Abstract
Cytochrome P450 (CYP) 4A and 4F enzymes metabolize arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE). Although CYP4A-derived 20-HETE is known to have prohypertensive and proangiogenic properties, the effects of CYP4F-derived metabolites are not well characterized. To investigate the role of CYP4F2 in vascular disease, we generated mice with endothelial expression of human CYP4F2 (Tie2-CYP4F2-Tr). LC/MS/MS analysis revealed 2-foldincreases in 20-HETE levels in tissues and endothelial cells (ECs), relative to wild-type (WT) controls. Tie2-CYP4F2-Tr ECs demonstrated increases in growth (267.1±33.4 vs. 205.0±13% at 48 h) and tube formation (7.7±1.1 vs. 1.6±0.5 tubes/field) that were 20-HETE dependent and associated with up-regulation of prooxidant NADPH oxidase and proangiogenic VEGF. Increases in VEGF and NADPH oxidase levels were abrogated by inhibitors of NADPH oxidase and MAPK, respectively, suggesting the possibility of crosstalk between pathways. Interestingly, IL-6 levels in Tie2-CYP4F2-Tr mice (18.6±2.7 vs. 7.9±2.7 pg/ml) were up-regulated via NADPH oxidase- and 20-HETE-dependent mechanisms. Although Tie2-CYP4F2-Tr aortas displayed increased vasoconstriction, vasorelaxation and blood pressure were unchanged. Our findings indicate that human CYP4F2 significantly increases 20-HETE production, CYP4F2-derived 20-HETE mediates EC proliferation and angiogenesis via VEGF- and NADPH oxidase-dependent manners, and the Tie2-CYP4F2-Tr mouse is a novel model for examining the pathophysiological effects of CYP4F2-derived 20-HETE in the vasculature.—Cheng, J., Edin, M. L., Hoopes, S. L., Li, H., Bradbury, J. A., Graves, J. P., DeGraff, L. M., Lih, F. B., Garcia, V., Shaik, J. S. B., Tomer, K. B., Flake, G. P., Falck, J. R., Lee, C. R., Poloyac, S. M., Schwartzman, M. L., Zeldin, D. C. Vascular characterization of mice with endothelial expression of cytochrome P450 4F2.
Keywords: CYP4F2, 20-HETE, oxidative stress, cellular proliferation, angiogenesis
Arachidonic acid (AA) is metabolized by enzymes of the cytochrome P450 (CYP) 4A and 4F subfamilies to generate 20-hydroxyeicosatetraenoic acid (20-HETE), which is a major eicosanoid of the microcirculation. With the exception of the pulmonary vasculature, the synthesis of 20-HETE has been localized to the vascular smooth muscle. Endothelial cells have limited capacity to produce 20-HETE (1, 2). In most vascular beds, such as the renal and cerebral microcirculation, 20-HETE promotes vasoconstriction by inhibiting the large-conductance calcium-activated potassium channels (3) and stimulating the L-type Ca2+ channels (4) to increase intracellular [Ca2+]. It also contributes to endothelial dysfunction, characterized by impaired vasorelaxation response to acetylcholine and uncoupling of endothelial nitric oxide synthase (eNOS) to reduce levels of bioactive nitric oxide (NO) (5, 6). Interestingly, in the pulmonary circulation, 20-HETE increases vasodilation by increasing NO production (7). This could be attributed to differences in properties between the pulmonary vasculature and other vascular systems. In all reported vascular beds and in cultured endothelial cells, 20-HETE is a potent inducer of reactive oxygen species (ROS), with superoxide being the main species (5, 6, 8, 9). Generation of superoxide under acute conditions is primarily a result of eNOS uncoupling, whereas under chronic conditions, 20-HETE-mediated superoxide production is a consequence of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase up-regulation (9). All of these effects together can contribute to the development of hypertension.
In addition to its prohypertensive properties, 20-HETE has been shown to induce angiogenesis and cell proliferation. Exogenous addition of 20-HETE stimulates the proliferation of various cell types, such as renal epithelial cells and endothelial cells (9–11). Guo et al. (9) showed that 20-HETE induces endothelial cell proliferation via ROS-mediated increases in vascular endothelial growth factor (VEGF) expression. Several studies have suggested that the effects of 20-HETE may parallel those of VEGF. Electrical stimulation-induced angiogenesis in skeletal muscle is blocked by chronic treatment with N-hydroxy-N′-(4-butyl-2-methylphenol) formamidine (HET0016), an inhibitor of CYP4A that prevents the synthesis of 20-HETE (12). Overexpression of CYP4A1 in vascular smooth muscle promotes endothelial sprouting in renal arterial microvessels (13). Chen et al. (10) demonstrated that HET0016 attenuates the angiogenic response to VEGF in the rat cornea and significantly inhibits corneal neovascularization. In recent years, 20-HETE has been implicated in various types of cancers. Implantation of tumor cells transfected with CYP4A11 into lungs of nude mice resulted in increased metastasis that was decreased by treatment with HET0016 (14).
20-HETE also possesses proinflammatory properties, which may or may not be interdependent on its proangiogenic properties. Endothelial cells transfected with CYP4A adenovirus displayed increased levels of IL-6 and intracellular cell adhesion molecule 1 (ICAM-1), as well as induction of NF-κB nuclear translocation (15). Ischemia/reperfusion-induced renal inflammation is attenuated by HET0016 or 20-6,15-hydroxyeicosadienoic acid (20-HEDE), the specific 20-HETE antagonist (16), suggesting a role for 20-HETE in acute kidney injury-related inflammation. Indeed, it is clear that 20-HETE plays a major role in the pathogenesis of vascular diseases.
The majority of the aforementioned studies have focused on the 20-HETE that is biosynthesized from AA by members of the CYP4A subfamily. These include CYP4A1, CYP4A2, CYP4A3, and CYP4A8 in the rat and CYP4A10, CYP4A12, and CYP4A14 in the mouse. However, the CYP4F subfamily is also capable of producing 20-HETE, although its actions in relation to 20-HETE are not as well characterized. In humans, CYP4F2 is the predominant 20-HETE synthase in leukocytes and kidneys (17, 18). Several studies have demonstrated the potential clinical significance of CYP4F2. A single-nucleotide polymorphism in CYP4F2 correlates with increased urinary excretion of 20-HETE (19), and hypertensive subjects in a Chinese cohort with CYP4F2 polymorphisms have elevated levels of urinary 20-HETE (20, 21). Recently, Liu et al. (22) generated transgenic mice overexpressing CYP4F2 in the kidney and showed that these mice had elevated levels of urinary 20-HETE and increased systolic blood pressure compared to their wild-type (WT) littermates. Notably, increases in 20-HETE levels are associated with vascular dysfunction, hypertension, and oxidative stress in humans (23, 24). In patients with advanced atherosclerotic cardiovascular disease, an inverse relationship between circulating 20-HETE levels and endothelial nitric oxide bioavailability, characterized by brachial artery flow-mediated dilation, was observed (25).
To further investigate the role of CYP4F-derived 20-HETE in the regulation of vascular function, we generated transgenic mice that express the human CYP4F2 cDNA in endothelial cells and exhibit increased 20-HETE biosynthesis. Our findings demonstrate that CYP4F2-derived 20-HETE increases endothelial oxidative stress, cellular proliferation, and angiogenesis via NADPH oxidase- and VEGF-dependent pathways and suggest that the Tie2-CYP4F2-Tr mouse is a novel model that can be used for studying the pathophysiological effects of vascular 20-HETE in various disease systems.
MATERIALS AND METHODS
Generation of Tie2-CYP4F2-Tr mice
Transgenic mice expressing CYP4F2 specifically in endothelial cells were developed on a C57BL/6 background, as described previously (26). The human CYP4F2 (GenBank NM_001082.3) cDNA sequence was subcloned downstream of the murine Tie2 promoter and upstream of the Tie2 full enhancer sequences, as described previously (26).
Measurement of mean arterial pressure (MAP)
Male Tie2-CYP4F2-Tr mice and their WT littermate controls (14–18 wk old) were anesthetized with 3% isoflurane and implanted with indwelling transducer-tipped radiotelemetry catheters (TA11PA-C20; Data Sciences International, St. Paul, MN, USA) in the right carotid artery, as described previously (27). After the surgery, the mice were allowed to recover for 1 wk. Baseline measurements were then obtained continuously for 2 h each morning over a 2-wk period. Averages of hourly measurements were recorded to depict that day's value.
Isolation of endothelial cells
Male Tie2-CYP4F2-Tr mice and their WT littermate controls (8–10 wk old) were sacrificed with a fatal dose of pentobarbital. Kidneys, lungs, livers, hearts, and aortas were collected, minced and digested with type I collagenase (2 mg/ml; Worthington Biochemical Corp., Lakewood, NJ, USA) at 37°C for 20 min. Homogenates were triturated 8 times through a 20-ml syringe and then incubated at 37°C for an additional 20 min. Afterward, the homogenates were triturated, filtered through a 70-μm mesh cell strainer, and centrifuged at 500 g for 10 min. Cell pellets were resuspended in phosphate-buffered saline (PBS) with 0.1% BSA and incubated with magnetic Dynabeads coated with anti-platelet/endothelial cell adhesion molecule 1 (PECAM-1; BD Biosciences, San Diego, CA, USA) for 30 min, after which they were washed and plated onto collagen-coated tissue culture dishes in endothelial cell growth medium [ECGM; Dulbecco's modified Eagle medium (DMEM) containing 100 μg/ml heparin, penicillin/streptomycin, 100 μg/ml endothelial cell growth supplement (Biomedical Technologies, Stoughton, MA, USA), 1× sodium pyruvate, 25 mM HEPES buffer and 1× nonessential amino acids] with 20% FBS. Cells were grown to 90% confluency, trypsinized, and incubated with anti-ICAM-2-coated Dynabeads (Invitrogen, Carlsbad, CA, USA) for a second selection. Passages 2–4 were used for all experiments. Cells were maintained in an incubator (5% CO2-95% O2) at 37°C.
Measurement of eicosanoids in cell culture media
Cells were plated on 10-cm2 tissue culture dishes. On the following day, they were incubated with serum-free ECGM for 4 h. The medium was collected from each sample, and the cells were incubated with serum-free ECGM containing 10 μM calcium ionophore A23187 for 10 min to stimulate AA release. The media were collected, and cells were lysed in tissue lysis buffer (50 mM Tris-HCl, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 0.25% sodium deoxycholate, and 1 mM NaF) containing 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor pellets (Roche, Basel, Switzerland), to determine protein concentration. Lipids were extracted from the media using Oasis HLB C18 columns (Waters Corp., Milford, MA, USA) and eluted in methanol/ethyl acetate. Samples were dried under nitrogen gas, resuspended in 50 μl 30% ethanol, and injected into an Agilent 1200 series capillary HPLC (Agilent Technologies, Santa Clara, CA, USA) coupled to an API 3000 triple quadrupole mass spectrometer (PE Sciex, Foster City, CA, USA), as described previously (28). Quantitation of eicosanoids was performed using the Analyst 5.1 software (Millipore, Billerica, MA, USA), and levels were normalized to the protein concentrations of cells.
