Abstract
Emulsion polymerization was used to synthesize poly butyl cyanoacrylate nanoparticles in presence of steric stabilizer dextran 70. Nanoparticles were characterized by particle size analysis, scanning electron microscopy and light microscopy. Polymerization factors affecting particle size and distribution such as dextran 70, polysorbate 80 (PS 80) and H+ concentration, polymerization time and temperature, and sonication were studied. Distinct concentrations of stabilizer were needed to produce proper nanoparticles. In this case, the appropriate value was 2 % of total volume. At pH 4 significant decrease in production efficiency demonstrated the substantial effect of H+ concentration on nanoparticles. Furthermore significant increases in particle size and distribution was observed at 50 °C compared to room temperature. 0.001 % (v/v) PS 80 represented notable influence on size and distribution. In addition, shaped nanoparticles were obtained by altering polymerization time from 5.5 h to 18 h. Finally, nanoparticle features were influenced by different factors. Appropriate manipulating of such factors can lead to obtaining desirable nanoparticles.
Keywords: Polybutyl cyanoacrylate, Nanoparticles, Drug delivery, Affecting factors
Introduction
Over the past two decades, cyanoacrylate nanoparticles have been studied focusing on their use in controlled drug delivery. Firstly the simplicity of production was described by Couvreur [1]. He illustrated that submicron particle size can be prepared by scattering alkyl cyanoacrylate monomers in HCl solution at low pH containing an emulsifier. The mechanism of polymerization is anionic based on the plan demonstrated by Behan in which the initiator anion was hydroxyl [2]. Studying several reaction variables comprehensively, Douglas recognized pH and monomer concentration as the most effective factors on the ultimate properties of the nanoparticles [3]. It was deciphered generally particles with less molecular weight (order of 2,000 Da) were formed in the pH range of 2.25–3.5. It was supposed that in this range of pH, oligomers were assembled to outline the nanoparticles. Furthermore, particle size was between 100 and 200 nm, which went up by increasing pH. In pH condition of higher than five, quick polymerization was happened, followed by formation of bulky shapeless accumulation with the inherent polymer holding wide multimodal molecular weight distributions.
In pH condition of higher than five, quick polymerization was happened. This followed by formation of bulky shapeless of polymer with different molecular weight.
As a result, it came from direct polymerization of the monomers supplemented to the reaction media, due to presence of higher concentration of hydroxyl. Vansnick [4] reported almost same results but broadly varying molecular weights and molecular weight distributions obtained by different methods of emulsification and stabilizing. Applying a variety of stabilizing agents resulted in production of ionic and nonionic surfactants, block copolymers and other emulsifiers [5–7]. Use of steric stabilizers such as dextran was also examined [3]. Muller described the production of particles of diverse size by means of the same reaction circumstances and reagents where the only variable was the monomer supply [8]. Behan showed that most efficient medium for particle formation was one with pH 2.5 at a temperature of 65 °C, with dextran 70 as a steric stabilizer [2]. Emulsion polymerization allows some intrinsic advantages like higher rate of polymerization. Furthermore, this technique lead to higher molecular weight and lower particle size. Consequently, emulsion polymerization stands alone among the approaches of free radical polymerization [9]. Considering mentioned points, we tried to produce poly butyl cyanoacrylate (PBCA) nanoparticles by emulsion polymerization. We studied production of PBCA nanoparticles by emulsion polymerization and investigated its characteristics by particle size analysis, scanning electron microscopy (SEM), and light microscopy. Additionally, influence of polymerization agents such as H+, polysorbate 80 (PS 80) and stabilizer concentration, polymerization time and temperature on particle size and distribution were studied. Results showed that nanoparticles were significantly influenced by dextran, H+ and PS 80 concentrations. In addition temperature reaction, sonication, and time of polymerization were effective factors.
