Abstract
New hopes in cancer treatment have been emerged using functional nanoparticles. In this work, we tried to synthesize gold nanoparticles and gold nanoparticles conjugated with DNA extracted from human breast cancer cells. After synthesizing, gold nanoparticles were mixed with nanoliposomal hydroxyurea and corresponding compounds were formed. They were described by UV–Visible spectrophotometry and Zeta sizer. Amount of drug loading into liposomes was determined by spectrophotometry and cytotoxicity effect on MCF-7 cells was measure by MTT assay. Drug loading was determined to be 70 %. Size, size distribution and Zeta potential of particles were 473 nm, 0.46 and −21 mV for control nanoliposomal ones and 351 nm, 0.38 and −25 mV for nanoliposomal particles containing hydroxyurea. This was 29 nm, 0.23 and −30 mV for gold nanoparticles and 502 nm, 0.41 and −38 mV for nanoliposomes containing drug loaded by gold nanoparticles conjugated with DNA. It was found that nano conjugated complex in concentrations less than 20 μM of hydroxyurea can improve efficiency compared with liposomal drug. In maximum concentration of drug (2,500 μM), cytotoxicity was equal to 95 %. In minimum concentration of drug (5 μM), cytotoxicity of liposomal drug and conjugated complex were 70 and 81 %, respectively which probably comes from increased drug entry into cells due to the presence of gold nanoparticles. Free drug resulted in toxicity of 32 % in 5 μM and 88 % in 2,500 μM. Results demonstrated higher drug efficiency in nanoparticle form compared with free form which can be used in in vivo studies.
Keywords: Gold nanoparticle, Liposome, Hydroxyurea, Drug delivery
Introduction
Medical application of multifunctional nanostructures is facing growing interest. Recent advances in engineering and technology has led to synthesizing various nanostructures such as quantum dots, nano shells and nanoparticles, paramagnetic nanoparticles, carbon nanotubes and lipid-based systems [1]. Such compounds result in decreasing side effect and increasing efficiency of chemotherapeutic agents [2]. In addition, they are used to cross biological barriers, protecting drug and releasing optimal dose [3]. Using nanoparticles as drug carrier refers to two important properties: They can penetrate the cell through tiny capillaries due to small size and effective accumulation of drug at target sites as well as sustained release of the drug in the target position for a period of a few days or a few weeks due to using biocompatible materials in synthesizing nanoparticles [4]. Liposomes are common drug carriers some of which like Doxil is verified by FDA [5]. Liposomes are phospholipid vesicles with hydrophobic tail and a hydrophilic head. Because of amphipathic properties, they can encapsulate water soluble drugs in internal phase and lipid soluble molecules in hydrophobic membrane [6]. Due to the physical and chemical properties of gold nanoparticles, they are focus of interest in medical field [7, 8]. They are used for various applications such as cell regulation, cell expression, chemotherapy and drug delivery. Liposomes are appropriate compounds for delivering gold nanoparticles in cell. Using liposomes can improve cellular absorption of gold nanoparticles. They can also provide possibility of targeted delivery due to easy linkage of ligands to surface of liposomes containing nanoparticles [9]. Hydroxyurea is an anti-cancer drug which is commonly used in treatment of myeloproliferative disorders in breast cancer [10, 11]. In spite of anti-cancer properties, it has adverse side effects, too. Drowsiness, nausea, vomiting and diarrhea are some of side effects. Also mucositis, constipation, anorexia, stomatitis, bone marrow toxicity, hair loss, skin changes, changes in liver enzymes, blood creatinine and urea are other side effects [10]. Considering mentioned points, we tried to synthesize nanoliposomal hydroxyurea as well as gold nanoparticles. Additionally, nanoparticles were mixed with liposomal drug (gold nanoparticle complex). On the other hand, gold nanoparticles were conjugated with DNA extracted from MCF-7 cells and mixed with liposomal drug. Cytotoxicity of all formulations was compared with free drug by MTT assay in addition to evaluating size, size distribution, Zeta potential and drug loading efficiency.
Materials and Methods
Materials
Isopropanol and ethanol were purchased from Merck. Cholesterol, hydroxyurea, phosphatidyl choline, H[AuCl4], Na3C6H5O7·2H2O and MTT were purchased from Sigma. Polyethylene glycol 3500 was purchased from Kimiyagarane emrooz (Iran) and RPMI 1640 culture medium from Invitrogen. MCF-7 cell line was supplied by Pasteur Institute of Iran. Water used through this study was in distillated form.
