Abstract
We report a novel strategy for the synthesis and capping of gold nanoparticles (GNPs) by tryptophan, glutamic acid and aspartic acid. The ratio of chloroaurate ions to amino acid was optimized in the reaction medium to obtain monodispersed GNPs. The size of nanoparticles and size distribution were controlled by sodium dodecyl sulfate which demonstrated high stability in aqueous solution over a period of time. GNPs were characterized by UV–Vis spectroscopy, dynamic light scattering and transmission electron microscopy.
Keywords: Gold nanoparticles, Amino acids, Stability, SDS
Introduction
Due to the unique optical electronic and catalytic properties of gold nanoparticles, they are the subject of substantial research, with applications in a wide variety of areas, including optoelectronic devices, ultrasensitive chemical and biological sensors, and as catalysts in chemical and photochemical reactions [1–6]. Thus controlling their physical and chemical properties are of significance which depends on their size [7, 8].
One of the most widely used precipitation methods for the synthesis of gold colloids in water, proposed by Turkevich, involves the reduction of an aqueous solution of chloroauric acid (HAuCl4) by trisodium citrate [7, 9]. The synthesized nanoparticles by this process are known to be stabilized by a physical adsorption of excess citrate ions from the medium [9]. Another process for synthesizing gold nanoparticles in a two-phase system was developed by Brust et al. who reported a simple method for the synthesis and capping of gold colloids with alkanethiols. The gold nanoparticles capped and stabilized with thiol-derivative monolayers are dispersed in organic solvents [7, 10]. Since then enormous researches have been performed on modifying the surface of gold colloids through changing the molecular structures of the thiolates on the particle surface [11–17]. The protection capping agent (thiols) is chemically bound to the surface of gold nanoparticles [7]. The byproducts of these reducing agents and organic solvents used in these techniques make them unsuitable for using in some bioanalytical applications.
The solubility of gold in water and polar organic solvents is determined by capping layer terminal functionality. Amine derivatives complex with gold nanoparticles are produced similar to thiol derivatives [18, 19]. Using amine chemistry provides the modification of gold nanoparticles surface by amino acids which can be performed by amine functionality [20, 21]. Dispersed in water, gold nanoparticles are widely applied in catalysis, sensors, and molecular markers and especially in bio applications such as bio-labeling and drug delivery [22–24].
In the present paper we report herewith a simple one-step method for synthesizing and capping gold nanoparticles using amino acids. As a result of this method the stability of nanoparticles is improved by adsorption of sodium dodecyl sulfate on their surfaces and thus the agglomeration is prevented. The properties of gold nanoparticles are also explained by transmission electron microscopy, thermal stability measurements, UV–Vis and dynamic light scattering.
Materials and Methods
Materials
Tetrachloroauric acid (HAuCl4), aspartic acid, glutamic acid, and tryptophan were purchased from Aldrich Chemicals. Sodium dodecyl sulfate (SDS) was purchased from Sigma Chemical Co., USA.
Synthesis of Gold Nanoparticles
1.5 ml aqueous solution of amino acid of different molarities (a: 1 mM, b: 5 mM, c: 10 mM, d: 15 mM, e: 20 mM, and f: 25 mM) was added to 25 ml of de-ionized water and the mixture was heated till boiling. Upon boiling, 5 ml of 5 mM HAuCl4 solution was added to this mixture and heating was continued at boiling conditions. After a few minutes, the reduction of the gold salt (Au3+) to GNPs (Au0) was confirmed by the appearance of color in the different colloidal solutions. When the color of the colloid stabilized, the reaction was rapidly quenched in ice.
SDS-Stabilized Gold Nanoparticles
The conditions used for the synthesis of SDS-stabilized gold nanoparticles were similar to previous step, with the concentration of 25 mM amino acid. But during synthesis, solution of 0.1 M SDS was added. Three different particles with different amino acids were synthesized by adding 0.05, 0.1 and 0.2 mmol of SDS to each of them.
Effect of Magnetic Stirrer on the Size of Gold Nanoparticles
In order to optimize the condition of production, magnetic stirrer was used for preparation of gold nanoparticles. Prepared gold nanoparticles with and without magnetic stirrer were analyzed by UV–Vis and DLS.
Instrumentation and Characterization of GNPs
UV–Vis Spectrophotometry
The optical properties of the gold colloidal solution were monitored on a Shimadzu dual beam spectrophotometer (Model 1601) in the range of 300–700 nm. Quartz cuvettes with 1 cm optical length were used for all measurements.
