Abstract
Alkaline phosphatase (ALP; E.C.3.I.3.1.) is an ubiquitous membrane-bound glycoprotein that catalyzes the hydrolysis of phosphate monoesters at basic pH values. Alkaline phosphatase is divided into four isozymes depending upon the site of tissue expression that are Intestinal ALP, Placental ALP, Germ cell ALP and tissue nonspecific alkaline phosphatase or liver/bone/kidney (L/B/K) ALP. The intestinal and placental ALP loci are located near the end of long arm of chromosome 2 and L/B/K ALP is located near the end of the short arm of chromosome 1. Although ALPs are present in many mammalian tissues and have been studied for the last several years still little is known about them. The bone isoenzyme may be involved in mammalian bone calcification and the intestinal isoenzyme is thought to play a role in the transport of phosphate into epithelial cells of the intestine. In this review, we tried to provide an overview about the various forms, structure and functions of alkaline phosphatase with special focus on liver/bone/kidney alkaline phosphatase.
Keywords: Enzymes, Isoenzymes, Alkaline phosphatase, L/B/K alkaline phosphatase, Liver alkaline phosphatase, Intestinal alkaline phosphatase, Placental alkaline phosphatase
Introduction
Alkaline phosphatases [ALP; orthophosphoric monoester phosphohydrolase (alkaline optimum) EC 3.1.3.1] are plasma membrane-bound glycoproteins [1, 2]. These enzymes are widely distributed in nature, including prokaryotes and higher eukaryotes [3–6], with the exception of some higher plants [7]. Alkaline phosphatase forms a large family of dimeric enzymes, usually confined to the cell surface [8, 9] hydrolyzes various monophosphate esters at a high pH optimum with release of inorganic phosphate [10, 11].
Mammalian alkaline phosphatases (ALPs) are zinc-containing metalloenzymes encoded by a multigene family and function as dimeric molecules. Three metal ions including two Zn2+ and one Mg2+ in the active site are essential for enzymatic activity. However, these metal ions also contribute substantially to the conformation of the ALP monomer and indirectly regulate subunit–subunit interactions [12].
Isoforms of Alkaline Phosphatase and Their Distribution
Human ALPs can be classified into at least four tissue-specific forms or isozyme mainly according to the specificity of the tissue to be expressed, termed as placental alkaline phosphatase (PLALP or Regan isozyme), Intestinal alkaline phosphatase (IALP), liver/bone/kidney alkaline phosphatase (L/B/K ALP), germ cell ALP (GCALP or NAGAO isozyme) [13]. The enzyme products of at least three ALP gene loci (placental, intestinal and L/B/K) [14–16] are distinguishable in man by a variety of structural, biochemical and immunologic methods [17–19].
Placental Alkaline Phosphatase
The human placental ALP gene was mapped to chromosome 2 [20]. A homology of 87 % is found with the IAP gene. There are, however, differences at their carboxyl terminal end [21]. Placental ALP is a heat stable enzyme present at high levels in the placenta. A trace amount of this isoenzyme can be detected in normal sera [22]. Part of the serum placental-type activity originates from neutrophils. The placental ALP gene can be re-expressed by cancer cells as the Regan isoenzyme. Placental ALP is a polymorphic enzyme, with up to 18 allelozymes resulting from point mutations, in contrast to the other ALP isoenzymes [23].
Intestinal Alkaline Phosphatase
The gene encoding for intestinal ALP (IAP) is a member of the gene family mapping to the long arm of chromosome 2 [24]. IAP is partially heat-stable isozyme present at high levels in intestinal tissue. In contrast to the other ALP isoenzymes, the carbohydrate side-chains of IAP are not terminated by sialic acid [25]. Distinct IALPs can be isolated from fetal and adult intestinal tissue, with the fetus forming a sialylated isoenzyme in contrast to the adult. The fetal and adult forms differ not only in the carbohydrate content but also in the protein moiety itself, suggesting that a separate ALP gene locus may exist in humans during fetal development. This embryonic gene can be reexpressed (in a modified form) by cancer cells and is designated as Kasahara isoenzyme [26].
Germ Cell Alkaline Phosphatase
The gene encoding for germ-cell ALP (GCAP, placental-like ALP) was also mapped to chromosome 2 [27]. It is heat-stable isozyme present at low levels in germ cells [2] embryonal and some neoplastic tissues [28, 29]. It encodes testis/thymus ALP and can be expressed in the placenta at low levels [30]. GCAP in testis appears to be localized to the cell membrane of immature germ cells and, like the other ALP isoenzymes, is attached to the cell membrane by means of a phosphatidyl-inositol-glycan anchor. Like the placental ALP gene, it can be reexpressed by cancer cells (or NAGAO isozyme) [31].
Liver/Bone/Kidney Alkaline Phosphatase
The heat-labile isozyme represents the liver/bone/kidney or tissue nonspecific (TNSALP) form [1, 2]. It is expressed in many tissues throughout the body and is especially abundant in hepatic, skeletal, and renal tissue. Slight differences in electrophoretic mobility and thermo stability between the L/B/K ALPs from various tissues are attributed to differences in post-translational modification, although it is possible that their protein moieties are encoded by separate but related genes [19].
Liver/bone/kidney or tissue-nonspecific alkaline phosphatase (TNSALP) is encoded as a single genetic locus, mapped to the short arm of chromosome 1 [32, 33]. Komoda and Sakagishi [25] postulated a hypothesis regarding the physiological role of the sugar moieties in ALPS: they would protect the enzyme from rapid removal from the circulation through binding by the asialoglycoprotein receptors of the liver.
The evolution of the ALP gene family has presumably involved the duplication of a primordial tissue-nonspecific ALP gene to create the TNSALP gene and an intermediate IAP gene, followed by additional duplications of the latter to create intestinal, placental, and germ-cell ALP genes. Only humans and great apes have placental ALP; all other mammals have IAP [34].