Analysis of cell growth
Renal endothelial cells were cultured on 12-well plates at a density of 20,000 cells/well, in the presence or absence of 20-HEDE (10 nM), sodium 4,5-dihydroxybenzene-1,3-disulfonate (Tiron; 1 mM), anti-VEGF-neutralizing antibody (VEGF Ab; 500 ng/ml), 7-(1,3-benzoxazol-2-ylsulfanyl)-3-benzyl-3H-[1, 2, 3]triazolo[4,5-d]pyrimidine, 1,3-benzoxazol-2-yl-3-benzyl-3H-[1, 2, 3]triazolo[4,5-d]pyrimidin-7-yl sulfide (VAS2870; 10 μM), 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene (U0126; 10 μM), 3-[2-(4-adamantan-1-yl-phenoxy)-acetylamino]-4-hydroxybenzoic acid methyl ester (LW6; 10 μM), and IL-6-blocking antibody (IL-6 Ab; 500 ng/ml, clone MP5-20F3, BD Biosciences). Cells were trypsinized after 24 and 48 h and counted using a hemacytometer. Accuracy of cell counts was confirmed using the Coulter particle counter (Beckman Coulter, Brea, CA, USA).
Matrigel tube formation assay
Multiwell (24-well) plates were coated with thawed Matrigel (BD Biosciences; 300 μl/well) and incubated at 37°C for 1 h to allow for polymerization of the gel. Renal endothelial cells were plated at a density of 48,000 cells/200 μl in ECGM with 1% FBS in the presence and absence of 20-HEDE (10 nM), Tiron (1 mM), VEGF Ab (500 ng/ml), VAS2870 (10 μM), LW6 (10 μM), and U0126 (10 μM), and incubated at 37°C for 16 h. Tubes were imaged at 5× using an inverted bright-field microscope, and quantitated using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Measurement of superoxide levels
Renal endothelial cells were plated on 96-well plates at a density of 5,000 cells/well and incubated for 24 h in serum-free ECGM with or without 20-HEDE (10 nM). Afterward, cells were washed with 1× HBSS and incubated with dihydroethidium (DHE; 5 μM) for 20 min. DHE fluorescence was measured using a Bio-Tek spectrophotometer (Bio-Tek Instruments, Inc., Winooski, VT, USA) at excitation and emission wavelengths of 530 and 620 nm, respectively.
Quantitation of VEGF release from cells
Renal endothelial cells were plated on 6-well plates and incubated with or without 20-HEDE (10 nM) and VAS2870 (10 μM) in serum-free ECGM for 16 h. The reaction medium was collected from each sample, and VEGF levels were measured using the mouse VEGF ELISA kit (Invitrogen), following the manufacturer's instructions.
Bacterial lipopolysaccharide (LPS)-induced inflammation in mice and cells
Tie2-CYP4F2-Tr mice and WT littermate controls (6–8 wk old) were treated with LPS (strain 0111:B4, 1 mg/kg body weight; Sigma, St. Louis, MO, USA) or saline for 4 h, after which the lungs were collected for analyses of proinflammatory molecules. Isolated renal endothelial cells were seeded onto 6-well plates and treated with LPS (10 ng/ml) or PBS in serum-free medium for 4 h. Posttreatment, cells were collected and lysed in buffer RLT (Qiagen, Valencia, CA, USA) for RNA extractions and analyses of proinflammatory molecules. For lung-specific inflammation, mice (10–12 wk old) were administered LPS (50 μg) via oropharyngeal aspiration. After 4 h, the lungs were lavaged twice with Hank's buffered saline solution. Total cell counts in the bronchoalveolar lavage fluid (BALF) were obtained using the Coulter particle counter. Cell differentials and neutrophil infiltration were analyzed by researchers who were blinded to the experimental groups.
RNA extraction and real-time RT-PCR analysis
Cells were lysed in buffer RLT, and RNA was extracted using the RNEasy mini kit (Qiagen), following the manufacturer's protocol. The elution volume for each sample was 30 μl, and quantitation of samples was performed using a Beckman DU 640 spectrophotometer (Beckman Coulter). RNA was reverse transcribed to cDNA using the ABI high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA), as described previously (26). Expression of human CYP4F2, murine VEGFa, Flt, Kdr, Cybb, Cyp4a10, Cyp4a12, Cyp4a14, Cyp4f13, Cyp4f14, Cyp4f18, IL-6, TNF-α, VCAM-1, and murine GAPDH were quantified by real-time quantitative RT-PCR using TaqMan Assays on Demand (Applied Biosystems). Expression of all genes was normalized to murine GAPDH using the 2−ΔΔCt method (29).
Western blot analysis
Renal endothelial cells were plated on 6-well plates and incubated with or without 20-HEDE in serum-free ECGM for 16 h, after which they were lysed in tissue lysis buffer (50 mM Tris-HCl, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 0.25% sodium deoxycholate, and 1 mM NaF) containing 1 mM Na3VO4, 1 mM PMSF, and protease inhibitor pellets. Quantitation of proteins was measured using the Bradford assay. Samples (20 μg) were loaded onto 12% SDS Tris-glycine gels (Invitrogen) and run for 3 h at 125 V. Proteins from the gel were transferred onto 0.45 μm nitrocellulose membranes via semidry transfer for 1 h. Blots were blocked in 10% milk, and antibodies against CYP4F2, hypoxia-inducible factor 1α (HIF-1α), phospho-p44/42 mitogen-activated protein kinase (MAPK), p44/42 MAPK, p67phox, phospho-IκB, or IκB (Cell Signaling, Danvers, MA, USA) were used to probe for expression levels of the respective proteins. Protein expression was normalized to GAPDH. Densitometry of proteins was performed using the ImageJ software.
Measurement of IL-6 in serum and cell culture media
Levels of IL-6 in serum and cell culture media were determined with a Bio-Plex mouse IL-6 singleplex kit (Bio-Rad, Hercules, CA, USA) using fluorescently labeled microsphere beads and a Bio-Plex suspension array system, according to the manufacturer's instructions.
Immunofluorescence
Frozen kidney sections (7 μm thick) from WT and Tie2-CYP4F2-Tr mice were cut onto slides and stained to confirm endothelial-specific expression of human CYP4F2. Briefly, slides were dried for 4 h at room temperature and incubated in 1 mg/ml sodium borohydride overnight. The sections were then rinsed with PBS, fixed with methanol and 1% H2O2 for 15 min at 4°C, washed, and permeabilized with 0.8% Triton X-100 in PBS for 15 min. After rinsing 3 times, sections were blocked with CAS-Block for 30 min and then incubated with the primary antibody against CYP4F2 (Abcam, Cambridge, MA, USA) for 1 h. Following 3 rinses with PBS, the sections were incubated with secondary antibodies for 1 h. A drop of DAPI was added to the sections at the end prior to mounting with DakoCytomation fluorescent mounting medium (DakoCytomation, Glostrup, Denmark). Slides were viewed under the confocal microscope at 20× magnification.
Wire myograph
Microdissected aortas were mounted on wires in myograph chambers (J.P. Trading, Aarhus, Denmark) for measurement of isometric tension, as described previously (5, 6). For vasoconstriction measurements, the vessels were constricted with increasing doses of phenylephrine (10−9 to 5×10−5 M; Sigma). For vasorelaxation measurements, the vessels were preconstricted with 5 × 10−6 M phenylephrine, after which the vasodilator responses to increasing doses of acetylcholine (10−9 to 5×10−5 M; Sigma) were recorded.
Statistical analysis
Significance of results (means±sem) was determined via Student's t test and/or 1-way or 2-way ANOVA, followed by the Newman-Keuls post hoc test. Values of P < 0.05 were determined to be significant.
RESULTS
CYP4F2 expression and 20-HETE levels are elevated in Tie2-CYP4F2-Tr tissues and endothelial cells
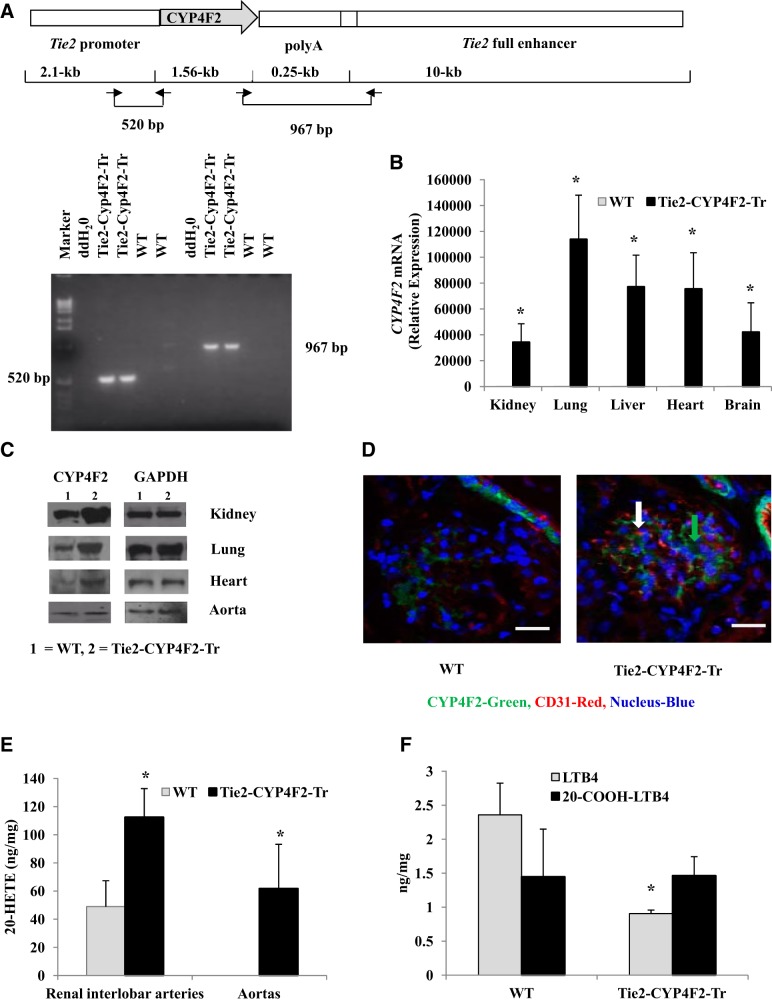
As shown in Fig. 1A, the Tie2 promoter was used to generate transgenic mice expressing the human CYP4F2 cDNA in endothelial cells. Two sets of primer pairs yielding product sizes of 520 and 967 bp were used to genotype the mice. Real-time RT-PCR analysis showed a significant amount of human CYP4F2 mRNA in Tie2-CYP4F2-Tr (Ct∼23) tissues and endothelial cells, with minimal expression in WT (Ct>40; Figs. 1B and 2A). Western blot analysis of tissues and cell lysates also revealed increased amounts of CYP4F protein in Tie2-CYP4F2-Tr tissues and endothelial cells compared to WT (Figs. 1C and 2B). Immunofluorescence images from kidney sections exhibited increased staining of CYP4F protein (green) in Tie2-CYP4F2-Tr mice, especially in the glomerular endothelial cells. Some staining of CYP4F protein was observed in WT sections due to the fact that the antibody cross-reacts with mouse CYP4F isoforms (Fig. 1D).
