Abstract
The newborn heart adapts to postnatal life by shifting from a fetal glycolytic metabolism to a mitochondrial oxidative metabolism. Abcc9, an ATP-binding cassette family member, increases expression concomitant with this metabolic shift. Abcc9 encodes a membrane-associated receptor that partners with a potassium channel to become the major potassium-sensitive ATP channel in the heart. Abcc9 also encodes a smaller protein enriched in the mitochondria. We now deleted exon 5 of Abcc9 to ablate expression of both plasma membrane and mitochondria-associated Abcc9-encoded proteins, and found that the myocardium failed to acquire normal mature metabolism, resulting in neonatal cardiomyopathy. Unlike wild-type neonatal cardiomyocytes, mitochondria from Ex5 cardiomyocytes were unresponsive to the KATP agonist diazoxide, consistent with loss of KATP activity. When exposed to hydrogen peroxide to induce cell stress, Ex5 neonatal cardiomyocytes displayed a rapid collapse of mitochondria membrane potential, distinct from wild-type cardiomyocytes. Ex5 cardiomyocytes had reduced fatty acid oxidation, reduced oxygen consumption and reserve. Morphologically, Ex5 cardiac mitochondria exhibited an immature pattern with reduced cross-sectional area and intermitochondrial contacts. In the absence of Abcc9, the newborn heart fails to transition normally from fetal to mature myocardial metabolism.—Fahrenbach, J. P., Stoller, D., Kim, G., Aggarwal, N., Yerokun, B., Earley, J. U., Hadhazy, M., Shi, N.-Q., Makielski, J. C., McNally, E. M. Abcc9 is required for the transition to oxidative metabolism in the newborn heart.
Keywords: cardiomyopathy, neonatal heart failure, sulfonylurea receptor, mitochondria
The heart undergoes metabolic adaptation in the neonatal period to accommodate to the comparatively higher oxygen environment and use fatty acids as fuel substrate (1, 2). The maturing cardiomyocytes become more reliant on fatty acid oxidation and the mitochondrial genesis of ATP, and this conversion is accompanied by subcellular morphological changes. In the early postnatal mouse heart, there is marked hyperplasia for the first 3 d of life (3). Following hyperplasia, there is cardiomyocyte hypertrophy with increased organization of sarcomeres along with the maturation of the sarcoplasmic reticulum and T tubules to regulate intracellular calcium handling more effectively. Between 3 and 7 d of life, the mitochondria increase in size forming interconnections with the myofibrils and sarcoplasmic reticulum and increasing intermitochondrial contacts. This maturation facilitates ready transfer of phosphate from phosphocreatine to ATP, forming energetic microdomains, which link intracellular energy pathways and cellular architecture (4).
ATP-sensitive potassium (KATP) channels are multimeric complexes that include a regulatory subunit, known as the sulfonylurea receptor (SUR), and an inwardly rectifying potassium (Kir) channel, Kir6.1 or Kir6.2. SUR2 is a member of the ATP-binding cassette (ABC) transporter family, and like other ABC proteins, is a multitransmembrane protein with two intracellular nucleotide-binding domains. SUR2-containing KATP channels are enriched in the sarcolemma, where they sense intracellular ADP content and trigger the opening or closing of the potassium channel. Because of their ability to alter membrane potential in response to the intracellular energy state, KATP channels are critical in cells during periods when there is high energy demand (5, 6).
In cardiomyocytes, the opening of plasma membrane KATP channels during cell stress, such as ischemia, conserves energy by shortening the action potential duration and attenuating contraction (5–7). KATP channels colocalize with metabolic enzymes such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH), lactate dehydrogenase, creatine kinase, and aldolase (8–10). KATP channels are also found at the mitochondrial membrane, although their protein components have been debated (11, 12). The ability of sarcolemmal KATP agonists and antagonists to respectively increase or decrease mitochondrial KATP channel had led some investigators to suggest that both SUR and Kir subunits contribute to the mitochondrial channel (13, 14). However, these agents also have off-target effects, including the capacity to modulate reactive oxygen species, which, in turn, can alter mitochondrial function independent of KATP channels (15, 16).
Abcc9, the gene encoding SUR2, also encodes a smaller 55-kDa protein (SUR2-55; ref. 17). SUR2-55 contains a portion of the first transmembrane domain linked to the carboxyl-terminal nucleotide-binding domain. Antibodies to the carboxyl terminus show the SUR2-55 protein to be highly enriched in mitochondria (18). When coexpressed heterologously with Kir6.2, the SUR2-55 protein is localized in mitochondria, and coexpression results in a functional channel that retains nucleotide sensitivity but loses sensitivity to sulfonylurea drugs (17, 18). A previously generated disruption of the Abcc9 gene, exon 14–18 deletion (Ex14/18 herein), does not express full-length SUR2 protein, but does retain expression of SUR2-55 (18, 19). Ex14/18 mice survive until adulthood (20). We now deleted exon 5 of Abcc9 to abolish both full-length SUR2 and SUR2-55. Homozygous exon 5 deletion (Ex5) mice died in early life with progressive cardiac dysfunction in the first weeks of life. We identified both functional and structural mitochondrial defects in Ex5 cardiomyocytes, consistent with a role for SUR2-KATP channels in the maturation of energetic domains required for normal adult cardiac metabolism.