Materials and Methods
Butyl cyanoacrylate (BCA) monomer was prepared from Evobond®Tong Shen Enterprise Co., Ltd. (Taiwan). Dextran 70 kDa, HCl and NaOH were purchased from Merck (Germany). All other chemicals and reagents met the analytical grade.
Nanoparticles Preparation and Characterization
PBCA nanoparticles were obtained by anionic polymerization at pH below three with minor modification described previously [8]. Briefly, 1 % BCA monomer (V/V) was added dropwise to a 0.01 N HCl solution containing 2 % dextran 70 kDa as it was stirring by magnetic stirrer. After 4.5 h of polymerization, the nanoparticle suspension was neutralized with 0.1 N sodium hydroxide and polymerization process was continued for one more hour. In process of producing of particles, PS 80 was added before introducing monomer to the reaction (to attain 0/001 % concentration of total volume). PBCA nanoparticle pellets were obtained after centrifugation (GRX-220 high speed refrigerated centrifuge, TOMY, Saitama, Japan) at 21,500 rpm, 4 °C for 50 min. In addition, the effect of sonication (Bandelin Sonorex Digitec, Germany) on nanoparticles obtained by dextran 1 % was evaluated The particles were stored in 2 ml vials at 4 °C after addition of mannitol (200 mg/ml). PBCA powder of nanoparticles was achieved by lyophilisation process (Edwards High Vacuum, Manor Royal, Crawley, Sussex, England). Different types of nanoparticles were produced successfully as mentioned in Table 1. The mean diameter and the size distribution (polydispersity index, PDI) of particles were determined by photon correlation spectroscopy (PCS) using a Malvern Zetasizer nano-Zs Zen 3600 (UK). Particles were analyzed accurately by SEM (XL30, Philips, The Netherlands) and light microscopy (Nikon Eclipse E200) for visual observation.
Table 1.
Various conditions used for nanoparticle preparation
| Variables | |||||
|---|---|---|---|---|---|
| Dextran concentration (%) | PS 80 concentration (%) | Polymerization time (h) | pH | Temperature (°C) | |
| A1 | 2 | 0.001 | 5.5 | 2 | RT |
| A2 | 2 | 0.001 | 18 | 2 | RT |
| A3 | 2 | 0 | 18 | 2 | RT |
| A4 | 2 | 0.001 | 14 | 2 | RT |
| A5 | 2 | 0 | 14 | 2 | RT |
| A6 | 2 | 0 | 5.5 | 2 | 50 |
| A7 | 2 | 0.001 | 5.5 | 2 | 50 |
| A8 | 0.1 | 0 | 5.5 | 2 | RT |
| A9 | 2 | 0.001 | 5.5 | 4 | RT |
| A10 | 1 | 0.001 | 5.5 | 2 | RT |
Results
Considering results, it is evident that particle properties are significantly influenced by different factors applied in this work (Table 2). PBCA nanoparticles were formed by dropwise addition of monomer into the acidic polymerization medium. The medium contained dextran 70 as a steric stabilizer.
Table 2.
Size and PDI of produced nanoparticles under different conditions
| Samples | Size (nm) | PDI |
|---|---|---|
| A1 | 345 | 0.234 |
| A2 | 333 | 0.214 |
| A3 | 374 | 0.255 |
| A4 | 340 | 0.233 |
| A5 | 378 | 0.270 |
| A6 | 380 | 0.318 |
| A7 | 340 | 0.280 |
The appearance of reaction medium get started to change from nearly colorless to milky about 10 min after initiation of process. After centrifugation Pellets obtained in presence of PS 80 were larger in comparison with pellets produced without PS 80. In addition, surfactant-applied nanoparticles afforded more clear supernatant compare to pellet of nanoparticles acquired with no PS 80 (Fig. 1).
Fig. 1.
