Abstract
In this study feruloylated oligosaccharides (FOs) was released from maize bran by hydrochloric acid hydrolysis, and feruloyl arabinose (F-Ara) was obtained by D301 macroporous resin chromatography followed by polyamide resin purification from FOs. After structural identification, the antioxidant activity of F-Ara was evaluated in vitro by DPPH and superoxide radical scavenging activity assay, reducing power assay and chelating activity assay. The results show that F-Ara exhibited antioxidant activity in vitro when compared to standard antioxidants such as butylated hydroxyanisole, ferulic acid and L-ascorbic acid. The antioxidant activity depends on the concentration and increases with increasing dose of sample. The present study suggests that F-Ara possesses promising future for its strong reducing power, chelating activity and free radical-scavenging activity. Therefore, it can be a natural and efficient antioxidant used in food, medicine and cosmetic.
Keywords: Antioxidant activity, Feruloyl arabinose, Free radical, Maize bran
Introduction
Maize is one of the major crops in China. The maize industry produces a large number of maize bran every year, which is generally used for animal feed or discarded as agricultural waste. However, it still contains useful substances such as phenolic compounds (e.g., ferulic acid (FA) and p-coumaric acid) (Saulnier et al. 2001). FA is the most predominant phenolic compound in maize bran and represents up to 3.1% of the dry weight of maize bran (Bunzel et al. 2001; Zhao and Moghadasian 2008). It is well known that FA has many physiological functions, including anti-oxidant, anti-microbial, anti-inflammatory, anti-thrombosis, and anti-cancer activities (Ou and Kwok 2004). Therefore, maize bran is promising for producing FA and its derivatives as functional substances.
In maize bran, FA is ester-linked at the C-5 position to α-l-arabinosyl residues which are substituents of the xylan backbone (Saulnier et al. 1995). Following mild acid hydrolysis, relatively weak glycosidic linkages are split and the ester linkages involving FA remained (Saulnier et al. 1999). The major products of hydrolysis are referred to as feruloylated oligosaccharides (FOs). Because of the presence of FA residues, interest in these oligosaccharides is motivated by their biological activities. One of the most active domains of the research is on their antioxidant activity. In normal rat erythrocytes, FOs show in vitro antioxidant activity against hemolysis induced by free radicals (Yuan et al. 2005a). In diabetic rats, FOs are a suitable in vivo antioxidant for protection against oxidative damage (Ou et al. 2007) In human lymphocytes, FOs exhibited significant protective effect against oxidative DNA damage in cells induced by H2O2 under in vitro conditions (Wang et al. 2008).
However, research has mainly focused on the mixture of FOs, limited information is available on the extract active fraction in FOs responsible for the observed antioxidant activity. Ohta et al. (1997) reported that antioxidant activity of 5-O-(trans-feruloyl)-L-Araf (feruloyl arabinose, F-Ara) isolated from FOs from maize bran is stronger than FA in the LDL oxidation system. In this article, we assessed the antioxidant activity of F-Ara using different in vitro test systems. The antioxidant activity was evaluated with respect to scavenging of DPPH and superoxide radical, reducing power and chelating activity.
Materials and methods
Maize bran was obtained from an animal feed company in Shijiazhuang, Hebei, China. The bran was milled and passed through a 0.5 mm sieve. Heat-stable α-amylase Termamyl 120 L (EC 3.2.1.1 from Bacillus licheniformis, 120 KNU/g) was purchased from Novo Nordisk, Bagsvaerd, Denmark. Protease papain (EC 3.4.22.2 from papaya, 600 KNU/g) was purchased from Yuantian, Guangzhou, China. D301 (weak alkali styrene-type anion exchange resin) was obtained from Zhengguang Industrial Co., Ltd, Zhejiang, China. Polyamide resin was obtained from Sijiashenhua, Zhejiang, China. 1,1-Diphenyl-2-picryl hydrazyl (DPPH) was purchased from Sigma Chemicals Co., St. Louis, MO, USA. All other reagents and chemicals used in the experiment were of analytical grade.