MATERIALS AND METHODS
Generation of Ex5 mice
The 5-kb 5′ homology arm included Abcc9 exons 3 and 4 and was inserted at SacII and NotI restriction sites (forward: 5′-AGCTATGCACCCCTTAACTAAAGCCACC-3′ and reverse: 5′-GAGAGAACATGTGAGCACACACATCG-3′) of the positive/negative selection PGKneolox2DTA targeting vector. The 3′ homology arm was also 5 kb and included coding exons 5 and 6. It was inserted at SalI and HindIII restriction sites (forward: 5′-GTGGAGATTACAAACGGAATGGAGAACTC-3′ and reverse: 5′-AGTAACCACTACAGAAGCTTCATCGGG-3′). A loxP consensus sequence was placed at an endogenous KasI restriction site located 400 bp downstream of exon 5 to flank exon 5 with loxP sites. An SphI restriction site was added to the 5′ end of this loxP site for subsequent identification of the targeted allele (Supplemental Fig. S1). R1/E mouse embryonic stem cells (ESCs; passage 11; American Type Culture Collection, Manassas, VA, USA) were electroporated with the Abcc9 targeting vector. ESCs were plated onto inactivated mouse ESCs (Millipore, Billerica, MA, USA) and selected with G418 (250 μg/ml). Individual ESC colonies were isolated and expanded for screening by Southern blot analysis. Isolated genomic DNA from 271 expanded ESC clones was digested with ApaI and StuI restriction enzymes, resolved on 0.8% agarose gels, and transferred to a nylon membrane. A 500-bp 32P-labeled probe that binds genomic DNA outside of the 5′ homology arm was used to identify correctly targeted Abcc9 ESC clones. ESCs heterozygous for the Abcc9-targeted allele were injected into C57BL6/J blastocysts and implanted into pseudopregnant females. Male chimeric mice were bred to C57BL6/J females, and the resulting offspring were screened by polymerase chain reaction (PCR) for germline transmission of the Abcc9 targeted allele (forward: 5′-GGAGGATTGGGAAGACAATAGCA-3′ and reverse: 5′-CTAGAGAACCTTAGCCCTGTTGCAGG-3′). Mice heterozygous for the Abcc9-targeted allele were crossed with 129 protamine Cre recombinase transgenic mice (stock no. 4746; Jackson Laboratory, Bar Harbor, ME, USA) to remove the neomycin cassette and exon 5. Resulting male offspring carrying the Abcc9-targeted allele were bred to 129SvJ females and then interbred to generate homozygous mice for study. Littermate controls were used for all experiments. Animals were housed, treated, and handled in accordance with guidelines set forth by the University of Chicago's Institutional Animal Care and Use Committee, the Animal Welfare Act regulations, and the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Northern blot analysis
Northern blots containing 20 mg of e16, P1, P7, P14, and 3-mo-old mouse heart total RNA were obtained from Zyagen Laboratories (San Diego, CA, USA). A 462-bp segment of Abcc9 mRNA including exons 2–5 (forward: CAGGCTGTTGGTAGCTCAAGTGCAC and reverse: GATGTTTCAATGTTATGATAATAGACG) was PCR-amplified and radioactively labeled with a Stratagene Prime-It kit (Agilent Technologies, Santa Clara, CA, USA). Northern blots were incubated with probe overnight at 65°C and imaged with Storm 860 phosphoimager (GE Health Care Life Science, Pittsburgh, PA, USA).
Reverse-transcription PCR (RT-PCR) and quantitative RT-PCR (RT-qPCR)
Heart tissue was harvested from 5- to 8-d-old neonatal cardiomyocytes, and the mRNA was isolated for 20 mg of tissue using an RNeasy kit (Qiagen, Germantown, MD, USA). Tissue was disrupted with a tissue homogenizer. cDNA was prepared using a SuperScriptIII kit (Invitrogen, Carlsbad, CA, USA). Standard PCR was used to amplify a region, including exon 5 of the Abcc9 locus using primers to exons 2 and 6 (forward: CAGGCTGTTGGTAGCTCAAGTGCA and reverse: ATCTCCACAGCCATCAGCAGCCAATT). Amplicons were resolved on a 2% agarose gel and sequenced.
For RT-qPCR, exon spanning primers yielding amplicons of ∼150 bp were used for each mRNA sequence. RT-qPCR was preformed with a Bio-Rad PCR machine using SYBRGreenER, according to the manufacturer's instructions. To attain the Tm for each transcript, the raw fluorescent signals were analyzed and normalized to the EEF1A1 transcript levels (21). Ex5 mRNA transcripts were considered up-regulated if the fold change of Ex5/wild-type (WT) mRNA transcript was >1.5.