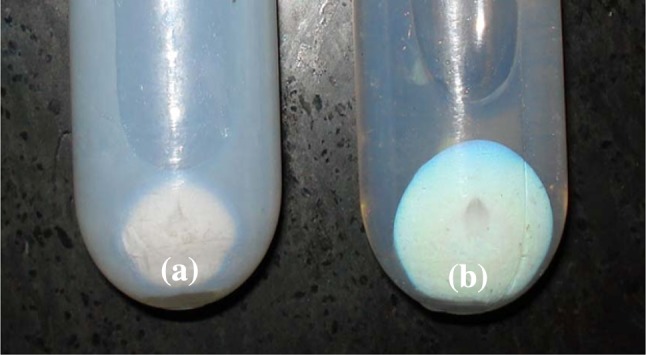
Picture shows two different types of pellets and their supernatants. Sample A2 (image on right side) constructed with ps 80 and A3 manufactured without ps 80 (image on left side). Larger pellet and clearer supernatant (b) obtain at the present of ps 80
Particle size and PDI of nanoparticles at optimum conditions (A2) were 333 nm and 0.214 respectively (Fig. 2).
Fig. 2.
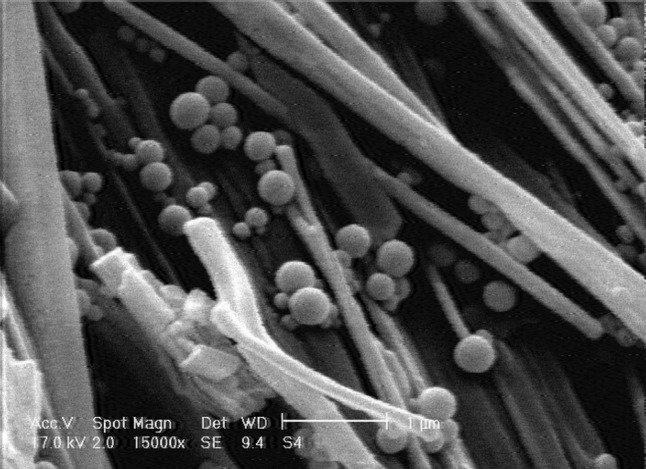
SEM micrograph of nanoparticle A2 (magnification ×15,000)
PBCA nanoparticles produced by dextran concentration of 0.1 % (v/v) (A8) coagulated rapidly and were colloidally unstable (Fig. 3). Alterations in pH intensified effective factors on particle configuration. Polymerization process at pH 4 (A9) offer some aggregates of polymer appeared as crystalline shapes under light microscopy (Fig. 4).
Fig. 3.

SEM micrograph of nanoparticle A8. Lessen the concentration of stabilizer to 0.1 % resulted in the coalescence of unstable formed nanoparticles and produce aggregates of polymer (magnification ×4,544)
Fig. 4.

The effect of pH on the quality of nanoparticles (A9). Some polymeric crystalline forms were produced because of pH raise from 2 to 4 (magnification ×100)
An unexpected event was observed for nanoparticles used dextran at concentration of 1 % (A10). For these nanoparticles after freezing step (−20 °C) and getting ready for lyophilisation process, cylindrical shapes were configured (Fig. 5).
Fig. 5.
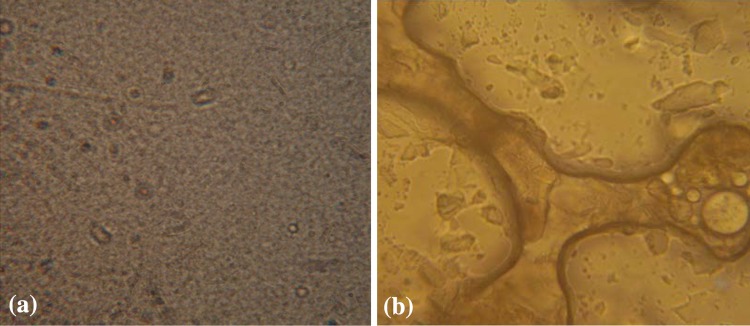
Light microscopy of nanoparticles produced with 1 % dextran concentration (A10). Freezing-point depressions to −20 resulted in disruption of nanoparticle configuration from normal (a) to sloppy crystalline shapes (b) (magnification ×400)
Then sonication was applied for 15 min using an ultrasonic water bath. Ultrasound waves break them to small segments distinctively different from the original ones (Fig. 6).