Synthesizing Pegylated Nanoliposomal Hydroxyurea
To synthesizing pegylated nanoliposomal hydroxyurea, lecithin, cholesterol and polyethylene glycol (portion of 500, 50 and 25 mg) were mixed in 50 ml ethanol 98 % (water bath, 40 °C) and stirred (300 rpm, 1 h). After mixing, a homogenous and yellow suspension was formed and ethanol was evaporated by rotary evaporator (Heidolph, Germany) in 50 °C and 90 rpm. 10 ml phosphate buffer (pH 7.2) and hydroxyurea (20 mg) were added to resultant gel and stirred. The suspension was sonicated (60 Hz, Bandelin Sonorex Digitec) for 10 min and homogenized by homogenizer (10 min, 7,500 rpm).
Determining Drug Loading Efficiency
To determine drug loading efficiency, liposomal hydroxyurea was centrifuged at 21,000 rpm and 4 °C for 30 min. The supernatant was separated and its light absorbance was measured in 215 nm (SHIMADZU spectrophotometer, UV—1601PC). Drug loading efficiency was calculated by below formula:
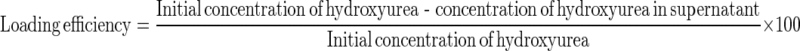 |
For standard curve, different concentrations of hydroxyurea were prepared and absorbance was measured in 215 nm.
Preparing Gold Nanoparticles
Gold nanoparticles were prepared according to previous studies [12]. Briefly, H[AuCl4] was used as gold salt while Na3C6H5O7·2H2O was the reducing agent. Gold salt solution (5 mM) was heated to boiling point. It was yellow in this stage. After adding sodium citrate, it was converted to citric acid which causes changing the color from yellow to transparent colorless and then black and totally red [13]. In all stages, it was heating and stirring. When color change was fulfilled, it was stirred to cool down. Nanoparticles containing amino acid passed the same process, although aspartic acid (final concentration of 10 μM) was added to reaction medium before adding gold salt.
Extracting DNA for Conjugating with Gold Nanoparticle
Extracting DNA from MCF-7 cells was carried out according to Strauss method [14]. Total concentration of DNA was 30 μg/ml.
Conjugating Gold Nanoparticles with DNA
Resultant DNA (100 μl and 30 μg/ml) was incubated with gold nanoparticle (10 mM and 2 ml) for 48 h (100 rpm, room temperature). Spectrophotometry was applied to confirm conjugation.
Preparing Complex of Gold Nanoparticles and Nano Conjugates
Nano conjugates were added to definite portions of liposomal suspension. At the end, concentration of gold nano particles was 100 μM. It was vortexed for 10 min and sonicated for 20 min. The process was the same for gold nanoparticle complex.
Describing Nanoparticles by Size, Size Distribution and Zeta Potential
To describe nanoparticles by size, size distribution and Zeta potential, Zeta sizer (nano-Zs Zen 3600, Malvern instruments UK) was used.
MTT Assay for Evaluating Cytotoxicity
To evaluate cytotoxicity effect of hydroxyurea and comparing different formulations, MTT assay was used. MCF-7 cells were poured in 96-well plate (1 × 104 cell per well) in RPMI 1640 culture medium. Culture medium containing 10 % fetal bovine serum and 1 % antibiotic (penicillin/streptomycin) were incubated at 37 °C with 10 % CO2. After 24 h, the supernatant was removed and cells were treated with concentration of 0, 5, 10, 20, 39.78, 156 and 312 μM nanoliposomal as well as free hydroxyurea. After incubating for 48 h, 100 μl MTT (0.5 mg/ml PBS, pH 7.4) was added to each well. Incubation was carried out for 3 h in 37 °C. Then MTT was removed and 200 μl isopropanol 100 % was added to each well in order to solve formazan crystals. Absorbance was measured at 570 nm by Elisa reader (BioTek Instruments, VT, USA). Experiments were triplet and repeated for 3 times. Cell cytotoxicity was determined by calculating portion of absorbance of treated cells to control cells.