Immunofluorescence microscopy and immunoblotting
Hearts were frozen in liquid nitrogen-cooled isopentane, sectioned (10 μm), and fixed for 10 min in ice-cold methanol. After a 1-h block (5 or 10% FBS for SUR2 or perilipin 5 staining, respectively, 0.1% Triton in PBS, sections were incubated overnight at 4°C in primary antibodies diluted 1:500 in blocking buffer. After a PBS wash, slides were incubated for 1 h at room temperature with Gar Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted 1:3000 in blocking buffer. VectaShield with DAPI (Vector Laboratories, Burlingame, CA, USA) was used to view nuclei and mount slides. Fluorescent images were viewed and collected using the Axioskop, AxioCam, and AxioVision microscope, camera, and software systems (Carl Zeiss, Oberkochen, Germany). All images shown were taken with identical exposure conditions. The anti-SUR2 antibody, transmembrane domain 1 (TMD1), was raised in rabbits against the peptide sequence YEEQKKKAADHPNRTPSIWL and purified, as described previously (22, 23). The perilipin 5 antibody was purchased from Novus Biologics (cat. no. NB110-60509; Novus Biologics Littleton, CO, USA).
To obtain the microsomal fractions, WT or Ex5 neonatal hearts were homogenized with a glass Dounce in 2 ml of isolation solution (50 mM Tris-HCl, 1 mM EDTA, 1 mM β-mercaptoethanol, and 1 mM PMSF, supplemented with Halt protease inhibitor; Sigma-Aldrich, St. Louis, MO, USA). The homogenate was centrifuged for 5 min at 500 rpm on an Eppendorf 5317C centrifuge (Eppendorf, Hamburg, Germany) to remove the mitochondrial and nuclear fractions. The supernatant was then centrifuged for 10 min at 50,000 rpm on a Beckman L8-55MR ultracentrifuge (Beckman Coulter, Fullerton, CA, USA). The microsomal pellet was then resuspended in 230 μl of the isolation solution. Protein samples were separated on a 8% SDS-PAGE gel and transferred to a PVDF membrane. Membranes were blocked with 6% milk and probed with primary antibody overnight at 4°C. After washing 3 times with TBST, membranes were probed with secondary antibody and imaged with chemiluminescence. The mitochondrial fractions were prepared from ∼10 neonatal hearts of each genotype, and the BNJ-39 antibody was used for blotting (18). The primary antibodies for mitofusion 2, succinate dehydrogenase A (SDHA), peroxisome proliferator-activated receptor γ coactivator 1-α (PCG-1α), hypoxia-inducible factor 1α (HIF-1α), renal outer medullary potassium 1 (ROMK1), dynamin-related protein 1 (DRP1), and Kir6.2 were from Abcam (ab50843, ab66484, and ab54481; Abcam, Cambridge, MA, USA), Novus Biologicals (NB100-134), Alomone Laboratories (APC-001; Alomone Laboratories, Jerusalem, Israel), and Santa Cruz Biotechnology (sc-32898 and sc-11228; Santa Cruz Biotechnology, Dallas, TX, USA).
Histology and electron microscopy
For study of the intact thorax, skin was removed from isolated tissue, followed by fixation in 10% buffered formalin and storage at 4°C. Tissue was dehydrated, paraffin embedded, serial sectioned at a thickness of 7 μM, and then stained with hematoxylin and eosin. Photomicrographs were taken using the Axioskop, AxioCam, and AxioVision microscope, camera, and software systems (Carl Zeiss). Cardiac mitochondrial morphology was visualized with electron microscopy. Hearts were harvested from 8-d-old WT, Ex14/18, and Ex5 mice and immediately fixed in PBS with 2.5% glutaraldehyde. The tissue was postfixed in 1% OsO4 for 1 h at 4°C, rinsed, dehydrated in a graded ethanol series, and embedded in EMbed-812 (Electron Microscopy Services, Hatfield, PA, USA). Tissue was sectioned and stained with 1% uranyl acetate, followed by lead citrate. Tissue sections were then photographed with a Tecnai electron microscope (FEI, Hillsboro, OR, USA).
Mouse neonatal ventricular myocyte isolation
Neonatal mice (3–5 d old) were sacrificed, and hearts were immediately placed in ice-cold PBS and trimmed of atria. Hearts were minced and incubated in a Krebs-Herch solution (113 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM Na2HPO4, 1.2 mM MgSO4, 12 mM NaHCO3, 10 mM KHCO3, 10 mM HEPES, 30 mM taurine, and 0.013 mM CaCl2) containing Liberase (0.2 mg/ml; Roche Applied Science, Indianapolis, IN, USA) for 20 min at 37°C. Single cells were obtained by gently pipetting the tissue, then transferring cells to DMEM supplemented with 5% FBS. To decrease fibroblast contamination, cells were preplated for 1 h; nonadherent cells were then collected and plated on glass coverslips coated with laminin (Sigma-Aldrich).
