscillatory EEG feedback processing has included reward versus loss. While ERP studies suggest that outcomes worse than expected that are loss or no-win evoke comparable FRNs (Hajcak, Moser, Holroyd, & Simons, 2006; Holroyd, Hajcak, & Larsen, 2006), we do not assume this to be true at the level of oscillatory activity. As well, our use of a chance-based task limits generalization of our findings to this type of task. Future work might examine EEG oscillations in learning feedback tasks, which may be able to speak to which aspects of oscillatory activity relate to learning rate across development.
Our ERSP effects did not survive FDR correction. Thus, statistically speaking, the effects we report here should be considered exploratory. We set a higher bar here than is typically done with the examination of oscillations with the decision to use .5 Hz by 16 ms measurements. However, the uncorrected effects we report here could be used to guide future studies in terms of relevant frequency by time windows.
We chose to parse our sample into three groups based on chronological age for several reasons. First, our previous paper with a subset of this sample (Crowley et al., in press) suggested this was a viable way to characterize age trends. Second, recent adolescent reward processing work suggests that some age related changes could be non-linear and a group based approach we felt was better for exploring this possibility. Third, the frequency by time array resulting from an ERSP or ITC analysis was better suited to group based comparisons for our implementation of FDR. We might have considered pubertal development as another option, or one to be considered in conjunction with chronological age. Despite this, we did identify age related changes in feedback processing, even among our 13-14 and 15-17-year-old groups, across both ERSP and ITC, with ITC effects surviving a more robust FDR statistical threshold. Recent adolescent neuroimaging studies reward processing and feedback learning have collapsed across these age ranges (Cohen et al., 2010; Galvan et al., 2006; van de Vijver et al., 2011), but our data suggest there may be important maturational changes in reward processing across adolescence. Another consideration was the effects of menstrual cycle on reward processing (Dreher et al., 2007). Few if any studies examining the FRN and reward-related feedback control for the effects of luteal phase. This question, while important, is probably best suited to a study that explicitly assesses females with a regular menstruation cycle, at different luteal phases. Of some relevance, we did not observe sex effects in this study, for ITC or ERSP. Finally, because our sample ranged from 10-17 years, we can only generalize to these age groups. An adult sample is clearly warranted for comparisons to our older group.
Conclusion
Our methodological approach and data processing pipeline provide some general guidelines for constraining future studies of oscillatory feedback processing in children and adolescents. They suggest that a more fine-grained look at the theta band, in terms of frequency and time, may provide a window into subcomponent processes within feedback processing and in relation to age trends. A growing body of work implicates the FRN as a marker of depressive symptoms (Foti & Hajcak, 2009) and also a correlate of risk taking (Crowley et al., 2009). Possibly, these effects reflect a common aspect of hedonic function. We have identified consistent sources of variation in the theta band for reward feedback in both ITC and less strongly for ERSP, that could serve as starting points for examination as neural correlates of hedonic function and phenotypic expression across development.
Highlights.
Fine-grained analysis of time-frequency decomposition with False Discovery Rate.
Greater EEG theta power/phase inter-trial coherence for non-rewards in adolescents.
A lack of differentiation in child reward vs. non-reward inter-trial EEG coherence.
A relative increase in inter-trial coherence for non-rewards in early adolescence.
Acknowledgements
This research was supported by NARSAD Young Investigator Award (MJC), Yale Interdisciplinary Research Consortium on Stress, Self-Control and Addiction Pilot project funding (MJC) through 1UL1RR024925-01 (R. Sinha); NIDA grants K01 DA034125 (MJC), RO1-DA-06025 (LCM), DA-017863 (LCM) and KO5 (LCM), and a grant from the Gustavus and Louise Pfeiffer Research Foundation (LCM). This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The mean FRN latencies across the three age groups were 289ms, 285ms, 275ms, respectively in our previous paper (Crowley et al., in press), thus we would capture activity coincident with the FRN in window used in the present study (0-578ms).