Figure 1.
Characterization of mice with endothelial-specific expression of CYP4F2. A) The human CYP4F2 cDNA was subcloned between the Tie2 promoter and Tie2 enhancer. Genotyping was done using two primer pairs yielding product sizes of 520 and 967 bp. B) CYP4F2 mRNA levels in tissues from Tie2-CYP4F2-Tr and WT mice. C) CYP4F protein expression in tissues from Tie2-CYP4F2-Tr and WT mice. D) Immunofluorescence (×20) of WT and Tie2-CYP4F2-Tr kidney sections, showing increased CYP4F staining in endothelial cells of Tie2-CYP4F2-Tr mice. Scale bars = 50 μm. CD31, red staining (white arrow); CYP4F2, green staining (green arrow). E) 20-HETE was extracted from the renal interlobar arteries and aortas with ethyl acetate and analyzed by LC/MS/MS. F) LTB4 and 20-COOH-LTB4 levels in renal interlobar arteries from WT and Tie2-CYP4F2-Tr mice. Results are expressed as means ± sem; n = 4–12. *P < 0.05 vs. WT.
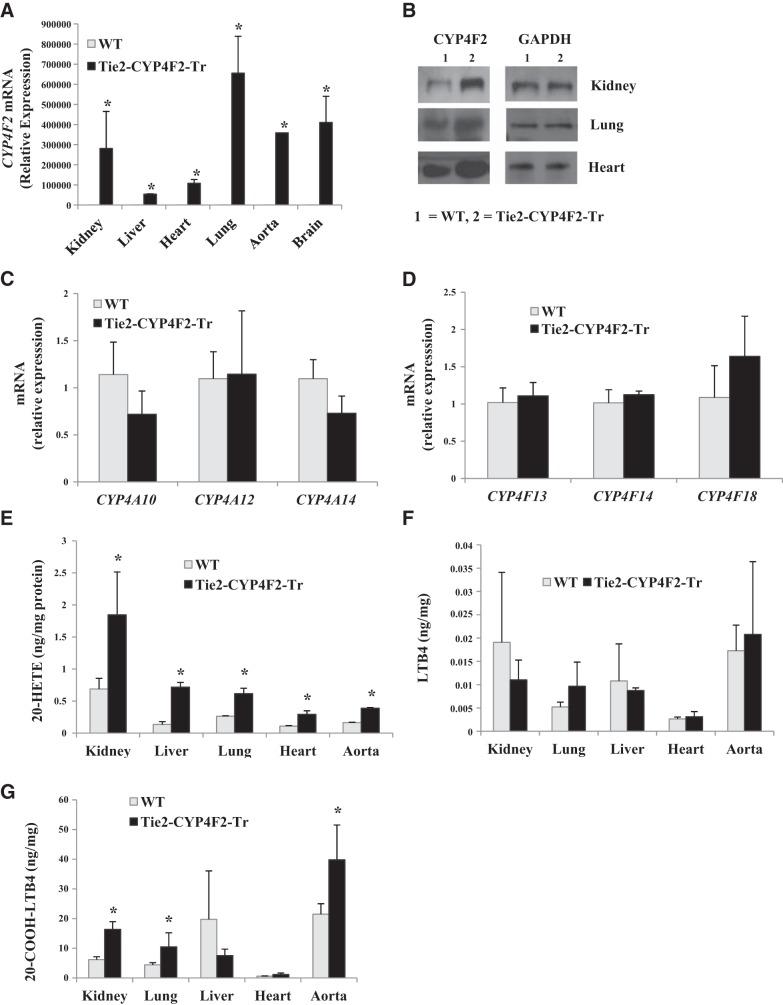
Figure 2.
CYP expression and eicosanoid levels in endothelial cells. Endothelial cells were isolated from WT and Tie2-CYP4F2-Tr mice using PECAM-1- and ICAM-2-coated magnetic beads. A) CYP4F2 mRNA levels in Tie2-CYP4F2-Tr and WT cells isolated from various tissues. B) CYP4F protein expression in Tie2-CYP4F2 and WT cells isolated from various tissues. C) Mouse CYP4A subfamily mRNA levels in Tie2-CYP4F2-Tr and WT renal cells. D) Mouse CYP4F subfamily mRNA levels in Tie2-CYP4F2-Tr and WT renal cells. E–G) Levels of 20-HETE (E), LTB4 (F), and 20-COOH-LTB4 (G) were determined in cell media by LC/MS/MS. Results are expressed as means ± sem; n = 6. *P < 0.05 vs. WT.
Levels of the mouse CYP4 subfamily isoforms (CYP4A10, CYP4A12, CYP4A14, CYP4F13, CYP4F14, and CYP4F18) in tissues and endothelial cells were unaffected by introduction of the CYP4F2 transgene (Fig. 2C, D, and Supplemental Fig. S1A, B). Levels of other mouse CYPs, such as CYP2J5 (Supplemental Fig. S1C) and CYP2C44 (Supplemental Fig. S1D), were also similar in WT and Tie2-CYP4F2-Tr tissues.
20-HETE levels were significantly increased in the renal interlobar arteries and aortas from Tie2-CYP4F2-Tr mice compared to WT (Fig. 1E). Levels of 20-HETE were also increased ∼2-fold in culture media of Tie2-CYP4F2-Tr endothelial cells isolated from kidney, liver, lung, heart, and aorta compared with WT (Fig. 2E).
In addition to metabolizing AA, CYP4F2 has the ability to convert leukotriene B4 (LTB4) to 20-OH-LTB4, which is rapidly degraded to 20-COOH-LTB4. LTB4 levels in renal interlobar arteries from Tie2-CYP4F2-Tr mice were significantly lower than those of WT mice (Fig. 1F). 20-COOH-LTB4 levels were not significantly different between WT and Tie2-CYP4F2-Tr mice (Fig. 1F). Surprisingly, while there were no differences in levels of LTB4 between WT and Tie2-CYP4F2-Tr cells (Fig. 2F), 20-COOH-LTB4 levels were significantly elevated in endothelial cells isolated from Tie2-CYP4F2-Tr kidney, lung, and aorta compared to WT (Fig. 2G). In addition, levels of 14,15-epoxyeicosatrienoic acid (EET) were comparable between WT and Tie2-CYP4F2-Tr cells (Supplemental Fig. S1E), suggesting that human CYP4F2 does not affect the activity of CYP epoxygenase enzymes.
A closer look at both male and female Tie2-CYP4F2-Tr mice revealed that their body weights, kidney-to-body weight ratios, and heart-to-body weight ratios were not significantly different from those of WT mice (Supplemental Fig. S2A, B). Litter sizes were also similar to those of WT mice; an average of 6.34 mice/litter was observed across 35 total litters.
Vascular reactivity, endothelial function, and MAP in Tie2-CYP4F2-Tr mice
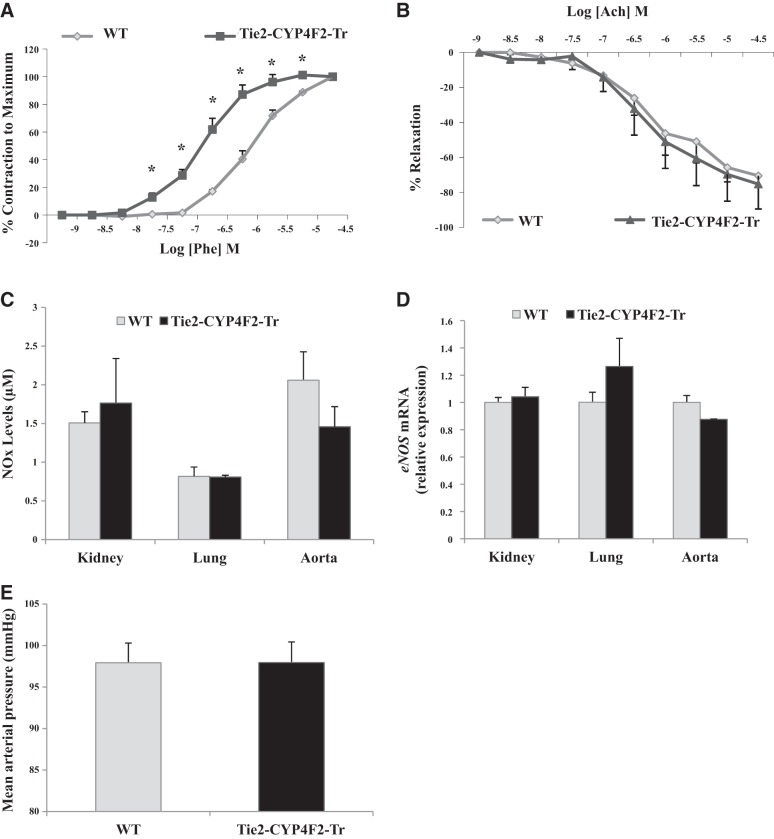
A prominent effect of 20-HETE in the vasculature is its ability to increase vasoconstriction and impair vasorelaxation (5, 6). To assess vascular reactivity and function, aortas from WT and Tie2-CYP4F2-Tr mice were isolated and mounted in wire myograph chambers. Aortas from Tie2-CYP4F2-Tr mice displayed increased phenylephrine-induced vasoconstriction responses compared to aortas from WT mice (Fig. 3A). In contrast, acetylcholine-induced vasorelaxation appeared to be preserved in Tie2-CYP4F2-Tr aortas (75.2±14.2 vs. 70.5±6.7% maximal relaxation; Fig. 3B). Furthermore, no differences in bioavailable NOx or eNOS expression were observed in various tissues from Tie2-CYP4F2-Tr mice relative to WT (Fig. 3C, D).
Figure 3.