Cellular electrophysiology
Whole-cell patch recording was performed at room temperature (19–22°C) using an Axopath 200B amplifier and pClamp 9.0 software (Axon Instruments, Union City, CA, USA). Borosilicate glass patch pipettes (World Precision Instruments, Sarasota, FL, USA) were pulled with a resistance of 2–4 MΩ when filled with recording solutions. The bath solution contained 4 mM KCl, 140 mM NaCl, 10 mM HEPES, 1 mM CaCl2, 10 mM glucose, 1.2 mM MgCl2, and 50 mM nifedipine, at pH 7.4, with KOH. Nifedipine was added to block the L-type Ca2+ current. The pipette solution contained 140 mM KCl, 20 mM HEPES, 5 mM EGTA, 2 mM MgCl2, and 1 mM UDP, at pH 7.25, with KOH. The whole-cell current was generated by clamp pulses from a holding potential of −80 mV to voltages ranging from −120 to 20 mV in 20-mV steps for 200 ms.
Mitochondrial membrane potential measurements
Isolated neonatal cardiomyocytes plated on glass coverslips were incubated for 15 min at room temperature with the 50 nM tetramethylrhodamine ethyl ester perchlorate (TMRE; Invitrogen) in Tyrode solution (135 mM NaCl, 5.4 mM KCl, 2 mM CaCl2, 1.2 mM MgCl2, 5 mM HEPES, and 10 mM glucose, pH 7.35 with NaOH) then washed twice with Tyrode solution. Images were obtained every 5 s with a Zeiss Axio Observer Z1 inverted microscope using an LD Plan-Neofluar ×40 objective at 37°C and analyzed with ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA). H2O2 (150 mM) or diazoxide (100 mM; Sigma-Aldrich) was applied with constant perfusion in respective experiments.
Oxygen consumption
Isolated neonatal cardiomyocytes from an individual heart (0.75×106 cells in 0.5 ml) were loaded into a Mitocell MT200 chamber, and the oxygen concentration was measured with a 782 oxygen meter (Strathkelvin Instruments, North Lanarkshire, UK) before and after addition of 10 μM pinacidil (Sigma-Aldrich). Neonatal cells were treated with oligomycin and FCCP to measure bioenergetic reserve as described previously (24).
Fatty acid oxidation
Fatty acid oxidation rate was measured as described previously (25). Briefly, 0.15 × 106 P4-5 WT and Ex5 cardiomyocytes were plated on laminin-coated XF24 cell culture plates (Seahorse Bioscience, North Billerica, MA, USA) and cultured for 48 h in medium. To prepare palmitate-BSA for fatty acid oxidation studies, sodium palmitate (Sigma-Aldrich) was dissolved in 65°C 150 mM NaCl. Fatty acid-free BSA from fraction V (Millipore) was separately dissolved in 37°C 1× PBS under constant agitation. The palmitate-BSA solution was attained by slowly adding palmitate solution to the BSA solution to a concentration of 1 mM. Fatty acid-free BSA and palmitate-BSA solutions were portioned into aliquots in glass vials and stored at −20°C. The oxygen consumption rate was measured with a Seahorse XF24 analyzer, according to the manufacturer's protocol (Seahorse Bioscience) before and after 200 μM palmitate-BSA application.
Free fatty acid (FFA) uptake
Isolated neonatal cardiomyocytes were plated on glass coverslips coated with a 0.02% gelatin and 0.001% fibronectin solution. Cells were perfused with Tyrode solution for 5 min prior to application of 1-μm fluorescently labeled palmitate (BODIPY FL C16; cat. no. D-3821; Invitrogen). Images were obtained every 5 s with a Zeiss Axio Observer Z1 inverted microscope using a LD Plan-Neofluar ×63 objective using a GFP filter cube (cat. no. 41001; Chroma, Bellows Falls, VT, USA). To determine the FFA uptake rate, the mean fluorescent signal was calculated for each time point using ImageJ software, and the uptake rate was determined as the slope of the best fit line for all the time points.
TUNEL assay
Ventricular sections from 7-d-old WT and Ex5 hearts were processed with an in situ cell death detection kit (cat. no. 11684795910; Roche Applied Science), according the manufacturer's instructions. To image all nuclei, sections were mounted in Vectashield containing DAPI (cat. no. H-1200; Vector Laboratories). Slides were imaged on a Zeiss Axio Observer Z1 inverted microscope.
Reactive oxygen species assay
WT and Ex5-isolated neonatal cardiomyocytes cultured on glass coverslips were incubated with 10 μM dihydroethidium (37291; Sigma-Aldrich) for 10 min at 37°C and imaged with a Zeiss Axio Observer Z1 inverted microscope.
Echocardiography
Percentage left ventricular fraction shortening in adult and neonatal mice was quantified with transthoracic echocardiography using a Vevo 770 ultrasound imager (VisualSonics, Toronto, ON, Canada). Mice were placed on a 37°C warming pad and lightly sedated with 1% isoflurane. Conscious imaging was performed for neonatal mice. Two-dimensional parasternal short-axis M-mode images were acquired and analyzed offline. Percentage left ventricular fractional shortening was calculated as [(diastolic left ventricular internal diameter − systolic left ventricular internal diameter)/diastolic left ventricular internal diameter] × 100 (ref. 26).