References
- Arnett Jeffrey. Reckless behavior in adolescence: A developmental perspective. Developmental Review. 1992;12(4):3–39-373. [Google Scholar]
- Asada H, Fukuda Y, Tsunoda S, Yamaguchi M, Tonoike M. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neuroscience Letters. 1999;274(1):2–9-32. doi: 10.1016/s0304-3940(99)00679-5. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57(1):2–89-300. [Google Scholar]
- Buzsáki G. Rhythms of the Brain. Oxford University Press; New York: 2006. [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28(1):6–2-77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Eisenberg I, Guitart-Masip M, Huys Q, Frank MJ. Frontal Theta Overrides Pavlovian Learning Biases. Journal of Neuroscience. 2013;33(19):8–541-8548. doi: 10.1523/JNEUROSCI.5754-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Figueroa CM, Cohen MX, Frank MJ. Frontal theta reflects uncertainty and unexpectedness during exploration and exploitation. Cerebral Cortex. 2012;22(11):2–575-2586. doi: 10.1093/cercor/bhr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJ. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49(4):3–198-3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49(2):2–20-238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nat Neurosci. 2010;13(6):6–69-671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Fell J. Oscillatory activity and phase-amplitude coupling in the human medial frontal cortex during decision making. Journal of Cognitive Neuroscience. 2009;21(2):3–90-402. doi: 10.1162/jocn.2008.21020. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35(2):9–68-978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Crutcher C, Bailey CA, Lejuez CW, Mayes LC. Risk-taking and the feedback negativity response to loss among at-risk adolescents. Developmental Neuroscience. 2009;31(1-2):1–37-148. doi: 10.1159/000207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Hommer R, South M, Molfese PJ, Fearon RMP, Mayes LC. A developmental study of the feedback-related negativity from 10-17 years: Age and sex effects for reward vs. non-reward. Developmental Neuropsychology. 2013:4–83-495. doi: 10.1080/87565641.2012.694512. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, McCreary S, Miller K, Mayes LC. Implementation of false discovery rate for exploring novel paradigms and trait dimensions with ERPs. Developmental Neuropsychology. 2012;37(6):5–59-577. doi: 10.1080/87565641.2012.694513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–-22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–-21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- DiClemente Ralph J., Hansen William Bunker, Ponton Lynn E. Handbook of adolescent health risk behavior. Plenum Press; New York, NY: 1996. [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences. 2007;104(7):2–465-2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Mock B, Kray J. Developmental differences in learning and error processing: evidence from ERPs. Psychophysiology. 2009;46(5):1–043-1053. doi: 10.1111/j.1469-8986.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology. 2009;81(1):1–-8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Frank Michael J., Claus Eric D. Anatomy of a decision: Striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychological Review. 2006;113(2) doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6–885-6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Developmental Science. 2007;10(2):F–8-F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gehring William J., Willoughby Adrian R. Medial prefrontal cortex and rapid processing of monetary gains and losses. Science. 2002;295:2–279-2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):4–68-484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Groppe DM, Urbach TP, Kutas M. Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review. Psychophysiology. 2011;48(12):1–711-1725. doi: 10.1111/j.1469-8986.2011.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71(2):1–48-154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Li SC, Müller V, Lindenberger U. Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. Journal of Cognitive Neuroscience. 2011;23(3):5–79-592. doi: 10.1162/jocn.2010.21475. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):6–79-709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Research. 2006;1105(1):9–3-101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holroyd Clay B., Nieuwenhuis Sander, Yeung Nick, Cohen Jonathan D. Errors in reward prediction are reflected in the event-related brain potential. NeuroReport. 2003;14(18):2–481-2484. doi: 10.1097/00001756-200312190-00037. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology. 1999;110(6):1–149-1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kahneman Daniel, Tversky Amos. Prospect Theory: An Analysis of Decision under Risk. Econometrica. 1979;47(2):2–63-291. [Google Scholar]
- Lage-Castellanos A, Martinez-Montes E, Hernandez-Cabrera JA, Galan L. False discovery rate and permutation test: an evaluation in ERP data analysis. Statistics in Medicine. 2010;29(1):6–3-74. doi: 10.1002/sim.3784. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A. Analysis of dynamic brain oscillations: methodological advances. Trends in Neurosciences. 2007;30(7):3–65-373. doi: 10.1016/j.tins.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin Will M., Zvolensky Michael J., Pedulla Christina M. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence. 2003;26(4):4–75-479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Luu Phan, Tucker Don M., Derryberry Douglas, Reed Marjorie, Poulsen Catherine. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14(1):4–7-53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Mackiewicz Seghete KL, Cservenka A, Herting MM, Nagel BJ. Atypical spatial working memory and task-general brain activity in adolescents with a family history of alcoholism. Alcoholism: Clinical and Experimental Research. 2013;37(3):3–90-398. doi: 10.1111/j.1530-0277.2012.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig Scott, Debener Stefan, Onton Julie, Delorme Arnaud. Mining event-related brain dynamics. Trends in Cognitive Sciences. 2004;8(5):2–04-210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J, Cucurell D, Cunillera T, Garcia R, Andres-Pueyo A, Munte TF, Rodriguez-Fornells A. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46(1):2–41-248. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Müller SV, Möller J, Rodriguez-Fornells A, Münte TF. Brain potentials related to self-generated and external information used for performance monitoring. Clinical Neurophysiology. 2005;116(1):6–3-74. doi: 10.1016/j.clinph.2004.07.009. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):3–29-337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci. 2002;5(2):9–7-98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433(7028):8–73-876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146(4):1–435-1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual Review of Neuroscience. 2000;23:4–73-500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Sutton Richard S., Barto Andrew G. Reinforcement learning : an introduction. MIT Press; Cambridge, Mass: 1998. [Google Scholar]
- van de Vijver I, Ridderinkhof KR, Cohen MX. Frontal oscillatory dynamics predict feedback learning and action adjustment. Journal of Cognitive Neuroscience. 2011;23(12):4–106-4121. doi: 10.1162/jocn_a_00110. [DOI] [PubMed] [Google Scholar]
- van den Bos W, Cohen MX, Kahnt T, Crone EA. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex. 2012;22(6):1–247-1255. doi: 10.1093/cercor/bhr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology. 2007;17(2):2–51-257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Zottoli TM, Grose-Fifer J. The feedback-related negativity (FRN) in adolescents. Psychophysiology. 2012;49(3):4–13-420. doi: 10.1111/j.1469-8986.2011.01312.x. [DOI] [PubMed] [Google Scholar]