Abstract
Purpose
Breast implant–associated anaplastic large-cell lymphoma (ALCL) is a recently described clinicopathologic entity that usually presents as an effusion-associated fibrous capsule surrounding an implant. Less frequently, it presents as a mass. The natural history of this disease and long-term outcomes are unknown.
Patients and Methods
We reviewed the literature for all published cases of breast implant–associated ALCL from 1997 to December 2012 and contacted corresponding authors to update clinical follow-up.
Results
The median overall survival (OS) for 60 patients was 12 years (median follow-up, 2 years; range, 0-14 years). Capsulectomy and implant removal was performed on 56 of 60 patients (93%). Therapeutic data were available for 55 patients: 39 patients (78%) received systemic chemotherapy, and of the 16 patients (28%) who did not receive chemotherapy, 12 patients opted for watchful waiting and four patients received radiation therapy alone. Thirty-nine (93%) of 42 patients with disease confined by the fibrous capsule achieved complete remission, compared with complete remission in 13 (72%) of 18 patients with a tumor mass. Patients with a breast mass had worse OS and progression-free survival (PFS; P = .052 and P = .03, respectively). The OS or PFS were similar between patients who received and did not receive chemotherapy (P = .44 and P = .28, respectively).
Conclusion
Most patients with breast implant–associated ALCL who had disease confined within the fibrous capsule achieved complete remission. Proper management for these patients may be limited to capsulectomy and implant removal. Patients who present with a mass have a more aggressive clinical course that may be fatal, justifying cytotoxic chemotherapy in addition to removal of implants.
INTRODUCTION
Non-Hodgkin's lymphomas can involve the breasts, either by arising in the breasts or secondarily as a result of disseminated disease.1 The most common types of lymphoma that involve the breasts are diffuse large B-cell lymphoma and extranodal marginal zone lymphoma.1 T-cell lymphoma involves the breasts far less often, representing fewer than 10% of all instances.2 Within this overall context, in recent years an association between breast implants and anaplastic large-cell lymphoma (ALCL), a subtype of T-cell lymphoma, has been observed. Since the first case of breast implant–associated ALCL described in 1997 by Keech and Creech,3 several case reports and small case series have been reported, and the United States Food and Drug Administration recently reported 60 registered instances of breast implant–associated ALCL.4,5 As the number of reported cases continues to grow, the association between breast implants and ALCL has become more striking. Also, anaplastic large-cell lymphoma rarely occurs in the breast in the absence of breast implants. In aggregate, these findings suggest the possibility of an etiologic relationship between breast implant–associated ALCL and breast implants.
The neoplastic nature of breast implant–associated ALCL is reflected in the morphology of the anaplastic cells, an aberrant T-cell immunophenotype, and the finding of monoclonal rearrangements of the T-cell receptor genes. Furthermore, in a few instances in which cells were cultured, clonal cytogenetic abnormalities were detected.6 Very limited clinical follow-up is available, and therefore the natural history of this disease is unknown. Some studies, based on short-term clinical follow-up, have suggested that breast implant–associated ALCL is clinically indolent.7,8 Rare case reports, in contrast, have raised concerns that this lymphoma can be aggressive.9,10 As a result, the optimal approach for managing patients with breast implant–associated ALCL is controversial. Longer clinical follow-up of these patients might be useful to better define the natural history of breast implant–associated ALCL, and a review of management modalities applied to these patients could also help in clarifying appropriate management.
In this study, we report clinical follow-up of patients with breast implant–associated ALCL, including an update of patients whose data were published previously in the literature as well as newly identified patients who were not previously reported in the literature. These data contribute to a better understanding of the natural history of patients with breast implant–associated ALCL and may be helpful in designing the most appropriate approach for patient management.