Statistical analysis
Data are reported as means ± sd. Statistical analysis was conducted using GraphPad Prism 4.0 (GraphPad, San Diego, CA, USA). Student t tests (2 experimental groups) or 1-way ANOVA (>2 experimental groups, 1 variable) were used where appropriate. Significance was set at a level of P ≤ 0.05.
RESULTS
Deletion of Abcc9 exon 5 ablates KATP channel components and activity
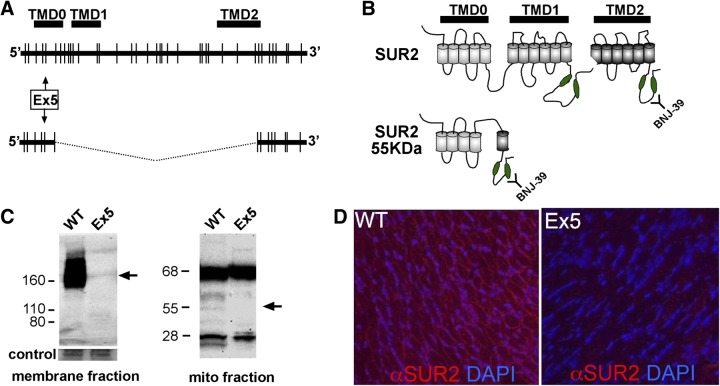
To ablate both SUR2 and SUR2-55, exon 5 was deleted from the Abcc9 gene by homologous recombination. The large form of SUR2 is encoded by 41 exons and encodes 3 multi-TMDs (Fig. 1). Exon 5 encodes a portion of TMD0 and is normally included in both the large form SUR2, as well as SUR2-55. The long form of SUR2 migrates as a broad band at 130–170 kDa due to alternative splicing and variable glycosylation, and it is absent from homozygous Ex5 mice (Fig. 1C). SUR2-55, which is normally enriched in mitochondrial preparations from cardiac tissue, was absent from neonatal Ex5 mitochondrial fractions (Fig. 1C). RT-PCR verified that exon 5 was removed from the Abcc9 transcript (Supplemental Fig. S1). Deletion of exon 5 induced a frameshift and disrupted coding of SUR2 and SUR2-55. Immunofluorescence microscopy of Ex5 hearts using an anti-SUR2 antibody demonstrated a loss of SUR2 and SUR2-55 immunoreactivity (Fig. 1D). The SUR2 partner, Kir6.2, was unaltered in Ex5 hearts, as was the ROMK channel, a described component of a mitochondrial KATP channel (Supplemental Fig. S1 and refs. 27, 28).
Figure 1.
Deletion of Abcc9 exon 5 disrupts SUR2 and SUR2-55. A) Splice forms from Abcc9. The Abcc9 gene spans 40 exons and encodes a protein with 3 TMDs. A smaller mRNA is produced that links exon 6 to exon 31. Deleting exon 5 targets both of these gene products. B) The long form of SUR2 includes 3 TMDs and 2 large intracellular loops that contain nucleotide-binding domains (green ovals). SUR2-55 includes most of TMD0 linked to TMD2 and the carboxyl-terminal nucleotide binding domain. The BNJ-39 antibody recognizes the carboxyl terminus. C) Immunoblotting of membrane fractions prepared from neonatal hearts. Full-length SUR2 migrates as a broad band (130–170 kDa, arrow) and is absent from homozygous Ex5 hearts. Mitochondrial fractions were prepared from neonatal hearts and show the absence of the SUR2-55 protein (arrow). The 68- and 28-kDa reactive proteins were still produced (18). D) Immunofluorescence microscopy shows a loss of immunoreactivity to SUR2 in neonatal Ex5 hearts (red).
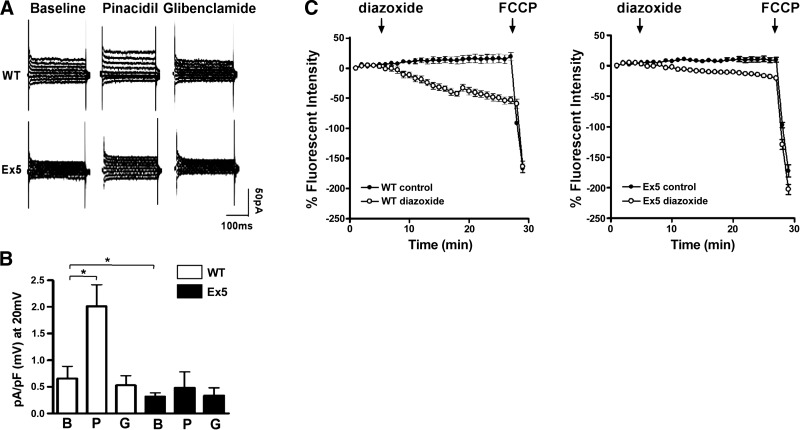
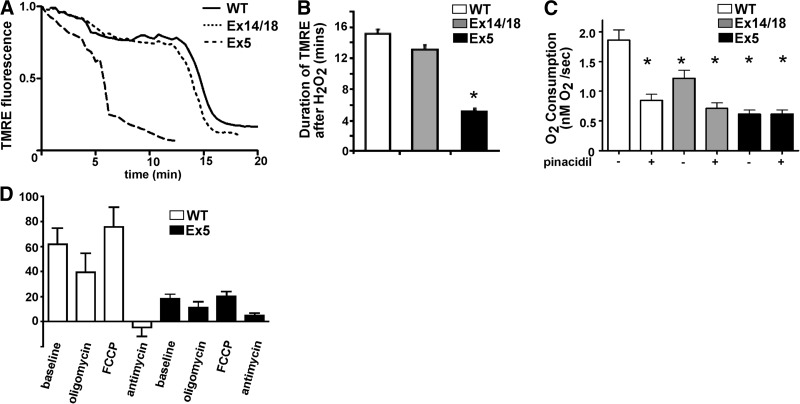
To demonstrate that ablation of Abcc9 induced a loss of functional KATP channels in neonatal cardiomyocytes, cells were isolated from newborn hearts, and channel activity was measured. Whole-cell KATP currents were compared in WT and Ex5 neonatal cardiomyocytes. At baseline, Ex5 cardiomyocytes had approximately half the basal KATP current compared to WT cardiomyocytes (0.31±0.03 vs. 0.66±0.1 pA/pF, P<0.05; Fig. 2A), indicating that Abcc9-encoded proteins contribute significantly to basal KATP activity in neonatal myocytes. The