Transmission Electron Microscopy
Samples for TEM analysis were prepared by placing a drop of the gold colloidal solutions on carbon-coated copper TEM grids. The sample deposited on the grid was allowed to dry in air for a few minutes before analysis. The morphology and the size of the prepared gold nanoparticles were determined by JEOL-JEN 2010 transmission electron microscope operated at an accelerating voltage of 200 kV.
Dynamic Light Scattering (DLS)
Determination of particles’ size distribution was carried out with Zetasizer (Malvern, UK) by illuminating the gold colloidal solution with He–Ne Laser (633 nm) in a sample cell.
Thermal Stability Measurements
TGA profiles of amino acids and amino acids-coated GNPs were monitored by Seiko TG/DTA 32 at a heating rate of 20 °C/min in the temperature range of 24–700 °C under a nitrogen flow of 40 ml/min.
Results
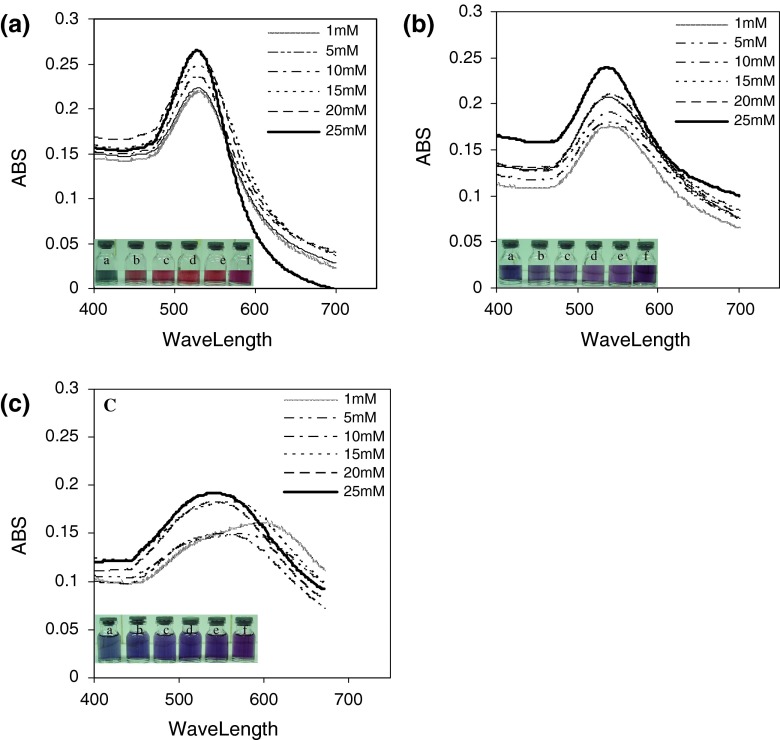
The UV–Vis spectra of gold colloids reduced with different concentrations of aspartic acid, glutamic acid and tryptophan (a: 1 mM, b: 5 mM, c: 10 mM, d: 15 mM, e: 20 mM, and f: 25 mM) are illustrated in Fig. 1. The maximum wavelength of produced gold nanoparticles are 535, 533, 531, 531, 530, 528 nm with aspartic acid, 543, 540, 540, 539, 539, 536 nm with glutamic acid and 596, 575, 561, 550, 541, 541 nm with tryptophan, respectively. Figure 1 shows glassy vials of solutions a–f which indicates variation of optical properties of gold nanoparticles. The colloidal gold solutions in vials ‘a–f’ exhibit gradual increase in reddish color. The change in color of colloidal GNPs solution from red to blue as a consequence of aggregation is a well-understood phenomenon [25].
Fig. 1.