Fig. 6.

Light microscopy of nanoparticles produced at 1 % dextran concentration. Nanoparticles after freezing performance (a), and those nanoparticles after 30 min sonication (b) (magnification ×100)
Nanoparticle features were improved with by increasing time of polymerization as well. PDI as one of the main features of nanoparticles was significantly decreased when the time of polymerization increased from 5.5 to 18 h. In addition, PS 80 played a key role in quality of nanoparticles. In all cases in presence of surfactant, size and PDI were significantly decreased. Moreover, temperature raising temperature, lead to an increase in size and PDI of particles.
Discussion
Drug delivery applications of PACA nanoparticles have been broadly studied for 25 years due to advantages such as low toxicity and biodegradability [1, 10]. BCA a member of alkyl cyanoacrylates group is a highly reactive monomer and anionic polymerization is one of the three mechanisms that can theoretically arise about this compound [11]. BCA—a member of alkyl cyanoacrylates group—is a highly reactive monomer. Anionic polymerization is one of most common mechanisms that could theoretically happened about this monomer [11].
Different factors can effect size and PDI properties of PBCA nanoparticles. Particle size distribution is expressed as PDI values and recognized to be between 0 and 1. Monodisperse emulsions typically have PDI values of less than 0.2 while largely distributed ones demonstrate values more than 0.5 [12]. Results of this study clearly showed that process conditions have a substantial effect on properties of nanoparticles. While many factors affect the quality of PBCA nanoparticles, we demonstrated that pH, dextran 70, PS 80, sonication, temperature, and time of polymerization plays key role in some cases.
Change in pH range from 2 to 4 and dextran concentration from 2 to 1 and 0.1 % have no nanoparticles formation. So nanoparticles A8–A10 had not provided distinct size and PDI values.
Among the obtained nanoparticles, biggest size belongs to A6 that produced under highest temperature in this study. Moreover PDI values about this nanoparticle has reached the peak. As long as size is considered A2 nanoparticle was the best because of its small size. Hydrodynamic size of A2 was equal to 333 nm. Nanoparticle A2 also presents the highest homogeneity. This nanoparticle has the narrowest PDI value equal to 0.214.
Although the procedure run with dextran 1 % gave nanoparticles, freezing step for lyophilization process lead to deformation of nanoparticles production of crystalline shapes which can be observed under light microscopy. To the best of our knowledge, this event has been reported for first time. Dextran molecule as a steric stabilizer contributes in process of nanoparticle formation. Such phenomenon may be described by cryoprotectant effect of this compound. In the absence of dextran, formed nanoparticles coagulated rapidly and were colloidally unstable [2]. We also observed aggregates of nanoparticles at dextran concentration of 0.1 %. This may suggest that a specific concentration of dextran is needed to prohibit development of aggregation. Formation of crystalline shapes at pH = 4 presented an excessive speed of polymerization to some extent that most of the BCA monomers had no enough time for dispersion and large amorphous agglomerates were formed. Decreasing PDI and size of nanoparticles in the presence of surfactant highlighted the positive role of this substance. This may be originated from intensifying effect of this matter on increasing number of activated monomers and micelle cores which results in lessening the molecular weight of polymers as well as their particle size [13]. Additionally, higher temperature presented a favorable effect on particle formation. The temperature effect may be useful to apply during nanoparticles stabilization process by the dextran. The efficient rotation radius of dextran would correspondingly increase with temperature which can improve steric stabilization [2]. Polymerization time as an effective factor led to longer chains and potentially larger particles formation. Finally, nanoparticle features were influenced by different factors. Appropriate manipulating of such factors can lead to obtaining desirable nanoparticles.