KATP channel opener pinacidil produced a robust current increase in WT but not Ex5 cardiomyocytes, consistent with a reduction in KATP function in Ex5 cardiomyocytes (Fig. 2B). To determine whether Ex5 cardiomyocytes had functional mitochondrial KATP channels, the mitochondrial membrane potential in Ex5 and WT neonatal cardiomyocytes was visualized with the mitochondria potential-sensitive dye, TMRE. Application of diazoxide, a mitochondrial KATP agonist, resulted in the expected decrease of mitochondrial potential in WT cardiomyocytes, but diazoxide had little effect in Ex5 cardiomyocytes (39±5 vs. 5±4%, P<0.05), consistent with reduced or absent mitochondrial KATP function in Ex5 cardiomyocytes (Fig. 2C).
Figure 2.
Ex5 cardiomyocytes have attenuated KATP channel activity. A, B) KATP current was measured in WT and Ex5 neonatal cardiomyocytes at baseline (B) and during the application of the KATP channel opener pinacidil (P) and the sulfonylurea blocker glibenclamide (G). A) Raw current traces from Ex5 cardiomyocytes showed little response to either pinacidil or glybenclamide compared to WT cardiomyocytes. B) WT cardiomyocytes displayed a pinacidil-responsive KATP current compared to baseline [0.69±0.19 (n=13) vs. 1.69±0.33 (n=11)]. Ex5 cardiomyocytes lacked this current [0.49±0.15 (n=11) vs. 0.57±0.15 (n=10); P>0.05]. A glibenclamide-sensitive KATP current was only seen in WT cardiomyocytes [0.69±0.19 (n=13) vs. 0.51±0.14 (n=10)]. *P < 0.05, 1-way ANOVA. C) To test mitochondrial KATP channel function, WT and Ex5 neonatal cardiomyocytes were loaded with the mitochondrial voltage-sensitive dye TMRE, and the mitochondrial response to a KATP opener, diazoxide, was measured. TMRE fluorescence declined during diazoxide application to WT cells, suggesting a robust mitochondrial KATP channel response, while Ex5 mitochondria exhibited a significantly attenuated response consistent with reduction of mitochondrial KATP activity.
Loss of Abcc9 results in neonatal lethality from cardiomyopathy
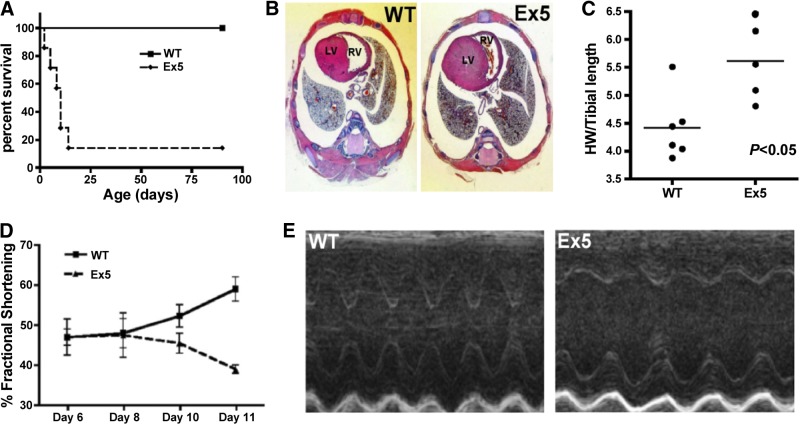
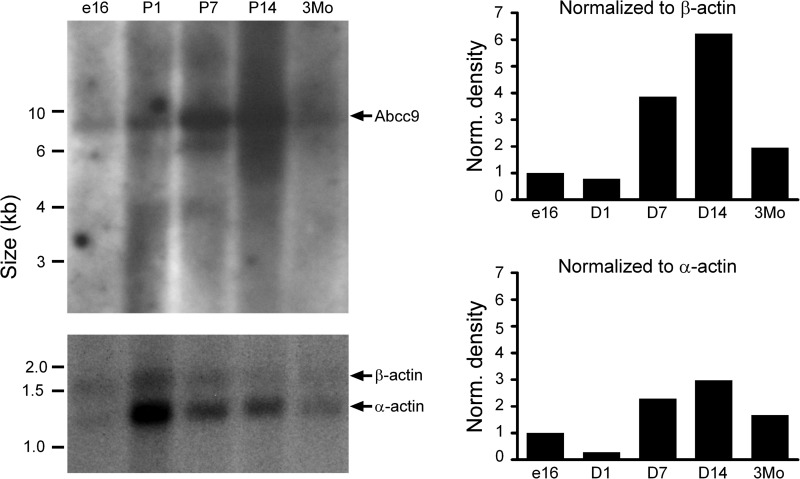
Ex5 mice died within 14 d of birth (Fig. 3A). Ex5 mice had enlarged hearts compared to WT littermate controls, and this was corroborated by an increased heart weight to tibia length ratio (5.6±0.3 vs. 4.4±0.2 mg/mm, P<0.05; Fig. 3B, C). Echocardiography was performed on neonatal mice on d 6–11 after birth. During this window, Ex5 mice exhibited a progressive decrease in ejection fraction compared to littermate controls (Fig. 3D). Representative M-mode echocardiograms from d 11 WT and Ex5 neonatal mice demonstrate decreased cardiac fraction shortening (59.3±2.8 vs. 69.7±1.0%, P<0.05; Fig. 3E). No changes in nonfasting serum glucose levels were detected between d 5–7 WT and Ex5 littermates (171.6±25.0 vs. 168±11.7 mg/dl, P>0.05). TUNEL assays from postnatal d 7 Ex5 and WT ventricles did not show an increase in apoptosis. Because of the early lethality and cardiomyopathy in Abcc9 hearts, we revisited the expression of Abcc9 in normal developing hearts, finding that Abcc9 mRNA expression increased dramatically between d 7 and 14 after birth. When normalized to nonsarcomeric β-actin, the peak of Abbc9 expression was between d 7 and 14. Sarcomeric α-actin peaks earlier, at d 1, so that marked sarcomerogenesis precedes the increase in Abcc9 expression (Fig. 4).
