Abstract
Background and purpose
In patients with metal-on-metal (MoM) hip prostheses, pain and joint effusions may be associated with elevated blood levels of cobalt and chromium ions. Since little is known about the kinetics of metal ion clearance from the body and the rate of resolution of elevated blood ion levels, we examined the time course of cobalt and chromium ion levels after revision of MoM hip replacements.
Patients and methods
We included 16 patients (13 female) who underwent revision of a painful MoM hip (large diameter, modern bearing) without fracture or infection, and who had a minimum of 4 blood metal ion measurements over an average period of 6.1 (0–12) months after revision.
Results
Average blood ion concentrations at the time of revision were 22 ppb for chromium and 43 ppb for cobalt. The change in ion levels after revision surgery varied extensively between patients. In many cases, over the second and third months after revision surgery ion levels decreased to 50% of the values measured at revision. Decay of chromium levels occurred more slowly than decay of cobalt levels, with a 9% lag in return to normal levels. The rate of decay of both metals followed second-order (exponential) kinetics more closely than first-order (linear) kinetics.
Interpretation
The elimination of cobalt and chromium from the blood of patients who have undergone revision of painful MoM hip arthroplasties follows an exponential decay curve with a half-life of approximately 50 days. Elevated blood levels of cobalt and chromium ions can persist for at least 1 year after revision, especially in patients with high levels of exposure.
After a dramatic rise in initial popularity, the use of metal-on-metal (MoM) hip arthroplasties has declined precipitously, both in hip resurfacings and conventional hip replacement, due to an alarming incidence of adverse inflammatory reactions (van der Weegen et al. 2011, NJR 2012).
There are now serious concerns about potential adverse biological effects, both local and systemic, arising from wear debris generated by MoM articulations (Matthies et al. 2011). The nano-scale particles generated through wear of MoM bearings can enter the reticulo-endothelial system and cross over into the circulation as early as 5 days after implantation (Daniel et al. 2007). The specific surface area (surface area to mass ratio) of these particles makes them susceptible to corrosion in vivo (Hart et al. 2010), leading to elevated levels of cobalt and chromium ions in the blood, usually ranging from 5 to 10 times normal values (Jacobs et al. 1996, Brodner et al. 1997, Skipor et al. 2002, Dunstan et al. 2005, Daniel et al. 2009, Hart et al. 2009, van der Weegen et al. 2011). Possible complications from long-term elevated metal ion levels include immune reactions (Pandit et al. 2008), necrosis (Campbell et al. 2010), toxicity (Keegan et al. 2007, Tower et al. 2010, Corradi et al. 2011), chromosomal aberrations (Ladon et al. 2004), and carcinogenicity (Case et al. 1994). However, the most common short-term complication is joint pain associated with inflammatory reactions (Milosev et al. 2005). In many cases where there is pain with soft-tissue masses, often in association with elevated levels of cobalt and chromium ions, removal of the implanted components is necessary. This form of failure is more common in female patients than in male patients, and it has been reported in 1–20% of cases at 5 years (Schmidt et al. 1996) depending on the design of the prosthesis.
Although revision is performed in the hope that the elevation of ion levels and symptoms will resolve, little is known about the kinetics of storage and turnover of these ions in the body. We examined the kinetics of cobalt and chromium ion decay after revision of MoM hip replacements. Our principal goals were to characterize the decay curves of cobalt and chromium ions after revision procedures and to determine when blood concentrations return to levels below the MHRA action level of 7 ppb, in order to determine exposure risk (MHRA 2010). We hypothesized that: (1) removal of MoM components results in a rapid drop in the level of metal ions in the blood followed by a slow steady-state decline; (2) chromium concentrations will decrease more slowly than cobalt concentrations; (3) the total body exposure to ions is orders of magnitude higher with poorly functioning or loose implants than with well-functioning components.
Patients and methods
With institutional review board approval, 16 patients (13 females) were enrolled in this study following revision hip surgeries performed during the period October 1999 to December 2008. Each patient underwent primary MoM total hip arthroplasty (13 resurfacing and 3 modular) at an average of 4.9 (2.1–10.9) years before revision. For inclusion in the study, patients were required to have had a painful MoM hip prosthesis without proven infection or fracture. The average age of the patients in the study group was 62 (47–74) years. The average head size was 47 (36–54) mm and the average cup size was 53 (48–60) mm. This cohort was a subgroup of a larger cohort (39 hips) of revision MoM hip arthroplasties that has been reported previously (Liddle et al. 2013). The patients in this cohort were those from the first cohort who were prepared to undergo the repeated blood ion measurements required for the present study.
Whole-blood samples were collected in trace-element blood tubes on successive days of each patient’s hospitalization for the revision procedure, and then at follow-up visits performed at 6 weeks, 3 and 6 months, and 1 year after revision surgery. Blood samples were also collected for trace-element analysis if additional blood was drawn for other reasons during the follow-up period. 106 blood samples (on average 6.6 (4–13) samples per patient) were collected during the course of the study over an average follow-up period of 5.8 (0.1–20.5) months. Each sample was taken, stored, and processed in a standardized fashion, which has been described previously (Hart et al. 2011b). The concentrations of cobalt and chromium ions in each blood sample were measured using standard operating procedures using dynamic reaction-cell inductively coupled plasma mass spectrometry (De Smet et al. 2008, Heisel et al. 2008, Sampson et al. 2012).