Absorption spectra of different colloidal gold solutions reduced with different concentrations of a aspartic acid, b glutamic acid, and c tryptophan (a 1 mM, b 5 mM, c 10 mM, d 15 mM, e 20 mM, and f 25 mM)
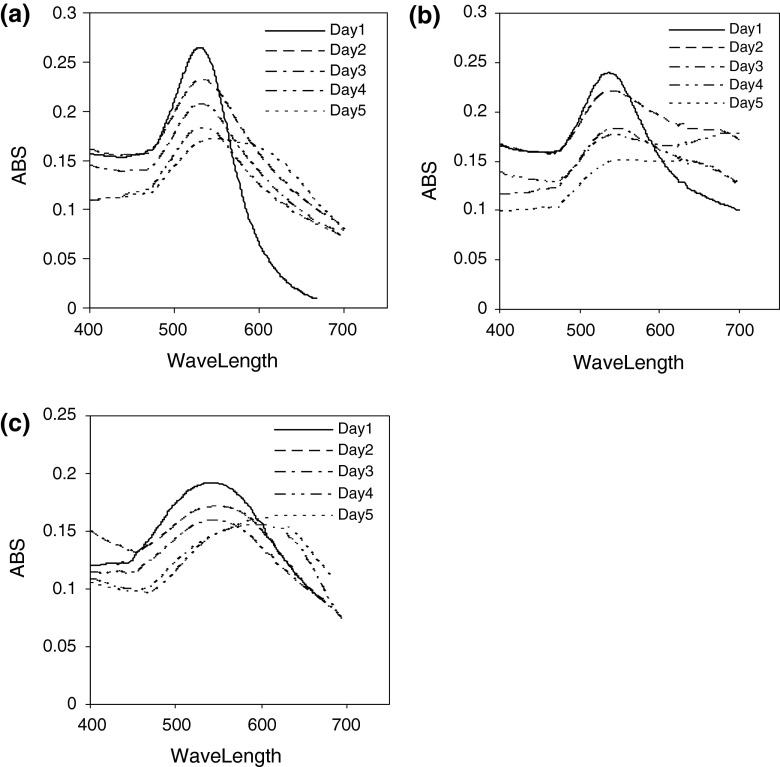
Figure 2 shows the UV–Vis spectra recorded from the gold colloidal solution reduced by aspartic acid, glutamic acid and tryptophan during 5 days. A strong absorption is observed for all spectra in the first day. This resonance corresponds to excitation of surface plasmon vibrations in the gold nanoparticles [26]. The absorption bands of gold colloidal solutions, however, are broadened, indicating aggregation of the gold nanoparticles.
Fig. 2.
UV–Vis spectra recorded from gold particles prepared with a aspartic acid, b glutamic acid, and c tryptophan during 5 day
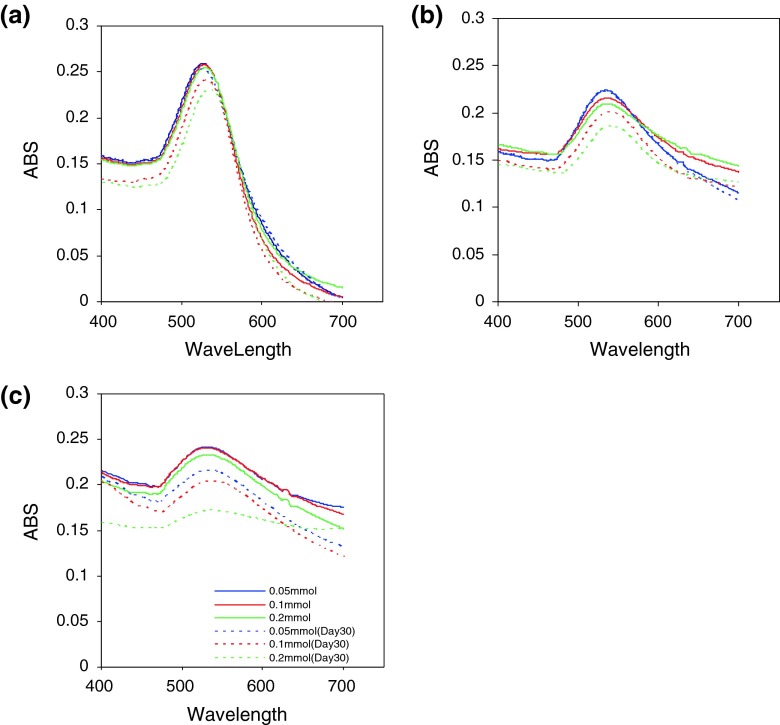
Figure 3 illustrates the UV–Vis spectra of the gold nanoparticles prepared by SDS stabilizer during 30 days. The UV–Vis spectra recorded from the colloidal gold solutions prepared with different concentrations of SDS solution in days 1 and 30 after the test indicate that, increase in SDS concentration would leads to decrease in absorbance and increase in wavelength.
Fig. 3.