Vascular function and blood pressure. A) Vasoconstriction responses of isolated aortas from Tie2-CYP4F2-Tr and WT mice to increasing doses of phenylephrine (5×10−9 to 5×10−5 M). B) Vasorelaxation responses of phenylephrine-constricted aortas from Tie2-CYP4F2-Tr and WT mice to increasing doses of acetylcholine (5×10−9 to 5×10−5 M). C) NOx levels in lysed tissues (50 μg protein) were assessed using the NO quantitation kit based on the Griess reaction. D) eNOS mRNA levels in tissues from Tie2-CYP4F2-Tr and WT mice were measured using real-time RT-PCR. E) WT and Tie2-CYP4F2-Tr mice were implanted with radiotelemetry catheters to measure blood pressure at baseline. Results are expressed as means ± sem; n = 3–16. *P < 0.05 vs. WT.
MAP was measured invasively in WT and Tie2-CYP4F2-Tr mice via radiotelemetry. No differences in MAP were observed between the two groups (Fig. 3E).
Tie2-CYP4F2-Tr endothelial cells have increased oxidative stress
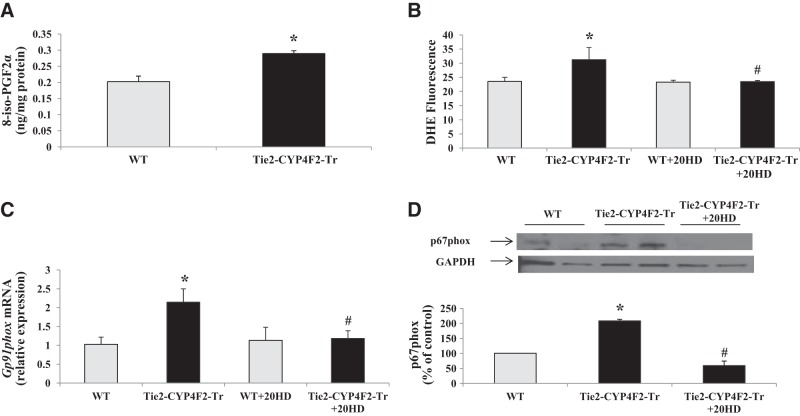
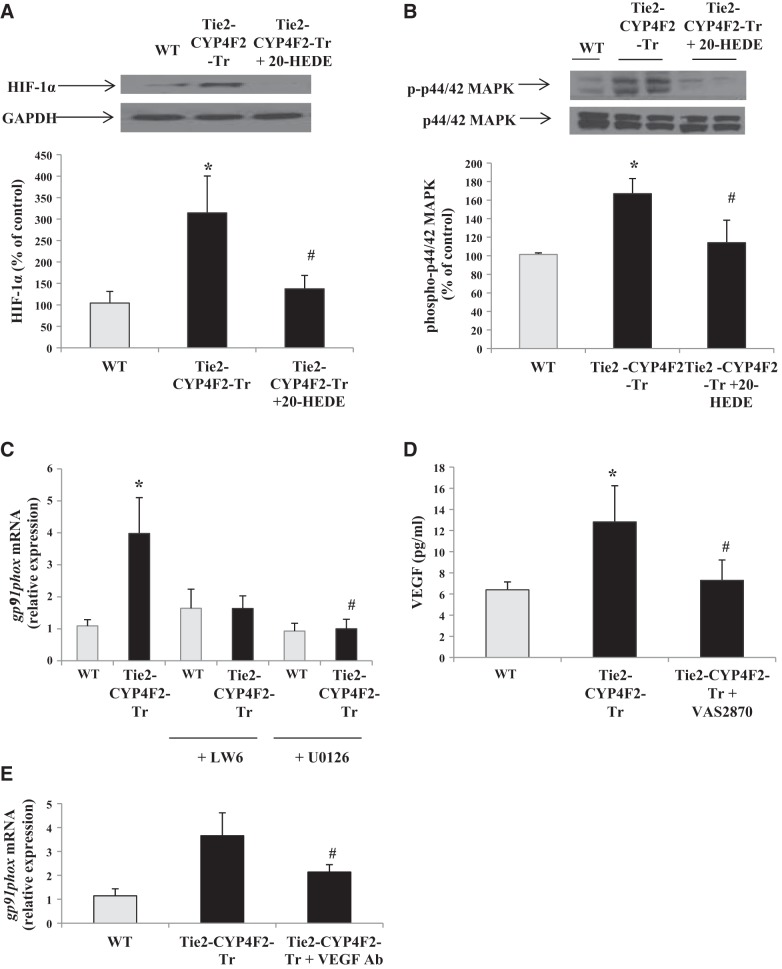
20-HETE is a potent inducer of superoxide generation in vivo and in vitro (5, 30). In our study, liquid chromatography-tandem mass spectrometry (LC/MS/MS) analysis revealed that Tie2-CYP4F2-Tr cells, which express CYP4F2 and produce high levels of 20-HETE, had increased levels of 8-iso-PGF2α, a marker of oxidative stress, compared to WT cells (Fig. 4A). Consistent with this observation, Tie2-CYP4F2-Tr kidney cells had significantly higher levels of superoxide compared with WT cells, as detected by measuring DHE fluorescence (Fig. 4B). Superoxide levels in Tie2-CYP4F2-Tr cells were reduced with the addition of 20-HEDE, an antagonist of 20-HETE, to levels similar to that in WT cells.
Figure 4.
Markers of oxidative stress, superoxide, and NADPH oxidase in endothelial cells. Renal endothelial cells were isolated from WT and Tie2-CYP4F2-Tr mice using magnetic beads coated with PECAM-1 and ICAM-2. A) Levels of 8-iso-PGF2α were measured via LC/MS/MS. B) Cells were plated on 96-well plates, treated with or without 20-HEDE (10 nM) for 16 h, and incubated with DHE (5 μM) for 20 min. Superoxide levels were detected using a spectrophotometer (excitation: 530 nm, excitation: 620 nm). C) gp91phox mRNA levels in endothelial cells were measured via real-time RT-PCR. D) p67phox protein expression was assessed using Western blot analysis. Results are means ± sem; n = 7–9. *P < 0.05 vs. WT; #P < 0.05 vs. Tie2-CYP4F2-Tr.
Among all superoxide-producing enzymes, NADPH oxidase has been shown to play a key role in 20-HETE-mediated oxidative stress under chronic conditions (6, 8). Message RNA levels of gp91phox, the catalytic subunit of NADPH oxidase, were increased ∼2.3-fold in Tie2-CYP4F2-Tr cells compared to WT. Treatment of the transgenic cells with 20-HEDE lowered gp91phox expression (Fig. 4C). Western blot analysis of Tie2-CYP4F2-Tr cell lysates showed an increase in the expression of p67phox, a subunit of NADPH oxidase that is critical for enzyme assembly and activation; this increase in expression was also prevented by treatment with 20-HEDE (Fig. 4D). Together, these results suggest that the increase in superoxide levels observed in Tie2-CYP4F2-Tr cells is driven by an effect of 20-HETE on up-regulating the expression of NADPH oxidase.
Tie2-CYP4F2-Tr mice exhibit a cytokine-specific proinflammatory phenotype
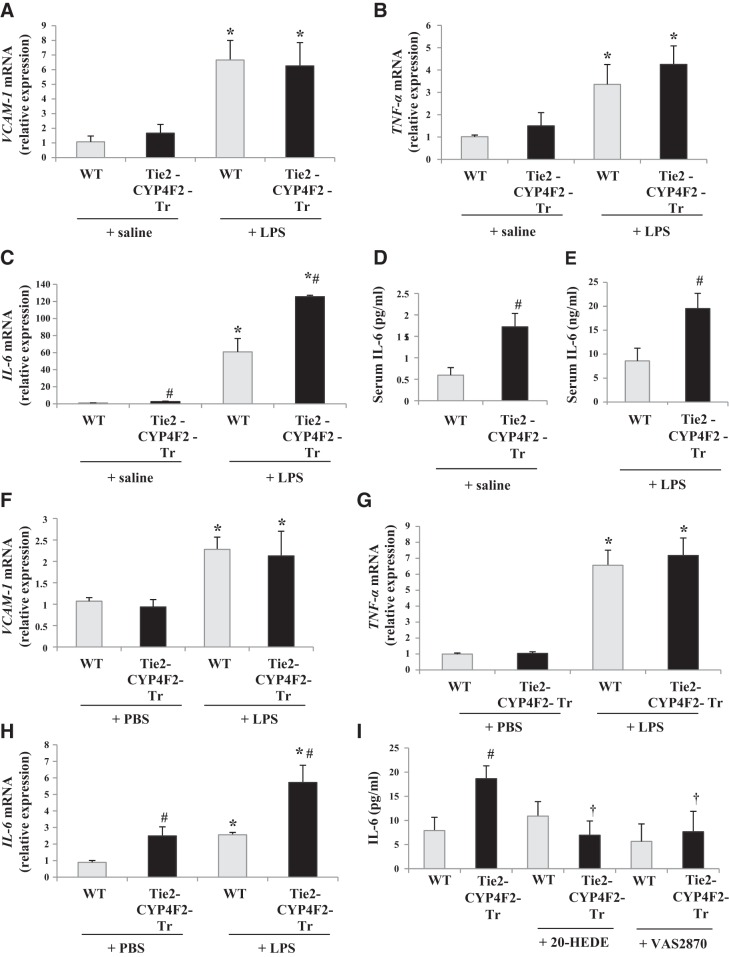
Ishizuka et al. (15) previously demonstrated the proinflammatory actions of CYP4A-derived 20-HETE. We examined whether Tie2-CYP4F2-Tr mice have increased inflammation compared to WT mice by isolating lungs and measuring levels of proinflammatory molecules, such as VCAM-1, TNF-α, and IL-6, via real-time RT-PCR.
Lung mRNA levels of VCAM-1 and TNF-α were comparable between WT and Tie2-CYP4F2-Tr mice under basal conditions (Fig. 5A, B). Treatment of mice with 1 mg/ml LPS i.p. for 4 h increased the levels of these factors by 3- to 6-fold, with no significant differences between WT and Tie2-CYP4F2-Tr lungs. Interestingly, basal mRNA levels of IL-6 were significantly higher in Tie2-CYP4F2-Tr lungs compared to WT. LPS also induced a greater increase in IL-6 levels in lungs from Tie2-CYP4F2-Tr compared to WT (Fig. 5C). IL-6 levels in serum were analyzed and were also found to be elevated in both saline- and LPS-treated Tie2-CYP4F2-Tr relative to WT, indicating a systemic inflammatory response (Fig. 5D, E).
Figure 5.