Structure of the Gene
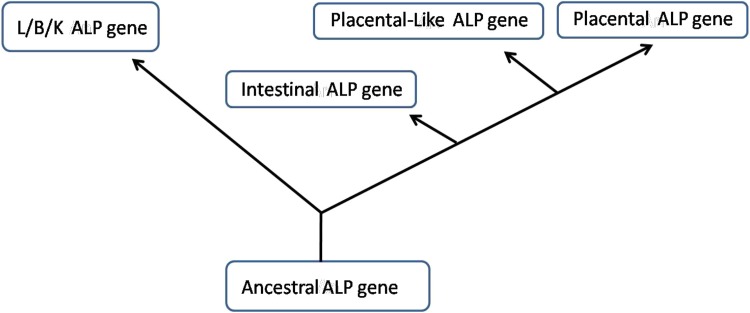
Alkaline phosphatase is a membrane-bound metalloenzyme that consists of a group of isoenzymes. Each isoenzyme is a glycoprotein encoded by different gene loci [35]. It is believed that all of the human ALP genes evolved from a single ancestral gene. Figure 1 shows a rough outline of the deduced evolutionary tree of ALP [19].
Fig. 1.
Illustration showing the postulated evolutionary relationships of the human liver/bone/kidney, intestinal, placental and placental-like genes [18]
Three ALP genes at chromosome 2q34–37 are expressed in essentially a tissue-specific manner and produce a placental, placental-like and intestinal ALP isoenzyme (PALP, PLALP and IALP respectively). The fourth ALP gene that is L/B/K ALP maps to the distal short arm of human chromosome 1, bands p34–p36.1 encodes a family of proteins [9, 35, 36]. Differential glycosylation of TNSALP gives rise to tissue-specific isoforms that differ from one another only by post-translational modification; these secondary ALP isoenzymes are present throughout the body, but individually are most abundant in hepatic, skeletal and renal tissue [37]. Accordingly, they are collectively called liver/bone/kidney or tissue-nonspecific ALP (TNSALP) [4].
Types and chromosomal locations of the ALP gene with their accession numbers are shown in Table 1. The L/B/K gene appears to be at least five times longer than each of the other three genes. The overall difference in length is due to very much longer introns in the L/B/K ALP gene (Fig. 2). The introns in the intestinal, placental and placental-like genes are all quite small (74–425 bp). The complete cDNA sequence of L/B/K ALP is known (Fig. 3) and the gene consists of 12 exons, compared with 11 in each of the other genes. The coding exons are 2–12. The additional exon is at the 5′ end in the non-coding region. Exons 2–12 are contained within 25 kb of DNA. The distance between exons 1 and 2 is at least an additional 25 kb of DNA. Thus, the entire gene is comprised of at least 50 kb of DNA [38].
Table 1.
Nomenclature of human ALP isozymes and gene including chromosomal location, gene size and accession numbers
| Gene | Protein name | Common name | Chromosomal location | Accession no. |
|---|---|---|---|---|
| ALPL | TNAP | Tissue-nonspecific alkaline phosphatase; TNSALP; liver/bone/kidney type AP | Chr1: 21581174–21650208 | NM_000478 |
| ALPP | PLAP | Placental alkaline phosphatase; PLALP | Chr2: 233068964–233073097 | NM_001632 |
| ALPI | IAP | Intestinal alkaline phosphatase; IALP | Chr2: 233146369–233150245 | NM_001631 |
| ALPP2 | GCAP | Germ cell alkaline phosphatase; GCALP | Chr2: 233097057–233100922 | NM_031313 |
Fig. 2.
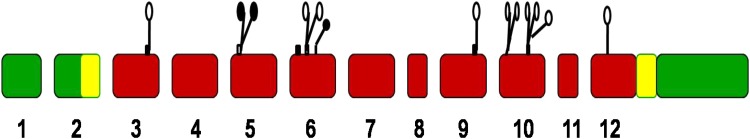
Relationship between exon organization and polypeptide structure of the L/B/K ALP gene. L/B/K ALP gene exons 1–12 are shown as large rectangles. Untranslated regions are indicated by green colour. The signal peptide at the amino terminus and the hydrophobic stretch of amino acids at the carboxyl terminus in exons 2 and 12, respectively, are shown in yellow. Regions which comprise the active pocket that are conserved in intestinal ALP, placental ALP, and E. coli ALP are shown as follows: small rectangles above the exons indicate conserved units of amino acid sequence which exist as discrete units of secondary structure in E. coli ALP (black for & sheets, white for a-helices); the open circles indicate metal ligands, and the closed circles indicate residues that directly interact with incoming substrate
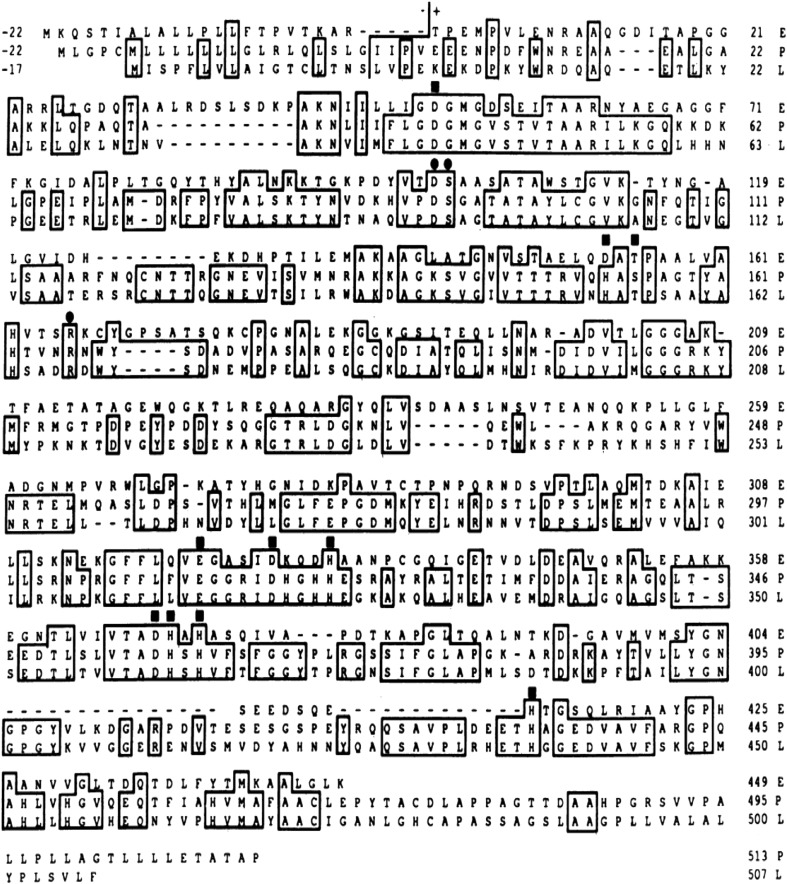
Fig. 3.