Results
Size, Size Distribution and Zeta Potential for Different Nanoparticles
Control and drug containing nanoliposomes were synthesized successfully. Mixing drug containing liposome and gold nanoparticles and nano conjugates lead to formation of two drug complexes. Gold nanoparticles had the minimum size and size distribution of 35 nm and 0.23; however conjugating these particles to DNA, increased the size significantly. Nano conjugates complex had the maximum size of 502 nm. Zeta potential was negative for all formulations. Nano conjugates demonstrated the lowest Zeta potential of −15 mV and the highest Zeta potential of −38 mV belonged to nano conjugates complex. Size distribution for all compounds was evaluated to be appropriate. Gold nanoparticles had minimum size distribution whereas nanoliposomal particles had the maximum. As it is evident in Table 1, nanoparticle properties notably depend on formulation.
Table 1.
Properties of synthesized formulations
| Formulation | Properties | ||
|---|---|---|---|
| Size (nm) | Size distribution | Zeta potential (mV) | |
| Control liposome | 473 | 0.46 | −21 |
| Liposomal hydroxyurea | 351 | 0.38 | −25 |
| Gold nanoparticle | 35 | 0.23 | −30 |
| Nano conjugate | 305 | 0.33 | −15 |
| Liposomal drug–nano conjugate complex | 502 | 0.41 | −38 |
Verifying Conjugation of Gold Nanoparticles to DNA
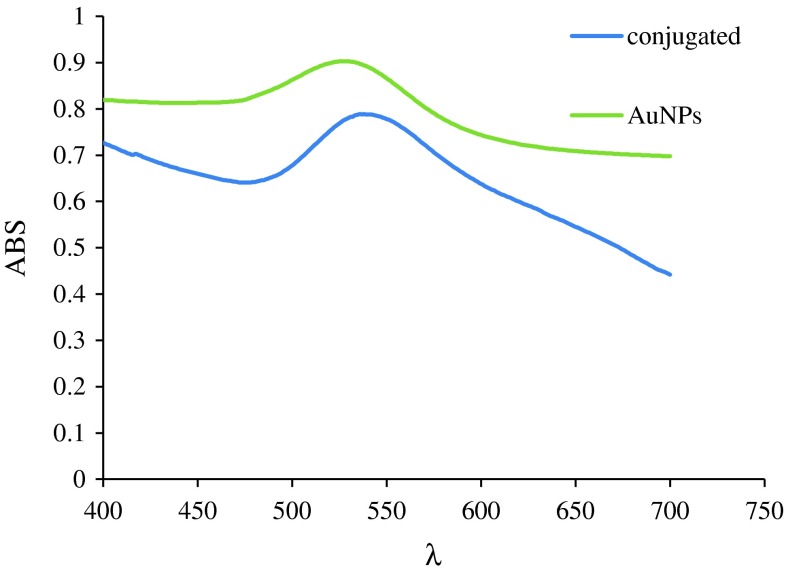
Results of UV–Visible spectrophotometry which is indicated in Fig. 1, verifies conjugation.
