Abstract
PURPOSE
To describe new options for diagnosis and severity grading of dry eye disease
DESIGN
Perspective on technological advancements to identify tear dysfunction and their value in diagnosing and grading dry eye disease.
METHODS
Evidence is presented on new and evolving technologies to measure tear stability, composition and meniscus height and their role in dry eye diagnosis and therapeutic efficacy grading is assessed.
RESULTS
Evolving concepts regarding pathogenesis and new technologies to evaluate the tears and ocular surface have improved the ability to diagnose, classify and grade the severity of dry eye disease. New technologies include noninvasive imaging of tear stability and tear meniscus height as a measure of tear volume and tear composition (osmolarity, lacrimal factors, inflammatory mediators, growth and differentiation factors). Approved tests, such as tear osmolarity and tear imaging, are being integrated into clinical practice and may eventually supplant certain traditional tests that have greater variability and less sensitivity. Other tests, such as molecular assays of tears and conjunctival cells are currently being used in studies investigating pathogenesis and therapeutic mechanism of action. They may eventually translate to routine clinical practice.
CONCLUSIONS
New technologies have emerged that can noninvasively evaluate the tears and measure disease-associated compositional changes. These tests are being integrated into clinical practice and therapeutic trials for diagnosis, classification and severity grading of dry eye disease.
Introduction
The past few years have provided a number of advancements in the understanding of dry eye disease particularly with regard to new testing options for diagnosis and grading of severity of this condition. The term keratoconjunctivitis sicca was created to describe the predominantly aqueous deficient dry eye found in Sjogren disease.1 Although historically the term dry eye has been applied, alternate terminology of dysfunctional tear syndrome was recommended by the Delphi panel in 2006 to emphasize that aqueous deficiency was not the only cause of tear dysfunction.2 The International Workshop on Dry Eye (DEWS) expanded the classification of dry eye disease in 2007.3 This Perspective describes the rationale and clinical application of new methods of diagnosis and evaluation of Tear Dysfunction present in Dry Eye Disease and Dysfunctional Tear Syndrome.
Current definition and classification of Dry Eye Disease/Dysfunctional Tear Syndrome
International Workshop on Dry Eye (DEWS) definition and classification
The Report of the International Workshop on Dry Eye Disease (DEWS) was published in 2007 and defines dry eye as a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface.3 It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.3 The classification system proposed by the same workshop (Figure 1) identified separate categories of aqueous deficient dry eye and evaporative dry eye as distinct etiological entities, both of which may occur in any given patient. Recent research has revealed that both mechanisms frequently coexist and that the evaporative form is more frequent than aqueous deficiency alone.3,4,5
Figure 1.
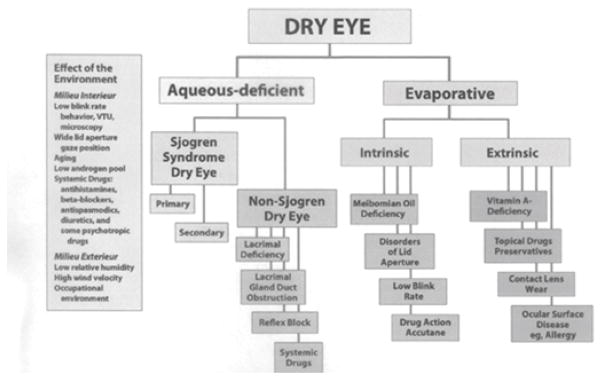
International Workshop on Dry Eye: Classification of Dry Eye Disease
(Adapted from: The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75–92)
A further thesis of the DEWS Report was that elevated tear osmolarity and inflammation of the ocular surface/lacrimal system were critical elements in the pathogenesis of the disease.6 Recent research confirms the importance of both elevated tear film osmolarity, and inflammation, as well as the interrelationship between those features 7,8 Methods for measurement of both osmolarity and inflammation have improved and are now available as point-of-service testing.9,10
Identifying Disease of the Lacrimal Functional Unit
The Lacrimal Functional Unit regulates production, distribution and clearance of tears to meet ocular surface demands.11 Disruption of this integrated functional unit at any point can result in tear instability, clinically significant tear dysfunction and ocular surface inflammation.12 Both lacrimal gland disease/dysfunction with tear hyposecretion or meibomian gland disease with increased tear evaporation can reduce tear volume. In contrast, diseases that interfere with tear distribution and clearance, such as conjunctivochalasis, commonly have normal or even elevated tear volume, but an abnormal tear composition that is associated with tear instability and ocular surface epithelial disease.12–14 Ocular irritation and ocular surface dye staining, traditional measures to diagnose and gauge severity of tear dysfunction, are common to all conditions affecting the lacrimal functional unit. Tests to accurately evaluate tear stability and measure tear production/volume may improve the ability to identify disease in the lacrimal functional unit and diagnostic classification of tear dysfunction. Furthermore, there is increasing evidence suggesting that molecular assays to measure lacrimal secreted tear constituents or levels of inflammatory/differentiation factors in the conjunctival epithelium may also be useful parameters for diagnosis and severity grading.