UV–Vis spectra of GNPs prepared with 0.05, 0.1, 0.2 mmol of SDS at the first day and thirtieth day. a Aspartic acid, b glutamic acid, and c tryptophan
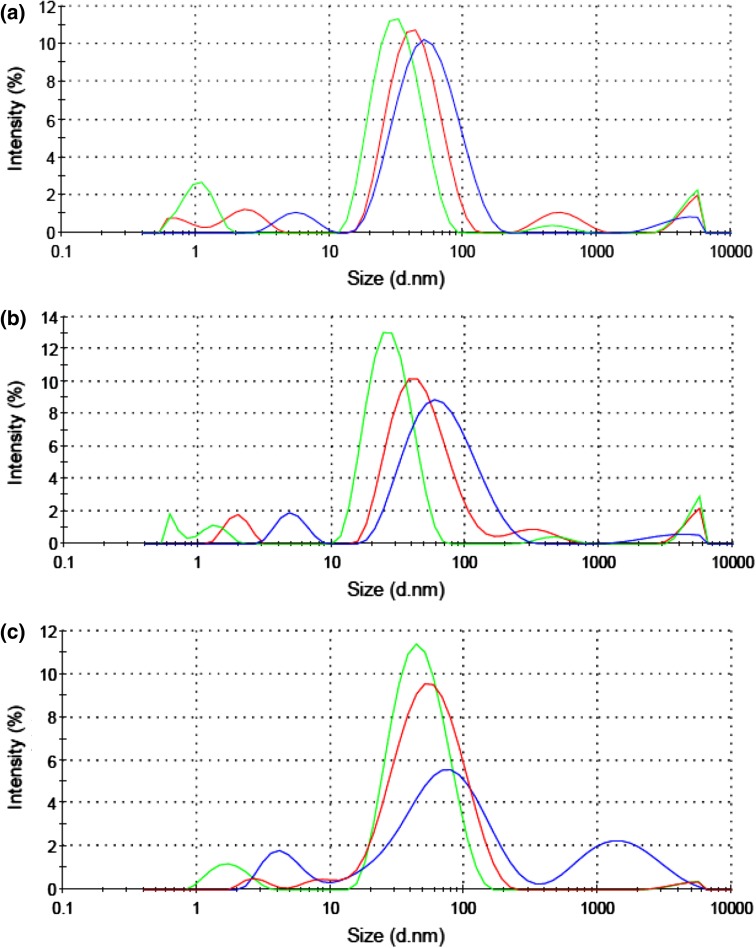
The measurement of particles’ size was carried out by DLS. Results of DLS for gold nanoparticles prepared by 0.05, 0.1 and 0.2 mmol of SDS solution are 29.85, 39.54 and 42.63 nm for aspartic acid; 30.98, 40.10 and 45.29 nm for glutamic acid; and 35.67, 42.39 and 51.86 nm for tryptophan, respectively (Fig. 4).
Fig. 4.
DLS spectra for gold nanoparticles stabilized with 0.05 (green), 0.1 (red), and 0.2 mmol (blue) of SDS. Samples were synthesized with a aspartic acid, b glutamic acid, and c tryptophan. In general, the particle size distribution decreases with increasing SDS concentrations
The UV–Vis spectra recorded from the colloidal gold solutions prepared with and without magnetic stirrer are shown in Fig. 5. It is inferred from the figure that magnetic stirrer has a significant role in size and distribution of produced nanoparticles. UV–Vis spectroscopy results indicate that the wavelengths of nanoparticles prepared with magnetic stirrer are 526, 531 and 533 nm for aspartic acid, glutamic acid and tryptophan, respectively. Nevertheless the wavelengths of nanoparticles prepared without magnetic stirrer are 530, 534 and 536 nm for aspartic acid, glutamic acid and tryptophan, respectively.
Fig. 5.
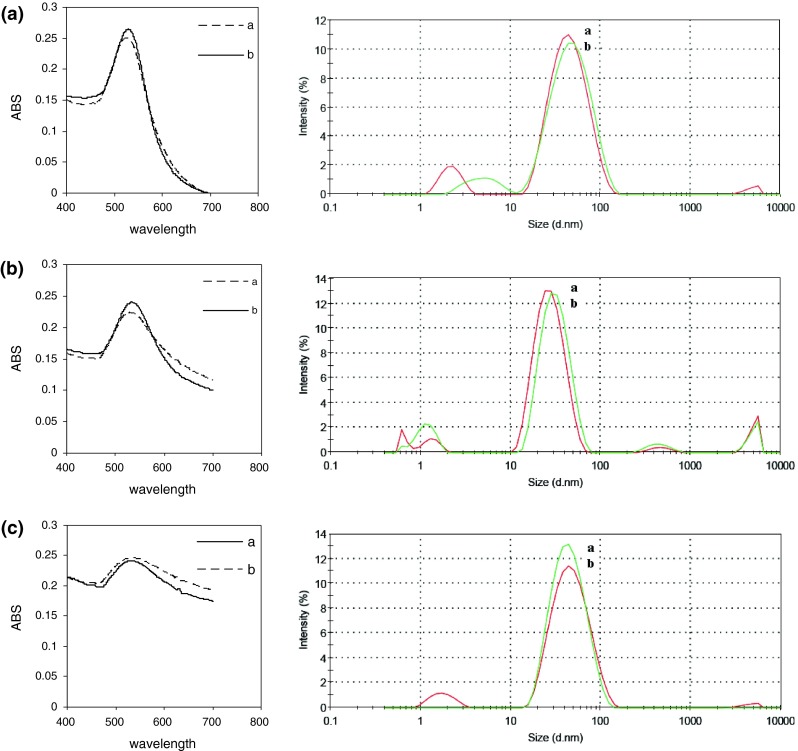
UV–Vis spectra of gold nanoparticles prepared a with magnet and b without magnet. Histograms of particle size distribution determined by DLS are shown on the right-hand side. a Aspartic acid, b glutamic acid, and c tryptophan
The particles’ size distribution curve using DLS, shown on the right-hand side of the images further confirmed the UV–Vis results (Fig. 5). The results point out that the size of gold nanoparticles prepared with magnetic stirrer is less than those which prepared without magnetic stirrer.