Levels of proinflammatory molecules. WT and Tie2-CYP4F2-Tr mice were treated with either saline or bacterial LPS (1 mg/kg bw i.p.) for 4 h. Lungs were collected and processed for real-time RT-PCR. A–C) Lung mRNA levels of VCAM-1 (A), TNF-α (B), and IL-6 (C). D, E) Serum was collected and analyzed for levels of IL-6 in saline-treated (D) and LPS-treated (E) mice. F–H) Renal endothelial cells were plated on 6-well plates and treated with PBS or LPS (10 ng/ml) for 4 h. Cellular mRNA levels of VCAM-1 (F), TNF-α (G), and IL-6 (H) were measured. I) Cells were treated with 20-HEDE (10 nM) or VAS2870 (10 μM) for 16 h, after which IL-6 levels in the medium were measured by Bio-Plex assay. Results are means ± sem; n = 4–8. *P < 0.05 vs. respective saline/PBS; #P < 0.05 vs. WT; †P < 0.05 vs. Tie2-CYP4F2-Tr.
Similar results were observed in endothelial cells treated with and without LPS. There were no differences in mRNA levels of VCAM-1 or TNF-α in WT and Tie2-CYP4F2-Tr cells (Fig. 5F, G); however, IL-6 levels were increased in Tie2-CYP4F2-Tr cells compared to WT, in the presence and absence of LPS (Fig. 5H). IL-6 has been linked to oxidative stress and NADPH oxidase (31, 32). As shown in Fig. 5I, IL-6 levels in Tie2-CYP4F2-Tr cell media were decreased on pretreatment of the cells with 20-HEDE or VAS2870, a newly characterized and specific inhibitor of NADPH oxidase (33). This suggests that the up-regulation of IL-6 in Tie2-CYP4F2-Tr cells is mediated through 20-HETE and NADPH oxidase.
Although 20-HETE has been implicated in NF-κB activation (15), which can increase IL-6 production, no differences in the expression of phosphorylated IκB (p-IκB) were observed in Tie2-CYP4F2-Tr cells, compared to those in WT cells. In addition, treatment of Tie2-CYP4F2-Tr cells with 20-HEDE had no effect on p-IκB levels (Supplemental Fig. S2E).
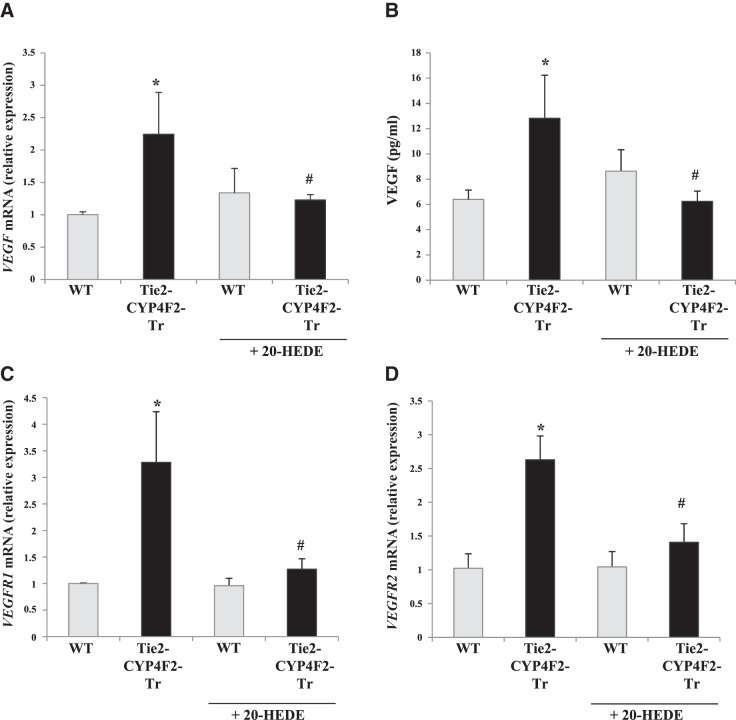
Expression of VEGF and its receptors are elevated in Tie2-CYP4F2-Tr cells
A recent study showed that 20-HETE-induced superoxide generation up-regulates VEGF expression (9). Tie2-CYP4F2-Tr cells had higher mRNA levels of VEGF-A, the isoform most commonly found in endothelial cells, compared to WT cells (Fig. 6A). Release of VEGF from the cells was quantified using an ELISA kit and was increased in culture media from Tie2-CYP4F2-Tr cells compared to WT (Fig. 6B). Treatment of the transgenic cells with 20-HEDE prevented the increase in VEGF-A mRNA and VEGF protein levels.
Figure 6.
Expression of VEGF and its receptors in endothelial cells. Renal endothelial cells were isolated from WT and Tie2-CYP4F2-Tr mice using magnetic beads coated with PECAM-1 and ICAM-2. Cells were plated on 12-well plates and treated with or without 20-HEDE (10 nM) for 16 h. A) VEGF-A mRNA levels were assessed via real-time RT-PCR. B) VEGF levels in the culture media were determined using a mouse VEGF ELISA kit. C, D) VEGFR1 mRNA levels (C) and VEGFR2 mRNA levels (D) were measured via real-time RT-PCR. Results are means ± sem; n = 6. *P < 0.05 vs. WT; #P < 0.05 vs. Tie2-CYP4F2-Tr.
VEGF-A signals through two receptors, VEGFR1 and VEGFR2, from which it elicits many effects, such as cell proliferation, angiogenesis, and vascular permeability (34). Levels of both VEGFR1 and VEGFR2 were increased in Tie2-CYP4F2-Tr cells relative to WT in 20-HETE-dependent manners (Fig. 6C, D).
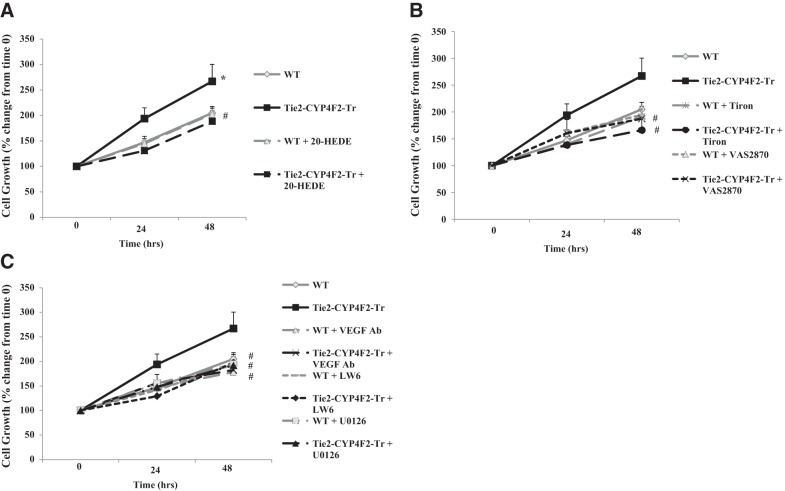
Proliferation of Tie2-CYP4F2-Tr endothelial cells is 20-HETE, superoxide, and VEGF dependent
Proliferation of Tie2-CYP4F2-Tr endothelial cells, expressed as percentage of control growth on day 0, was increased by 194 ± 21 and 267 ± 33% at 24 and 48 h, respectively. This proliferation was significantly higher than that observed in WT endothelial cells (147±12 and 205±13%, respectively). Proliferation of Tie2-CYP4F2-Tr endothelial cells was decreased to WT levels with 20-HEDE treatment, suggesting that the increase in cell growth is driven by 20-HETE (Fig. 7A). In addition, the increased proliferation of Tie2-CYP4F2-Tr endothelial cells was blunted in the presence of Tiron, a superoxide scavenger, or VAS2870, an inhibitor of NADPH oxidase, at both 24 and 48 h, thus implicating NADPH oxidase-dependent superoxide generation in this process (Fig. 7B).
Figure 7.
Endothelial cell proliferation. Renal endothelial cells were isolated from WT and Tie2-CYP4F2-Tr mice using magnetic beads coated with PECAM-1 and ICAM-2. Cells were plated on 12-well plates in the presence or absence of 20-HEDE (10 nM; A), Tiron (1 mM) and VAS2870 (10 μM) (B), and VEGF-neutralizing antibody (VEGF Ab; 500 ng/ml), LW6 (10 μM), or U0126 (10 μM) (C), and the cells were counted at 24 and 48 h. Results are means ± sem; n = 4–8. *P < 0.05 vs. WT; #P < 0.05 vs. Tie2-CYP4F2-Tr.
Proliferation of Tie2-CYP4F2-Tr endothelial cells was also reduced to WT levels by treatment with a VEGF-neutralizing antibody, suggesting that the VEGF pathway contributes to the increase in cell numbers (Fig. 7C). VEGF is regulated by hypoxia-inducible factor 1α (HIF-1α) and binds to VEGFR2 to initiate the activation of MAPK kinase (MEK)-MAPK signaling to stimulate cell proliferation and angiogenesis (34). Tie2-CYP4F2-Tr cell proliferation was reduced to WT levels in the presence of U0126, the inhibitor of MEK. Similar results were achieved in cells treated with LW6, an inhibitor of HIF-1α (Fig. 7C). Because superoxide has been linked to VEGF up-regulation, cells were cotreated with VAS2870 and the VEGF-neutralizing antibody. No further reductions in Tie2-CYP4F2-Tr cell numbers were observed with this combination (Supplemental Fig. S3A). In addition, treatment with a blocking antibody of IL-6 had no effect on Tie2-CYP4F2-Tr cell growth (Supplemental Fig. S3B). No effects of the inhibitors on WT endothelial cells were observed.
Together, these results suggest that the increased cell proliferation observed in endothelial cells with elevated CYP4F2 expression is 20-HETE, NADPH oxidase, VEGF, MAPK, and HIF-1α dependent.