In 2010, the Medicines and Healthcare Products Regulatory Agency in the UK issued a safety alert which recommended on-going surveillance of patients with blood concentrations of cobalt or chromium, or both, of 7 ppb or greater (MHRA 2010). Based on this recommendation, we divided our study group into 2 subgroups. The first subgroup comprised 12 patients with initial post-revision metal concentrations of 7 ppb or greater; they were followed for an average of 7 months with an average of 7.2 blood measurements during the post-revision surveillance period. The second group, consisting of 4 patients with initial concentrations of less than 7 ppb, was followed for an average of 3 months with an average of 5 blood draws during that period.
The metal ion concentrations of each blood sample were plotted for each patient as a function of the time since revision. To standardize comparisons between different patients, the data on each plot were interpolated to yield concentrations of cobalt and chromium present at 7, 30, and 180 days post-revision. In the case of the high-concentration subgroup, the time required for ion concentrations to drop to 7 ppb was also recorded. Plots were also prepared expressing ion levels as a percentage of the initial values measured at revision and interpolated to calculate the period until blood levels dropped to 50% of the levels at revision. 10 of the cases in the high-concentration subgroup had 4 or more blood draws distributed over more than 50 days after revision. For these cases, plots of ln[ion] against time and 1/[ion] against time were also generated to test for first- and second-order decay kinetics.
The area under the curve for ion concentration vs. time was calculated for the patients with very high blood ion levels who entered steady-state decay well above 7 ppb, in order to compare metal ion load to that in a well-functioning implant. The line of best fit was fitted to each data set, and the integrated value of cumulative exposure (in ppb-years) was calculated and compared to that in a typical patient with a blood ion concentration of 2 ppb. This threshold value of 2 ppb was based on the work of Hart et al. (2009), who measured the ion concentrations of 88 unilateral BHR hips 3–5 years postoperatively.
Results
16 patients with a mean age of 62 (47–74) years were enrolled into the study (Table 1). 12 of the 16 were resurfacing prostheses, and 9 of the 12 were Birmingham Hip Resurfacings. The other resurfacing prostheses were ASR (3 patients) and XL Magnum (1 patient). The remaining modular cases were Polarstem/BHR, Durom and 36-mm Pinnacle. All patients underwent revision to a non-MoM bearing couple; ceramic on ceramic in 9 of the16 cases and metal on ultra-high molecular weight polyethylene in 7 of the 16. 1 patient (with a stemmed prosthesis) was revised to a modular revision system; the remainder received “primary” cemented (Exeter; Stryker, Newbury, UK) or cementless (Furlong HAC; JRI, Sheffield, UK) femoral stems. In this cohort, there have been no re-admissions, reoperations, or significant complications.
Table 1.
Patient demographics and information regarding prostheses
| Patient no. | Age | Sex | First prosthesis | Modular | Head size | Cup size | Inclination | Version | Survival time | Revision prosthesis |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Articulation | Cemented | ||||||||||
| 1 | 47 | F | BHR | N | 42 | 50 | 43 | 39 | 65 | CoC | N |
| 2 | 58 | F | BHR | N | 46 | 52 | 42 | 20 | 25 | CoC | N |
| 3 | 60 | F | BHR | N | 46 | 52 | 36 | 26 | 68 | MoP | N |
| 4 | 61 | M | BHR | N | 46 | 52 | 43 | 22 | 131 | MoP | N |
| 5 | 72 | F | XL Magnum | N | 44 | 50 | 38 | -5 | 22 | CoC | N |
| 6 | 73 | F | Durom | Y | 50 | 56 | 55 | 37 | 38 | CoC | Y |
| 7 | 74 | F | Polarstem/BHR | Y | 50 | 56 | 37 | 19 | 49 | MoPa | N |
| 8 | 69 | F | ASR | N | 43 | 48 | 55 | 27 | 62 | MoP | N |
| 9 | 63 | F | ASR | N | 47 | 54 | 55 | 48 | 29 | CoC | Y |
| 10 | 69 | M | BHR | N | 54 | 60 | 73 | 41 | 63 | MoP | Y |
| 11 | 50 | F | BHR | N | 50 | 56 | 71 | 42 | 31 | CoC | Y |
| 12 | 66 | F | BHR | N | 42 | 50 | 66 | 34 | 68 | CoC | N |
| 13 | 64 | M | BHR | N | 50 | 56 | 57 | 43 | 92 | CoC | Y |
| 14 | 61 | F | Pinnacle | Y | 36 | 52 | 68 | -3 | 56 | MoP | N |
| 15 | 55 | F | BHR | N | 46 | 50 | 64 | 30 | 72 | MoP | N |
| 16 | 57 | F | ASR | N | 53 | 60 | 70 | 43 | 45 | CoC | N |
CoC: ceramic on ceramic; MoP: metal on polyethylene.
aRevision stem (all other are primary stems)
MRI findings were available for 9 patients, 4 of whom showed fluid-filled masses. Loose components without gross osteolysis were present in 2 of 9 patients, osteolysis in 4 patients, synovitis in 5 patients, pseudotumor in 5 patients, and destructive muscle loss in 3 patients.