DNA sequence and deduced amino acid sequence of the L/B/K ALP cDNA. Numbers preceded by + or–refer to amino acid positions. All other numbers refer to nucleotide positions. Asterisks occur at 10-base intervals. Amino acids -17 to -1 comprise a putative signal peptide. A vertical line precedes amino acid +1, the amino-terminal residue found in the mature protein. Amino acid residues that have been determined by protein sequence analysis of purified liver ALP are underlined. Five potential N-linked glycosylation signals, Asn-Xaa-Thr/Ser, are boxed. A 12-bp direct repeat in the 3′ untranslated region of the cDNA is labeled by arrows. A single poly(A) addition signal AATAAA is underlined twice [10]
The sequences at the 5′ and 3′ ends of each intron are in agreement with the consensus sequence for intron-exon boundaries of other eukaryotic genes. All introns begin with the dinucleotide GT and end with AG. Intron number 1, at least 25 kb in length, interrupts the 5′ untranslated sequence 105 bp upstream of the initiation methionine codon. All other introns interrupt the gene within protein coding regions. Exon 12, about 1,025 bp, contains 263 nucleotides of coding sequence, the termination codon, and the entire 3′ untranslated region. At the end of exon 12, there are putative 3′-mRNA processing signals that are commonly found in other eukaryotic genes; the mRNA cleavage/polyadenylation site is flanked by the sequence AATAAA about 12 bp upstream, and a G/T-rich region about 12 bp downstream.
Characterization and Discrimination of the ALPs
Many different biochemical and immunological methods have been used to discriminate between and selectively assay the different ALPS at the enzyme and protein level. Three general methods have proved particularly useful: thermostability studies; differential inhibition with various aminoacids, small peptides and other low molecular weight substances; and immunologic methods [39].
Thermostability
The intestinal and L/B/K ALPs are rapidly inactivated at temperature >65 °C (Table 2). In contrast, placental and placental-like ALPS are remarkably thermostable. They may be heated at 65 °C for an hour or more without loss of activity. However, the intestinal ALP is somewhat more thermostable than the L/B/K ALP. It has also been shown that in serum, liver ALP is slightly, though significantly, more thermostable than bone ALP [39].
Table 2.
Relative thermostabilities of human ALPs [39]
| Human ALP | 56 °C (min) | 65 °C (min) |
|---|---|---|
| L/B/K | 7.4 | 1.0 |
| Intestinal | >60.0 | 6.5 |
| Placental and plac-like | – | >60.0 |
Time in minute required to give 50 % inactivation of different human ALPs at 56 °C and 65 °C
Inhibition Studies
Various low molecular weight substances show differential inhibition of the different ALPs. Table 3 summaries the effects with five inhibitors which have been extensively used. The L/B/K ALPs are more sensitive to inhibition with l-homoarginine (Har) than placental, placental-like or intestinal ALPs. In contrast, placental, placental-like and intestinal ALPS are about 30 times more sensitive to inhibition with l-phenylalanine (Phe) than the L/B/K ALPs. r,-Phenylalanyl-glycyl-glycine (Pgg) gives sharp differential inhibition between placental, intestinal and L/B/K ALPs. It also differentiates between placental ALP and placental-like ALP, which with this inhibitor more nearly resembles intestinal ALP. l-Leucine (Leu) characteristically gives much stronger inhibition with placental-like ALP than with the other ALPs. Levamisole (Leva) is a particularly potent inhibitor of L/B/K ALP, but has little inhibitory effect on the other ALPs [39].
Table 3.
Effects of various inhibitors on different Huaman ALPs [19]
| Inhibitors | ALP | |||
|---|---|---|---|---|
| L/B/K ALP | Intestinal | Placental | Plac-like | |
| l-Plenylalanine (Phe) | 31 | 0.8 | 1.1 | 0.8 |
| l-Homoarginine (Har) | 2.7 | 40 | >50 | 36 |
| l-Phenylalanineglycylglycine (Pgg) | 30.6 | 3.7 | 0.1 | 2.9 |
| l-Leucine (Leu) | 13.1 | 3.6 | 5.7 | 0.6 |
| Levamisole (Leva) | 0.03 | 6.8 | 1.7 | 2.7 |
Concentrations (nmol/l) of various inhibitors required to produce 50 % inhibition of different human ALPs under standardized conditions
Immunologic Studies
Antisera raised in rabbits against purified placental ALP cross-react with placental- like ALP and intestinal ALP, but not with L/B/K ALP. Complementary results are obtained with antisera raised against intestinal ALP or L/B/K ALP. These findings demonstrate that some, though not all, of the antigenic determinants detected on placental ALP are also present on intestinal ALP, but the placental and placental like ALPs are immunologically very similar. Some but not other monoclonals, raised against placental and intestinal ALPs react with both ALPs and some, though not other, monoclonals differentiate the placental and placental-like ALP. Combinations of these various biochemical and immunological techniques have been used to devise methods which give precise analytical information about the quantities of each of the ALPs when they are present together in a tissue extract or body fluid such as serum or amniotic fluid.
l-Phenylalanyl-glycyl-glycine (Pgg) gives sharp differential inhibition between placental, intestinal and L/B/K ALPs. Leva is particularly a potent inhibitor of L/B/K ALP, but has little inhibitory effect on other ALPs. It should be noted that these various inhibitors are stereospecific and uncompetitive [19].