PATIENTS AND METHODS
We performed a literature search for all reports of breast implant–associated ALCL published between 1997 and December 2012 and we evaluated these reports to determine whether the lymphomas fulfilled the diagnostic criteria for breast implant–associated ALCL.2,11 Breast implant–associated ALCL is defined as a neoplasm of large lymphoid cells with abundant cytoplasm and pleomorphic nuclei of T-cell lineage that uniformly expresses CD30 and is negative for anaplastic lymphoma kinase (ALK) protein or translocations involving the ALK gene at chromosome 2q23. In most patients, the tumor presents as an effusion around the breast implant. The tumor is not identified grossly but neoplastic cells are detected microscopically within the effusion or lining the fibrous capsule surrounding the implant. In a minority of patients, the tumor cells form a mass that is detected by radiologic or gross pathologic examination.12 In our study, we included patients who presented with these described features, and we excluded reports of patients with ALK-negative ALCL whose disease was confined to skin, patients with breast tumors not adjacent to the fibrous capsule around an implant, or patients who had concomitant systemic disease at time of presentation.
The corresponding authors of all published cases of patients with breast implant–associated ALCL were contacted and invited to contribute updated pathologic and clinical information as well as treatment and follow-up data. During the course of our study, we encountered in our consultation practice additional patients with breast implant–associated ALCL and these patients are also included in this study. This study was approved by the institutional review board of MD Anderson Cancer Center.
Overall survival (OS) was calculated from time of diagnosis to death from any cause or to last follow-up for living patients. Progression-free survival (PFS) was calculated from time of diagnosis to progression or death or time of last clinical follow-up if patients remained progression-free. Overall and progression-free survival curves of different groups were analyzed by using the Kaplan-Meier survival curves and differences were compared using the log-rank (Mantel-Cox) test. All statistical analyses were performed using SAS 9.3 for Windows. All differences with P ≤ .05 were considered to be statistically significant. Patients who died or developed tumor progression as documented in the previously published case reports were also included, even if no further follow-up was obtained for this patient subset because the clinical follow-up was definitive.
RESULTS
Clinical Findings
Since the first report in 1997 through December 2012, 50 patients with breast implant–associated ALCL were indexed in PubMed. Corresponding authors responded with additional clinical follow-up for 28 patients2,3,6,12–21 and 16 patients reported previously9–12,22–26 had a definitive end point; however, no follow-up was obtained for six reported patients. Together with the 16 new patients seen in consultation either at MD Anderson Cancer Center or diagnosed at different institutions in Australia, a total of 60 patients represent our study group and their clinicopathologic features are listed in Table 1.
Table 1.
Breast Implant–Associated ALCL (1997-2012): Clinicopathologic Features of 60 Patients
| Clinical Features | No. | % |
|---|---|---|
| Age, years | ||
| Median | 52 | |
| Range | 28-87 | |
| Side | ||
| Right | 31 | |
| Left | 20 | |
| Bilateral | 1 | |
| Reason for implants | ||
| Cosmetic | 34 | |
| Breast cancer | 26 | |
| Stage I | 5 | |
| Stage II | 3 | |
| Stage III | 1 | |
| Carcinoma in situ | 6 | |
| Stage, NA | 11 | |
| Therapy for breast cancer | ||
| Surgical approach | 22 | |
| Radical mastectomy | 9 | |
| Mastectomy | 8 | |
| Lumpectomy | 2 | |
| NA | 4 | |
| Chemotherapy or radiation | ||
| Yes | 15 | |
| No | 5 | |
| NA | 6 | |
| Type of implant | 51 | |
| Silicone | 23 | |
| Saline | 28 | |
| Texture of implant | ||
| Textured | 21 | |
| NA | 39 | |
| Interval to lymphoma diagnosis, years | 59 | |
| Median | 9 | |
| Mean | 10.9 | |
| Range | 1-32 | |
| Clinical presentation | ||
| Effusion | 42 | |
| Mass | 18 | |
| Tumor size, cm | 9 | |
| Mean | 3.2 | |
| Median | 2 | |
| Range | 0.5-10 | |
| Not specified | 3 | |
| NA | 6 | |
| Axillary lymphadenopathy | 29 | |
| Yes | 10 | 34 |
| Positive | 6 | |
| Negative | 4 | |
| No | 19 | 66 |
| Stage of disease at presentation | 59 | |
| I | 49 | 83 |
| II | 6 | 10 |
| IV | 4 | 7 |
| NA | 1 | |
Abbreviations: ALCL, anaplastic large cell lymphoma; NA, not available.