Noninvasive measurement of tear stability and tear volume
Tear stability has been traditionally measured by fluorescein tear break-up time; however, several instruments have been introduced in the past decade that use image analysis software to evaluate tear smoothness and stability noninvasively in sequentially captured images of ring mires reflected off the precorneal tear layer. Studies using these devices have found more rapid and extensive ring distortion (break-up) in tear dysfunction that correlates with severity of corneal epithelial disease.15,16 These noninvasive devices appear to have advantages over the conventional invasive fluorescein break-up method because they evaluate more data points and the software can detect alterations that may not be visually apparent. It is likely that these non-invasive methods will gain popularity for clinical evaluation and as therapeutic endpoints.
Until recently, there have been no commercially available clinical tests to evaluate tear meniscus dimensions as a measure of tear volume. Clinicians have relied on the Schirmer test as a indirect measure of tear production and volume; however, it is widely recognized that this test is variable and capable of inducing reflex tearing that can mask low basal tear production and volume. Biomicrosopic measurement of tear meniscus height and use of reflective meniscography have not gained widespread acceptance.16 Noninvasive direct high-resolution measurement of tear meniscus dimension by anterior segment optical coherence tomography represents a major advance in the ability to gauge tear volume in unstimulated basal conditions.13 A number of published studies have used optical coherence tomography to compare tear volume to clinical ocular surface disease parameters and have found that optical coherence tomography has the potential to sub classify tear dysfunction.18,19 Optical coherence tomography-measured inferior tear meniscus height was found to be a surrogate for tear volume. This parameter was reduced in patients with aqueous tear deficiency and correlated with clinical parameters, including non-invasive tear break up time and severity of corneal fluorescein staining.18 An inferior tear meniscus height <0.30mm was found to have sensitivity of 67% and specificity of 81% for dry eye using the Japanese Dry Eye criteria.20 The ability of this technology to stratify tear dysfunction based on tear volume may improve diagnostic classification for epidemiological studies, therapeutic decision-making and clinical trials. Alex and colleagues found a moderate statistically significant inverse correlation between optical coherence tomography measured tear meniscus height and severity of corneal fluorescein staining in response to a low humidity environment.21
Measurement of compositional changes of tear film
Osmolarity of the tear film has been measured in the research setting for many years. Determination of osmolarity can be measured by freezing point depression, vapor pressure, or electrical impedance/conductance of a solution. The Clifton osmometer was most often used to measure freezing point depression but was cumbersome in the handling of microvolumes of tear and prolonged duration of measurement.22 The Advanced Instruments Nanoliter Osmometer (Advanced Instruments, Inc, Norwood, MA), although less cumbersome, was also hampered by the manipulation of small quantities of tear with micropipettes.21 The Wescor vapor pressure osmometer (Wescor, Inc, Logan, UT) was easy to use for larger volumes of fluid but not reproducible for small samples of clinically obtainable tears,23,24 The TearLab instrument (TearLab, Inc, San Diego, CA) accurately measures osmolarity of 50 nanoliter microvolumes of tears in a rapid assay based upon lab-on-a-chip measurement of electrical impedance/conductance.9 The TearLab instrument is most adaptable to use in the clinic setting and has received Food and Drug Administration clearance for such use. This technique is highly accurate with a variance of less than 1% in control solutions.
Elevated osmolarity of the tear film occurs in all types of dry eye and thus cannot differentiate aqueous deficient from evaporative dry eye. It is, nonetheless, a reliable indicator of dry eye and now can be measured in the clinic setting in a rapid assay with the TearLab system.
Reduced levels of various constituent proteins in the tear film have been documented in tear dysfunction. Historically these proteins have been measured by agar diffusion, radial immunodiffusion, electrophoresis, enzyme linked immunosorbent assay and mass spectroscopy. 26–29 One of the first proteins found to be reduced in dry eye disease was lysozyme. It was measured by the degree of diffusion into a gel of agar infused with micrococcus lysodiekticus bacteria over a 3 day incubation.27 Lactoferrin has also been shown to be reduced in tears with tear dysfunction by both radial immunodiffusion and enzyme linked immunosorbent assay testing.28,29 High performance liquid chromatography further has revealed reduced lactoferrin levels29 as has mass spectroscopy.30 Elevated levels of matrix metalloproteinases have been measured in dry eye disease and probably reflect related inflammation.31,32
More sophisticated testing by proteomic analysis has been applied to evaluation of tear proteins and promises to amplify our understanding of both normal and abnormal tear physiology.33–37 The complexity of the multiple proteins in tears in both health and disease requires comprehensive and precise measurement of individual proteins and their interactions.33 At the present time up to 1543 different proteins have been identified in normal tears.35 Patterns of altered protein composition in dry eye also have been identified.36 Although the sophisticated equipment needed to perform such proteomic testing is still limited to academic centers, reports of techniques applicable to the clinical setting are emerging.36,38
Measurement of inflammatory/differentiation biomarkers
As noted, biomarkers are an evolving option for diagnosis and severity grading of tear dysfunction. In 1998, the National Institutes of Health Biomarkers Definitions Working Group defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”39 Biomarkers assessing lacrimal gland function and ocular surface health and differentiation have been identified. Immunoassays and biochemical methods have been used to detect proteins secreted by the lacrimal glands (e.g. lactoferrin, epidermal growth factor) or produced by epithelial or inflammatory cells on the ocular surface. Studies have reported reduced concentrations of lacrimal proteins, increased concentrations of inflammatory mediators and altered concentrations of mucins or differentiation-associated proteins (e.g. MUC5AC) in patients with aqueous tear deficiency.27–31, 40–42 Elevated S100AB and A9 peptides were detected in tears of patients with meibomian gland disease.43 Increased matrix metalloproteinase-9 activity in the tears has been found in aqueous tear deficiency and meibomian gland disease.31,45 Potential problems with tear assays that increase variability of test results include inability to obtain an adequate sample in patients with low tear volume or induction of reflex tearing during sample collection.