Figure 3.
Early neonatal lethality and cardiac dysfunction in Ex5 mice. A) Kaplan-Meier survival curve shows that Ex5 mice die within 14 d of birth, indicating that SUR2 and SUR2-55 are necessary for survival. B) Histological analysis shows that Ex5 mice had enlarged hearts compared to WT littermate controls (LV, left ventricle; RV, right ventricle). C) Ex5 mice have an increased heart weight to tibia length ratio compared to WT (5.6±0.3 vs. 4.4±0.2 mg/mm, P<0.05). D) Echocardiography was performed on neonatal mice from postnatal d 6 to 11. Ex5 mice exhibited a progressive decrease in ejection fraction compared to littermate controls during this time period. E) Representative M-mode echocardiograms from d 11 WT and Ex5 neonatal mice demonstrate a decline in cardiac fractional shortening (39.3±2.8 vs. 59.7±1.0%, P<0.05).
Figure 4.
Abcc9 increases its expression in the newborn heart. Northern blot analysis shows the increase in Abbc9 expression in postnatal d 7 and 14 in the mouse heart.
Rapid loss of mitochondrial membrane potential and reduced oxygen consumption in Ex5 neonatal cardiomyocytes
To determine functional consequences to the mitochondria from disrupting Abcc9, the mitochondrial response to cell stress and O2 consumption were examined. We also included in this analysis neonatal cardiomyocytes from Ex14/18 mice, which lack full-length SUR2 but retain the SUR2-55 (17). The mitochondrial resistance to cell stress was examined by applying 150 μM H2O2 to TMRE-loaded WT, Ex14/18, and Ex5 neonatal cardiomyocytes (Fig. 5A). Both WT and Ex14/18 cardiomyocytes displayed a similar duration of H2O2 resistance, suggesting that the full-length SUR2 is dispensable for cardioprotection in this setting (15.1±0.6 vs. 13.1±0.6 s, P>0.05; Fig. 5B). However, Ex5 mitochondrial membrane potential rapidly collapsed in comparison to WT and Ex14/18 mitochondria (5.2±0.3 s, P<0.05; Fig. 5B).
Figure 5.
Loss of KATP channels results in increased susceptibility to stress and reduced O2 consumption. A, B) Mitochondrial resistance to cell stress was tested by application of 150 μM H2O2 to TMRE-loaded cardiomyocytes. Ex14/18 mice lack full-length SUR2 but retain SUR2-55; Ex14/18 mice survive into adulthood (20). Duration of TMRE fluorescence after H2O2 exposure was similar between WT and Ex14/18 neonatal cardiomyocytes (15.1±0.6 vs. 13.1±0.6 s, P>0.05). However, Ex5 mitochondrial membrane potential rapidly collapsed in comparison to WT and Ex14/18 mitochondria (5.2±0.3 s). *P < 0.05. C) O2 consumption was measured from isolated neonatal cardiomyocytes using a Clark electrode. Ex5 mitochondria exhibited a decreased O2 consumption rate and lacked pinacidil responsiveness compared to WT and Ex14/18 mitochondria. *P < 0.05. D) Mitochondrial reserve was measured by uncoupling mitochondria with FCCP. Ex5 cardiomyocytes showed no capacity to increase O2 consumption after FCCP treatment.