Acknowledgments
The financial support by the Research Council of Pasteur Institute of Iran is gratefully acknowledged.
References
- 1.Couvreur P, Kante B, Roland M, Guiot P, Bauduin P, Speiser P. Polycyanoacrylate nanocapsules as potential lysosomotropic carriers: preparation, morphological and sorptive properties. J Pharm Pharmacol. 1979;31:331–332. doi: 10.1111/j.2042-7158.1979.tb13510.x. [DOI] [PubMed] [Google Scholar]
- 2.Behan N, Birkinshaw C, Clarke N. Poly n-butyl cyanoacrylate nanoparticles: a mechanistic study of polymerisation and particle formation. Biomaterials. 2001;22:1335–1344. doi: 10.1016/S0142-9612(00)00286-6. [DOI] [PubMed] [Google Scholar]
- 3.Douglas SJ, Illum L, Davis SS, Kreuter J. Particle size and size distribution of poly(butyl-2-cyanoacrylate) nanoparticles: I. Influence of physicochemical factors. J Colloid Interface Sci. 1984;101:149–158. doi: 10.1016/0021-9797(84)90015-8. [DOI] [Google Scholar]
- 4.Luc V, Couvreur P, Christiaens-Leyh D, Roland M. Molecular weights of free and drug-loaded nanoparticles. Pharm Res. 1985;2:36–41. doi: 10.1023/A:1016366022712. [DOI] [PubMed] [Google Scholar]
- 5.Seijo B, Fattal E, Roblot-Treupel L, Couvreur P. Design of nanoparticles of less than 50 nm diameter: preparation, characterization and drug loading. Int J Pharmaceut. 1990;62:1–7. doi: 10.1016/0378-5173(90)90024-X. [DOI] [Google Scholar]
- 6.Kreuter J, Mills SN, Davis SS, Wilson CG. Poly butyl-cyanoacrylate nanoparticles for the delivery of 75-SE-norcholesterol. Int J Pharmaceut. 1983;16:105–113. doi: 10.1016/0378-5173(83)90133-3. [DOI] [Google Scholar]
- 7.EL-Egakey MA, Bentele V, Kreuter J. Molecular-weights of polycyanoacrylate nanoparticles. Int J Pharmaceut. 1983;13:349–352. doi: 10.1016/0378-5173(83)90084-4. [DOI] [Google Scholar]
- 8.Muller RH, Lherm C, Herbot J, Blunk T, Couvreur P. Alkylcyanoacrylate drug carriers: I. Physicochemical characterization of nanoparticles with different alkyl chain length. Int J Pharmaceut. 1992;84:1–11. doi: 10.1016/0378-5173(92)90209-K. [DOI] [Google Scholar]
- 9.Bovey FA, Kolthoff IM, Medalia AI, Meehan EJ. Emulsion polymerization. New York: Interscience publishers; 1955. pp. 1–22. [Google Scholar]
- 10.Al Khouri FN, Roblot-Treupel L, Fessi H, Devissaguet JP, Puisieux F. Development of a new process for the manufacture of polyisobutyl-cyanoacrylate nanocapsules. Int J Pharmaceut. 1986;28:125–132. doi: 10.1016/0378-5173(86)90236-X. [DOI] [Google Scholar]
- 11.Gilbert RG. Emulsion polymerization: a mechanistic approach. London: Academic Press; 1995. p. 30. [Google Scholar]
- 12.Wu M, Dellacherie E, Durand A, Marie E. Poly(n-butyl cyanoacrylate) nanoparticles via miniemulsion polymerization (1): dextran-based surfactants. Colloids Surf B. 2009;69:141–146. doi: 10.1016/j.colsurfb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Guise V, Drovin JY, Benoit J, Mahuteau P, Dumont P, Couvreur P. Vidarabine-loaded nanoparticles: a physicochemical study. Pharm Res. 1990;7:736–741. doi: 10.1023/A:1015819706491. [DOI] [PubMed] [Google Scholar]