As an alternative to tear based assays, differentiation factors and inflammatory mediators can also be assayed in conjunctival cells removed from the conjunctival surface with a brush or membrane. Proteins have been measured by immunostaining or flow cytometry and levels of mRNA transcripts by polymerase chain reaction.31,45,46 Cellular expression of biomarkers provide a snapshot of the cell status since they reflect events at the instant of collection, whereas, tear sampling annoyingly influenced by the potential for reflex tearing, may provide a more blurred picture.
Table 1 lists biomarkers that have been found to have moderate to strong correlation with clinical parameters, or have changed in response to treatment in clinical trials.
Table 1.
Biomarkers of Dry Eye Disease with Moderate to High Clinical Correlation or Responding to Treatment*
| Marker | Clinical Correlation | Reference |
|---|---|---|
| HLA-DR | Decreased with CsA and Tofacitinab treatment | 45,56 |
| MMP-9 | Symptom severity, corneal fluorescein staining, conjunctival lissamine green staining | 31 |
| Tear EGF | Ocular surface rose Bengal staining, corneal fluorescein staining, conjunctival lissamine green staining | 40,65 |
| Tear IL-6 | Ocular surface rose bengal staining, corneal fluorescein staining, conjunctival lissamine green staining | 40,65 |
| Tear IL-8, MIP-1α, IL-1β | Corneal fluorescein staining, conjunctival lissamine green staining, | 40 |
| Tear CXCL9 (I-TAC) | Basal tear secretion, keratoepitheliopathy, goblet cell density | 66 |
| Tear proteins S100A8 and A9 Lactoferrin Lipocalin |
In subjects with MGD Grittiness, transient blur Eye pain and tearing Tearing, lid heaviness | 43 |
| MUC16 mRNA MUC16 cellular MUC5AC tears |
Tear meniscus Lissamine green staining, Dry eye symptom questionnaire Lissamine green staining |
67 |
Correlation coefficient ≥ 0.35
HLA-DR= human leukocyte antigen DR, MMP-9=matrix metalloproteinase 9, EGF=epidermal growth factor, IL-6=interleukin 6, IL-1= interleukin 1, MIP-1α= macrophage inflammatory protein 1, MGD: meibomian gland disease, CXCL9= chemokine (C-X-C motif) ligand 9, MUC= mucin
Until recently, assays to measure inflammatory biomarkers mediators in tears and conjunctival epithelium have been performed in research studies at academic centers to evaluate risk factors and pathogenesis. A semi quantitative commercial immunoassay to measure tear matrix metalloproteinase-9 has been approved in Europe, Canada and most recently in the US.10
Role of Osmolarity Testing
The early testing of elevated osmolarity in dry eye disease concentrated on determining a referent level the threshold of which would provide evidence of tear dysfunction. The work of Farris and Gilbard identified a reliable level of osmolarity in dry eye disease with acceptable sensitivity and specificity.22,47 They also observed variability between eyes and with repeated measurement, but did not pursue the diagnostic implications of this variability.48 Their work was primarily performed in moderate to severe dry eye patients and thus a referent level of 312 mOsm/ml was recommended as the diagnostic threshold.22,47 Subsequent meta-analysis of multiple studies of osmolarity refined the recommended threshold for diagnosis of dry eye disease to 316 mOsm/ml.49
More recent investigation has identified the validity of tear osmolarity as one of the best objective measures of dry eye.50,51 A hallmark of normal tears is the lack of variability between eyes and with repeated measurement.51 Further, the observed variability between eyes or multiple measurements in the same eye has established a means of identifying those patients with early/mild tear dysfunction before manifestations of more advanced disease occur.51 The current explanation for the development of tear dysfunction is that when the normal homeostasis of the tear film is disturbed, variability of tear osmolarity ensues and as the severity of the disorder progresses, a higher level of osmolarity is established with more variability and sustained elevated osmolarity.25
The TearLab Osmometer allows the practical application of measurement of tear osmolarity. The recommended protocol is to obtain the sample of tear from the inferior tear meniscus at the lateral lid using the tip of the handpiece of the TearLab instrument prior to any other examination or intervention, particularly avoiding instillation of any solution or medication. [Figure 2] The handpiece is then placed in the recording platform per the manufacturer’s recommendation. A calculated value of osmolarity is available in about 20 seconds. Both eyes should be measured independently because variability of elevation of osmolarity between eyes increases with severity of disease but does not occur in normal eyes.7 [Figure 3]. Interpretation of the results is by noting whether there is a level of 308 mOsm/ml in either eye or a variance between eyes of 8 mOsm/ml. If those parameters are met or if a subsequent measurement demonstrates variability of measured value, a diagnosis of tear dysfunction/dry eye disease can be made. Caveats for measurement are that the instrument should be housed in a temperature-controlled room (room temperature) away from heat source or cooling vent.