Metal ion decay
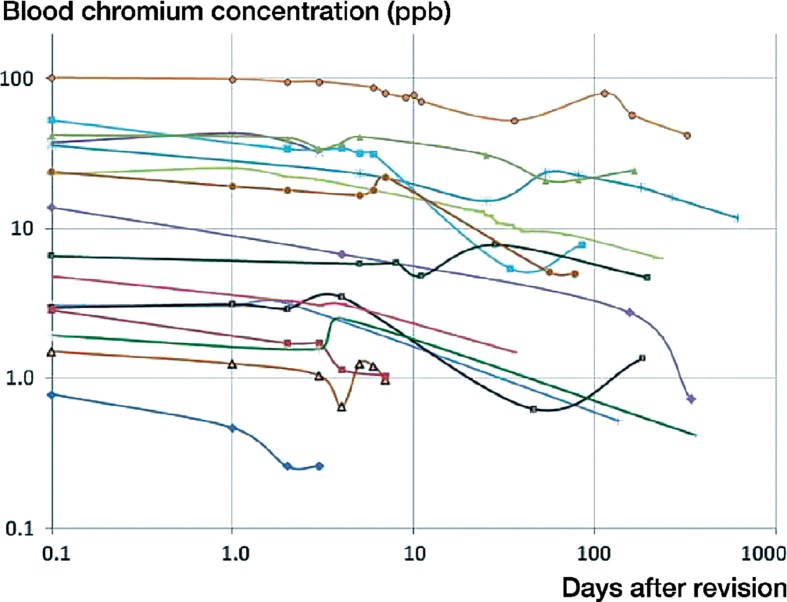
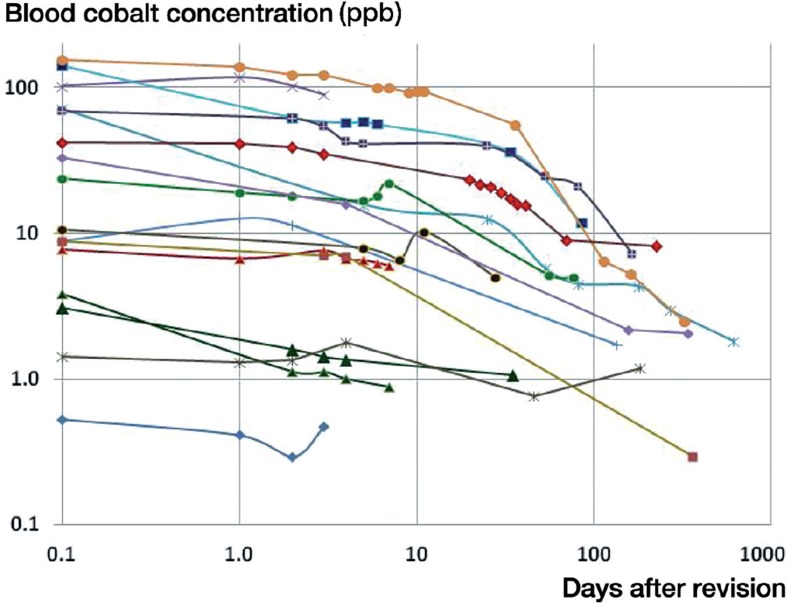
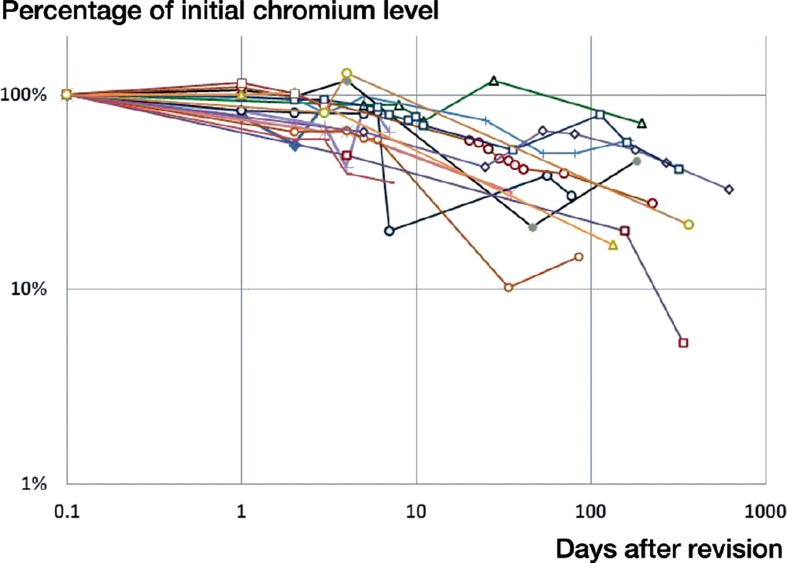
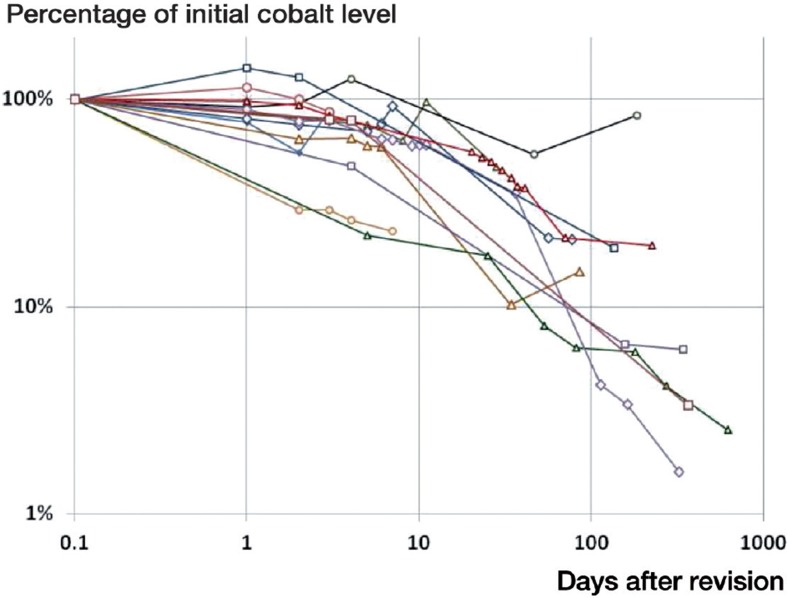
On the day of revision of the MoM implants, the average blood ion concentrations of chromium and cobalt were 22 ppb and 43 ppb, respectively, for all 16 patients (Table 2). For the 12 patients with initial cobalt and/or chromium levels in excess of 7 ppb, the average whole-blood ion concentration at the time of revision was 28 ppb for chromium (95% CI: 12–45) and 56 ppb for cobalt (95% CI: 27–86). These values are 106 and 157 times higher than established controls for concentrations of chromium and cobalt in serum (Muñiz et al. 2001). In this subgroup, average chromium and cobalt levels fell to 20 ppb and 28 ppb after 7 days, and to 14 ppb and 22 ppb at 30 days, based on data available from 11 and 10 patients (Figures 1 and 2). This corresponds to 69% and 50% of initial levels at 7 days, and 48% and 39% at 30 days (Figures 3 and 4). In the 6 patients with blood ion values at 180 days or more, chromium ions remained at 15 ppb at 180 days postoperatively (56% of the initial value), while cobalt ion levels dropped to an average of 5 ppb (corresponding to 10% of initial levels).
Table 2.
Whole-blood concentrations of chromium and cobalt ions
| Chromium, ppb |
Cobalt, ppb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. | Initial value | 7 days | 30 days | 180 days | Final value | Initial value | 7 days | 30 days | 180 days | Final value | Observation (days postop.) |
| Low metal ion cases | |||||||||||