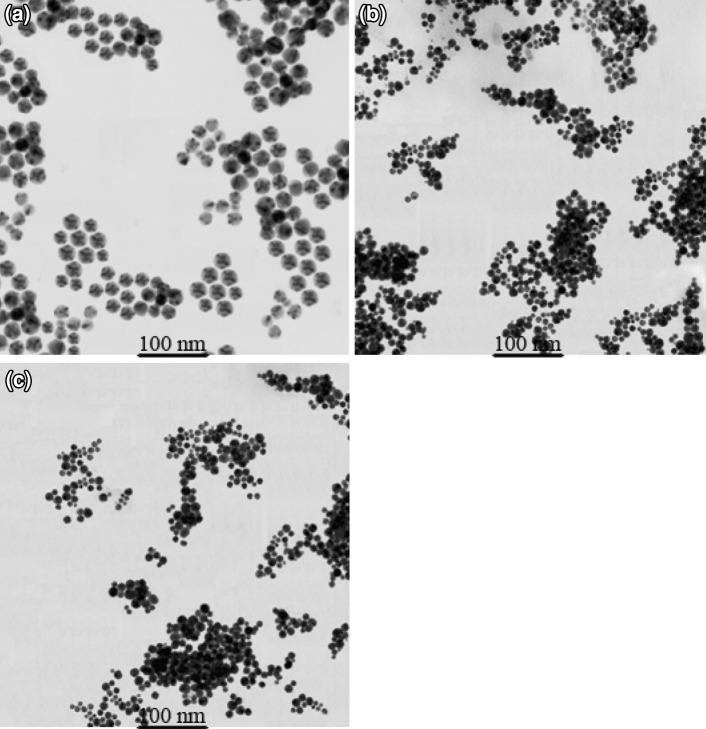
TEM images of GNPs solutions in Fig. 6 reveal that the particles are roughly spherical in shape. Monodispersed particles prepared with aspartic acid, glutamic acid and tryptophan have an average size of 30 ± 5, 15 ± 5 and 10 ± 5 nm, respectively.
Fig. 6.
TEM images of gold nanoparticles preparation with a aspartic acid, b glutamic acid, and c tryptophan
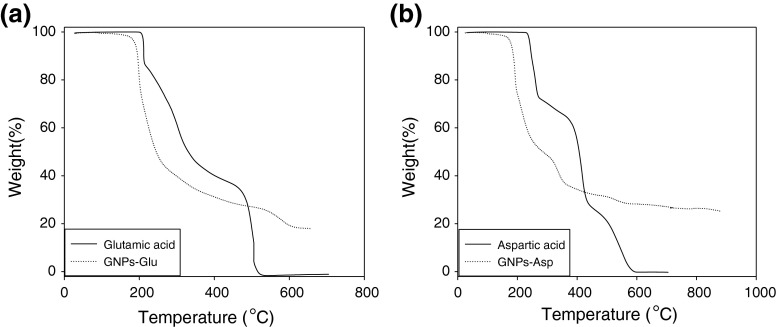
TGA was used for the study of amino acid-coated gold nanoparticles. Figure 7 shows TGA profiles recorded from the glutamic acid–capped gold nanoparticles and pure glutamic acid (curve a), aspartic acid–capped gold nanoparticles and pure aspartic acid (curve b).