Figure 2.
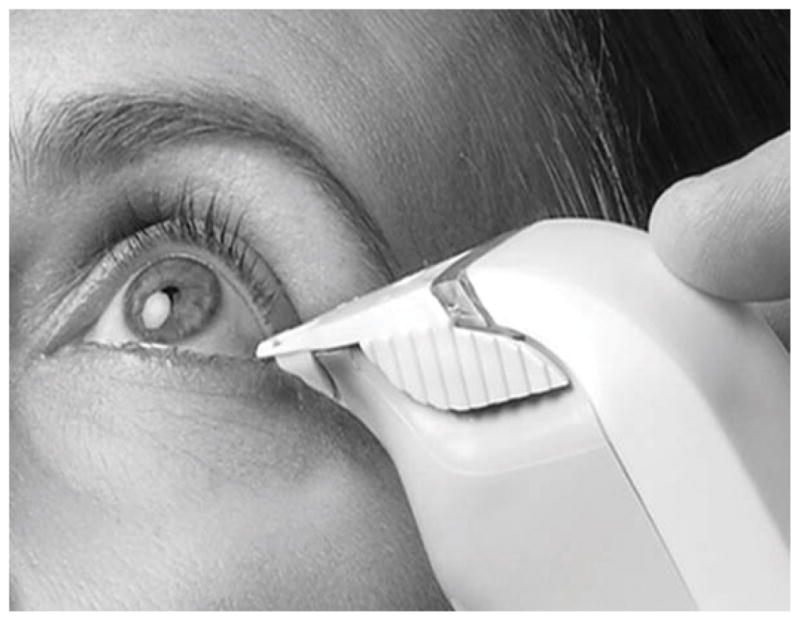
TearLab Osmometer Instrument in clinical use
(Courtesy of TearLab, Inc, San Diego, CA)
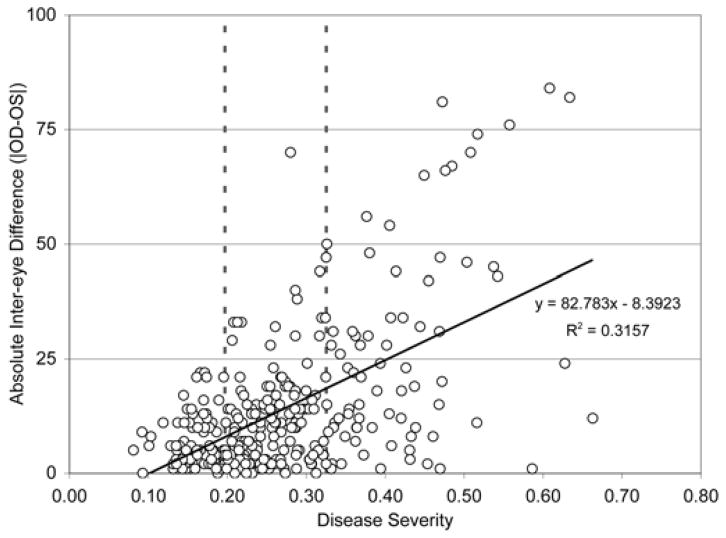
Figure 3.
Inter-eye variability of tear osmolarity in dry eye disease.
There is increasing variability of tear osmolarity with increasing severity of dry eye disease. Absolute inter-eye difference is in mOsm/L
(Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792–798.)