Preparation of maize bran insoluble fiber
Maize bran (100 g) was dried in an oven for 4 h at 105 °C and subsequently ground to pass a 60-mesh sieve. After defatted by n-hexane, maize bran was suspended in water (1000 mL) and the heat-stable α-amylase (7.5 mL) was added. Beakers were heated in a boiling water bath for 1 h and shaken gently every 5 min. The pH was adjusted to 7.5, and samples were incubated with protease (1 g) at 60 °C for 30 min with continuous agitation. After cooling the samples to room temperature, the suspension was centrifuged at 3000 g for 10 min, and the residue was washed with hot water (70 °C) until no cloudiness was evident, and was finally dried at 40 °C overnight in an oven to get maize bran insoluble fiber (Bunzel et al. 2001).
Isolation of feruloylated oligosaccharides
Mild acid hydrolysis of maize bran insoluble fiber was carried out as described by Allerdings et al. (2005), but with minor modifications. Insoluble fiber (100 g) was treated with 50 mmol/L HCl (1.5 L) under reflux for 3 h at 100 °C. After centrifugation (3000 g, 10 min), the supernatant was filtered (<10 μm) on a sintered glass funnel to get maize bran hydrolysate.
One hundred and fifty milliliter of the hydrolysate was applied to a column (30 × 4.5 cm) of D301 resin (previously washed with 5%NaOH and then 5%HCl). Elution was carried out with 8 column volumes of H2O, 5 column volumes of 60% (v/v) EtOH/H2O, and 8 column volumes of EtOH. The fraction eluted with 60% EtOH/H2O was evaporated at 40 °C under vacuum to dryness, then dissolved in water (10 mL) and applied to a column (30 × 4.5 cm) of polyamide resin. Elution was carried out at room temperature and a flow rate of 5 mL/min was maintained. A gradient was used, successively, comprising 10, 20, 30, 40, 50, and 60% (v/v) EtOH/H2O in 250 mL, respectively. The absorbance of the eluent was monitored continuously at 320 nm with an UV detector and fractions were collected in each tube every minute. Fractions corresponding to separate peaks of the chromatogram were pooled, concentrated (40 °C in vacuum) for further analysis.
Identification of feruloyl arabinose
Molecular weights of F-Ara were determined using electro-spray ionization mass spectrometer (ESI-MS) (mass spectrometer API4000 Q TRAP (ion-source: gas auxiliary electro-spray ionization), Applied Biosystems Co., USA).
Esterified FA from F-Ara was determined according to the method of Yuan et al. (2005b). Sample was analyzed for FA using high-performance liquid chromatography (HPLC) using a ZORBAX SB C18 column (150 mm × 4.6 mm, 5 μm). FA was identified by comparison of its relative retention time with standard compound (FA).The isolated FOs were also analyzed by HPLC in the same chromatographic condition.
Monosaccharide composition was analyzed by high-performance anion-exchange chromatography using a pulse amperometric detector (HPAEC-PAD) as described by Yuan et al. (2005a). The sample was analyzed by HPAEC-PAD on a Dionex BioLC system using a Amino Pac PA10 column (2 mm × 250 mm). Monosaccharide composition was identified by comparison to relative retention times of authentic standards (arabinose and xylose).
Determination of antioxidant activity of feruloyl arabinose
The scavenging activity of F-Ara on DPPH radicals was measured according to the method of Yuan et al. (2005a) with minor modifications. FA and butylated hydroxyanisole (BHA) were used as reference materials. An aliquot of 0.5 mL of sample solution at different concentrations (0.05–2 mmol/L) was mixed with 2 mL of Tris–HCl buffer (100 mmol/L, pH 7.4) and 2.5 mL of 0.2 mmol/L ethanolic solution of DPPH. The reaction mixture was shaken well and incubated for 30 min in the dark at room temperature, and the absorbance of the resulting solution was measured at 517 nm against 50% (v/v) aqueous ethanol using a UV-9600 UV/VIS Recording Spectrophotometer (Rayleigh Analytical Instruments, Beijing, China). The radical-scavenging activity of the tested samples was measured as a decrease in the absorbance of DPPH and was calculated by the following equation:
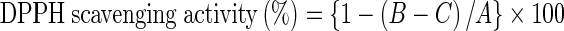 |
where A, B and C are the initial absorbance of the blank, the absorbance of test sample and DPPH solution, and the absorbance of test sample without the DPPH solution, respectively.