Homology Between Different Isoforms
The complete amino acid sequences of ALP proteins are now known (Fig. 4). A computer-assisted comparison of E. coli (471 amino acids) [40], human placental (535 amino acids) [41] and human L/B/K ALP (524 amino acids) precursor proteins is shown in Fig. 4. Amino acid positions that are identical in all three proteins, or in the two human proteins, are depicted in boxed (Fig. 4). Gaps have been introduced into the protein sequences to maximize alignment of homologous regions.
Fig. 4.
Comparison of the amino acid sequences of E. coli (E), human placental (P), and human L/B/K (L) ALP precursor proteins. Gaps that have been introduced into the sequences to maximize pairing of homologous amino acids are indicated by -Amino acid +1 corresponds to the first residue in each of the mature proteins. Amino acids that are identical in all three proteins or in the two human proteins are boxed. Amino acids are shown in the single-letter code [10]
At the amino acid level, the tissue-specific ALP isoenzymes are 86–98 % identical to one another [9, 42], but 52–56 % identical when compared with TNSALP [4, 35]. IALP, PALP and GCALP are highly homologous with >90 % identical amino acid sequences, whereas TNSALP is significantly more diverse. At the DNA level, L/B/K and placental ALP are 60 % homologous in the coding regions but no homology is detected between the cDNA in the 5′ and 3′ untranslated regions. As expected, there is less homology between E. coli and mammalian ALPs. Thus, E. coli ALP is 25 % homologous to L/B/K ALP and 29 % homologous to placental ALP over the 47 % amino acids of the E. coli enzyme [37].
Several areas are highly conserved in all three ALP polypeptides. These are the same regions detected by Millan, 1986 [27] and Kam et al. 1985 [43] in their comparisons of placental and E. coli ALPs. These areas represent conservation of amino acids that comprise the active site region in the E. coli ALP [44]. There are also several regions that are conserved only between the human L/B/K and placental ALPs, presumably representing functions of mammalian ALPs not present in E. coli. Two N-linked glycosylation signals at homologous sites occur in the L/B/K and placental ALPs, though the L/B/K enzyme contains three additional glycosylation signals that are absent in placental ALP.
Molecular Modeling of L/B/K ALP Protein
Two zinc atoms are present in the active site and one calcium atom is present in the metal-binding site. Calcium is the natural ion that binds to the metal-binding domain. The effects of calcium on ALP activity should be reconsidered including in the analysis of the presence of calcium site. The precise biological role of calcium in TNSALP remains to be addressed. It is very interesting to observe that with the evolution and the specialization of the enzyme function, new features have been added: in E. coli, where there is no skeleton to mineralize, there is no calcium site in its ALP [9].
The model of TNSALP shows that the active site valley located on both sides of the active site contains a large number of polar residues. Thus, the hydrophobic residues, Trp168, Tyr169, and Tyr206 are surrounded by ionic residues. A basic residue, Arg433, is present close to the active site. The hydrophobic pocket is not conserved in TNSALP. However, the tyrosine, which enters in the active site of the other monomer (Tyr367 in PLALP), is conserved in TNSALP (Tyr371). This reinforces the idea that Tyr371 may contribute to the allosteric properties shared by the two enzymes. All residues that are essential for the hydrolytic activity of the bacterial and the other mammalian phosphatases are preserved in TNSALP, but those that confer substrate specificity are different.
The structural features that comprise the N-terminal helix involved in the dimer interface, the 76 residues of the calcium-binding-domain residues 211–289), and the interfacial “crown-domain” formed by the insertion of a 60-residue segment (371–431) from each monomer occur in TNSALP. Within the crown domain, a unique surface loop not present in the E. coli enzyme that extends from amino acids 400–430. This loop has been shown to play an important role in defining the conformation and stability of the ALP molecule. The loop is also partially responsible for the interaction of ALPs with extracellular matrix proteins, such as collagen. The TNSALP model shows that this loop is highly accessible and located at the very tip of the crown domain. This loop is responsible for the unique property of mammalian ALPs of being uncompetitively inhibited by a number of amino acids and small peptides (Fig. 5) [11].
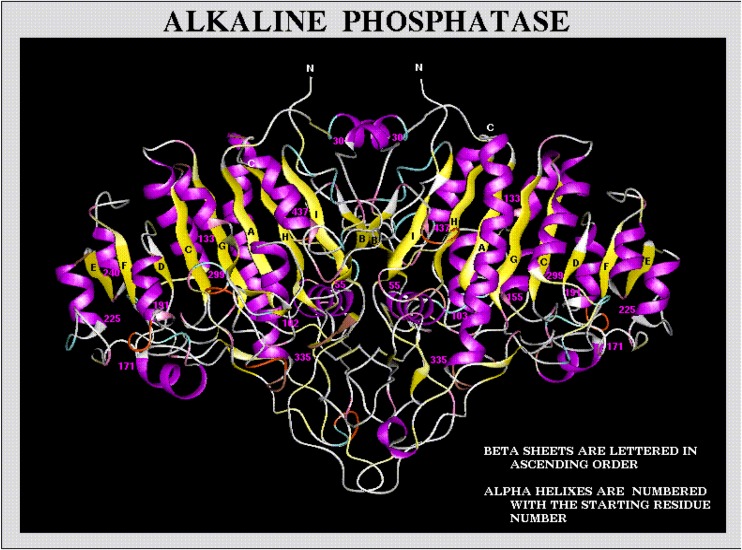
Fig. 5.