The value of measuring tear osmolarity is not only for diagnosis, but also for grading severity of disease and possible monitoring of therapy. In fact, in one clinical therapeutic trial, only tear osmolarity was a reliably responsive parameter of tear dysfunction/dry eye disease.51 In the authors experience, the reduction in tear osmolarity precedes by 2–4 weeks the subjective improvement of the condition when treating with topical cyclosporine. A recent report questions the reliability of change in osmolarity as a therapeutic monitor despite evidence from other clinical trials.52,53
Role of measuring lacrimal and inflammatory biomarkers
There are several potential roles for measuring lacrimal and inflammatory biomarkers. This information may improve the ability to diagnose the impact of tear dysfunction on the ocular surface, particularly in early disease where signs of inflammation or abnormal differentiation may be detected in the absence of ocular surface dye staining. Concentrations of lacrimal gland secreted factors in tears may improve ability to identify lacrimal gland dysfunction. The biomarkers may improve the ability to gauge disease severity and guide therapeutic decision-making. For example, when high levels of matrix metalloproteinases are present, therapies that decrease production or activity of matrix metalloproteinases would be indicated. If increased levels of T helper (Th) cytokines, such as IL-17A or IFN-γ are detected, the treatment with T cell immunomodulatory therapy, such as cyclosporine A would be appropriate.54,55 Finally, biomarkers may prove useful in future clinical trials to improve the ability to identify responders, investigate mechanism of action and monitor therapeutic efficacy. Certain inflammatory markers, such as class II human leukocyte antigen have shown to be sensitive efficacy parameters in therapeutic trials, and they may prove to be less variable than ocular surface dye staining.44,56
Role of evaluation of tear film stability
Instability of the tear film is a defined feature of tear dysfunction tear dysfunction/dry eye disease. The stability of the tear film historically in clinical practice has been assessed by the tear film break up test conducted either after fluorescein instillation or non-invasively.15,26,57 The test assesses the time taken for a random break to appear in the tear film after a spontaneous blink within a deliberately extended blink interval. In normal subjects, the values reported for tear break up are greater than the normal blink interval during standard conditions of temperature and humidity. The relationship between the blink interval and break up time can be characterized by determining the ocular protection index, which is the breakup time divided by the blink interval.58 A value for the ocular protection index of <1 indicates that breakup of the film is occurring within the blink interval. With a value of ≥ 1, the break up time recorded with blink suppression exceeds the blink interval and the eye is protected from desiccation throughout the blink cycle. In early dry eye disease the ocular protection index is initially > 1 and falls towards 1 as tear osmolarity is driven up, either by a raised evaporation rate (as in evaporative dry eye) or by evaporation from a reduced preocular tear film volume (as in aqueous deficient dry eye). Later, as the disease progresses and the ocular protection index falls below 1, hyperosmolarity is amplified locally by the increase in evaporation at the site of tear break-up and heightened by the defective lipid layer, expanding centrifugally as the breakup enlarges. This mechanism will raise the osmolarity within the exposed epithelial cells locally, subjacent to the breakup, the effect being greater, the earlier the onset of breakup. Modeling considerations suggest that a wave of hyperosmolarity, whose peak is at the starting point of the break up, spreads outwards as the break up expands, reaching levels that depend on the duration of the break up and the ambient conditions. Local tear instability, giving rise to local drying, can be an independent starting point for tear hyperosmolarity and dry eye disease. Tear hyperosmolarity can be initiated by a loss of ocular surface wettability, leading to tear break-up and an ocular protection index < 1.
Normally, tear dynamics are tightly regulated to maintain a smooth and optically effective precorneal tear film while performing a range of tasks, but in dry eye disease these homeostatic mechanisms break down, provoking tear film instability and visually disturbing optical aberrations.3,37 Clinically, dry eye patients have fluctuating vision, a decrease in contrast sensitivity, and an increase in forward scatter and glare.59,60 Many approaches have been used to study tearfilm stability and its effect on visual function. However, a functional measure of visual acuity has been developed and may ultimately be adaptable to the clinical venue 61–63.
Role of evaluating tear volume
Direct measurement of tear volume will assist in classifying patients with tear instability into aqueous deficient and aqueous sufficient subgroups. Patients with aqueous tear deficiency appear to benefit from therapies that improve lacrimal gland function, stimulate tear production, increase tear volume or treat the secondary conjunctival squamous metaplasia and goblet loss that is associated with lacrimal gland hyposecretion.63–65 In contrast, patient with normal or increased tear volume may need treatments to improve tear clearance, distribution and stability or decrease ocular surface inflammation. These patients may develop bothersome epiphora or worsening ocular surface or lid margin inflammation if they receive punctal occlusion therapy. Additionally, optical coherence tomography provides the opportunity to evaluate the effects of conjunctivochalasis on the tear meniscus and distribution of tears along the lower lid margin.14
Future options
As technology advances there will undoubtedly be new methods for measuring analytes in microvolumes of tears or by identifying biomarkers on the ocular surface. Once validation of the technique is achieved in laboratory work, the application of such techniques to the clinical setting as an integral part of the ocular evaluation will also require validation. Given the multifactorial nature of dry eye disease/tear dysfunction, we can expect to see an expanding number of candidate markers of the disorder.
Acknowledgments
Financial Support: NIH Grant EY11915 (SCP), NEI/NIH core Grant for Vision Research EY-002520-37, an unrestricted grant from Research to Prevent Blindness, New York, NY (SCP), the Oshman Foundation, Houston, TX (SCP), the William Stamps Farish Fund, Houston, TX (SCP), Hamill Foundation, Houston, TX (SCP).