The superoxide radical-scavenging activity was estimated using the spectrophotometric monitoring of the inhibition of pyrogallol autoxidation as described by Li et al. (2008) with some modifications. Pyrogallol solution (0.2 mL and 45 mmol/L) was added into a tube containing F-Ara (0.3 mL and 0.25–8 mmol/L) previously dissolved in phosphate buffer (2.7 mL and 0.05 mmol/L, pH 8.2) at 25 °C. The mixture was incubated at 25 °C for 3 min and the optical density (OD) was measured at 420 nm using a spectrophotometer. The antioxidant activity was determined as the percentage of inhibiting pyrogallol autoxidation, which was calculated from OD in the presence or absence of pyrogallol and F-Ara. FA and l-ascorbic acid (Vitamin C, VC) were used as controls.
The reducing power of F-Ara was determined according to the method of Gulcin et al. (2003) with some modification. The sample (0.75 mL) at different concentrations (0.1–1 mmol/L) was mixed with 0.75 mL of 200 mmol/L sodium phosphate buffer (pH 6.6) and 0.75 mL of 1% potassium ferricynide and the mixture was incubated at 50 °C for 20 min. Then, 0.75 mL of 10% trichloroacetic acid was added, and the mixture was centrifuged at 3000 g for 10 min. The upper layer (1.5 mL) was mixed with 1.5 mL of deionized water and 1.5 mL of 0.1% ferric chloride. Finally, the absorbance was measured at 700 nm against a blank (containing all reagents except the test sample). FA and VC were used as controls. The reducing power of the tested sample increased with the absorbance value.
The chelating activity of F-Ara on Fe2+ was estimated by the method of Dinis et al. (1994) with modifications. In brief, 1 ml of sample solution (0.03–0.5 mmol/L) was mixed with 3.7 ml of deionized water and 0.1 ml of 2 mmol/l FeCl2. The reaction was initiated by the addition of 0.2 ml of 5 mmol/l ferrozine, followed by shaking vigorously and left to react at room temperature for 10 min. The absorbance was measured spectrophotometrically at 562 nm. EDTA and FA served as the positive controls, and a sample without test materials served as the negative control. All tests were run in triplicate and averaged. Chelating activity of F-Ara on Fe2+ was calculated as follows:
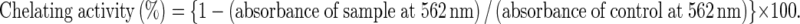 |
Statistical analysis
All the tests were done in triplicate and data were reported as the mean values and standard deviation. Data were analyzed by an analysis of variance and significant differences between means were determined by Duncan’s multiple range tests. Differences in the statistical tests were considered significant when P < 0.05.
Results and discussion
Isolation and identification of feruloyl arabinose
The isolation procedure of FOs involved mild acidic hydrolysis of maize bran fiber, pre-separation of the hydrolyzate using a D301 resin column and further separation by polyamide resin chromatography. Owing to the complex structure of maize bran arabinoxylan which decreases the access of carbohydrolases to the xylan backbone (Allerdings et al. 2006), the method of mild acidic hydrolysis was chosen for preparing FOs. A treatment with 50 mmol/L HCl at 100 °C for 3 h was applied since it gave appreciable amounts of FOs with less release of free FA. The hydrolysate was loaded onto a D301 resin column which was eluted with water, 60% EtOH/H2O, and EtOH. The fraction obtained by eluting with 60% EtOH/H2O contained approximately 80% of esterified FA of the hydrolysate and was, therefore, selected to isolate FOs using polyamide resin chromatography.
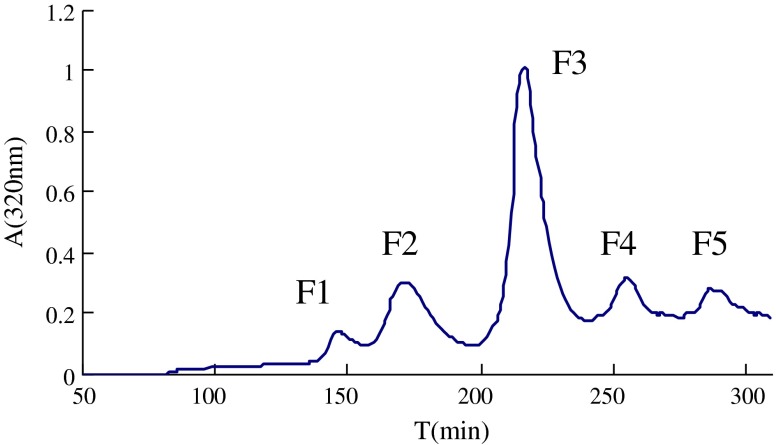
Five fractions (F1–F5) were eluted by a stepwise gradient using ethanol–water from a polyamide resin column (Fig. 1) and analyzed by HPLC. Fractions F1, F4 and F5 yielded several peaks of absorbance at 320 nm in chromatogram of HPLC, which indicate a poor quality in purity. Fraction F3 gave the only one peak of absorbance at 320 nm indicating the purity of F3 reaching HPLC-purity level. Using the described isolation procedure, about 600 mg of F3 were isolated out of 100 g insoluble maize fiber. Fractions corresponding to F3 were pooled, identified for structure, and evaluated for antioxidant activity.