All patients were women with a median age of 52 years (range, 28 to 87 years). The right breast was affected in 37 patients, the left in 20, and both sides were affected in one patient19; this data was not available for two patients. The reasons for breast implants were cosmetic in 34 patients and as part of reconstructive surgery, mostly for breast carcinoma, in 26 patients. The stage of breast carcinoma was known for 15 patients. Breast implants were filled with saline (n = 28) or silicone (n = 23); this information was not available for nine patients. The surface of the implants was not known for 39 patients, whereas 21 patients received textured breast implants. The time interval from implantation to diagnosis of ALCL was available for 59 patients, and it ranged from 1 to 32 years (median, 9 years; mean, 10.9 years). The most frequent clinical presentation was effusion in 42 patients and a distinct mass (usually with associated effusion around implant) in 18 patients.
The presence of axillary lymphadenopathy was specifically addressed in 29 patients and was detected in 10 patients (34%). Lymph nodes were biopsied or excised for pathologic evaluation in eight patients: four were positive for lymphoma and four were negative. In the remaining two patients, axillary lymph nodes were considered to be positive by clinical and radiologic assessment and were not sampled for pathologic evaluation. Staging data were available for 59 patients (98%): 49 patients (83%) had stage I, six (10%) had stage II, and four (7%) had stage IV disease.
Pathologic Findings
The capsule was excised in 56 patients and initially was left in place in four patients. Gross examination could not identify a mass in 42 patients, but a distinct mass was detected in 18 patients.2,3,9,10,12,21–24,26 The dimensions of the masses were available for nine patients and ranged from 0.5 to 10 cm (mean, 3.2 cm; median, 2 cm).2,12,21,26 In one additional patient, the mass was noted as “large,”10 and in two more patients nodules were described. Information on size was not available in six patients.
Histologic examination revealed that the tumor was confined within the capsule in 42 patients. The ALCL cells were present as small clusters within the effusion and/or lining the fibrous capsule, but without growth as a distinct tumor mass. In 18 patients, a distinct mass of tumor cells within the thickness of the capsule or beyond the capsule was found. In these patients, there were confluent sheets or loose clusters of ALCL cells with a variable amount of necrosis or sclerosis. In both patient subsets, the lymphoma cells were large and anaplastic and included cells with horseshoe-shaped nuclei (so-called hallmark cells).
All tumors were uniformly and strongly positive for CD30 (n = 60) and had a T-cell immunophenotype (n = 60). All 57 tumors tested for ALK were negative. Twenty-nine tumors were assayed for T-cell receptor γ chain gene rearrangements by polymerase chain reaction methods; 28 tumors (97%) were monoclonal and one (3%) was polyclonal.
Therapy and Clinical Follow-Up
A summary of the therapies employed for all 60 patients is listed in Table 2. Fifty-six (93%) of 60 patients underwent capsulectomy. In addition, five patients also had mastectomy (one patient's was radical) and five patients underwent axillary lymph node dissection. Information for adjuvant therapy was available for 55 patients. Chemotherapy was administered to 39 (71%) of 55 patients and local radiation therapy was given to 31 (55%) of 56 patients. Details of the chemotherapeutic regimens and schedules were available for 32 patients and were unavailable for seven patients; the data are summarized in Table 2. Twenty-six (47%) of 55 patients received both chemotherapy and radiation therapy and four patients received radiation therapy only (one at time of local relapse). Autologous stem-cell transplantation was performed in eight patients. Twelve patients opted out of therapy beyond implant removal and capsulectomy; they are undergoing watchful waiting. Thus, including the four patients who did receive radiation therapy only, a total of 16 patients did not receive chemotherapy.
Table 2.