Footnotes
Contribution of authors: Interpretation of the data (GNF, SCP); Preparation, review, and approval of the manuscript (GNF, SCP).
BOTH AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST. GNF is consultant to Bausch and Lomb, Eleven Biotherapeutics, Insite Pharmaceuticals, Kala, Parion and R-Tech-Ueno. He owns stock in TearLab, Inc. SCP is consultant to Allergan, Bausch and Lomb, Glaxo Smith Kline and Oculeve and receives research support from Allergan, Bausch and Lomb and Glaxo Smith Kline.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gary N. Foulks, Department of Ophthalmology and Vision Science, University of Louisville, Louisville, KY
Stephen C. Pflugfelder, Ocular Surface Center, Cullen Eye Institute, Cullen Eye Institute, Department of Ophthalmology, Baylor College of Medicine, Houston, TX
References
- 1.Sjogren HSC. Zur kenntnis der keratoconjunctivitis sicca (Keratitis filiformis) bei Hypofunktion der Tranendrusen. Acta Ophthalmologica (Kbh) 1933;(Supp 2):1–151. [Google Scholar]
- 2.Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, et al. Dysfunctional tear syndrome. A Delphi approach to treatment recommendations. Cornea. 2006;25(8):90–7. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 3.The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 4.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea. 2012;31(5):472–478. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- 5.Bron AJ, Yokoi N, Gafney E, Tiffany JM. Predicted phenotypes of dry eye: Proposed consequences of its natural history. Ocul Surf. 2009;7(2):78–92. doi: 10.1016/s1542-0124(12)70299-9. [DOI] [PubMed] [Google Scholar]
- 6.Report of the Pathogenesis Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 7.Lemp MA, Bron AJ, Baudouin C, Benítez Del Castillo JM, Geffen D, Tauber J, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792–798. doi: 10.1016/j.ajo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005;31(5):186–193. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51(12):6125–6130. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- 10.Sambursky R, Davitt WF, 3rd, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013;131(1):24–28. doi: 10.1001/jamaophthalmol.2013.561. [DOI] [PubMed] [Google Scholar]
- 11.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: The interaction between ocular surface and lacrimal glands. Cornea. 1998;17(6):584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Stern ME, Schaumburg CS, Dana R, Calonge M, Niederkorn JY, Pflugfelder SC. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010;3(5):425–42. doi: 10.1038/mi.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumus K, Pflugfelder SC. Increasing prevalence and severity of conjunctivochalasis with aging detected by anterior segment optical coherence tomography. Am J Ophthalmol. 2013;155(2):238–242. doi: 10.1016/j.ajo.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Ward SK, Wakamatsu TH, Dogru M, Ibrahim OM, Kaido M, Ogawa Y, et al. The role of oxidative stress and inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2010;51(4):1994–2002. doi: 10.1167/iovs.09-4130. [DOI] [PubMed] [Google Scholar]
- 15.Gumus K, Crockett CH, Rao K, Yeu E, Weikert MP, Shirayama M, et al. Non invasive assessment of the tear film stability in patients with tear dysfunction using the Tear Film Stability Analysis System (TSAS) Invest Ophthalmol Vis Sci. 2011;52(1):456–61. doi: 10.1167/iovs.10-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong J, Sun X, Wei A, Cui X, Li Y, Qian T, et al. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea. 2013;32(5):716–21. doi: 10.1097/ICO.0b013e3182714425. [DOI] [PubMed] [Google Scholar]
- 17.Yokoi N, Bron A, Tiffany J, Brown N, Hsuan J, Fowler C. Reflective meniscometry: a non-invasive method to measure tear meniscus curvature. Br J Ophthalmol. 1999;83(1):92–7. doi: 10.1136/bjo.83.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Palakuru JR, Aquavella JV. Correlations among upper and lower tear menisci, nonivasive tear break-up time, and the Schirmer test. Am J Ophthalmol. 2008;145(5):795–800. doi: 10.1016/j.ajo.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu X, Gong L, Lu Y, Jin H, Robitaille M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjögren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmol. 2012;90(5):e359–66. doi: 10.1111/j.1755-3768.2012.02413.x. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim OM, Dogru M, Takano Y, Wakamatsu TH, Tsubota K, Fujishima H. Application of visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology. 2010;117(10):1923–9. doi: 10.1016/j.ophtha.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 21.Alex A, Edwards A, Hays JD, Kerkstra M, Shih A, de Paiva CS, et al. Factors predicting the ocular surface response to desiccating environmental stress. Invest Ophthalmol Vis Sci. 2013;54(5):3325–32. doi: 10.1167/iovs.12-11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbard JP, Farris RL, Santamaria J., 2nd Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96(4):677–81. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- 23.Yildiz EH, Fan VC, Banday H, Ramanathan LV, Bitra RK, Garry E, et al. Evaluation of a new tear osmometer for repeatability and accuracy, using 0.5-microL (500-Nanoliter) samples. Cornea. 2009;28(6):677–80. doi: 10.1097/ICO.0b013e318198396b. [DOI] [PubMed] [Google Scholar]