Fig. 1.
Polyamide resin chromatogram of feruloylated oligosaccharides obtained by D301 macroporous resin chromatochraphy
Analysis of F3 by ESI-MS gave a sodium adduct ion with m/z 349.3 [M + Na]+ in positive ion mode and a deprotonated ion with m/z 325.5[M–H]– in negative ion mode, indicating a molecular mass of 326, corresponding to one FA and one arabinose.
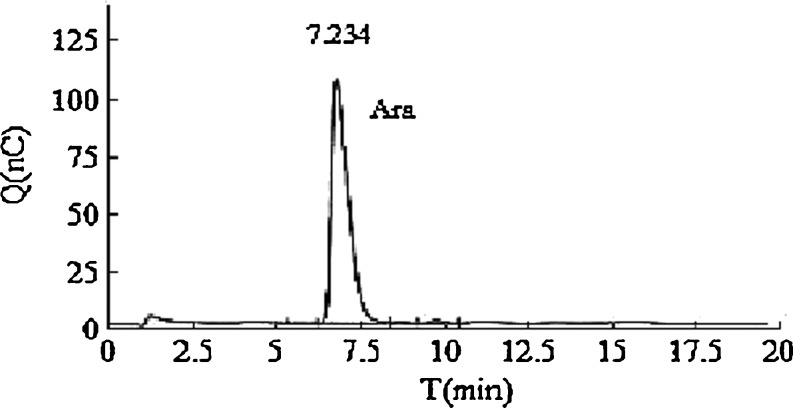
The hydrolyzed products of F3 by treatment with mild alkaline were analyzed by HPLC. Comparison of its relative retention time with standard compound (FA) confirmed the presence of FA. The glycosyl residue composition of the de-esterified products of F3 was analyzed by HPAEC-PAD. The results showed that only arabinose was discovered in the monosaccharide composition of F3 (Fig. 2).
Fig. 2.
Ion chromatogram of monosaccharide composition of fraction F3
Thus, compared with the published literature (Saulnier et al. 1995; Allerdings et al. 2005; Ishii 1997), the structure of F3 was determined to be 5-O-(trans-feruloyl)-L-Araf (F-Ara).
1,1-Diphenyl-2-picryl hydrazyl radical-scavenging activity
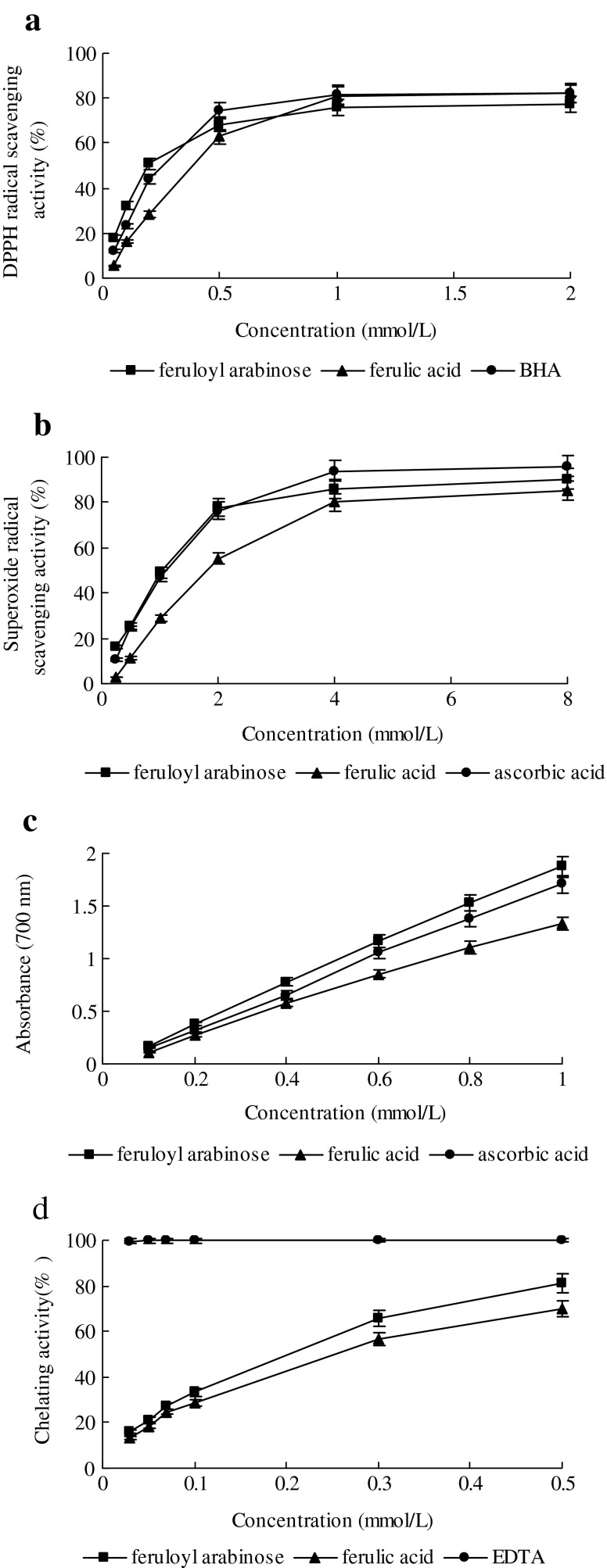
DPPH assay is considered a valid accurate, easy and economic method to evaluate radical scavenging activity of antioxidants, since the radical compound is stable and need not be generated. The assay is based on the measurement of the scavenging capacity of antioxidants towards DPPH. The odd electron of nitrogen atom in DPPH is reduced by receiving a hydrogen atom from antioxidants to the corresponding hydrazine (Kedare and Singh 2011). The profile of scavenging activities of F-Ara and reference materials on DPPH is shown in Fig. 3a. All of test samples were capable of scavenging DPPH radicals in a concentration-dependent manner. The scavenging effect of F-Ara, FA and BHA on DPPH radicals significantly (P < 0.05) increased from 0.05 to 1 mmol/L and subsequently followed by a much slower increase from 1 to 2 mmol/L. At a concentration of 2 mmol/L, the DPPH radical-scavenging activity of F-Ara, FA and BHA reached 77.3 ± 2.6%, 82.0 ± 3.1% and 82.1 ± 2.8%, respectively,, indicating that F-Ara, FA and BHA showed similar DPPH radical scavenging activity (P > 0.05).