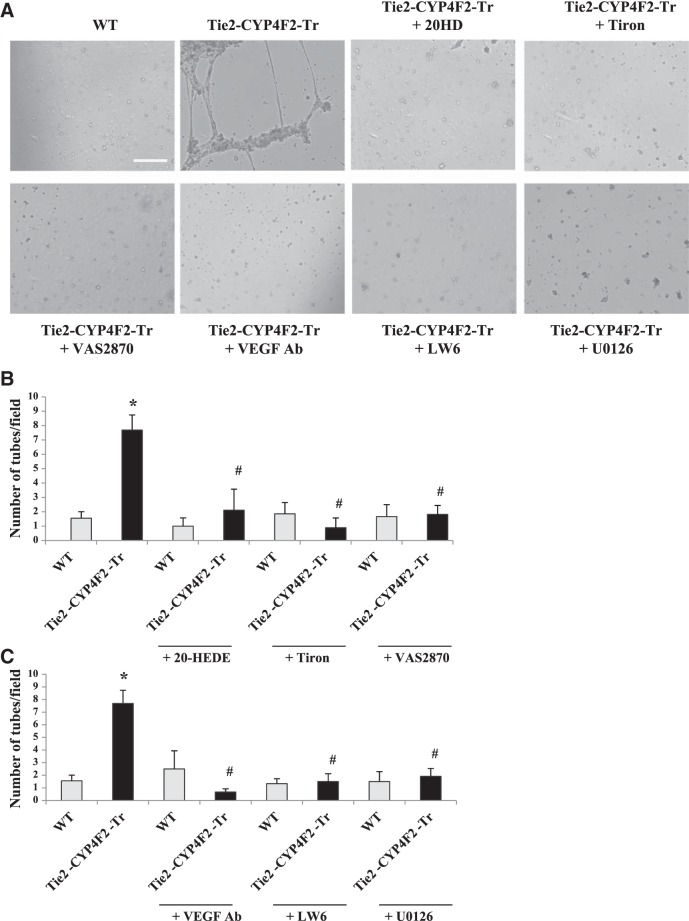
Tube formation is increased in Tie2-CYP4F2-Tr endothelial cells
Increased endothelial cell proliferation can be associated with angiogenesis, a process that involves the formation of new blood vessels. The Matrigel tube formation assay was used to determine the degree of in vitro angiogenesis in WT and Tie2-CYP4F2-Tr cells (Fig. 8A). Interestingly, Tie2-CYP4F2-Tr cells produced a significantly higher number of tubes per field (9.0±1.6) compared to WT cells (1.9±0.9).
Figure 8.
Tube formation. Renal endothelial cells were isolated from WT and Tie2-CYP4F2-Tr mice using magnetic beads coated with PECAM-1 and ICAM-2. Cells were added to wells containing Matrigel in the presence or absence of 20-HEDE (10 nM), Tiron (1 mM), VAS2870 (10 μM), VEGF-neutralizing antibody (VEGF Ab; 500 ng/ml), LW6 (10 μM), or U0126 (10 μM). After 16 h, the wells were imaged using an inverted bright-field microscope (×5). Images (A) and quantitation (B, C) of tube markers are depicted. Scale bar = 250 μm. Results are means ± sem; n = 4–8. *P < 0.05 vs. WT; #P < 0.05 vs. Tie2-CYP4F2-Tr.
To determine the factors involved in the tube formation response, cells were incubated with 20-HEDE, Tiron, or VAS2870 and added to polymerized gels. As shown in Figs. 8A, B, inhibiting 20-HETE, superoxide, or NADPH oxidase decreased the number of tubes per field made by Tie2-CYP4F2-Tr cells to 2.1 ± 1.5, 0.9 ± 0.7, and 0.7 ± 0.3, respectively. None of the inhibitors had any effects on tube formation by WT cells. Tube formation in the presence of the VEGF-neutralizing antibody was also significantly reduced (Fig. 8A, C). Similarly, treatment of Tie2-CYP4F2-Tr endothelial cells with either LW6 or U0126 decreased tube formation.
Together, these results support the concept that increased tube formation in Tie2-CYP4F2-Tr endothelial cells is also 20-HETE, NADPH oxidase, VEGF, MAPK, and HIF-1α dependent.
Protein expression levels of HIF-1α and phospho-p44/42 MAPK are elevated in Tie2-CYP4F2-Tr endothelial cells
To further investigate the signaling pathways affected in Tie2-CYP4F2-Tr endothelial cells, Western blot analyses were performed. HIF-1α expression was increased by 314.4 ± 86.1% in Tie2-CYP4F2-Tr endothelial cells compared to WT cells (Fig. 9A). Treatment with 20-HEDE lowered HIF-1α expression in Tie2-CYP4F2-Tr cells, suggesting that 20-HETE mediates the observed increase in HIF-1α. In addition, Tie2-CYP4F2-Tr cells exhibited increases in p44/42 MAPK phosphorylation that were attenuated when the cells were treated with 20-HEDE (Fig. 9B). These results provide further evidence to suggest that the increased 20-HETE in Tie2-CYP4F2-Tr endothelial cells modulates its effects via activation of the VEGF signaling pathway.
Figure 9.
Signaling mechanisms responsible for oxidative stress, cell proliferation and angiogenesis. Renal endothelial cells were isolated from WT and Tie2-CYP4F2-Tr mice using magnetic beads coated with PECAM-1 and ICAM-2. A, B) Protein expression of HIF-1α (A) and phosphorylated p44/42 MAPK (B) was assessed in cells treated with/without 20-HEDE (10 nM) via Western blot analysis. C) Cells were treated with or without LW6 (10 μM) and U0126 (10 μM), and mRNA levels of gp91phox were determined. D) VEGF levels in the culture media of cells treated with or without VAS2870 (10 μM) were measured via ELISA. E) Cells were treated with/without the VEGF-neutralizing antibody (VEGF Ab; 500 ng/ml), and mRNA levels of gp91phox were determined. Results are means ± sem; n = 4–6. *P < 0.05 vs. WT; #P < 0.05 vs. Tie2-CYP4F2-Tr.
Increased NADPH oxidase expression in Tie2-CYP4F2-Tr endothelial cells is MAPK dependent
Our results show that Tie2-CYP4F2-Tr endothelial cells display increased proliferation and tube formation that are NADPH oxidase and VEGF dependent. Whether NADPH oxidase and VEGF are working together to elicit these effects is unknown. There is evidence that 20-HETE-induced VEGF/MAPK signaling pathways are mediated by superoxide (9). To investigate the relationship between these components, we examined the effects of LW6 and U0126 on expression of the gp91phox subunit of NADPH oxidase. Expression of gp91phox was increased in Tie2-CYP4F2-Tr cells relative to WT cells (Fig. 9C). Treatment of Tie2-CYP4F2-Tr cells with either LW6 or U0126 decreased levels of NADPH oxidase to WT levels. LW6 and U0126 pretreatment had no effect on NADPH oxidase expression in WT cells. Interestingly, the increased VEGF levels in Tie2-CYP4F2-Tr cell media were reduced by pretreatment of the cells with VAS2870 (Fig. 9D). No effects of VAS2870 on VEGF levels in WT cells were observed. Furthermore, pretreatment with the VEGF-neutralizing antibody attenuated levels of NADPH oxidase in Tie2-CYP4F2-Tr cells, although these levels were still significantly greater than those of WT cells (Fig. 9E). Together, these data suggest that induction of NADPH oxidase is, at least in part, MAPK dependent. In Tie2-CYP4F2-Tr cells, NADPH oxidase regulates VEGF, and VEGF partially regulates NADPH oxidase.
DISCUSSION
In humans, the major 20-HETE synthases are CYP4A11 and CYP4F2 (17). In liver, kidney and leukocytes, CYP4F2 is the primary source of 20-HETE (17, 18). Ward et al. (19) showed that a single nucleotide polymorphism in CYP4F2, but not CYP4A11, is associated with increased urinary excretion of 20-HETE and hypertension. This suggests that CYP4F2-derived 20-HETE may affect human health. Unfortunately, little is known about the role of CYP4F2-derived 20-HETE in vascular disease, since most studies have focused on CYP4A-derived 20-HETE. Several recent studies have demonstrated that transgenic mice with kidney-specific expression of CYP4F2 are hypertensive and have increased urinary levels of 20-HETE (22) and that the ratio of 20-HETE to EETs in these mice is altered in androgen-induced hypertension (35). A greater understanding of the CYP4F2-20-HETE system is needed in order to determine its overall effect on human health and disease. In the current study, we focused on the effects of CYP4F2-derived 20-HETE within the vasculature, specifically in endothelial cells that have been shown to be a major target for the bioactions of 20-HETE (5, 6).
The endothelial cells have been shown to express CYP4F2 (36); however, they have limited capacity to generate 20-HETE in culture (1). Thus, synthetic 20-HETE or its analogs are used to characterize its effects in the endothelium. Because 20-HETE is a relatively unstable lipid, alterations in the actual concentrations used may occur when solutions of 20-HETE have been reconstituted multiple times. In some studies, endothelial cells are transfected with CYP4A adenoviruses to increase endogenous 20-HETE levels (15); however, adenoviral transfection can also influence a number of proinflammatory signaling pathways, and there are no reports of CYP4F-transfected endothelial cells. To remedy these issues and to examine the endothelial effects of CYP4F2-derived 20-HETE, we generated mice with endothelial expression of human CYP4F2 using the Tie2 promoter (Tie2-CYP4F2-Tr).
Tie2-CYP4F2-Tr mice appear normal, in terms of litter size, body weight, and heart/kidney-to-body weight ratios. They express human CYP4F2 in various tissues and in isolated endothelial cells. The expression of CYP4F2 in vivo remains constant over time; older transgenic mice express similar amounts of human CYP4F2 as younger mice. There are no differences in the expression of other CYP enzymes, including members of the mouse CYP2J, CYP2C, CYP4A, and CYP4F subfamilies. Most important, isolated endothelial cells and vessels from the mice exhibit increased 20-HETE biosynthesis. Levels of 20-HETE found in cells and vessels from Tie2-CYP4F2-Tr mice are similar to those achieved in CYP4A-transfected cultured cells (15) and vessels from rats transduced with an endothelial-specific CYP4A2 lentivirus (37). In addition, endothelial 20-HETE levels remain elevated regardless of cell passage number.
No differences in MAP between Tie2-CYP4F2-Tr and WT mice were observed. This is not surprising, as 20-HETE is a complex molecule, which has antihypertensive effects in kidney tubules via inhibition of cotransporters and channels that lead to increased sodium excretion (18). It is possible that the CYP4F2-derived 20-HETE that is generated in the endothelium diffuses to the tubules, thus canceling out any prohypertensive vascular effects that may be present. Furthermore, two important characteristics of hypertension are an increase in vascular reactivity and a decrease in endothelium-dependent vasodilation. While we observed an increase in phenylephrine-induced vasoconstriction in the transgenic aortas, acetylcholine-induced vasorelaxation responses were unchanged. In addition, there were no changes in eNOS expression or bioavailable NO levels in transgenic mice. All of these factors may contribute to the normotensive phenotype of the Tie2-CYP4F2-Tr mice.