- 24.Gokhale M, Stahl U, Jalbert I. In situ osmometry: validation and effect of sample collection technique. Optom Vis Sci. 2013;90(4):359–65. doi: 10.1097/OPX.0b013e31828aaf10. [DOI] [PubMed] [Google Scholar]
- 25.Eldridge DC, Sullivan BD, Berg MD, Lemp MA, Durrie DS. Longitudinal variability of tear film osmolarity in normal and dry eye patients. Invest Ophthalmol Vis Sci. 2010;51(5):3379. [Google Scholar]
- 26.Methodologies to Diagnose and Monitor Dry Eye Disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):108–152. doi: 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 27.van Bijsterveld OP. Proceedings: The lysozyme agar diffusion test in the sicca syndrome. Ophthalmologica. 1973;167(5):429–32. doi: 10.1159/000306987. [DOI] [PubMed] [Google Scholar]
- 28.Lucca JA, Nunez JN, Farris RL. A comparison of diagnostic tests for keratoconjunctivitis sicca: lactoplate, Schirmer, and tear osmolarity. CLAO J. 1990;16(2):109–12. [PubMed] [Google Scholar]
- 29.Ohashi Y, Ishida R, Kojima T, Goto E, Matsumoto Y, Watanabe K, et al. Abnormal protein profiles in tears with dry eye syndrome. Am J Ophthalmol. 2003;136(2):291–9. doi: 10.1016/s0002-9394(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 30.Green-Church KB, Nichols KK, Kleinholz NM, Zhang L, Nichols JJ. Investigation of the human tear film proteome using multiple proteomic approaches. Mol Vis. 2008 Mar 7;14:456–70. [PMC free article] [PubMed] [Google Scholar]
- 31.Chotikavanich S, de Paiva CS, de-Quan Li, Chen JJ, Bian F, Farley WJ, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50(7):3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acera A, Rocha G, Vecino E, Lema I, Durán JA. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008;40(6):315. doi: 10.1159/000150445. [DOI] [PubMed] [Google Scholar]
- 33.Grus FH, Joachim SC, Pfeiffer N. Proteomics in ocular fluids. Proteomics Clin Appl. 2007;1(8):876–88. doi: 10.1002/prca.200700105. [DOI] [PubMed] [Google Scholar]
- 34.Jacob JT, Ham B. Compositional profiling and biomarker identification of the tear film. Ocul Surf. 2008;6(4):175–85. doi: 10.1016/s1542-0124(12)70178-7. [DOI] [PubMed] [Google Scholar]
- 35.Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, et al. In-depth analysis of the human tear proteome. J Proteomics. 2012;16:75(13):3877–85. doi: 10.1016/j.jprot.2012.04.053. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8(11):4889–905. doi: 10.1021/pr900686s. [DOI] [PubMed] [Google Scholar]
- 37.Karns K, Herr AE. Human tear protein analysis enabled by an alkaline microfluidic homogeneous immunoassay. Anal Chem. 2011;83(21):8115–22. doi: 10.1021/ac202061v. [DOI] [PubMed] [Google Scholar]
- 38.Versura P, Bavelloni A, Blalock W, Fresina M, Campos EC. A rapid standardized quantitative microfluidic system approach for evaluating human tear proteins. Mol Vis. 2012;18:2526–37. [PMC free article] [PubMed] [Google Scholar]
- 39.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Biomarkers Definitions Working Group. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 40.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198–205. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehm N, Riechardt AL, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest ophthalmol Vis Sci. 2011;52(10):7725–30. doi: 10.1167/iovs.11-7266. [DOI] [PubMed] [Google Scholar]
- 42.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43(4):1004–1011. [PubMed] [Google Scholar]
- 43.Tong L, Zhou L, Beuerman RW, Zhao SZ, Li XR. Association of tear proteins with Meibomian gland disease and dry eye symptoms. Br J Ophthalmol. 2011;95(6):848–852. doi: 10.1136/bjo.2010.185256. [DOI] [PubMed] [Google Scholar]
- 44.Afonso A, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1β concentration and fluorescein tear clearance. Invest Ophthalmol Vis Sci. 1999;40(11):2506–12. [PubMed] [Google Scholar]
- 45.Brignole F, Pisella PJ, De Saint Jean M, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001;42(1):90–5. [PubMed] [Google Scholar]