A ribbon diagram of L/B/K ALP protein structure. (http://biochem.dental.upenn.edu/)
Intracellular Calcium and L/B/K ALP
The three dimensional structural model of human TNSALP was proposed by Mornet in 2001 [9]. According to this model, one of the feature that differentiate ALP of mammals from that of E. coli is the acquisition of a calcium binding site during evolution in addition to two zinc and one magnesium binding sites indispensable for ALP activity. Human alkaline phosphatase has four metal binding sites -two for zinc, one for magnesium, and one for calcium ion. Calcium helps to stabilize a large area that includes loops 210–228 and 250–297 [45]. The calcium atom in TNSALP is assumed to be coordinated by four amino acid residues (Glu218, Phe273, Glu274 and Asp289) and a water molecule. Calcium binding is crucial for the proper folding and correct assembly of newly synthesized TNSALP molecule. It has been demonstrated that loss of calcium binding potency has a deleterious effect on biosynthesis of the TNSALP molecule. It might result in misfolded ALP molecule. There is increasing evidence that many misfolded proteins are retained in the ER or moved from cis-golgi to the ER as part of the quality control system, thus permitting only properly folded and assembled proteins to move to their final destination However, the physiological importance of this calcium binding site of TNSALP remains obscure [46].
Physiological Functions of ALP
Since its first description by Suzuki and colleagues [47] in 1907, alkaline phosphatase (ALP) has been investigated continuously and extensively. But little is known regarding the physiological function of ALPs in most tissues except that the bone isoenzyme has long been thought to have a role in normal skeletal mineralization [48]. The natural substrates for TNSALP appear to include at least three phosphor compounds: phosphoethanolamine (PEA), inorganic pyrophosphate (PPi), and pyridoxal-5′-phosphate (PLP), as evidenced by increased plasma and/or urinary levels of each in subjects with hypophosphatasia [49, 50], but this is uncertain. Indeed, a variety of mechanisms have been proposed to explain the role of ALP in bone mineralization [51]. However, apart from its role in normal bone mineralization, the other functions of L/B/K remains obscure both in physiological and neoplastic conditions.
Alkaline Phosphatase in Health and Diseases
The activity of liver and bone alkaline phosphatases in serum has been applied extensively in routine diagnosis. Values for each isoenzyme in healthy individuals of different ages are reported together with results obtained in various diseases. Data from normal subjects shows that bone alkaline phosphatase contributes about half the total alkaline phosphatase activity in adults. The normal serum range of alkaline phosphatase is 20 to 140U/L. The enzyme alkaline phosphatase is an important serum analyte and its elevation in serum is correlated with the presence of bone, liver, and other diseases [52]. High ALP levels can show that the bile ducts are obstructed. Levels are significantly higher in children and pregnant women. Also, elevated ALP indicates that there could be active bone formation occurring as ALP is a byproduct of osteoblast activity (such as the case in Paget’s disease of bone) or a disease that affects blood calcium level (hyperparathyroidism), vitamin D deficiency, or damaged liver cells [53]. Levels are also elevated in people with untreated Celiac Disease [54]. Placental alkaline phosphatase is elevated in seminomas [55]. Lowered levels of ALP are less common than elevated levels. Some conditions or diseases such as hypophosphatasia, postmenopausal women receiving estrogen therapy because of osteoporosis, men with recent heart surgery, malnutrition, magnesium deficiency, hypothyroidism, severe anemia, children with achondroplasia and cretinism, children after a severe episode of enteritis, pernicious anemia, aplastic anemia, chronic myelogenous leukemia, wilson’s disease may lead to reduced levels of alkaline phosphatase. In addition, the drugs such as oral contraceptives have been demonstrated to reduce alkaline phosphatase [56].
Deficiency in TNSALP leads to hypophosphatasia (HPP), an inborn error of metabolism characterized by epileptic seizures in the most severe cases, caused by abnormal metabolism of pyridoxal-5′-phosphate (the predominant form of vitamin B6) and by hypomineralization of the skeleton and teeth featuring rickets and early loss of teeth in children or osteomalacia and dental problems in adults caused by accumulation of inorganic pyrophosphate (PPi) [57]. Subjects with hypophosphatasia have generalized deficiency of TNSALP activity and suffer from defective bone mineralization (rickets or osteomalacia), yet placental and intestinal ALP isoenzyme activity is normal. The most severe cases are lethal in infancy, with virtually complete absence of L/B/K ALP in all tissues [58]. Severe forms of the disease are transmitted as an autosomal recessive trait. Identification of a missense mutation in the TNSALP gene in one typical case of the severe perinatal (lethal) form of hypophosphatasia established this link between TNSALP and skeletal mineralization [59, 60].
Several studies have indicated the involvement of ALPs in cellular events such as the regulation of protein phosphorylation, cell growth, apoptosis and cellular migration during embryonic development. ALP genes are regulated by distinct signals as shown by clear differences in their expression profiles [2]. Ectopic expression of ALPs have been associated with a variety of human cancers. The expression pattern of ALP isozymes are altered in malignant tissues, for example, PALP and GCALP are over expressed in cells derived from breast cancer and choriocarcinoma, respectively. PALP is a marker of cancer of ovary, testis, lung, and gastrointestinal tract. Plasma TNALP levels can indicate the presence of osteosarcomas, Paget’s disease and osteoblastic bone metastates [36]. Enhanced expression of IALP has also been reported in hepatocellular carcinoma [61]. The aberrant expression of ALP genes in cancer [11, 62] has led to the suggestion that ALP isozymes may be involved in tumorigenesis [6]. ALPL itself represents a new tumor suppressor gene homozygously inactivated in meningiomas [63]. Higher ALP activities reported in breast cancer patients [64].