Fig. 7.
a, b show representative thermogravimetric analysis profile of a glutamic acid-coated-GNPs and pure glutamic acid, and b aspartic acid-coated-GNPs and pure aspartic acid
The glutamic acid-coated GNPs display two weight losses in the temperature intervals 190–270 °C (66 % weight loss), and 535–605 °C (7 % weight loss), while pure aspartic acid shows two sharp weight losses in the temperature intervals 210–340 °C (55 % weight loss), and 490–520 °C (32 % weight loss). The aspartic acid-coated GNPs display three weight losses in the temperature intervals 180–240 °C (44 % weight loss), 320–355 °C (10 % weight loss) and 500–560 °C (2 % weight loss), while pure aspartic acid shows three sharp weight losses in the temperature intervals 235–270 °C (27 % weight loss), 395–430 °C (33 % weight loss) and 505–580 °C (21 % weight loss).
Discussion
Gold colloids were prepared by reducing tetrachloroauric acid with amino acid. The reduction of HAuCl4 occurred through the transfer of electrons from the amine group of amino acid to the Au3+ ion leading to the formation of Au0. Gold colloids are known to absorb specific band of light due to surface plasmon resonance (SPR). The width of the absorption band and the position of the maximum absorption peak depend on the morphology of the particles (size, shape and uniformity), coagulation among the particles, and also the dielectric environment [27–29].
A comparison of the peak of the absorption spectra of the colloids show a red-shift in the absorption peak with decreasing molar ratio due to a nominal increase in particle size and an increase in the number of non-spherical particles. As a result, the UV–Vis spectra showed that nanoparticles reduced by the amino acid having 25 mM concentration (in which the ratio of amino-acid to gold is 7.5) has shorter wavelength. This concentration was selected as the proper one for reducing and coating nanoparticles.
The variation in optical properties of gold nanoparticles in glass vials containing solutions ‘a–f’ is shown in Fig. 2. The colloidal gold solutions reduced by aspartic acid exhibit gradual increase in reddish color, while in glutamic acid and tryptophan the solutions tend to change from blue to violet. The different colors ranging from red to blue depends on the size of the particles, red being the smallest and blue being the largest [30].
The UV–Vis spectra recorded from the gold colloidal solution reduced by aspartic acid, glutamic acid and tryptophan showed sharp peaks (Fig. 2), but gradually the peaks became broader, indicating aggregation of the gold nanoparticles. Thereby the SDS stabilizer was chosen to optimize preparation conditions of nanoparticles. The UV–Vis spectra recorded from the gold nanoparticles showed that the optimum result was attained using 0.05 mmol of SDS solution during 30 days. It is because that it has more stability and the most absorption in shorter wavelength than other concentrations. Also, DLS results endorsed that nanoparticles prepared by 0.05 mmol of SDS solution have smaller size than that of other concentrations.
On the other hand, studying the nanoparticles prepared with and without magnetic stirrer led to conclusion that those particles prepared by magnetic stirrer have smaller size. As shown in the UV–Vis spectra (Fig. 6) when the magnetic stirrer is used, the maximum absorption of nanoparticles colloids appears in shorter wavelength. This was also confirmed by DLS results from which the nanoparticles diameter was smaller when magnetic stirrer was used.
As mentioned earlier, the peak position can be correlated to the average size of the particle. The main apparent trend is such that the plasmon peaks position follows as: tryptophan > glutamic acid > aspartic acid. As expected, characteristic of the surface plasmon resonance of gold nanoparticles prepared with aspartic acid, glutamic acid and tryptophan was consistent well with the TEM results.