Fig. 1.
Spectrophotometry of gold nanoparticles (green) and nano conjugates (blue)
As indicated in Fig. 1, two distinct spectrums were obtained. Green one belongs to gold nanoparticles and blue one to nano conjugates.
Drug Loading Efficiency
Drug loading efficiency was calculated considering hydroxyurea standard curve. It was estimated to be 70 %.
Cytotoxicity of Different Formulations
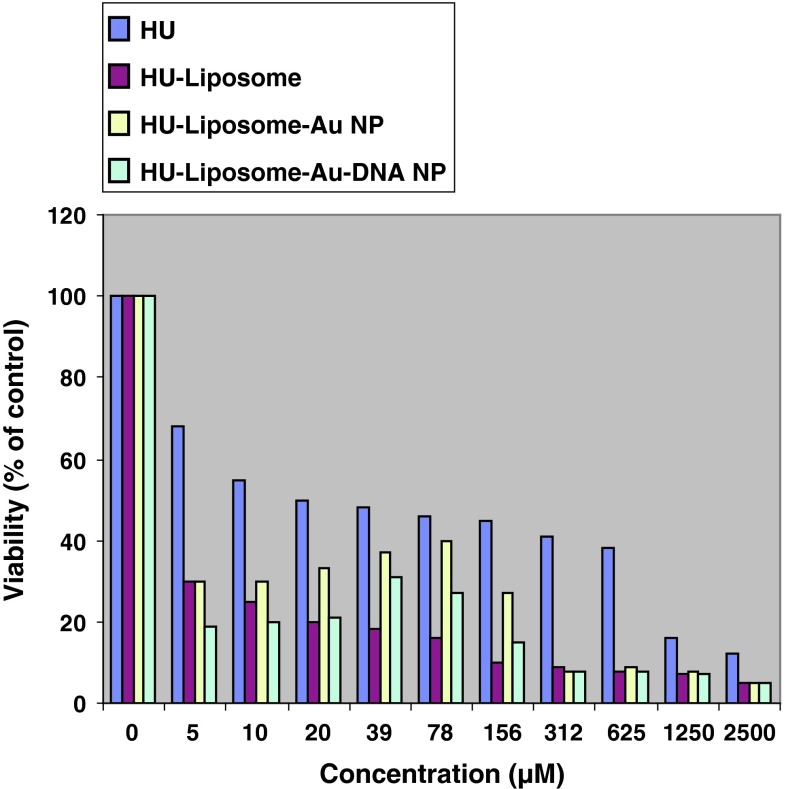
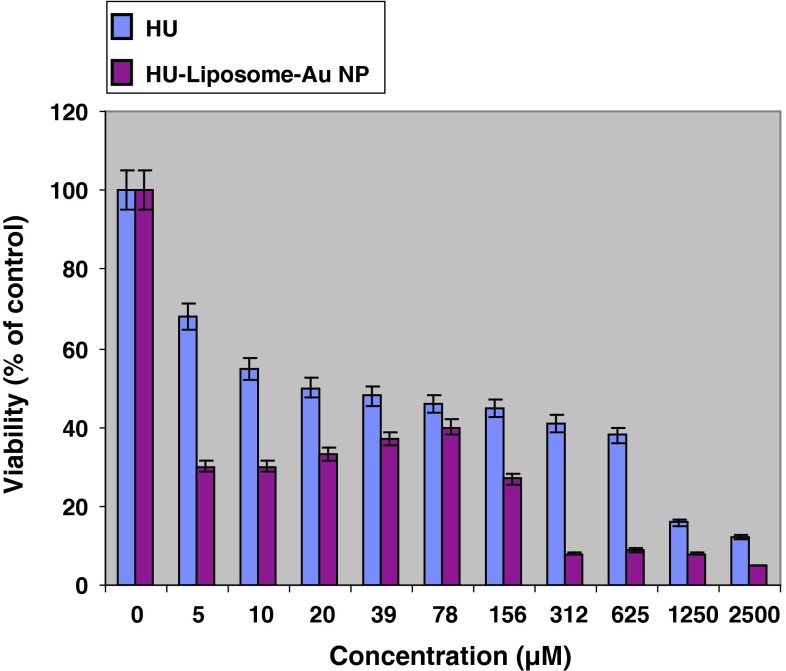
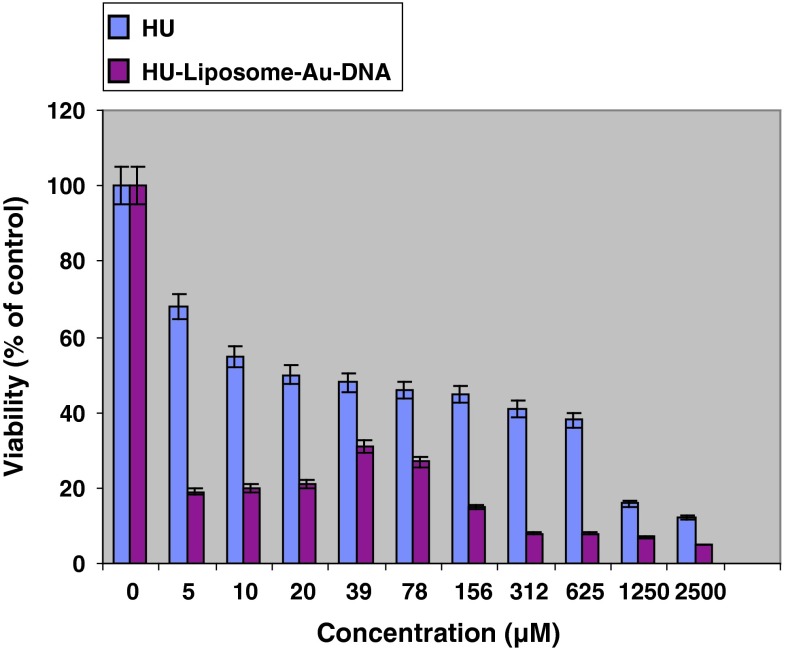
It was observed that non drug containing liposomes have no cytotoxicity effect even in high concentrations. In addition, all drug containing formulations showed higher cytotoxicity compared with free drug (Fig. 2). Although all drug containing formulations including hydroxyurea-loaded liposome (Fig. 3) and two other nanodrug complexes demonstrated nearly same cytotoxicity in maximum concentration of 2,500 μM, nano conjugate complex showed considerably high toxicity in minimum concentration. In all formulations, cytotoxicity reduced by decreasing drug concentration; however it represented an ascending trend in spite of decreasing drug concentration in concentrations of less than 80 μM for gold nanoparticle complex (Fig. 4) and 40 μM for nano conjugate complex (Fig. 5). It should be added that, in mentioned concentrations of nano conjugate complex, concentration of gold nanoparticles was 1.5 μM while it was 3 μM for gold nanoparticle complex.