Therapy of 60 Patients With Breast Implant–Associated ALCL Diagnosed Between 1997 and 2012
| Treatment | No. of Patients |
|---|---|
| Surgical procedure | |
| Capsulectomy* | 56 |
| Mastectomy | 5 |
| Plus axillary lymph node dissection | 5 |
| None | 4 |
| Chemotherapy | |
| Yes | 39 |
| No | 16 |
| NA | 5 |
| Radiation therapy | |
| Yes | 31 |
| No | 25 |
| NA | 4 |
| Chemotherapy plus radiation | 26 |
| Chemotherapy, no radiation | 13 |
| No chemotherapy | 16 |
| Watchful waiting | 12 |
| Radiation only | 4 |
| SCT† | 8 |
| Autologous | 6 |
| As salvage therapy | 4 |
| No SCT | 39 |
| NA | 13 |
| Chemotherapy details | |
| Chemotherapy, not specified | 7 |
| CHOP and CHOP-like | 31 |
| CHOP | 30 |
| CHOEP | 1 |
| CHOP and ICE‡ | 3 |
| CHOP, ICE,‡ and CY‡ | 1 |
| Hyper-CVAD‡ | 1 |
| No. of cycles | |
| 6 | 22 |
| 5 | 1 |
| 4 | 1 |
| 3 | 4 |
| NA | 11 |
Abbreviations: ALCL, anaplastic large cell lymphoma; CVAD, cyclophosphamide, vincristine, doxorubicin, dexamethasone; CHOP, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone/prednisolone; CHOEP, CHOP and etoposide; CY, cytoxan; ICE, ifosfamide, carboplatin, etoposide; NA, not available; OS, overall survival; PFS, progression-free survival; SCT, stem-cell transplantation.
Two instances of capsulectomy performed after chemotherapy failure.
Six patients with autologous SCT; four patients received SCT as salvage therapy.
Chemotherapy after relapse or CHOP failure.
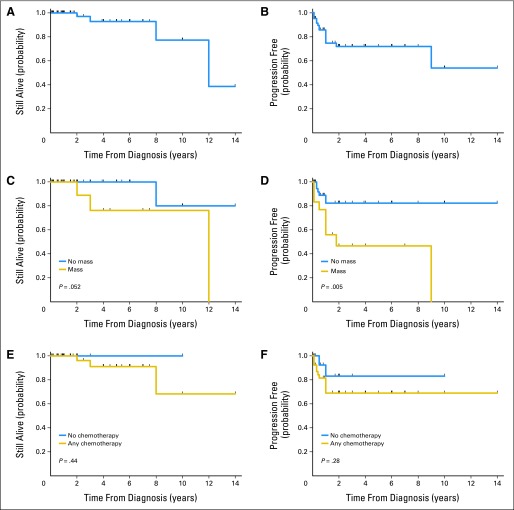
Longer or updated clinical follow-up or a definitive end point was obtained for all 60 patients and is listed in Table 3. The follow-up period for the 60 patients in this study ranged from 0.1 to 14 years, with median and mean follow-up times of 2 and 3.1 years, respectively. The median OS for all patients is 12 years (Fig 1A). The median OS for 18 patients with mass was also 12 years, but OS was not reached for patients without tumor mass (P = .052; Fig 1C); however statistical significance was reached for the 3- and 5-year survival rates (P = .0308). The median PFS for all patients could not be estimated because the survival curve remained above 50% (Fig 1B); median PFS was 1.8 years for patients with a mass and was not reached for patients without a mass (P = .005; Fig 1D). The 3- and 5-year OS rates were 97% and 92%, respectively.
Table 3.
Follow-Up and Outcome of 60 Patients With Breast Implant–Associated ALCL Diagnosed Between 1997 and 2012
| Survival | Value |
|---|---|
| Follow-up, years | |
| Original publication | |
| Median | 0.8 |
| Mean | 1.3 |
| SD | 1.8 |
| Range | 0-9 |
| Updated follow-up | |
| Median | 2 |
| Mean | 3.1 |
| SD | 3.2 |
| Range | 0.1-14 |
| OS, years | |
| Median OS | 12 |
| OS patients with no mass | Not reached |
| OS patients with mass | 12 |
| OS patients with no mass > OS patients with mass | P = .052 |
| Survival | |
| Survival with no mass, % of patients | |
| 3-year | 100 |
| 5-year | 100 |
| Survival with mass, % of patients | |
| 3-year | 82 |
| 5-year | 75 |
| Overall survival, % of patients | |
| 3-year | 97 |
| 5-year | 92 |
| OS patients no mass > OS patients mass | |
| 3-year | P = .0308 |
| 5-year | P = .0308 |
| PFS | |
| Median PFS | Not reached |
| PFS patients with mass | 1.8 |
| PFS patients without mass | Not reached |
| PFS patients with no mass > PFS patients with mass | P = .005 |
| Effect of chemotherapy, patients who received chemotherapy v patients who did not receive chemotherapy | |
| OS (n = 55); deaths (n = 3) | P = .44 |
| PFS (n = 55); events (n = 15) | P = .28 |
Abbreviations: ALCL, anaplastic large-cell lymphoma; OS, overall survival; PFS, progression-free survival; SD, standard deviation.