Finally, recent study proposes a new role for TNSALP in the toxic effect of extracellular tau protein. The extracellular tau remains in a dephosphorylated state. Hyperphosphorylated tau protein, the main component of intracellular neurofibrillary tangles present in the brain of Alzheimer’s disease (AD) patients, plays a key role in progression of the disease. An increase in TNSALP activity together with increase in protein and transcript levels were detected in Alzheimer’s disease patients as compared to healthy controls [56].
References
- 1.McComb RB, Bowers GN, Posen S. Alkaline phosphatase. New York: Plenum Publishing Corp; 1979. [Google Scholar]
- 2.Tsai LC, Hung MW, Chen YH, Su WC, Chang GG, Chang TC. Expression and regulation of alkaline phosphatases in human breast cancer MCF-7 cells. Eur J Biochem. 2000;267:1330–1339. doi: 10.1046/j.1432-1327.2000.01100.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang TC, Wang JK, Hung MW, Chiao CH, Tsai LC, Chang GG. Regulation of the expression of alkaline phosphatase in a human breast cancer cell line. Biochem J. 1994;303:199–205. doi: 10.1042/bj3030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whyte MP, Landt M, Ryan LM, Mulivor RA, Henthorn PS, Fedde KN, Mahuren JD, Coburn SP. Alkaline phosphatase: placental and tissue-nonspecific isoenzymes hydrolyze phosphoethanolamine, inorganic pyrophosphate, and pyridoxal 5′-phosphate substrate accumulation in carriers of hypophosphatasia corrects during pregnancy. J Clin Invest. 1995;95:1440–1445. doi: 10.1172/JCI117814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calhau C, Martel F, Hipolito-Reis C, Azevedo I. Effect of P-glycoprotein modulators on alkaline phosphataseactivity in cultured rat hepatocytes. Cell Physiol Biochem. 2000;10:195–202. doi: 10.1159/000016350. [DOI] [PubMed] [Google Scholar]
- 6.Sadeghirishi A, Yazdanparast R. Plasma membrane homing of tissue nonspecific alkaline phosphatase under the influence of 3-hydrogenkwadaphnin, an anti-proliferative agent from Dendrostellera lessertii. Acta Biochim Pol. 2007;54:323–329. [PubMed] [Google Scholar]
- 7.Shigenari A, Ando A, Baba R, Vaniamoti T, Katsuoka Y, Inoko H. Characterization of alkaline phosphatase genes expressed in seminoma by cDNA cloning. Cancer Res. 1998;58:5079–5082. [PubMed] [Google Scholar]
- 8.Benham F, Cottell DC, Franks M, Wilson PD. Alkaline phosphatase activity in human bladder tumor cell lines. J Histochem Cytochem. 1977;25:266–274. doi: 10.1177/25.4.870558. [DOI] [PubMed] [Google Scholar]
- 9.Mornet E, Stura E, Lia-Baldin AS, Stigbrand T, Menez A, Le Du MH. Structural evidence for a functional role of human tissue non specific alkaline phosphatase in bone mineralization. J Biol Chem. 2001;276:31171–31178. doi: 10.1074/jbc.M102788200. [DOI] [PubMed] [Google Scholar]
- 10.Weiss MJ, Henthorn PS, Lafferty MA, Slaughter C, Raducha M, Harris H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc Natl Acad Sci. 1986;83:7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma U, Singh SK, Pal D, Khajuria R, Mandal AK, Prasad R. Implication of BBM lipid composition and fluidity in mitigated alkaline phosphatase activity in renal cell carcinoma. Mol Cell Biochem. 2012;369:287–293. doi: 10.1007/s11010-012-1391-y. [DOI] [PubMed] [Google Scholar]
- 12.Hoylaerts MF, Manes T, Millan JL. Mammalian alkaline phosphatases are allosteric enzymes. J Biol Chem. 1997;272:22781–22787. doi: 10.1074/jbc.272.36.22781. [DOI] [PubMed] [Google Scholar]
- 13.Sligbrand T. Present status and future trends of human alkaline phosphatases. Prog Clin Biol Res. 1984;166:3–14. [PubMed] [Google Scholar]
- 14.Henthorn PS, Raducha M, Fedde KN, Lefferty MA, Whyte MP. Different missense mutations at the tissue nonspecific alkaline phosphatase genes locus in autosomal recessively inherited forms of mild and severe hypophosphatasia. Proc Natl Acad Sci. 1992;89:9924–9928. doi: 10.1073/pnas.89.20.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millan JL, Fishman WH. Biology of human alkaline phosphatase with special reference to cancer. Crit Rev Clin Lab Sci. 1995;32:1–39. doi: 10.3109/10408369509084680. [DOI] [PubMed] [Google Scholar]
- 16.Muller H, Yamazaki M, Michigami T, Kageyama T, Chonau E, Schneider P, Ozono K. Asp361Val mutant of alkaline phosphatase found in patients with dominantly inherited hypophosphatasia inhibits the activity of the wild-type enzyme. J Clinical Endocrinol Metabolism. 2000;85:743–747. doi: 10.1210/jcem.85.2.6373. [DOI] [PubMed] [Google Scholar]
- 17.Mulivor RA, Plotkin LI, Harris H. Developmental change in human intestinal alkaline phosphatase. Ann Hum Genet. 1978;42:1–13. doi: 10.1111/j.1469-1809.1978.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 18.McKenna MJ, Hamilton TA, Sussman HH. Comparison of human alkaline phosphatase isoenzymes. structural evidence for three protein classes. Biochem J. 1979;181:67–73. doi: 10.1042/bj1810067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris H. The harvey lectures: series 76. New York: Academic; 1986. pp. 95–123. [Google Scholar]
- 20.Raimondi E, Talarico D, Moro L, et al. Regional mapping of the human placental alkaline phosphatase gene (ALP) to 2q37 by in situ hybridization. Cyrogenet Cell Genet. 1988;47:98–99. doi: 10.1159/000132518. [DOI] [PubMed] [Google Scholar]
- 21.Henthom PS, Raducha M, Hadesch T, et al. Sequence and characterization of the human intestinal alkaline phosphatase gene. J Biol Chem. 1988;263:12011–12019. [PubMed] [Google Scholar]