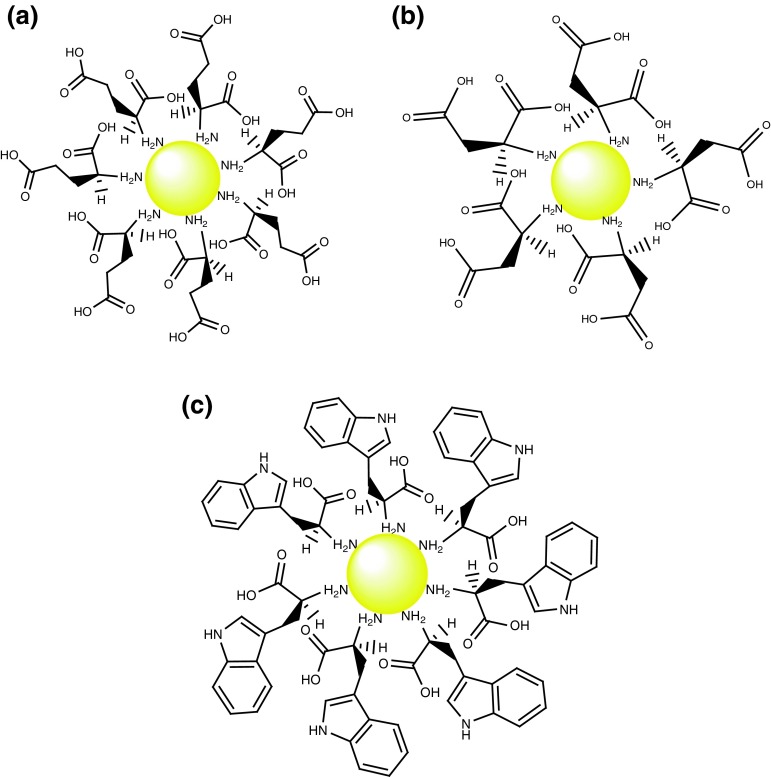
To apply nanoparticles in various processes such as diagnosis and therapeutics, they should be coated for more compatibility of the particles with biological systems. In the preparation method, amino acids initially act as reducing agent and then bind to GNPs surface. This is due to the fact that both Au and N atoms of amine group in the amino acid are soft [10, 16]. Figure 8 schematically demonstrates the amino acids binding to GNPs surface. As it is considered at the result of TGA (Fig. 7), the monotonic weight loss (ca. 44 %) in the temperature interval 190–270 °C (curve A) and 180–240 °C (curve B) are attributed to desorption of trapped water and amino acids molecules which are bounded by non-covalent interactions such as hydrogen bonding to coated-GNPs. In this study, the 7 % weight loss in the temperature interval 535–605 °C for glutamic acid-coated GNPs (curve A) and 12 % weight loss in the temperature interval 320–560 °C for aspartic acid-coated GNPs (curve B) are attributed to complete decomposition of the amino acid molecules bounded to the gold nanoparticles surface. These results clearly indicate that amino acid molecules are bounded to the surface of gold nanoparticles.
Fig. 8.
The schematic representation of coated-GNPs with a aspartic acid, b glutamic acid, and c tryptophan
Due to presence of carboxylic acid groups, the nanoparticles formed by aspartic acid and glutamic acid are proper to react with proteins. Lots of amine groups in proteins can react with carboxylic acids at the surface of GNPs and attach to them. The attachment of the functionalized nanoparticles to proteins creates stable gold–proteins complexes.
Conclusion
Synthesis of gold nanoparticles via reduction of tetrachloroauric acid using aspartic acid, glutamic acid, and tryptophan was reported. TEM images clearly showed the existence of nano-scale particles. Particles produced using glutamic acid were found to be larger on average than those produced in identical conditions using aspartic acid while the particles produced by tryptophan had the largest size.
Particles synthesis using amino acids was in proportion with the ratio of the amino acids used, as decreases in particles size were observed at high amino acid concentrations. The particle size was controlled with different concentrations of sodium dodecyl sulfate as stabilizer. The long-time stability was observed in 0.05 mmol SDS. The studies with TGA clearly showed that amino acids molecules were bounded to the surface of gold nanoparticles.
References
- 1.Mi W, Tian J, Jia J, Tian W, Dai J, Wang X. Characterization of nucleation and growth kinetics of the formation of water-soluble CdSe quantum dots by their optical properties. J Phys D Appl Phys. 2012;45:435303. doi: 10.1088/0022-3727/45/43/435303. [DOI] [Google Scholar]
- 2.Emory SR, Nie S. Screening and enrichment of metal nanoparticles with novel optical properties. J Phys Chem B. 1998;102:493–497. doi: 10.1021/jp9734033. [DOI] [Google Scholar]
- 3.Doose S, Tsay JM, Pinaud F, Weiss S. Comparison of photophysical and colloidal properties of biocompatible semiconductor nanocrystals using fluorescence correlation spectroscopy. Anal Chem. 2005;77:2235–2242. doi: 10.1021/ac050035n. [DOI] [PubMed] [Google Scholar]
- 4.Chan WCW, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 5.Goel A, Rani N. Effect of PVP, PVA and POLE surfactants on the size of iridium nanoparticles. Open J Inorg Chem. 2012;2:67–73. doi: 10.4236/ojic.2012.23010. [DOI] [Google Scholar]
- 6.Schmidt TJ, Noeske M, Gasteiger HA, Behm RJ. PtRu alloy colloids as precursors for fuel cell catalysts. J Electrochem Soc. 1998;145:925–931. doi: 10.1149/1.1838368. [DOI] [Google Scholar]
- 7.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 8.Liz-Marzan LM. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir. 2006;22:32–41. doi: 10.1021/la0513353. [DOI] [PubMed] [Google Scholar]