Fig. 3.
DPPH radical scavenging, superoxide radical scavenging, reducing power and chelating activity of F-Ara from maize bran. FA, BHA, VC and EDTA were used as reference antioxidants (n = 3)
The effective concentration for 50% scavenging (EC50) determined using the regression equation indicated that for 50% scavenging of DPPH radical, a sample concentration of 0.19 mmol/L of F-Ara is required. For FA and BHA, EC50 values were 0.37 and 0.23 mmol/L respectively. A higher DPPH radical-scavenging activity is associated with a lower EC50 value (Ozsoy et al. 2008). It was evident that F-Ara did show hydrogen-donating ability to act as antioxidants which could react with free radicals to convert them to more stable products and terminate the radical chain reaction.
Superoxide radical-scavenging activity
Numerous biological reactions generate superoxide radical which is a highly toxic species. Although it cannot directly initiate lipid oxidation, superoxide radical is a potential precursor of damaging oxygen species, such as hydroxyl radical, and thus the study of the scavenging of this radical is important (Jayasri et al. 2009). The scavenging activities of F-Ara and the controls on superoxide radicals are shown in Fig. 3b. It was found that F-Ara, FA and VC revealed considerable superoxide scavenging activities at all the concentrations,which increased with the increase of their concentrations. F-Ara and FA at 4 mmol/L exhibited 86.2 ± 1.6% and 79.9 ± 1.1% superoxide radical-scavenging activity, respectively. These values were significantly (P < 0.05) lower than that of the same dose of VC (93.9 ± 2.3%). The data indicate that superoxide radical-scavenging activity of those samples followed the order: VC > F-Ara > FA.