Fig. 2.
Cytotoxicity effect of hydroxyurea (blue) and hydroxyurea loaded into liposomal nanoparticle (red), hydroxyurea loaded into gold nanoparticles complex (yellow), and nano conjugate complex (green) on MCF-7 cell line after 48 h of incubation. Results are presented as mean ± 5 % error of three independent experiments. As displayed in the figure, all formulations have a higher cytotoxicity compared with free form. In maximum concentration (2,500 μM), cytotoxicity effect of all formulations were approximately the same while in concentrations of lower than 20 μM, nano conjugate complex applied highest cytotoxicity
Fig. 3.
Cytotoxicity effect of hydroxyurea and nanoliposomal hydroxyurea on MCF-7 cell line after 48 h of incubation. Results are presented as mean ± 5 % error of three independent experiments. As it is displayed in the figure, loading drug into liposomes has notably improved cytotoxicity
Fig. 4.
Cytotoxicity effect of hydroxyurea and hydroxyurea loaded into gold nanoparticle complex on MCF-7 cell line after 48 h of incubation. Results are presented as mean ± 5 % error of three independent experiments. Improving drug efficiency in nanoparticle form compared with free form is also evident in this figure. Furthermore it illustrates cytotoxicity reduction by decreasing drug concentration which was valid for concentrations higher than 80 μM. In lower concentrations of 80 μM (i.e. gold NP concentration was about 3 μM), it showed an increasement in cytotoxicity in spite of reducing drug concentration
Fig. 5.
Cytotoxicity effect of hydroxyurea and hydroxyurea loaded into nano conjugate complex on MCF-7 cell line after 48 h of incubation. Results are presented as mean ± 5 % error of three independent experiments. Reducing cytotoxicity by decreasing drug concentration was also observed for nano conjugates which was valid for concentrations higher than 40 μM. Concentrations lower than 40 μM (i.e. gold NPs concentration was lower than 2 μM) demonstrated enhancing cytotoxicity in spite of reducing drug concentration
Discussion
The most common problems of drug delivery systems include poor bioavailability, poor in vivo stability, low solubility and low intestinal absorption. The other challenge is weakness of targeted and continuous drug delivery system. In addition, low efficiency of treatment, side effects and plasma oscillations associated with this system is another obstacle. Applying nanotechnology in drug delivery is an effective solution to overcome such challenges. Nanostructures are employed in such systems. Nanostructures are able to avoid hydrolytic and enzymatic gastrointestinal degradation of encapsulated drugs and deliver a variety of drugs to various parts of body for continuous release [15–17]. Liposome is one type of such particles. In this study, we succeeded to synthesize liposome by reverse phase evaporation. Obtained liposomes had favorable properties such as appropriate size, size distribution and Zeta potential. The stability of liposomes was investigated. So after 2 months of synthesize, the resulting suspension was examined again by Zeta sizer and was determined to be unchanged. Furthermore, the effect of sonication and homogenization on the size of the liposomes was fundamental since after sonication and homogenization, the size was reduced to almost a quarter. Drug loading efficiency was calculated to be 70 %.
On the other hand, gold nanoparticles were synthesized in small size. The size was increased significantly by conjugating with DNA. All formulations demonstrated higher toxicity probably due to delayed release. Cytotoxicity was decreased by reducing drug concentration. This was valid up to 80 μM for gold nanoparticle complex and 40 μM for conjugated complex. In fewer concentrations, cytotoxicity was increased. The difference between such complexes and drug containing nanoliposomes comes from existence of gold nanoparticles and nano conjugates and cytotoxicity probably comes from existence of these particles. It should be added that certain concentration of nanoparticle is essential to achieve highest efficiency. This was 3 μM for gold nanoparticle complex and 1.5 μM for nano conjugated complex. This was presumably due to effect of gold nanoparticle on enhancing drug entry to cell. Additionally, it is possible that in such concentrations nanoparticles are in individually dispersed form and demonstrate higher level of activity. Negative charge of DNA, causes stability confirmation and higher dispersion. Furthermore, all formulations had high level of stability considering Zeta potentials. If all particles of suspension had same electrical charge, they would repel each other and formation of lump was not possible [18] which causes stability. The other notable point was existence of polyethylene glycol in formulations. It was able to improve efficiency and stability in blood [19]. Totally, considering impressive improvement of hydroxyurea properties, it is recommended to investigate possibility of above-mentioned achievements for in vivo studies.