- 9.Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;17(110):15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 10.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman RJ. Synthesis of thiol-derivatized gold nanoparticles in a twophase liquid-liquid system. J Chem Soc Chem Commun. 1994;801-2
- 11.Volkert AA, Subramaniam V, Ivanov MR, Goodman AM, Haes AJ. Salt-mediated self assembly of thioctic acid on gold nanoparticles. ACS Nano. 2011;5:4570–4580. doi: 10.1021/nn200276a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Templeton AC, Wuelfing WP, Murray RW. Monolayer-protected cluster molecules. Acc Chem Res. 2000;33:27–36. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]
- 13.Sperling RA, Parak WJ. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans R Soc A. 2010;368:1333–1383. doi: 10.1098/rsta.2009.0273. [DOI] [PubMed] [Google Scholar]
- 14.Low A, Bansal V. A visual tutorial on the synthesis of gold nanoparticles. Biomed Imaging Interv J. 2010;6:e9. doi: 10.2349/biij.6.1.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo Q, Worden JG. Monofunctional gold nanoparticles: synthesis and applications. J Nanopart Res. 2007;9:1013–1025. doi: 10.1007/s11051-006-9170-x. [DOI] [Google Scholar]
- 16.Sommer WJ, Weck M. Facile functionalization of gold nanoparticles via microwave-assisted 1,3 dipolar cycloaddition. Langmuir. 2007;23:11991–11995. doi: 10.1021/la7018742. [DOI] [PubMed] [Google Scholar]
- 17.Yonezawa T, Sutoh M, Kunitake T. Practical preparation of size-controlled gold nanoparticles in water. Chem Lett. 1997;26:619–620. doi: 10.1246/cl.1997.619. [DOI] [Google Scholar]
- 18.Kemp MM, Kumar A, Mousa S, Dyskin E, Yalcin M, Ajayan P, et al. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology. 2009;20:455104. doi: 10.1088/0957-4484/20/45/455104. [DOI] [PubMed] [Google Scholar]
- 19.Brown LO, Hutchison JE. Formation and electron diffraction studies of ordered 2-D and 3-D superlattices of amine-stabilized gold nanocrystals. J Phys Chem B. 2001;105:8911–8916. doi: 10.1021/jp011231a. [DOI] [Google Scholar]
- 20.Sastry M, Kumar A, Mukherjee P. Phase transfer of aqueous colloidal gold particles into organic solutions containing fatty amine molecules. Colloid Surf A. 2001;181:255–259. doi: 10.1016/S0927-7757(00)00784-6. [DOI] [Google Scholar]
- 21.Kumar A, Mandal S, Selvakannan PR, Pasricha R, Mandale AB, Sastry M. Investigation into the interaction between surface-bound alkylamines and gold nanoparticles. Langmuir. 2003;19:6277–6282. doi: 10.1021/la034209c. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Y, Patolsky F, Katz E, Hainfeld JF, Willner I. “Plugging into Enzymes”: nanowiring of redox enzymes by a gold nanoparticle. Science. 2003;299:1877–1881. doi: 10.1126/science.1080664. [DOI] [PubMed] [Google Scholar]
- 23.Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimines transfer of plasmid DNA into mammalian cells. Proc Natl Acad Sci USA. 2003;100:9138–9143. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnol. 2004;2:1–6. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayya KS, Patil V, Sastry M. On the stability of carboxylic acid derivatized gold colloidal particles: the role of colloidal solution pH studied by optical absorption spectroscopy. Langmuir. 1997;13:3944–3947. doi: 10.1021/la962140l. [DOI] [Google Scholar]
- 26.Zhang Y, Gao G, Qian Q, Cui D. Chloroplasts-mediated biosynthesis of nanoscale Au–Ag alloy for 2-butanone assay based on electrochemical sensor. Nanoscale Res Lett. 2012;7:1–8. doi: 10.1186/1556-276X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutta J, Hofmann H. Self organization of colloidal nanoparticles. Encycl Nanosci Nanotechnol. 2004;9:617–640. [Google Scholar]
- 28.Liz-Marzan LM. Nanometals: formation and color. Mater Today. 2004;7:26–31. doi: 10.1016/S1369-7021(04)00080-X. [DOI] [Google Scholar]
- 29.Link S, El-Sayed MA. Optical properties and ultrafast dynamics in metallic nanocrystals. Annu Rev Phys Chem. 2003;54:331. doi: 10.1146/annurev.physchem.54.011002.103759. [DOI] [PubMed] [Google Scholar]
- 30.Sugunan A, Dutta J. Nanoparticles for nanotechnology. PSI Jilid. 2004;4:50–57. [Google Scholar]