| 1 | 0.78 | na | na | na | 0.26 | 0.53 | na | na | na | 0.47 | 3 |
| 2 | 1.7 | 3.0 | 1.8 | na | 1.0 | 1.4 | 1.3 | 1.1 | na | 0.9 | 35 |
| 3 | 3 | 3.3 | 1.7 | 1.4 | 1.4 | 1.4 | 1.7 | 1.1 | 1.2 | 1.2 | 183 |
| 4 | 2.9 | 1 | na | na | 1 | 3.8 | 0.9 | na | na | 0.9 | 7 |
| High metal ion cases | |||||||||||
| 5 | 1.9 | 2.5 | 2.4 | 1.4 | 0.4 | 8.8 | 6.9 | 6.5 | 3.7 | 0.3 | 363 |
| 6 | 1.5 | 1.0 | na | na | 1.0 | 7.7 | 6.0 | na | na | 6.0 | 7 |
| 7 | 3.1 | 3.1 | 2.6 | na | 0.5 | 8.9 | 10.9 | 9.2 | na | 1.7 | 135 |
| 8 | 6.6 | 5.8 | 7.7 | 4.9 | 4.7 | 11 | 7.5 | 10 | 5.6 | 5 | 196 |
| 9 | 21.3 | 4.3 | 6.1 | na | 5 | 24 | 22.1 | 14 | na | 5 | 77 |
| 10 | 13.8 | 6.7 | 6.1 | 2.4 | 0.7 | 33 | 15.4 | 14 | 2.1 | 2.1 | 340 |
| 11 | 23 | 19.5 | 10.8 | 7.1 | 6.3 | 42 | 32 | 19 | 8.4 | 8.2 | 224 |
| 12 | 41.9 | 40 | 28.8 | na | 24.3 | 70 | 41.9 | 37 | na | 7.2 | 165 |
| 13 | 36.0 | 23 | 16.8 | 18.8 | 16.1 | 71 | 14 | 11 | 4.3 | 3.0 | 270 |
| 14 | 37.5 | na | na | na | 32.1 | 102 | na | na | na | 89 | 3 |
| 15 | 53 | 30 | 9.2 | na | 7.8 | 141 | 55 | 38 | na | 11.9 | 85 |
| 16 | 101.3 | 79.5 | 53 | 54.1 | 36.3 | 156 | 99.4 | 61 | 5 | 1.2 | 322 |
| Mean (high ion cases) | |||||||||||
| 28 | 20 | 14 | 15 | 11 | 56 | 28 | 22 | 4 | 12 | 182 | |
| 95% CI | 13–44 | 6.2–33 | 5.1–24 | 0.0–30 | 4.3–18 | 27.8–85 | 12.3–44 | 11.6–32 | 3.3–6.4 | –1.6 to 25 | 114–250 |
Reported at the time of revision (initial value), at 7, 30, and 180 days after revision, and at the final blood draw (last observation). Data for fixed time points have been interpolated from the nearest available measurements.