Fig 1.
Breast implant–associated anaplastic large-cell lymphoma. (A) Overall survival (OS) for all patients. (B) Progression-free survival (PFS) for 60 patients with long-term follow-up. (C) Comparison of OS between patients with and without grossly detected tumor mass. (D) Comparison of PFS between patients with and without grossly detected tumor mass. (E) Comparison of OS between patients who received and who did not receive chemotherapy. (F) Comparison of PFS between patients who received and who did not receive chemotherapy.
For the 42 patients with localized disease confined by the fibrous capsule, six patients (14%) experienced disease relapse; of which two patients had persistence of disease at last follow-up, one patient died of unrelated causes 7 years after relapse, and three patients achieved complete remission at last follow-up. Thus, overall, 39 (93%) of 42 patients without a mass achieved complete remission at last follow-up. In contrast, nine (50%) of 18 patients with a mass experienced disease relapse; of which three (17%) of 18 patients died, two (11%) of 18 had persistence of disease at last follow-up, and four (22%) of 18 achieved complete remission after chemotherapy. Thus, overall, 13 (72%) of 18 patients with a mass achieved complete remission at last follow-up.
For the entire patient cohort, no other significant statistical associations were detected when outcome was compared with clinical and pathologic information. There were no significant associations with patient age, side of lymphoma, reason for implants (cosmetic v reconstructive surgery), implant type (silicone v saline; textured v nontextured), time interval from implant to lymphoma, and treatment with chemotherapy. For the latter comparison, 39 (71%) of 55 patients received chemotherapy, 26 of whom also received local radiation therapy, and the remaining 16 patients (29%) did not receive chemotherapy, including 12 patients (22%) who chose watchful waiting and four (7%) who received local radiation therapy but not chemotherapy. A comparison between patients who received chemotherapy and patients who did not receive any chemotherapeutic regimen revealed no difference in OS (P = .44; Fig 1E) or PFS (P = .28; Fig 1F). The median and mean follow-up of the 16 patients who did not receive chemotherapy was 1.5 and 1.9 years, respectively (range, 0.1 to 10 years). The 12 patients who opted for watchful waiting had a median follow-up period of 1 year (range, 0.1 to 10 years).
Three patient deaths in this study occurred in the subset of patients who received chemotherapy; therapy information is not available for one patient. All 16 patients who did not receive chemotherapy were alive and in complete remission at last follow-up, including one patient who initially opted for watchful waiting and subsequently developed local relapse for which she was treated with local radiation therapy. This patient achieved complete remission and was free of disease at last follow-up, 1.5 years after initial diagnosis.
DISCUSSION
Breast implant–associated ALCL is a rare type of lymphoma that was initially described in 1997.3 The incidence of developing breast implant–associated ALCL among patients with breast implants is low and, according to a study in the Netherlands, the incidence is 1 in 500,000 women who have received breast implants.12 Systematic studies on the incidence of this disease in the United States are not available, and current reports may underestimate the incidence of this tumor, a trend that results in part because recommendations for pathologic examination of tissues excised during cosmetic surgery are not standardized. A survey of 413 institutions in 1999, mostly in the United States, showed that approximately 10% of institutions had policies that exempt submitting “mammary implants” for pathologic examination, and slightly over 60% of institutions had policies that recommend “mammary implants” for gross examination only.27
We present the longest clinical follow-up for a group of patients with breast implant–associated ALCL currently available in the literature. Our data show that most patients achieve complete remission, which can be considered as a cure of disease after initial therapeutic measures. Only four patients (6.7%) in this patient cohort died; three patients whose deaths were attributable to disease and one of unrelated causes. We also show an association between clinical presentation and subsequent disease course. In 42 patients who presented with disease confined within the fibrous capsule, 39 patients (93%) achieved complete remission, mostly with subsequent disease-free survival, and were alive at last clinical follow-up, whereas only one patient (2%) died of unrelated causes. In contrast, among 18 patients who presented with a mass, 13 patients (72%) achieved complete remission (P = .18) and, although some patients had recurrent or persistent disease, only three patients (17%) died as a result of their disease.