Contributor Information
Tahereh Zadeh Mehrizi, Phone: +98 939 112 7687, Email: t.mehrizi@yahoo.com.
Azim Akbarzadeh, Phone: +98-2166465406, FAX: +98-2166465132, Email: azimakbarzadeh1326@gmail.com.
References
- 1.Chithrani DB, Dunne M, Stewart J, Allen C, Jaffray DA. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomedicine. 2010;6:161–169. doi: 10.1016/j.nano.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Mozafari MR. Nanocarrier technologies. Front Nanother. 2006;237:1–12. [Google Scholar]
- 3.Costantino L, Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov Today. 2012;17:367–378. doi: 10.1016/j.drudis.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54:135–147. doi: 10.1016/S0169-409X(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 5.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1:297–315. doi: 10.2217/17435889.1.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Jamal WT, Kostarelos K. Liposome-nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomedicine (Lond) 2007;2:85–98. doi: 10.2217/17435889.2.1.85. [DOI] [PubMed] [Google Scholar]
- 7.Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 8.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 9.Mady MM, Fathy MM, Youssef T, Khalil WM. Biophysical characterization of gold nanoparticles-loaded liposomes. Phys Med. 2012;28:288–295. doi: 10.1016/j.ejmp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Liebelt EL, Balk SJ, Faber W, Fisher JW, Hughes CL, Lanzkron SM, Lewis KM, Marchetti F, Mehendale HM, Rogers JM, Shad AT, Skalko RG, Stanek EJ. NTP-CERHR expert panel report on the reproductive and developmental toxicity of hydroxyurea. Birth Defects Res B Dev Reprod Toxicol. 2007;80:259–366. doi: 10.1002/bdrb.20123. [DOI] [PubMed] [Google Scholar]
- 11.SE Alavi, MKM Esfahani, F Alavi, F Movahedi, A Akbarzadeh. Drug delivery of hydroxyurea to breast cancer using liposomes. Ind J Clin Biochem. 2012. doi:10.1007/s12291-012-0291-y. [DOI] [PMC free article] [PubMed]
- 12.Huang H, Yang X. Chitosan mediated assembly of gold nanoparticles multilayer. Coll Surf A Physicochem Eng Aspects. 2003;226:77–86. doi: 10.1016/S0927-7757(03)00382-0. [DOI] [Google Scholar]
- 13.Shipway AN, Katz E, Willner I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. Chem Phys Chem. 2000;1:18–52. doi: 10.1002/1439-7641(20000804)1:1<18::AID-CPHC18>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Strauss WM. Preparation of genomic DNA from mammalian tissue. Curr Protoc Mol Biol. 2001;Chapter 2:Unit2.2. [DOI] [PubMed]
- 15.Jung T, Kamm W, Breitenbach A, Kaiserling E, Xiao JX, Kissel T. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm. 2000;50:147–160. doi: 10.1016/S0939-6411(00)00084-9. [DOI] [PubMed] [Google Scholar]
- 16.Nimesh S, Manchanda R, Kumar R, Saxena A, Chaudhary P, Yadav V, Mozumdar S, Chandra R. Preparation, characterization and in vitro drug release studies of novel polymeric nanoparticles. Int J Pharm. 2006;323:146–152. doi: 10.1016/j.ijpharm.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 17.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/S0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 18.Paolino D, Fresta M, Sinha P, Ferrari M. Drug delivery systems. In: Webester JG, editor. Encyclopedia of medical devices and instrumentation. New Jersey: Wiley; 2006. pp. 437–495. [Google Scholar]
- 19.Wang X, Yang L, Chen ZG, Shin DM. Application of nanotechnology in cancer therapy and imaging. CA Cancer J Clin. 2008;58:97–110. doi: 10.3322/CA.2007.0003. [DOI] [PubMed] [Google Scholar]