Figure 1.
Variation in blood chromium concentration as a function of time since revision for the 16 patients enrolled in the study.
Figure 2.
Variation in blood cobalt concentration as a function of time since revision.
Figure 3.
Change in blood chromium as a percentage of the initial value at revision.
Figure 4.
Change in blood cobalt as a percentage of the initial value at revision.
The half-life of chromium decay was 57 days (95% CI: 6–108) (Figures 1 and 3). A temporary increase in chromium levels occurred in 6 patients, 5 of which occurred between postoperative days 53 and 113. 8 of the 16 patients had chromium ion levels in excess of 7 ppb. In 4 of these cases, chromium ion levels had not dropped below 7 ppb by the last blood draw at an average of 190 days after revision. In the other 4 cases, chromium levels dropped to 7 ppb after an average of 12 (1–27) weeks.
In 12 of the 16 patients, cobalt ion concentrations had dropped to less than 50% of the initial values at the time of the final blood draw (Figure 4). In these cases, the half-life of decay was 44 days (95% CI: 11–77). In 8 of the 12 patients with high initial ion levels, cobalt concentrations dropped to the 7 ppb level at an average of 63 days after revision (95% CI: 36–90 days). 1 of the remaining 4 patients had been followed for only 3 days after revision, while in the other 3 cases cobalt levels averaged 14 ppb at 158 days post-revision, corresponding to 16% of the initial values.
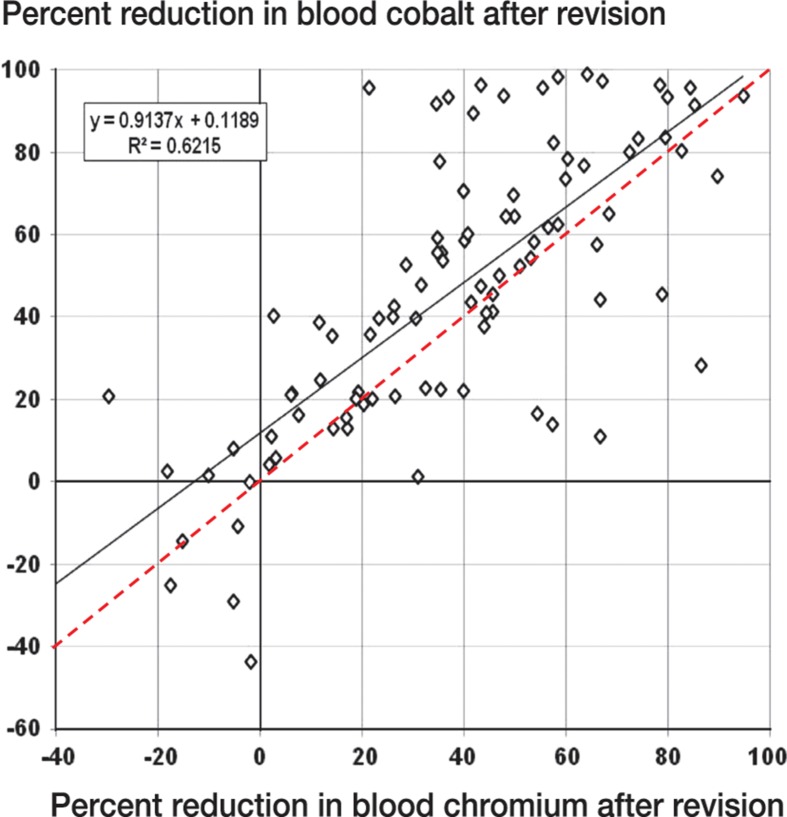
When we compared cobalt and chromium levels at each time point (a total of 96 observations), the drop in cobalt ion concentration exceeded the corresponding value for chromium for 71% of observations (Figure 5). Averaged over all time points, the average decrease in cobalt levels was 45%—as opposed to 36% for chromium, a difference of 9%.
Figure 5.
Plot of chromium concentration vs. cobalt concentration (% of initial value) for each patient and each time point. Solid line and equation correspond to the line of best fit. The red dashed line corresponds to Cr% = Co%.
We plotted ln[c(t)/cO] vs. time and 1/[c(t)/cO vs. time for the subset of 8 patients with multiple blood draws and longer post-revision follow-up in our cohort. A linear relationship between ln[c(t)/cO] and time indicates first-order decay kinetics, while a linear relationship between 1/[c(t)/cO] and time is indicative of second-order kinetics. Cobalt decay followed second-order kinetics more closely than first-order kinetics, although the correlation coefficients were similar (average values of r2 for 8 cases: 0.87 and 0.76, respectively). Chromium decay was less accurately described by either second-order or first-order kinetics, with both models generating similar correlation coefficients (r2 = 0.59 and 0.58).