- 22.Vergote IB, Abeler VM, Bormer OP, et al. CA125 and placental alkaline phosphatase as serum tumor markers in epithelial ovarian carcinoma. Tumor Biol. 1992;13:168–174. doi: 10.1159/000217761. [DOI] [PubMed] [Google Scholar]
- 23.Fishman WH, Inglis NR, Green S, et al. Immunology and biochemistry of regan isoenzyme of alkaline phosphatase in human cancer. Nature. 1968;219:697–699. doi: 10.1038/219697a0. [DOI] [PubMed] [Google Scholar]
- 24.Griffin CA, Smith M, Henthorn PS, et al. Human placental and intestinal alkaline phosphatase genes map to 2q34–q37. Am J Hum Genet. 1987;41:1025–1034. [PMC free article] [PubMed] [Google Scholar]
- 25.Komoda T, Sakagishi Y. The function of the carbohydrate moiety and alteration of carbohydrate composition in human alkaline phosphatase isoenzymes. Biochim Biophys Acta. 1978;523:395–406. doi: 10.1016/0005-2744(78)90042-6. [DOI] [PubMed] [Google Scholar]
- 26.Higashino K, Muratani K, Hade T, et al. Gene structure of alkaline phosphatases: purification and some properties of the fast migrating alkaline phosphatase in FL-amnion cells (the Kasahara isoenzyme) and its cDNA cloning. Clin Chim Acta. 1989;186:151–164. doi: 10.1016/0009-8981(90)90032-N. [DOI] [PubMed] [Google Scholar]
- 27.Millan JL. Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem. 1986;261:3112–3115. [PubMed] [Google Scholar]
- 28.Millan JL, Manes R. Seminoma-derived nagao isozyme is encoded by a germ cell alkaline phosphatase gene. Proc Natl Acad Sci. 1988;85:3024–3028. doi: 10.1073/pnas.85.9.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann MC, Jeltsch W, Brecher J, Walt H. Alkaline phosphatase isozymes in human testicular germ cell tumors, precancerous stage, and three related cell lines. Cancer Res. 1989;49:4696–4700. [PubMed] [Google Scholar]
- 30.Povinelli CM, Knoll BJ. Trace expression of the germ-cell alkaline phosphatase gene in human placenta. Placenta. 1991;12(663):8. doi: 10.1016/0143-4004(91)90500-f. [DOI] [PubMed] [Google Scholar]
- 31.Fishman WH. Clinical and biological significance of an isoenzyme tumor marker-PLAP. Clin Biochem. 1987;20:387–392. doi: 10.1016/0009-9120(87)90003-8. [DOI] [PubMed] [Google Scholar]
- 32.Smith M, Weiss MJ, Griffin CA, et al. Regional assignment of the gene for human liver/bone/kidney alkaline phosphatase to chromosome 1 p36.1–p34. Genomics. 1988;2:139–143. doi: 10.1016/0888-7543(88)90095-X. [DOI] [PubMed] [Google Scholar]
- 33.Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 2010;77:4–12. doi: 10.1272/jnms.77.4. [DOI] [PubMed] [Google Scholar]
- 34.Fishman WH. Alkaline phosphatase isozymes: recent progress. Clin Biochem. 1990;23:99–104. doi: 10.1016/0009-9120(90)80019-F. [DOI] [PubMed] [Google Scholar]
- 35.Weiss MJ, Ray K, Henthorn PS, Lamb B, Kadesch T, Harris H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1988;263:12002–12010. [PubMed] [Google Scholar]
- 36.Du MHL, Milla JL. Structural evidence of functional divergence in human alkaline phosphatases. J Biol Chem. 2002;277:49808–49814. doi: 10.1074/jbc.M207394200. [DOI] [PubMed] [Google Scholar]
- 37.Moss DW. Perspectives in alkaline phosphatase research. Clin Chem. 1992;38:2486–2492. [PubMed] [Google Scholar]
- 38.Kiledjian M, Kadesch T. Post-transcriptional regulation of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem. 1991;266:4207–4213. [PubMed] [Google Scholar]
- 39.Harris H. The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta. 1990;186:133–150. doi: 10.1016/0009-8981(90)90031-M. [DOI] [PubMed] [Google Scholar]
- 40.Bradshaw RA, Cancedda F, Ericsson LH, Neumann PA, Piccoli SP, Schlesinger MJ, Shriefer K, Walsh KA. Amino acid sequence of Escherichia coli alkaline phosphatase. Proc Natl Acad Sci. 1981;78:3473–3477. doi: 10.1073/pnas.78.6.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henthorn PS, Raducha M, Edwards YN, Weiss MJ, Slaughter C, Lafferty MA, Harris H. Nucleotide and amino acid sequences of human intestinal alkaline phosphatase: close homology to placental alkaline phosphatase. Proc Natl Acad Sci. 1984;84:1234–1238. doi: 10.1073/pnas.84.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss MJ, Cole DEC, Ray K, Whyte MP, Lafferty MA, Mulivor RA, Harris H. A missense mutation in the human liver/bone/kidney alkaline phosphatase gene causing a form of lethal hypophosphatasia. Proc Natl Acad Sci. 1988;85:7666–7669. doi: 10.1073/pnas.85.20.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kam W, Clauser E, Kim YS, Kan YW, Rutter WJ. Cloning, sequencing, and chromosomal localization of human term placental alkaline phosphatase cDNA. Proc Natl Acad Sci. 1985;82:8715–8719. doi: 10.1073/pnas.82.24.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sowadski JM, Handschumacher MD, Murthy HMK, Foster BA, Wyckoff HW. Refined structure of alkaline phosphatase from Escherichia coli at 2.8 A resolution. J Mol Biol. 1985;186:417–433. doi: 10.1016/0022-2836(85)90115-9. [DOI] [PubMed] [Google Scholar]
- 45.Llinas P, Masella M, Stigbrand T, Menez A, Stura EA, Le Du MH. Structural studies of human alkaline phosphatase in complex with strontium: implication for its secondary effect in bones. Protein Sci. 2006;15:1691–1700. doi: 10.1110/ps.062123806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoko I, Keiichi K, Masahiro I, Yoshihiro A, Shoji K, Kimimitsu O. Tissue-nonspecific alkaline phosphatase with an Asp289 → Val mutation fails to reach the cell surface and undergoes proteosome-mediated degradation. J Biochem. 2003;134:63–70. doi: 10.1093/jb/mvg114. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki U, Yoshimura K, Takashi M. Uber ein enzyme ―phytase‖ das anhydro-oxy-methylen-diphosphorsaure spaltet. Bull Coll Agri Tokyo Imp Univ. 1907;7:503–12.