To address the possible effect of chemotherapy, we compared OS and PFS of 39 patients who received chemotherapy versus 16 patients who did not receive chemotherapy; in this latter group there were four patients who received local radiation therapy only. Patients who received chemotherapy did not show better overall or progression-free survival when compared with patients who did not receive chemotherapy (P = .44 and P = .28, respectively). Clinical follow-up of patients who opted for watchful waiting after capsulectomy (n = 12) or who received radiation therapy only (n = 4) showed that all these patients achieved complete remission (median follow-up, 1.5 years; mean, 1.9 years; range, 0.1 to 10 years).
Based on the data we present that most patients with breast implant–associated ALCL achieve complete remission and have excellent progression-free survival, and that patients with high-stage disease or relapsed disease can often be treated successfully, we suggest that a conservative approach to patient management may be best. Perhaps the approach to patients with breast implant–associated ALCL might be similar to the model used for cutaneous CD30-positive T-cell lymphoproliferative disorders,28 and breast implant–associated ALCL could be designated as a CD30+ T-cell lymphoproliferative disorder associated with a breast implant. The diagnosis of ALCL could be reserved for patients with a tumor mass or advanced stage disease who are most likely to require aggressive therapy.
Despite the apparent strong association between breast implants and ALCL that typically surrounds the implants, suggesting an etiologic relationship, the cause of breast implant–associated ALCL is unknown. Breast implants have been used since the early 1960s and there are no reports of breast implant–associated ALCL between 1964 and 1991.29 Epidemiologic studies before 1996 did not find an association between breast implants and an increased risk of breast cancer,30 and the index case of breast implant–associated ALCL was first reported in 1997.3 Possible explanations include a long interval between implant and ALCL genesis, an increased number of women who receive implants as they became more accepted and accessible, or a change in the type of implant.
In summary, we present an updated analysis with longer clinical follow-up of 60 patients with breast implant–associated ALCL. These data suggest that there are two patient subsets. Most patients who present with an effusion around the implant, without a tumor mass, achieve complete remission and excellent disease-free survival. A smaller subset of patients presents with a tumor mass associated with the fibrous capsule and are more likely to have clinically aggressive disease. We suggest that patients without a mass may benefit from a conservative therapeutic approach, perhaps removal of the implant with capsulectomy alone, whereas patients with a tumor mass may need removal of the implants and systemic therapy that still needs to be defined. Although our study expands the follow-up and outcomes of most patients with breast implant–associated ALCL reported in the literature, the median follow-up time of 2 years for these patients is still short and it will be valuable to follow this patient cohort for a longer period of time.
Glossary Terms
- TCR (T-cell receptor):
A disulfide-linked heterodimer of highly variable α and β chains in complex with CD3 molecules on T-cell surfaces. In some subsets of T cells, disulfide-linked highly variable δ and γ chains are in complex with CD3 molecules. Thus, T cells carrying these receptors are designated either as α:β or δ:γ T cells, respectively.