Between postoperative day 0 and a median time of 6.5 months after revision, the blood ion load of these patients was comparable to 4 (1.4–47) years of chromium exposure in a well-functioning implant and 5 (3–9) years of cobalt exposure (Table 3).
Table 3.
Ion exposure data for 6 patients with high metal ion levels
| Patient. no | Years of cobalt exposure | Years of chromium exposure | Days postoperatively |
|---|---|---|---|
| 5 | 5.3 | 5.4 | 165 |
| 6 | 4.1 | 1.4 | 85 |
| 7 | 9.1 | 47 | 707 |
| 10 | 5.5 | 1.9 | 82 |
| 15 | 3.4 | 2.7 | 224 |
| 16 | 2.7 | 7.2 | 270 |
| Median values | 4.7 | 4.1 | 195 |
Discussion
Using data collected from the London Implant Retrieval Centre (LIRC), a tertiary center specializing in revision of MoM implants (Hart et al. 2009, 2010, 2011a, Matthies et al. 2011, Sampson et al. 2012), the present study provides new insights to aid in the management of patients after revision of painful MoM prostheses. These patients had multiple sequential blood draws to measure decline in their whole-blood metal ion concentrations. Close surveillance of metal ion decay after revision has not yet become standard in evaluation of problematic implants, but it may be able to guide treatment (Brodner et al. 1997, MacDonald et al. 2004, De Smet et al. 2008, Sampson et al. 2012).
Our study had several shortcomings, including the limited number of patients. Measurement of blood trace metal ion levels requires special preparation and testing compared to common laboratory tests, and as such is very expensive. Additionally, studies of this type in which revision patients must agree to frequent blood sampling often have a high attrition rate, which limits the number of cases analyzed. There was a large degree of variability in our patients, both in their initial metal concentrations and in the pattern of decay. Thus, it was not possible to define a “normal” pattern of decay representative of all patients. This may be due to the numerous differences between patients, both in their physiology and in the wear characteristics of their original implants. It also suggests that a larger sample may be required before a typical response becomes clear. When calculating the total-body metal ion exposure in patients with high metal concentrations, we used 2 ppb as a control. Although this value has been used as a reference level for well-functioning implants, its use may lack the rigorous precision needed for a scientific study (Skipor et al. 2002, Dunstan et al. 2005, Vendittoli et al. 2007, Daniel et al. 2009). Until the widespread acceptance of 2 ppb as “normal”, this remains a weakness of the study (MacDonald et al. 2004).
Postoperatively, there was a dramatic decrease in metal ion concentrations in the blood compared to values at the time of revision, with cobalt and chromium levels dropping by 50% and 31% at 1 week, and 61% and 52% at 1 month after the revision surgery. During the period of surveillance in this study, 8 of 12 patients had their cobalt ion levels dropping below 7 ppb at an average of 2 months. Only 4 patients had a drop in chromium ion levels to 7 ppb, in this case at an average of 3 months after revision. After the initial exponential decrease in ion concentration, the decay curve entered a steady state with abnormal elevations of metal ion levels in the blood for periods of up to 2 years after revision. Although in most patients metal ion concentrations dropped below 7 ppb within 3 months, in a minority of cases (4 of 12) chromium ion concentrations remained between 12 and 36 ppb at an average of 1 year after revision.
Chromium concentrations decreased more slowly than cobalt concentrations at both early and late time points after revision surgery.
In our patient population, average blood ion concentrations on the day of revision were 43 ppb for cobalt and 22 ppb for chromium, with large inter-patient variability. We attribute these differences to variations in the debris burden remaining in the tissues after debridement at revision (and its rate of corrosion in vivo), to differences between patients in the rate of renal clearance of ions in the blood, and to variations in the extent to which debris was bound and encapsulated in tissues and organs and therefore available for dissolution and excretion (Case et al. 1994, Urban et al. 2004, Hart et al. 2010). It is important to note that blood ion concentrations are singular pieces of information that may be of diagnostic significance when evaluating the performance of hip implants, but they cannot take the place of a thorough history and physical examination or radiographic evaluation. In fact, 1 of 3 patients with destructive muscle loss and 1 of 4 patients with massive osteolysis had cobalt and chromium blood levels below 7 ppb.
The average time in situ was 5 (1–11) years before revision. Although the average survivorship of MoM implants is much longer, the overall systemic exposure to cobalt and chromium at the time of revision was 156 and 107 times larger than control values. Interestingly, in half of our study population with ion measurements beyond 30 days, the levels of chromium ions increased between 2 and 4 months postoperatively. One possible cause of this increase could be mobilization of chromium ions stored in the organs triggered by a decrease in circulating chromium levels. Urban et al. (2004) noted accumulation of wear particles in the spleen and liver at autopsy or biopsy in 7 of 8 patients after revision of their hip replacement. Exposure to high doses of metallic salts in animals showed that urinary excretion of chromium lagged behind that of cobalt and nickel, and it accumulated in the lung, liver, kidney, spleen, and red blood cells (Merritt et al. 1989). Other possible explanations include a mobilization of ions from the operative joint to the blood in response to healing, reduced renal clearance of chromium, or an increase in exogenous chromium from food or supplements.