EC50 values, in scavenging abilities on superoxide radical, were comparable for F-Ara (1.05 mmol/L) and VC (1.08 mmol/L), and more effective (P < 0.05) than that of FA (1.77 mmol/L).
This assay is dependent on the reducing activity of test compound by a superoxide radical-dependent reaction, which releases chromophoric products. (Li et al. 2008). The results imply that F-Ara is a good superoxide scavenger and its capacity to scavenge superoxide may contribute to its antioxidant activity.
Reducing power
Fe3+ reduction is often used as an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant action (Dorman et al. 2003). The reducing properties are generally associated with the presence of reductones, the antioxidant action of which is based on the breaking of the free radical chain by donating a hydrogen atom (Shahidi 2000). Figure 3c shows the dose–response curves for the reducing powers of F-Ara from maize bran and reference materials. High absorbance indicates high reducing power (Kosanić et al. 2011). The reducing power of F-Ara increased from 0.169 ± 0.005 at 0.1 mmol/L to 1.873 ± 0.063 at 1 mmol/L. The reducing power of FA and VC increased from 0.112 ± 0.003 and 0.153 ± 0.005 at 0.1 mmol/L to 1.332 ± 0.045 and 1.705 ± 0.058 at 1 mmol/L, respectively. At a dosage of 0.6 mmol/L, F-Ara showed highest reducing values of 1.162 ± 0.031 compared with FA (0.855 ± 0.024) and VC (1.054 ± 0.037), suggesting that F-Ara had a noticeable effect on reducing Fe3+. The results were found statistically significant (P < 0.05).
A high correlation was observed between reducing power and antioxidant activity determined by scavenging of DPPH (r2 = 0.9669, 0.9452 for F-Ara and FA, respectively), and scavenging of superoxide radical (r2 = 0.9858, 0.9948 for F-Ara and FA, respectively). The results were in accordance with other investigators who have also reported that antioxidant properties are concomitant with the development of reducing power (Chung et al. 2005; Kanatt et al. 2007; Ozsoy et al. 2008).
In this study, the reducing power of the samples assessed implies that F-Ara was able to donate electron, hence it should be able to donate electrons to free radicals in actual biological or food systems, making the radicals stable and unreactive.
Chelating activity
F-Ara was assessed for its chelating ability on Fe2+ in free solution. It is well-known that Fe2+ can catalyze oxidative changes in lipid, protein, and other cellular components. Therefore, minimizing Fe2+concentration affords protection against oxidative damage. In this study, Ferrozine can quantitatively form complexes with Fe2+. In the presence of other chelating agents, the complex formation is disrupted with the result that the red color of the complex is decreased. Measurement of the rate of color reduction therefore allows estimation of the chelating activity of the coexisting chelator (Yamaguchi et al. 2000; Wu and Ng 2008). The Fe2+ chelating ability of F-Ara, FA and EDTA were presented in Fig. 3d. F-Ara and FA showed chelating activity as demonstrated by their effectiveness in inhibiting the formation of ferrous and ferrozine complex. The absorbance of Fe2+-ferrozine complex was dose dependently decreased from 0.03 to 0.5 mmol/L for F-Ara and FA. The chelating effect of F-Ara on ferrous ions was significantly (P < 0.05) higher (81.3 ± 2.3%) than that of FA(69.9 ± 1.6%) at 0.5 mmol/L. Neither of them appeared to be better chelators of Fe2+ than the positive control EDTA in this assay system. EDTA showed excellent chelating ability of 99.6 ± 0.8% at a concentration as low as 0.03 mmol/L.
As compared with the EC50 value (the effective concentration at which ferrous ions were chelated by 50%), F-Ara (0.19 mmol/L) showed more effective chelating ability on Fe2+ than FA (0.24 mmol/L), but much less than EDTA (<0.03 mmol/L). The higher Fe2+ chelating ability of F-Ara is of immense importance in the protective ability of F-Ara against oxidative stress, because it is usually too late to attempt to use hydroxyl radical scavengers for therapeutic purposes, because of the high reactivity of hydroxyl radical (Bayır et al. 2006).