Footnotes
Supported in part by Cancer Center Support Grant No. P30 CA016672 from the National Cancer Institute (data analyses).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Dennis P. O'Malley, Clarient/GE Healthcare (C) Consultant or Advisory Role: Glen S. Brooks, Sientra (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Roberto N. Miranda, Ken H. Young, L. Jeffrey Medeiros
Provision of study materials or patients: L. Jeffrey Medeiros
Collection and assembly of data: Roberto N. Miranda, Tariq N. Aladily, H. Miles Prince, Daphne de Jong, Luis E. Fayad, Mitual B. Amin, Nisreen Haideri, Govind Bhagat, Glen S. Brooks, David A. Shifrin, Dennis P. O'Malley, Chan Y. Cheah, Carlos E. Bacchi, Gabriela Gualco, Shiyong Li, John A. Keech, Ephram P. Hochberg, Matthew J. Carty, Summer E. Hanson, Eid Mustafa, Steven Sanchez, John T. Manning, Zijun Y. Xu-Monette, Jan Paul de Boer, Zaher Chakhachiro, Dongjiu Ye, Douglas Clark, L. Jeffrey Medeiros
Data analysis and interpretation: Roberto N. Miranda, Rashmi Kanagal-Shamanna, Alonso R. Miranda, Patricia Fox, Roland L. Bassett, Jorge J. Castillo, Brady E. Beltran, Ken H. Young, L. Jeffrey Medeiros
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Talwalkar SS, Miranda RN, Valbuena JR, et al. Lymphomas involving the breast: A study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol. 2008;32:1299–1309. doi: 10.1097/PAS.0b013e318165eb50. [DOI] [PubMed] [Google Scholar]
- 2.Miranda RN, Lin L, Talwalkar SS, et al. Anaplastic large cell lymphoma involving the breast: A clinicopathologic study of 6 cases and review of the literature. Arch Pathol Lab Med. 2009;133:1383–1390. doi: 10.5858/133.9.1383. [DOI] [PubMed] [Google Scholar]
- 3.Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–555. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Anaplastic large cell lymphoma (ALCL) in women with breast implants: Preliminary FDA findings and analyses. 2013. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm239995.htm.
- 5.US Food and Drug Administration. FDA medical device communication: Reports of anaplastic large cell lymphoma (ALCL) in women with breast implants. 2013. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm064461.htm.
- 6.Lechner MG, Lade S, Liebertz DJ, et al. Breast implant-associated, ALK-negative, T-cell, anaplastic, large-cell lymphoma: Establishment and characterization of a model cell line (TLBR-1) for this newly emerging clinical entity. Cancer. 2011;117:1478–1489. doi: 10.1002/cncr.25654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aladily TN, Medeiros LJ, Alayed K, et al. Breast implant-associated anaplastic large cell lymphoma: A newly recognized entity that needs further refinement of its definition. Leuk Lymphoma. 2012;53:749–750. doi: 10.3109/10428194.2011.639020. [DOI] [PubMed] [Google Scholar]
- 8.Ralfkiaer E, Willemze R, Paulli M, et al. Primary cutaneous CD30 T-cell lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al., editors. Tumours of the Hematopoietic and Lymphoid Tissues. ed 4. Lyon, France: International Agency for Research on Cancer; 2008. pp. 300–301. [Google Scholar]
- 9.Popplewell L, Thomas SH, Huang Q, et al. Primary anaplastic large-cell lymphoma associated with breast implants. Leuk Lymphoma. 2011;52:1481–1487. doi: 10.3109/10428194.2011.574755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carty MJ, Pribaz JJ, Antin JH, et al. A patient death attributable to implant-related primary anaplastic large cell lymphoma of the breast. Plast Reconstr Surg. 2011;128:112e–118e. doi: 10.1097/PRS.0b013e318221db96. [DOI] [PubMed] [Google Scholar]
- 11.Roden AC, Macon WR, Keeney GL, et al. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: An indolent T-cell lymphoproliferative disorder. Mod Pathol. 2008;21:455–463. doi: 10.1038/modpathol.3801024. [DOI] [PubMed] [Google Scholar]
- 12.Aladily TN, Medeiros LJ, Amin MB, et al. Anaplastic large cell lymphoma associated with breast implants: A report of 13 cases. Am J Surg Pathol. 2012;36:1000–1008. doi: 10.1097/PAS.0b013e31825749b1. [DOI] [PubMed] [Google Scholar]