Based on the values of the regression coefficients for decay of cobalt and chromium ions, a second-order model best fitted the kinetics of elution of cobalt ions after implant removal, indicating that the change in cobalt ion levels occurs through 2 independent processes. Cobalt is usually conserved by the kidneys in the normal population, but little is known about the kinetics of renal transport and excretion of metal ions. Both first- and second-order kinetics approximated the decay in chromium ion concentration. Our decay curves and the lag in chromium excretion compared to cobalt excretion shows that a single independent transport mechanism is unlikely. It is unknown whether second-order kinetics also describes excretion of ions from patients with well-functioning implants or from patients without implants, or whether the ion levels are sufficiently low for secretion to occur via a single-stage mechanism.
Patients with the highest initial ion levels were also exposed to markedly elevated concentrations of blood metal ions for extended periods after revision of their MoM components. Based on our measurements of the area under the curves of ion concentration vs. time, cumulative exposures in these patients in the first few months after revision were equivalent to several years in patients with well-functioning implants. It is important to note that this exposure comes even after removal of the source of MoM debris from the body, and that the metal ion exposure is presumed to be considerably higher while in situ. Despite the rapid decay in blood ion levels in many of our patients, the sheer magnitude of ion exposure from these poorly functioning implants is cause for concern. The long-term sequelae of metal ion exposure are unknown; however, given the level of chronic exposure, long-term follow-up of patients after revision of MoM implants appears to be warranted in order to detect any medical complications (Tower 2010).
Acknowledgments
The work presented here was a collaboration between all the authors, who contributed to and approved the manuscript.
The authors wish to acknowledge the assistance of Ms Denise Leon and Ms Sophie Fuller in preparing the manuscript.
The study was performed at the London Implant Retrieval Centre (LIRC) and the Institute of Orthopedic Research and Education (IORE) in Houston. All activities were funded using the internal resources of both organizations. The LIRC is funded by the British Orthopaedic Association (BOA) and is a joint venture between Imperial College London, the Royal National Orthopaedic Hospital, the British Orthopaedic Association, and 9 orthopedic companies (JRI, Corin, Mathys, Zimmer, Depuy, Finsbury, Stryker, Smith and Nephew, and Biomet).
No competing interests declared.
References
- Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Elevated serum cobalt with metal-on-metal articulating surfaces . J Bone Joint Surg (Br) 1997;79(2):316–21. doi: 10.1302/0301-620x.79b2.7326. [DOI] [PubMed] [Google Scholar]
- Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips . Clin Orthop. 2010;(468)((9)):2321–7. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, Solomon L. 4. Widespread dissemination of metal debris from implants . J Bone Joint Surg (Br) 1994;76(5):701–12. [PubMed] [Google Scholar]
- Corradi M, Daniel J, Ziaee H, Alinovi R, Mutti A, McMinn DJ. Early markers of nephrotoxicity in patients with metal-on-metal hip arthroplasty . Clin Orthop. 2011;469:1651–9. doi: 10.1007/s11999-010-1682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study . J Bone Joint Surg (Br) 2007;89:169–73. doi: 10.1302/0301-620X.89B2.18519. [DOI] [PubMed] [Google Scholar]
- Daniel J, Ziaee H, Pradhan C, McMinn DJ. Six-year results of a prospective study of metal ion levels in young patients with metal-on-metal hip resurfacings . J Bone Joint Surg (Br) 2009;91:176–9. doi: 10.1302/0301-620X.91B2.21654. [DOI] [PubMed] [Google Scholar]
- De Smet K, De Haan R, Calistri A, et al. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing . J Bone Joint Surg (Am) (Suppl 4) 2008;90:202–8. doi: 10.2106/JBJS.H.00672. [DOI] [PubMed] [Google Scholar]
- Dunstan E, Sanghrajka AP, Tilley S, Unwin P, Blunn G, Cannon SR, Briggs TW. Metal ionlevels after metal-on-metal proximal femoral replacements: a 30- year follow-up . J Bone Joint Surg (Br) 2005;87:628–31. doi: 10.1302/0301-620X.87B5.15384. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Skinner JA, Winship P, Faria N, Kulinskaya E, Webster D, Muirhead-Allwood S, Aldam CH, Anwar H, Powell JJ. Circulating levels of cobalt and chromium from metal-on-metal hip replacement are associated with CD8+ T-cell lymphopenia . J Bone and Joint Surg (Br) 2009;91(6):835–42. doi: 10.1302/0301-620X.91B6.21844. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Quinn PD, Sampson B, Sandison A, Atkinson KD, Skinner JA, Powell JJ, Mosselmans JF. The chemical form of metallic debris in tissues surrounding metal-on-metal hips with unexplained failure . Acta Biomater. 2010;6(11):4439–46. doi: 10.1016/j.actbio.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Ilo K, Underwood R, Cann P, Henckel J, Lewis A, Cobb J, Skinner J. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study . J Bone Joint Surg (Br) 2011a;93:315–20. doi: 10.1302/0301-620X.93B3.25545. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Sabah SA, Bandi AS, Maggiore P, Tarassoli P, Sampson B, Skinner A. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement . J Bone Joint Surg (Br) 2011b;93:1308–13. doi: 10.1302/0301-620X.93B10.26249. [DOI] [PubMed] [Google Scholar]
- Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer JP. Characterization of the running-in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis . J Bone Joint Surg (Am) (Suppl 3) 2008;90:125–33. doi: 10.2106/JBJS.H.00437. [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, Skipor AK, Doorn PF, Campbell P, Schmalzried TP, Black J, Amstutz HC. Cobalt and chromium concentrations in patients withmetal on metal total hip replacements . Clin Orthop (329 Suppl) 1996. pp. S256–S263. [DOI] [PubMed]
- Keegan GM, Learmonth ID, Case CP. Orthopaedic metals and their potential toxicity in the arthroplasty patient: a review of current knowledge and future strategies . J Bone Joint Surg (Br) 2007;89:567–73. doi: 10.1302/0301-620X.89B5.18903. [DOI] [PubMed] [Google Scholar]
- Ladon D, Doherty A, Newson R, et al. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty . J Arthroplasty (Suppl 3) 2004;19:78–83. doi: 10.1016/j.arth.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Liddle AD, Satchithananda K, Henckel J, Sabah SA, Vipulendran KV, Lewis A, Skinner JA, Mitchell A WM, Hart AJ. Revision of metal-on-metal hip arthroplasty in a tertiary center. A prospective study of 39 hips with between 1 and 4 years of follow-up . Acta Orthop. 2013;84(3):237–45. doi: 10.3109/17453674.2013.797313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SJ. Can a safe level for metal ions in patients with metal-on-metal total hip arthroplasties be determined? . J Arthroplasty (Suppl 3) 2004;19:71–7. doi: 10.1016/j.arth.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Matthies A, Underwood R, Cann P, Ilo K, Nawaz Z, Skinner J, Hart AJ. Retrieval analysis of 240 metal-on-metal hip components, comparing modular total hip replacement with hip resurfacing . J Bone Joint Surg (Br) 2011;93:307–14. doi: 10.1302/0301-620X.93B3.25551. [DOI] [PubMed] [Google Scholar]
- Medicines and Healthcare Products Regulatory Agency London: MHRA; 2010. Medical Device Alert. All metal-on-metal (MoM) hip replacements (MDA/2010/033) [Google Scholar]
- Merritt K, Crowe TD, Brown SA. Elimination of nickel, cobalt, and chromium following repeated injections of high dose metal salts . J Biomed Mater Res. 1989;23(8):845–62. doi: 10.1002/jbm.820230804. [DOI] [PubMed] [Google Scholar]
- Milosev I, Pisot V, Campbell P. Serum levels of cobalt and chromium in patients with Sikomet metal-metal total hip replacements . J Orthop Res. 2005;23(3):526–35. doi: 10.1016/j.orthres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Muñiz CS, Fernández-Martin JL, Marchante-Gayón JM, García Alonso JI, Cannata-Andía JB, Sanz-Medel A. Reference values for trace and ultratrace elements in human serum determined by double-focusing ICP-MS . Biol Trace Elem Res. 2001;82(1-3):259–72. doi: 10.1385/bter:82:1-3:259. [DOI] [PubMed] [Google Scholar]
- National Joint Registry (United Kingdom) http://www.njrcentre.org.uk/njrcentre/default.aspx
- Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings . J Bone Joint Surg (Br) 2008;90(7):847–51. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- Sampson B. Hart, A. Clinical usefulness of blood metal measurements to assess the failure of metal-on-metal orthopaedic implants . Ann Clin Biochem. 2012;49:118–31. doi: 10.1258/acb.2011.011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Weber H, Schön R. Cobalt chromium molybdenum metal combination for modular hip prostheses . Clin Orthop (329 Suppl) 1996. pp. S35–47. [DOI] [PubMed]
- Skipor AK, Campbell P, Patterson LM. Serum and urine metal levels in patients with metal-on-metal surface arthroplasty . J Mater Sci Mater Med. 2002;13:1227–34. doi: 10.1023/a:1021179029640. [DOI] [PubMed] [Google Scholar]
- Streicher RM. Metal-on-metal articulation in total hip arthroplasty: the case for using metal-on-metal . J Arthroplasty. 1998;13(3):343–5. doi: 10.1016/s0883-5403(98)90181-4. discussion 345-6. [DOI] [PubMed] [Google Scholar]
- Tower S. Arthroprosthetic cobaltism: Neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: A case report . J Bone Joint Surg (Am) 2010;92:1–5. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- Urban RM, Tomlinson MJ, Hall DJ, Jacobs JJ. Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in hip arthroplasty . J Arthroplasty (Suppl 3) 2004;19(8):94–101. doi: 10.1016/j.arth.2004.09.013. [DOI] [PubMed] [Google Scholar]
- van der Weegen W, Hoekstra HJ, Sijbesma T, Bos R, Schemitsch EH, Poolman RW. Survival of metal-on-metal hip resurfacing arthroplasty: a systematic review of the literature J Bone Joint Surg (Br) . 2011;93:298–306. doi: 10.1302/0301-620X.93B3.25594. [DOI] [PubMed] [Google Scholar]
- Vendittoli PA, Ganapathi M, Lavigne M. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty . J Bone Joint Surg (Br) 2007;89:989–90. doi: 10.1302/0301-620X.89B7.19972. [DOI] [PubMed] [Google Scholar]