We expected to observe increased inflammation in the Tie2-CYP4F2-Tr mice, as 20-HETE has been shown to activate the NF-κB pathway (15). Although basal and LPS-stimulated levels of TNF-α and VCAM-1 were unchanged in both tissues and isolated endothelial cells from Tie2-CYP4F2-Tr mice compared to WT controls, IL-6 levels were significantly increased in Tie2-CYP4F2-Tr tissues, sera, cells, and cell culture media. The lack of differences in TNF-α and VCAM-1 levels may be attributed, at least in part, to increased levels of 20-COOH-LTB4 in Tie2-CYP4F2-Tr cells. LTB4 is a proinflammatory molecule that is metabolized by CYP4F2 to form 20-OH-LTB4, an anti-inflammatory molecule that rapidly degrades to 20-COOH-LTB4, an inactive product. There were no differences in LTB4 levels between WT and Tie2-CYP4F2-Tr cells; however, levels were very low (0.01 ng/mg). Interestingly, Tie2-CYP4F2-Tr tissues exhibited significantly lower levels of LTB4 compared to WT. In addition, 20-COOH-LTB4 levels were comparable in Tie2-CYP4F2-Tr and WT mice, suggesting that LTB4 may be metabolized by other enzymes (38). Nonetheless, this is the first report to demonstrate a positive relationship between CYP4F2-derived 20-HETE and IL-6 levels, and our results suggest that Tie2-CYP4F2-Tr mice may be useful in studying IL-6-specific proinflammatory diseases.
The increase in IL-6 was related to oxidative stress and appeared to be dependent on NADPH oxidase activation in the transgenic mice. Oxidative stress, manifested as an increase in superoxide levels and increased activity of superoxide-generating enzymes, is a known effect of CYP4A-derived 20-HETE, as has been demonstrated in vitro in cultured cells, as well as in vivo in the vasculature (5, 6). In hypertensive humans, Ward et al. (23) demonstrated that levels of 8-isoprostane, a marker of oxidative stress, correlated with urinary excretion of 20-HETE. Tie2-CYP4F2-Tr cells display oxidative stress, as evidenced by increased levels of 8-isoprostane and superoxide. The increase in superoxide was 20-HETE dependent and was also associated with increased expression of NADPH oxidase subunits (gp91phox and p47phox; refs. 39, 40). NADPH oxidase has been demonstrated to contribute to 20-HETE-related oxidative stress in androgen-treated rats (6) and to the 20-HETE-mediated increase in superoxide levels in pulmonary endothelial cells (8).
Several studies have shown that exogenous addition of 20-HETE stimulates the proliferation of both vascular smooth muscle cells (41) and endothelial cells (9). Mice injected with CYP4A11-transfected A549 cells display increased angiogenesis characterized by increased microvessel density around tumors (14). In the current study, Tie2-CYP4F2-Tr endothelial cells had significantly elevated cell proliferation, as well as tube formation, an in vitro assay for angiogenesis. Moreover, they expressed higher mRNA levels of VEGF and its two receptors, VEGFR1 and VEGFR2. Secretion of VEGF into cell culture media was also elevated in transgenic cells. All were abolished by 20-HEDE, indicating that these effects are 20-HETE-mediated.
The effects of 20-HETE on cell proliferation have been shown to be mediated via a superoxide-induced increase in VEGF (9). In endothelial cells, VEGF binds to VEGFR2 and activates the MAPK signaling pathway to increase cell proliferation and vessel formation. The function of VEGFR1 is less clear, as some studies have shown that it counters the effects of VEGFR2, whereas other studies have shown that it works along with VEGFR2 to elicit its effects. VEGF is tightly regulated by HIF-1α, a hypoxic factor that can also be induced by 20-HETE under nonhypoxic conditions (42). In an effort to investigate the mechanisms by which CYP4F overexpression in endothelial cells causes increased cell proliferation and angiogenesis, we focused on the two main regulators of the VEGF pathway: HIF-1α and MAPK. Our results show that both HIF-1α and the p44/42 MAPK phosphorylation were elevated in transgenic cells. These increases were not observed in cells treated with 20-HEDE, suggesting that 20-HETE is driving these effects.
Using pharmacological inhibitors against various components in the VEGF and superoxide signaling pathways, we showed that both cell proliferation and tube formation in Tie2-CYP4F2-Tr cells were mediated via NADPH oxidase, VEGF, HIF-1α, and MAPK. Significant reductions in tube formation and cell numbers were seen in the presence of inhibitors of these components. Notably, similar reductions in cell number were observed in the presence of the VEGF-neutralizing antibody and the specific NADPH oxidase inhibitor. Our results suggest that, although oxidative stress is commonly associated with endothelial dysfunction and eNOS uncoupling, it may play a greater role in the angiogenesis and proliferation phenotypes in Tie2-CYP4F2-Tr mice. We postulate that increased CYP4F2 levels lead to increased production of 20-HETE, which induces cell proliferation and angiogenesis through NADPH oxidase activation and the VEGF signaling pathway.
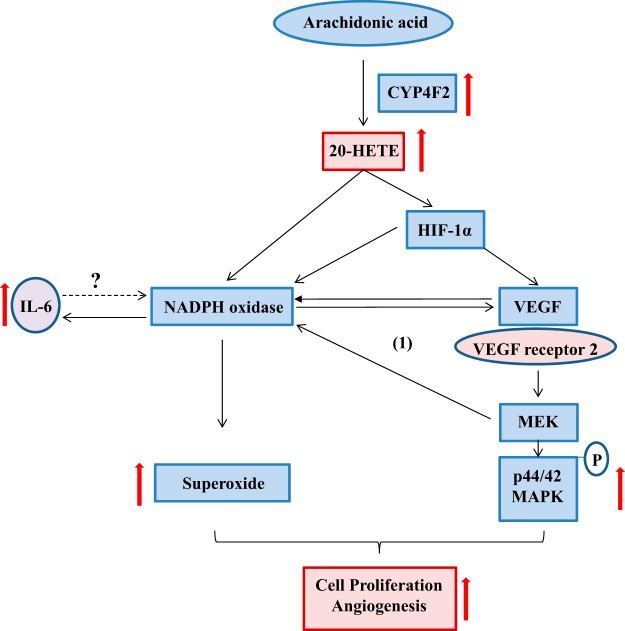
The exact actions of NADPH oxidase, VEGF, and MAPK require further elucidation in future studies. VEGF is regulated by NADPH oxidase, as Tie2-CYP4F2-Tr cells pretreated with VAS2870 had decreased levels of VEGF. In addition, the increase in NADPH oxidase was MAPK dependent and was also partially attenuated by the VEGF-neutralizing antibody. Thus, there may be a feed-forward mechanism that is contributing to these effects (Fig. 10). Further studies are needed to determine which VEGF receptors may be involved and whether IL-6 contributes to this process. Interestingly, IL-6 did not affect the proliferation of Tie2-CYP4F2-Tr cells, suggesting that it may not play a role in angiogenesis. Recently, 20-HETE has been implicated as a major contributor to the development of various cancers (14, 43, 44). Because of the integral contribution of CYP4F2 to 20-HETE biosynthesis in humans, these transgenic mice provide a novel tool to investigate the effect of increased 20-HETE biosynthesis on susceptibility to tumor development and metastases. Future studies examining the physiological effects of CYP4F2-induced angiogenesis in vivo, such as retinal microvascular density and wound healing, will be performed in these mice.
Figure 10.
Proposed schematic of the effects of CYP4F2 in endothelial cells. The proposed mechanisms by which introduction of the human CYP4F2 transgene induces cell proliferation and angiogenesis are depicted. Increased CYP4F2 expression results in elevated levels of 20-HETE, which stimulates NADPH oxidase and VEGF signaling pathways to induce cell proliferation and angiogenesis. A feed-forward mechanism (cycle 1) exists, wherein MEK contributes to NADPH oxidase up-regulation, and NADPH oxidase regulates VEGF. IL-6 is also up-regulated in an NADPH oxidase-dependent manner, although its exact link to cell proliferation and angiogenesis remains unknown.
In summary, we developed and characterized a novel mouse model in which the endothelial expression of human CYP4F2 leads to constitutively increased 20-HETE biosynthesis. The major phenotypic findings of constitutively elevated CYP4F2-derived 20-HETE are as follows: elevated oxidative stress and NADPH oxidase expression; increased IL-6 expression; and increased cell proliferation and angiogenesis via activation of NADPH oxidase and VEGF pathways. Findings from this study suggest that the Tie2-CYP4F2-Tr mice can be used to further our understanding of the physiological and pathological effects of 20-HETE in the vasculature both in vitro and in vivo.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the staff at Xenogen Biosciences (Cranberry, NJ, USA) for assistance with pronuclear injections and generation of the Tie2-CYP4F2-Tr mice.