- 13.Gualco G, Chioato L, Harrington WJ, Jr, et al. Primary and secondary T-cell lymphomas of the breast: Clinico-pathologic features of 11 cases. Appl Immunohistochem Mol Morphol. 2009;17:301–306. doi: 10.1097/PAI.0b013e318195286d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Do V, Shifrin DA, Oostendorp L. Lymphoma of the breast capsule in a silicone implant-reconstructed patient. Am Surg. 2010;76:1030–1031. [PubMed] [Google Scholar]
- 15.Li S, Lee AK. Silicone implant and primary breast ALK1-negative anaplastic large cell lymphoma, fact or fiction? Int J Clin Exp Pathol. 2009;3:117–127. [PMC free article] [PubMed] [Google Scholar]
- 16.Olack B, Gupta R, Brooks GS. Anaplastic large cell lymphoma arising in a saline breast implant capsule after tissue expander breast reconstruction. Ann Plast Surg. 2007;59:56–57. doi: 10.1097/SAP.0b013e31804d442e. [DOI] [PubMed] [Google Scholar]
- 17.Farkash EA, Ferry JA, Harris NL, et al. Rare lymphoid malignancies of the breast: A report of two cases illustrating potential diagnostic pitfalls. J Hematop. 2009;2:237–244. doi: 10.1007/s12308-009-0043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alobeid B, Sevilla DW, El-Tamer MB, et al. Aggressive presentation of breast implant-associated ALK-1 negative anaplastic large cell lymphoma with bilateral axillary lymph node involvement. Leuk Lymphoma. 2009;50:831–833. doi: 10.1080/10428190902795527. [DOI] [PubMed] [Google Scholar]
- 19.de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300:2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 20.Sahoo S, Rosen PP, Feddersen RM, et al. Anaplastic large cell lymphoma arising in a silicone breast implant capsule: A case report and review of the literature. Arch Pathol Lab Med. 2003;127:e115–e118. doi: 10.5858/2003-127-e115-ALCLAI. [DOI] [PubMed] [Google Scholar]
- 21.Hanson SE, Gutowski KA. Primary T-cell lymphoma associated with breast implant capsule. Plast Reconstr Surg. 2010;126:39e–41e. doi: 10.1097/PRS.0b013e3181dab2e0. [DOI] [PubMed] [Google Scholar]
- 22.Gaudet G, Friedberg JW, Weng A, et al. Breast lymphoma associated with breast implants: Two case-reports and a review of the literature. Leuk Lymphoma. 2002;43:115–119. doi: 10.1080/10428190210189. [DOI] [PubMed] [Google Scholar]
- 23.Bishara MR, Ross C, Sur M. Primary anaplastic large cell lymphoma of the breast arising in reconstruction mammoplasty capsule of saline filled breast implant after radical mastectomy for breast cancer: An unusual case presentation. Diagn Pathol. 2009;4:11. doi: 10.1186/1746-1596-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor KO, Webster HR, Prince HM. Anaplastic large cell lymphoma and breast implants: Five Australian cases. Plast Reconstr Surg. 2012;129:610e–617e. doi: 10.1097/PRS.0b013e3182450aae. [DOI] [PubMed] [Google Scholar]
- 25.Smith TJ, Ramsaroop R. Breast implant related anaplastic large cell lymphoma presenting as late onset peri-implant effusion. Breast. 2012;21:102–104. doi: 10.1016/j.breast.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Newman MK, Zemmel NJ, Bandak AZ, et al. Primary breast lymphoma in a patient with silicone breast implants: A case report and review of the literature. J Plast Reconstr Aesthet Surg. 2008;61:822–825. doi: 10.1016/j.bjps.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Zarbo RJ, Nakhleh RE. Surgical pathology specimens for gross examination only and exempt from submission: A College of American Pathologists Q-Probes study of current policies in 413 institutions. Arch Pathol Lab Med. 1999;123:133–139. doi: 10.5858/1999-123-0133-SPSFGE. [DOI] [PubMed] [Google Scholar]
- 28.Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: A report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653–3661. [PubMed] [Google Scholar]
- 29.Gabriel SE, O'Fallon WM, Beard CM, et al. Trends in the utilization of silicone breast implants, 1964-1991, and methodology for a population-based study of outcomes. J Clin Epidemiol. 1995;48:527–537. doi: 10.1016/0895-4356(94)00209-9. [DOI] [PubMed] [Google Scholar]
- 30.Silverman BG, Brown SL, Bright RA, et al. Reported complications of silicone gel breast implants: An epidemiologic review. Ann Intern Med. 1996;124:744–756. doi: 10.7326/0003-4819-124-8-199604150-00008. [DOI] [PubMed] [Google Scholar]