- 48.Wuthier RE, Register T. Role of alkaline phosphatase, a polyfunctional enzyme, in mineralizing tissues. In: Butler WT, editor. Chemistry and biology of mineralized tissues. Birmingham: EBSCO Media; 1985. pp. 113–124. [Google Scholar]
- 49.Whyte MP. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann N Y Acad Sci. 2010;1192:190–200. doi: 10.1111/j.1749-6632.2010.05387.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhu T, Gan YH, Liu H. Functional evaluation of mutations in the tissue-nonspecific alkaline phosphatase gene. Chin J Dent Res. 2012;15:99–104. [PubMed] [Google Scholar]
- 51.Moss DW. Aspects of the relationship between liver, kidney and bone alkaline phosphatase. In: Stigbrand T, Fishman WH, editors. Human alkaline phosphatases. New York: Alan R Liss; 1984. pp. 79–86. [PubMed] [Google Scholar]
- 52.Epstein E, Kiechle FL, Artiss JD, Zak B. The clinical use of alkaline phosphatase enzymes. Clin Lab Med. 1986;6:491–505. [PubMed] [Google Scholar]
- 53.Rodan GA, Rodan SB. In: Advances in bone and mineral research annual II. Peck WA, editor. Amsterdam: Excerpta Medica; 1984. pp. 244–285. [Google Scholar]
- 54.Preussner HT. Detecting coeliac disease in your patients. Am Fam Physician. 1998;57:1023–1034. [PubMed] [Google Scholar]
- 55.Lange PH, Millan JL, Stigbrand T, Vessella RL, Ruoslahti E, Fishman WH. Placental alkaline phosphatase as a tumor marker for seminoma. Cancer Res. 1982;42:3244–3247. [PubMed] [Google Scholar]
- 56.Schiele F, Vincent-Viry M, Fournier B, Starck M, Siest G. Biological effects of eleven combined oral contraceptives on serum triglycerides, gamma-glutamyltransferase, alkaline phosphatase, bilirubin and other biochemical variables. Clin Chem Lab Med. 1998;36:871–878. doi: 10.1515/CCLM.1998.153. [DOI] [PubMed] [Google Scholar]
- 57.Buchet R, Millán JL, Magne D. Multisystemic functions of alkaline phosphatases. Methods Mol Biol. 2013;1053:27–51. doi: 10.1007/978-1-62703-562-0_3. [DOI] [PubMed] [Google Scholar]
- 58.Mueller HD, Stinson RA, Mohyuddin F, Milne JK. Isoenzymes of alkaline phosphatase in infantile hypophosphatasia. J Lab Clin Med. 1983;102:24–30. [PubMed] [Google Scholar]
- 59.Sultana S, Al-Shawafi HA, Makita S, Sohda M, Amizuka N, Takagi R, Oda K. An asparagine at position 417 of tissue-nonspecific alkaline phosphatase is essential for its structure and function as revealed by analysis of the N417S mutation associated with severe hypophosphatasia. Mol Genet Metab. 2013;109:282–288. doi: 10.1016/j.ymgme.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Chang KC, Lin PH, Su YN, Peng SS, Lee NC, Chou HC, Chen CY, Hsieh WS, Tsao PN. Novel heterozygous tissue-nonspecific alkaline phosphatase (TNAP) gene mutations causing lethal perinatal hypophosphatasia. J Bone Miner Metab. 2012;30:109–113. doi: 10.1007/s00774-011-0282-8. [DOI] [PubMed] [Google Scholar]
- 61.Usoro NI, Omabbe MC, Usoro CAO, Nsonwu A. Calcium, inorganic phosphates, alkaline and acid phosphatase activities in breast cancer patients in Calabar, Nigeria. African Health Sci. 2010;10:9–13. [PMC free article] [PubMed] [Google Scholar]
- 62.Prasad R, Lambe S, Kaler P, Pathania S, Kumar S, Attari S, Singh SK. Ectopic expression of alkaline phosphatase in proximal tubular brush border membrane of human renal cell carcinoma. Biochim et Biophy acta. 2005;1741:240–245. doi: 10.1016/j.bbadis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Niedermayer I, Feiden W, Henn W, Steilen-Gimbel H, Steudel WI, Zang KD. Loss of alkaline phosphatase activity in meningiomas: a rapid histochemical technique indicating progression-associated deletion of a putative tumor suppressor gene on the distal part of the short arm of chromosome 1. J Neuropathol Exp Neurol. 1997;56:879–886. doi: 10.1097/00005072-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Kim JM, Kwon CH, Joh JW, Park JB, Ko JS, Lee JH, Kim SJ, Park CK. The effect of alkaline phosphatase and intrahepatic metastases in large hepatocellular carcinoma. World J Surg Oncol. 2013;11:40. doi: 10.1186/1477-7819-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]