- 46.Corrales RM, Narayanan S, Fernandez I, Mayo A, Galarreta DJ, Fuentes-Páez G, et al. Ocular mucin gene expression levels as biomarkers for the diagnosis of dry eye syndrome. Invest Ophthalmol Vis Sci. 2011;52(11):8363–8369. doi: 10.1167/iovs.11-7655. [DOI] [PubMed] [Google Scholar]
- 47.Gilbard JP, Farris RL. Tear osmolarity and ocular surface disease in keratoconjunctivitis sicca. Arch Ophthalmol. 1979;97(9):1642–6. doi: 10.1001/archopht.1979.01020020210003. [DOI] [PubMed] [Google Scholar]
- 48.Farris RL, Stuchell RN, Mandel ID. Tear osmolarity variation in the dry eye. Trans Am Ophthalmol Soc. 1986;84:250–68. [PMC free article] [PubMed] [Google Scholar]
- 49.Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47(10):4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki M, Massingale ML, Ye F, Godbold J, Elfassy T, Vallabhajosyula M, Asbell PA. Tear osmolarity as a biomarker for dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51(9):4557–61. doi: 10.1167/iovs.09-4596. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan BD, Crews LA, Sonmez B, de la Paz MF, Comert E, Charoenrook V, et al. Clinical utility of objective tests for dry eye disease: Variability over time and implications for clinical trials and disease management. Cornea. 2012;31(9):1000–1008. doi: 10.1097/ICO.0b013e318242fd60. [DOI] [PubMed] [Google Scholar]
- 52.Scuderi G, Contestabile MT, Gagliano C, Iacovello D, Scuderi L, Avitabile T. Effects of phytoestrogen supplementation in postmenopausal women with dry eye syndrome: a randomized clinical trial. Can J Ophthalmol. 2012;47(6):489–92. doi: 10.1016/j.jcjo.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 53.Amparo F, Jin Y, Hamrah P, Schaumberg DA, Dana R. What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease? Am J Ophthalmol. 2014;157(1):69–77. doi: 10.1016/j.ajo.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27(1):64–9. doi: 10.1097/ICO.0b013e318158f6dc. [DOI] [PubMed] [Google Scholar]
- 55.De Paiva CS, Raince JK, McClellan AJ, Shanmugam KP, Pangelinan SB, Volpe EA, Corrales RM, Farley WJ, Corry DB, Li DQ, Pflugfelder SC. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011;4(4):397–408. doi: 10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang JF, Yafawi R, Zhang M, McDowell M, Rittenhouse KD, Sace F, Liew SH, Cooper SR, Pickering EH. Immunomodulatory effect of the topical ophthalmic Janus kinase inhibitor tofacitinib (CP-690,550) in patients with dry eye disease. Ophthalmology. 2012;119(7):e43–50. doi: 10.1016/j.ophtha.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 57.Mengher LS, Pandher KS, Bron AJ. Non-invasive tear film break-up time: Sensitivity and specificity. Acta Ophthalmol (Copenh) 1986;64(4):441–444. doi: 10.1111/j.1755-3768.1986.tb06950.x. [DOI] [PubMed] [Google Scholar]
- 58.Ousler GW, 3rd, Hagberg KW, Schindelar M, Welch D, Abelson MB. The ocular protection index. Cornea. 2008;27(5):509–513. doi: 10.1097/ICO.0b013e31816583f6. [DOI] [PubMed] [Google Scholar]
- 59.Rieger G. The importance of the precorneal tear film for the quality of optical imaging. Br J Ophthalmol. 1992;76(3):157–158. doi: 10.1136/bjo.76.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ridder WH, 3rd, LaMotte J, Hall JQ, Jr, Sinn R, Nguyen AL, Abufarie L. Contrast sensitivity and tear layer aberrometry in dry eye patients. Optom Vis Sci. 2009;86(9):E1059–68. doi: 10.1097/OPX.0b013e3181b599bf. [DOI] [PubMed] [Google Scholar]
- 61.Kaido M, Dogru M, Ishida R, Tsubota K. Concept of functional visual acuity and its applications. Cornea. 2007;26(9 Suppl 1):S29–35. doi: 10.1097/ICO.0b013e31812f6913. [DOI] [PubMed] [Google Scholar]
- 62.Kaido M, Matsumoto Y, Shigeno Y, Ishida R, Dogru M, Tsubota K. Corneal fluorescein staining correlates with visual function in dry eye patients. Invest Ophthalmol Vis Sci. 2011;52(13):9516–9522. doi: 10.1167/iovs.11-8412. [DOI] [PubMed] [Google Scholar]
- 63.Kaido M, Ishida R, Dogru M, Tamaoki T, Tsubota K. Efficacy of punctum plug treatment in short break-up time dry eye. Optom Vis Sci. 2008;85(8):758–763. doi: 10.1097/OPX.0b013e3181819f0a. [DOI] [PubMed] [Google Scholar]
- 64.Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330–7. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- 65.Enríquez-de-Salamanca A, Castellanos E, Stern ME, Fernández I, Carreño E, García-Vázquez C, Herreras JM, Calonge M. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Molecular Vision. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 66.Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51(2):643–50. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gipson IK, Spurr-Michaud SJ, Senchyna M, Ritter R, 3rd, Schaumberg D. Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye. Cornea. 2011;30(12):1346–1352. doi: 10.1097/ICO.0b013e31820d852a. [DOI] [PMC free article] [PubMed] [Google Scholar]