Natural antioxidants are closely related in their medicinal and beneficial properties. Thus antioxidant capacity is a widely used parameter for assessing the bioavailability of materials. The antioxidant properties of materials should be evaluated in a variety of model systems using several indices to ensure the effectiveness (Ozsoy et al. 2008). In this study 4 antioxidant activity methods were employed to evaluate the antioxidant property of F-Ara. Interestingly, F-Ara exhibits a better ability to scavenge free radicals and metal ion as compared to FA in vitro. The result is in agreement with the previous study (Ohta et al. 1997). The effectiveness of F-Ara could come primarily from the hydrogen-donating ability of its hydrophobic ferulic acid moiety. The presence of electron donating groups on the benzene ring (3-methoxy and more importantly 4-hydroxyl) of ferulic acid moiety gives the additional resonance structures of the resulting phenoxyl radical, contributing to the stability of this intermediate or even terminating free radical chain reactions, and the carboxylic acid group in ferulic acid moiety with adjacent unsaturated C = C double bond can provide additional attack sites for free radicals (Wang et al. 2008). In addition, F-Ara also contain hydrophilic arabinose moiety, which might further intensify the stability of resonance structures of the resulting phenoxyl radical. Thereby the rate-limiting hydrogen abstraction reaction of F-Ara is promoted and the termination of free radical chain reactions achieve more rapidly.
Ohta et al. (1997) reported that orally administered F-Ara existed as the conjugated form of F-Ara (25%) and FA (75%) in the circulation system. The result demonstrates that F-Ara can be absorbed intact from digestive tract to circulation system and metabolized into conjugated F-Ara. The formation of FA conjugates could be due to the hydrolysis of F-Ara by intestinal esterases (Zhao and Moghadasian 2008). F-Ara from FOs is a nonionic chemical species that may pass through cell membranes with a high density of inner negative charges more easily than the negative free phenolic compounds (Ou et al. 2007). It is suggested that F-Ara in the circulation system possesses the potential to enter the cells and act as an antioxidant in cells. The antioxidant activity of F-Ara in cells is needed to further investigate.
Conclusion
In conclusion, this study indicated that F-Ara could be obtained by D301 macroporous resin chromatography followed by polyamide resin purification from FOs, which was released from maize bran by hydrochloric acid hydrolysis, and was capable of donating hydrogen to convert DPPH to stable product and directly quenching superoxide radical to terminate the radical chain reaction, acting as a reducing agent on reducing Fe3+ significantly, chelating metal ions to prevent such ions from participating in the initiation of lipid peroxidation and oxidative stress. Therefore, this study provided evidence on the potential health benefits of F-Ara.
Acknowledgments
This research was supported by Education Department of Guangdong Province, China: Project Number: cgzhzd0709.
Contributor Information
Qiling Lin, Email: linscott2010@gmail.com.
Shiyi Ou, Email: tosy@jnu.edu.cn.
References
- Allerdings E, Ralph J, Schatz PF, Gniechwitz D, Steinhart H, Bunzel M. Isolation and structural identification of diarabinosyl 8-O-4-dehydrodiferulate from maize bran insoluble fibre. Phytochemistry. 2005;66:113–124. doi: 10.1016/j.phytochem.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Allerdings E, Ralph J, Steinhart H, Bunzel M. Isolation and structural identification of complex feruloylated heteroxylan side-chains from maize bran. Phytochemitry. 2006;67:1276–1286. doi: 10.1016/j.phytochem.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Bayır H, Kochanek PM, Kagan VE. Oxidative stress in immature brain after traumatic brain injury. Dev Neurosci. 2006;28:420–431. doi: 10.1159/000094168. [DOI] [PubMed] [Google Scholar]
- Bunzel M, Ralph J, Marita JM, Hatfield RD, Steinhart H. Diferulates as structural components in soluble and insoluble cereal dietary fibre. J Agric Food Chem. 2001;81:653–660. doi: 10.1002/jsfa.861. [DOI] [Google Scholar]