This work was made possible by U.S. National Institutes of Health (NIH) grant DK-38226 and support from the Robert A. Welch Foundation (to J.R.F.), NIH grant GM-088199 (to C.R.L), NIH grant HL-34300 (to M.L.S.), Diversity Supplement Award HL-34300-26A1S1 (to V.G.), and funds from the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES050167 to K.B.T. and Z01 ES025034 to D.C.Z.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 20-HEDE
- 20-6,15- hydroxyeicosadienoic acid
- 20-HETE
- 20-hydroxyeicosatetraenoic acid
- AA
- arachidonic acid
- CYP
- cytochrome P450
- DHE
- dihydroethidium
- ECGM
- endothelial cell growth medium
- EET
- epoxyeicosatrienoic acid
- eNOS
- endothelial nitric oxide synthase
- HIF
- hypoxia-inducible factor
- ICAM
- intracellular cell adhesion molecule
- LC/MS/MS
- liquid chromatography-tandem mass spectrometry
- LPS
- lipopolysaccharide
- LTB4
- leukotriene B4
- MAP
- mean arterial pressure
- MAPK
- mitogen-activated protein kinase
- MEK
- mitogen-activated protein kinase kinase
- NADPH
- nicotinamide adenine dinucleotide phosphate
- NO
- nitric oxide
- PBS
- phosphate-buffered saline
- PECAM
- platelet/endothelial cell adhesion molecule
- PMSF
- phenylmethylsulfonyl fluoride
- ROS
- reactive oxygen species
- VEGF
- vascular endothelial growth factor
- WT
- wild type
REFERENCES
- 1. Cheng J., Wu C. C., Gotlinger K. H., Zhang F., Falck J. R., Narsimhaswamy D., Schwartzman M. L. (2010) 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IκB kinase-dependent endothelial nitric-oxide synthase uncoupling. J. Pharmacol. Exp. Ther. 332, 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen Y., Medhora M., Falck J. R., Pritchard K. A., Jr., Jacobs E. R. (2006) Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L378–L385 [DOI] [PubMed] [Google Scholar]
- 3. Miyata N., Roman R. J. (2005) Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J. Smooth Muscle Res. 41, 175–193 [DOI] [PubMed] [Google Scholar]
- 4. Zeng Q., Han Y., Bao Y., Li W., Li X., Shen X., Wang X., Yao F., O'Rourke S. T., Sun C. (2010) 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 299, H1109–H1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng J., Ou J. S., Singh H., Falck J. R., Narsimhaswamy D., Pritchard K. A., Jr., Schwartzman M. L. (2008) 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 294, H1018–H1026 [DOI] [PubMed] [Google Scholar]
- 6. Singh H., Cheng J., Deng H., Kemp R., Ishizuka T., Nasjletti A., Schwartzman M. L. (2007) Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50, 123–129 [DOI] [PubMed] [Google Scholar]
- 7. Jacobs E. R., Zhu D., Gruenloh S., Lopez B., Medhora M. (2006) VEGF-induced relaxation of pulmonary arteries is mediated by endothelial cytochrome P-450 hydroxylase. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L369–L377 [DOI] [PubMed] [Google Scholar]
- 8. Medhora M., Chen Y., Gruenloh S., Harland D., Bodiga S., Zielonka J., Gebremedhin D., Gao Y., Falck J. R., Anjaiah S., Jacobs E. R. (2008) 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L902–L911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo A. M., Arbab A. S., Falck J. R., Chen P., Edwards P. A., Roman R. J., Scicli A. G. (2007) Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J. Pharmacol. Exp. Ther. 321, 18–27 [DOI] [PubMed] [Google Scholar]
- 10. Chen P., Guo M., Wygle D., Edwards P. A., Falck J. R., Roman R. J., Scicli A. G. (2005) Inhibitors of cytochrome P450 4A suppress angiogenic responses. Am. J. Pathol. 166, 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park F., Sweeney W. E., Jia G., Roman R. J., Avner E. D. (2008) 20-HETE mediates proliferation of renal epithelial cells in polycystic kidney disease. J. Am. Soc. Nephrol. 19, 1929–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amaral S. L., Maier K. G., Schippers D. N., Roman R. J., Greene A. S. (2003) CYP4A metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 284, H1528–H1535 [DOI] [PubMed] [Google Scholar]
- 13. Jiang M., Mezentsev A., Kemp R., Byun K., Falck J. R., Miano J. M., Nasjletti A., Abraham N. G., Laniado-Schwartzman M. (2004) Smooth muscle-specific expression of CYP4A1 induces endothelial sprouting in renal arterial microvessels. Circ. Res. 94, 167–174 [DOI] [PubMed] [Google Scholar]
- 14. Yu W., Chen L., Yang Y. Q., Falck J. R., Guo A. M., Li Y., Yang J. (2011) Cytochrome P450 omega-hydroxylase promotes angiogenesis and metastasis by upregulation of VEGF and MMP-9 in non-small cell lung cancer. Cancer Chemother. Pharmacol. 68, 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishizuka T., Cheng J., Singh H., Vitto M. D., Manthati V. L., Falck J. R., Laniado-Schwartzman M. (2008) 20-hydroxyeicosatetraenoic acid stimulates nuclear factor-κB activation and the production of inflammatory cytokines in human endothelial cells. J. Pharmacol. Exp. Ther. 324, 103–110 [DOI] [PubMed] [Google Scholar]
- 16. Hoff U., Lukitsch I., Chaykovska L., Ladwig M., Arnold C., Manthati V. L., Fuller T. F., Schneider W., Gollasch M., Muller D. N., Flemming B., Seeliger E., Luft F. C., Falck J. R., Dragun D., Schunck W. H. (2011) Inhibition of 20-HETE synthesis and action protects the kidney from ischemia/reperfusion injury. Kidney Int. 79, 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Powell P. K., Wolf I., Jin R., Lasker J. M. (1998) Metabolism of arachidonic acid to 20-hydroxy-5,8,11, 14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. J. Pharmacol. Exp. Ther. 285, 1327–1336 [PubMed] [Google Scholar]
- 18. Lasker J. M., Chen W. B., Wolf I., Bloswick B. P., Wilson P. D., Powell P. K. (2000) Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J. Biol. Chem. 275, 4118–4126 [DOI] [PubMed] [Google Scholar]
- 19. Ward N. C., Tsai I. J., Barden A., van Bockxmeer F. M., Puddey I. B., Hodgson J. M., Croft K. D. (2008) A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension 51, 1393–1398 [DOI] [PubMed] [Google Scholar]
- 20. Deng S., Zhu G., Liu F., Zhang H., Qin X., Li L., Zhiyi H. (2010) CYP4F2 gene V433M polymorphism is associated with ischemic stroke in the male northern Chinese Han population. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 664–668 [DOI] [PubMed] [Google Scholar]
- 21. Liu H., Zhao Y., Nie D., Shi J., Fu L., Li Y., Yu D., Lu J. (2008) Association of a functional cytochrome P450 4F2 haplotype with urinary 20-HETE and hypertension. J. Am. Soc. Nephrol. 19, 714–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X., Zhao Y., Wang L., Yang X., Zheng Z., Zhang Y., Chen F., Liu H. (2009) Overexpression of cytochrome P450 4F2 in mice increases 20-hydroxyeicosatetraenoic acid production and arterial blood pressure. Kidney. Int. 75, 1288–1296 [DOI] [PubMed] [Google Scholar]
- 23. Ward N. C., Puddey I. B., Hodgson J. M., Beilin L. J., Croft K. D. (2005) Urinary 20-hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic. Biol. Med. 38, 1032–1036 [DOI] [PubMed] [Google Scholar]
- 24. Ward N. C., Rivera J., Hodgson J., Puddey I. B., Beilin L. J., Falck J. R., Croft K. D. (2004) Urinary 20-hydroxyeicosatetraenoic acid is associated with endothelial dysfunction in humans. Circulation 110, 438–443 [DOI] [PubMed] [Google Scholar]
- 25. Schuck R. N., Theken K. N., Edin M. L., Caughey M., Bass A., Ellis K., Tran B., Steele S., Simmons B. P., Lih F. B., Tomer K. B., Wu M. C., Hinderliter A. L., Stouffer G. A., Zeldin D. C., Lee C. R. (2013) Cytochrome P450-derived eicosanoids and vascular dysfunction in coronary artery disease patients. Atherosclerosis 227, 442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee C. R., Imig J. D., Edin M. L., Foley J., DeGraff L. M., Bradbury J. A., Graves J. P., Lih F. B., Clark J., Myers P., Perrow A. L., Lepp A. N., Kannon M. A., Ronnekleiv O. K., Alkayed N. J., Falck J. R., Tomer K. B., Zeldin D. C. (2010) Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 24, 3770–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Athirakul K., Bradbury J. A., Graves J. P., DeGraff L. M., Ma J., Zhao Y., Couse J. F., Quigley R., Harder D. R., Zhao X., Imig J. D., Pedersen T. L., Newman J. W., Hammock B. D., Conley A. J., Korach K. S., Coffman T. M., Zeldin D. C. (2008) Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB J. 22, 4096–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edin M. L., Wang Z., Bradbury J. A., Graves J. P., Lih F. B., DeGraff L. M., Foley J. F., Torphy R., Ronnekleiv O. K., Tomer K. B., Lee C. R., Zeldin D. C. (2011) Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 25, 3436–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 30. Wang J. S., Singh H., Zhang F., Ishizuka T., Deng H., Kemp R., Wolin M. S., Hintze T. H., Abraham N. G., Nasjletti A., Laniado-Schwartzman M. (2006) Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ. Res. 98, 962–969 [DOI] [PubMed] [Google Scholar]
- 31. Wassmann S., Stumpf M., Strehlow K., Schmid A., Schieffer B., Bohm M., Nickenig G. (2004) Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 94, 534–541 [DOI] [PubMed] [Google Scholar]
- 32. Behrens M. M., Ali S. S., Dugan L. L. (2008) Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J. Neurosci. 28, 13957–13966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ten Freyhaus H., Huntgeburth M., Wingler K., Schnitker J., Baumer A. T., Vantler M., Bekhite M. M., Wartenberg M., Sauer H., Rosenkranz S. (2006) Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 71, 331–341 [DOI] [PubMed] [Google Scholar]
- 34. Olsson A. K., Dimberg A., Kreuger J., Claesson-Welsh L. (2006) VEGF receptor signalling—in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 [DOI] [PubMed] [Google Scholar]
- 35. Liu X., Wu J., Liu H., Lai G., Zhao Y. (2012) Disturbed ratio of renal 20-HETE/EETs is involved in androgen-induced hypertension in cytochrome P450 4F2 transgenic mice. Gene 505, 352–359 [DOI] [PubMed] [Google Scholar]
- 36. Cheng J., Garcia V., Ding Y., Wu C. C., Thakar K., Falck J. R., Ramu E., Schwartzman M. L. (2012) Induction of angiotensin-converting enzyme and activation of the renin-angiotensin system contribute to 20-hydroxyeicosatetraenoic acid-mediated endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 32, 1917–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inoue K., Sodhi K., Puri N., Gotlinger K. H., Cao J., Rezzani R., Falck J. R., Abraham N. G., Laniado-Schwartzman M. (2009) Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am. J. Physiol. Renal Physiol. 297, F875–F884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hankin J. A., Murphy R. C. (2000) The metabolism of leukotriene B4 in Lewis lung carcinoma porcine kidney cells. Am. J. Respir. Crit. Care Med. 161, S81–S87 [DOI] [PubMed] [Google Scholar]
- 39. Zalba G., San Jose G., Moreno M. U., Fortuno M. A., Fortuno A., Beaumont F. J., Diez J. (2001) Oxidative stress in arterial hypertension: role of NAD(P)H oxidase. Hypertension 38, 1395–1399 [DOI] [PubMed] [Google Scholar]
- 40. Lassegue B., Griendling K. K. (2010) NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 30, 653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uddin M. R., Muthalif M. M., Karzoun N. A., Benter I. F., Malik K. U. (1998) Cytochrome P-450 metabolites mediate norepinephrine-induced mitogenic signaling. Hypertension 31, 242–247 [DOI] [PubMed] [Google Scholar]
- 42. Guo A. M., Scicli G., Sheng J., Falck J. C., Edwards P. A., Scicli A. G. (2009) 20-HETE can act as a nonhypoxic regulator of HIF-1α in human microvascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 297, H602–H613 [DOI] [PubMed] [Google Scholar]
- 43. Alexanian A., Rufanova V. A., Miller B., Flasch A., Roman R. J., Sorokin A. (2009) Down-regulation of 20-HETE synthesis and signaling inhibits renal adenocarcinoma cell proliferation and tumor growth. Anticancer Res. 29, 3819–3824 [PMC free article] [PubMed] [Google Scholar]
- 44. Alexanian A., Miller B., Roman R. J., Sorokin A. (2012) 20-HETE-producing enzymes are up-regulated in human cancers. Cancer Genomics Proteomics 9, 163–169 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.