- Chung Y, Chen S, Hsu C, Chang C, Chou S. Studies on the antioxidative activity of Graptopetalum paraguayense E. Walther. Food Chemistry. 2005;91:419–424. doi: 10.1016/j.foodchem.2004.06.022. [DOI] [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetoaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Dorman HJD, Peltoketo A, Hiltunen R, Tikkanen MJ. Characterization of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003;83:255–262. doi: 10.1016/S0308-8146(03)00088-8. [DOI] [Google Scholar]
- Gulcin Y, Buyukokuroglu ME, Oktay M, Kufrevioglu OY. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn subsp. pallsiana (Lamb.) Holmboe. J Ethnopharmacol. 2003;86:51–58. doi: 10.1016/S0378-8741(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Ishii T. Structure and functions of feruloylated polysaccharides. Plant Science. 1997;127:111–127. doi: 10.1016/S0168-9452(97)00130-1. [DOI] [Google Scholar]
- Jayasri MA, Mathew L, Radha A. A report on the antioxidant activity of leaves and rhizomes of Costus pictus D. Don. Int J Integr Biol. 2009;5:20–26. [Google Scholar]
- Kanatt SR, Chander R, Sharma A. Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007;100:451–458. doi: 10.1016/j.foodchem.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosanić M, Ranković B, Vukojević J. Antioxidant properties of some lichen species. J Food Sci Technol. 2011;48:584–590. doi: 10.1007/s13197-010-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Ohta T, Sembokum N, Kuchii A, Egashira Y, Sanada H (1997) Antioxidant activity of corn bran cell-wall fragments in the LDL oxidation system. J Agric Chem 45:1644-1648
- Ou SY, Kwok KC. Ferulic acid: pharmaceutical functions, preparation and applications in foods. J Sci Food Agric. 2004;84(11):1261–1270. doi: 10.1002/jsfa.1873. [DOI] [Google Scholar]
- Ou SY, Jackson M, Jiao X, Chen J, Wu JZ, Huang XS. Protection against oxidative stress in diabetic rats by wheat bran feruloyl oligosaccharides. J Agric Food Chem. 2007;55:3191–3195. doi: 10.1021/jf063310v. [DOI] [PubMed] [Google Scholar]
- Ozsoy N, Can A, Yanardag R, Akev N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008;110:571–578. doi: 10.1016/j.foodchem.2008.02.037. [DOI] [Google Scholar]
- Saulnier L, Vigouroux J, Thibault J-F. Isolation and partial characterization of feruloylated oligosaccharides from maize bran. Carbohydr Res. 1995;272:241–253. doi: 10.1016/0008-6215(95)00053-V. [DOI] [PubMed] [Google Scholar]
- Saulnier L, Crepeau MJ, Lahaye M, Thibault J-F, Garcia-Conesa MT, Kroon PA, Williamson G. Isolation and structural determination of two 5,5′-diferuloyl oligosaccharides indicate that maize heteroxylans are covalently cross-linked by oxidatively coupled ferulates. Carbohydr Res. 1999;320:82–92. doi: 10.1016/S0008-6215(99)00152-4. [DOI] [Google Scholar]
- Saulnier L, Marot C, Elgorriaga M, Bonnin E, Thibault J-F. Thermal and enzymatic treatments for the release of free ferulic acid from maize bran. Carbohydr Polym. 2001;45:269–275. doi: 10.1016/S0144-8617(00)00259-9. [DOI] [Google Scholar]
- Shahidi F. Antioxidants in food and food antioxidants. Nahrung. 2000;44:158–163. doi: 10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun B, Cao Y, Song S, Tian Y. Inhibitory effect of wheat bran feruloyl oligosaccharides on oxidative DNA damage in human lymphocytes. Food Chem. 2008;109:129–136. doi: 10.1016/j.foodchem.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Wu S, Ng L. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. LWT. 2008;41:323–330. doi: 10.1016/j.lwt.2007.03.003. [DOI] [Google Scholar]
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa K. Antioxidative and antiglycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem. 2000;48:180–185. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- Yuan X, Wang J, Yao H, Chen F. Free radical-scavenging capacity and inhibitory activity on rat erythrocyte hemolysis of feruloyl oligosaccharides from wheat bran insoluble dietary fiber. LWT. 2005;38:877–883. doi: 10.1016/j.lwt.2004.09.012. [DOI] [Google Scholar]
- Yuan X, Wang J, Yao H. Feruloyl oligosaccharides stimulate the growth of Bifidobacterium bifidum. Anaerobe. 2005;11:225–229. doi: 10.1016/j.anaerobe.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Moghadasian MH. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem. 2008;109:691–702. doi: 10.1016/j.foodchem.2008.02.039. [DOI] [PubMed] [Google Scholar]