A Clark electrode was used to measure the O2 consumption rate before and after application of the potassium channel opener pinacidil in WT, Ex14/18, and Ex5 cardiomyocytes. At baseline, WT cardiomyocytes had the greatest O2 consumption rate, while Ex5 cardiomyocytes had the lowest O2 consumption rate (Fig. 5C). WT and Ex14/18 cardiomyocytes were sensitive to pinacidil and displayed a reduction in O2 consumption (WT: 1.86±0.41 vs. 0.85±0.2, n=6, P<0.05; Ex14/18: 1.21±0.28 vs. 0.71±0.19, n=4, P<0.05), while Ex5 cardiomyocytes showed no responsiveness to O2 consumption (Ex5: 0.49±0.52 vs. 0.49±0.51, n=4, P>0.05) (Fig. 5C). We treated Ex5 cardiomyocytes with FCCP, a mitochondrial uncoupling agent, to measure bioenergetic reserve (24). In Ex5 cardiomyocytes, mitochondrial uncoupling with FCCP did not increase O2 consumption, consistent with the failure to respond to stress (Fig. 5D). The decreased resistance of Ex5 cardiomyocytes to cell stress and reduced basal O2 consumption reflect loss of SUR2-55 and its role in mitochondrial KATP function.
Smaller mitochondria and reduced fatty acid oxidation in Ex5 neonatal cardiomyocytes
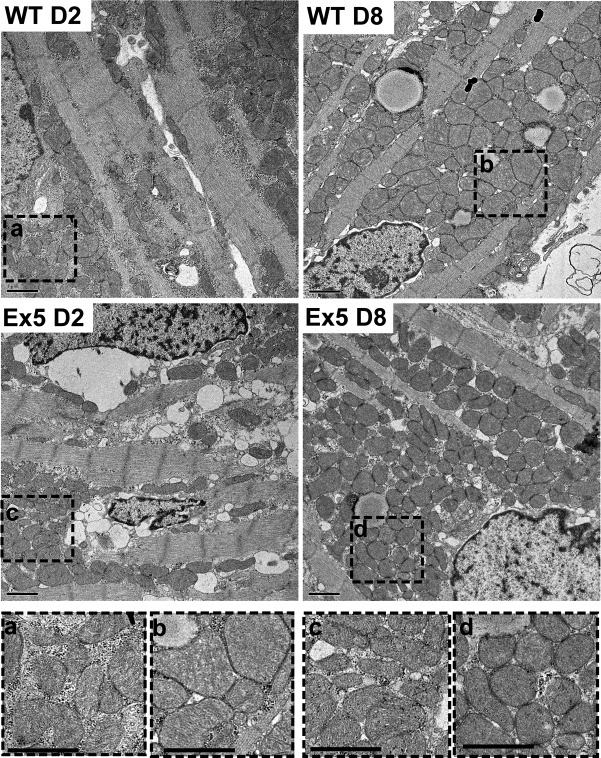
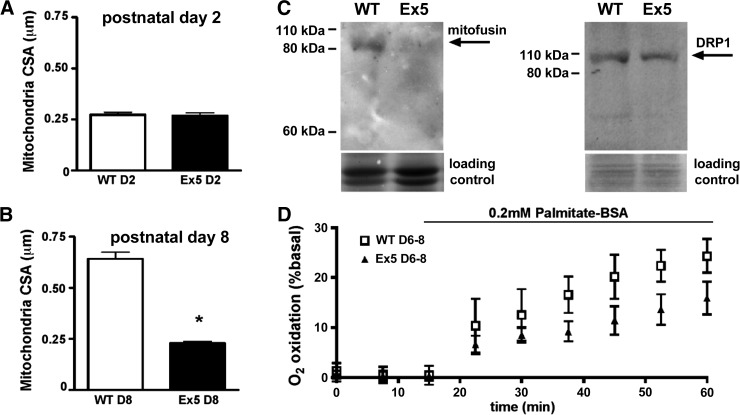
To explore whether the defects in mitochondria function correlated with mitochondrial morphological changes, WT and Ex5 neonatal cardiomyocytes were examined at postnatal d 2 and 8. During this window, cardiomyocytes gain sarcomeres with a corresponding increase in the mitochondrial network (4). Electron microscopic images showed intact cristae, and at d 2, mitochondrial cross-sectional area was similar between Ex5 and WT (0.27±0.01 vs. 0.27±0.01 μm2, P>0.05; Figs. 6 and 7A). By d 8, mitochondria from WT cardiomyocytes had increased cross-sectional area, consistent with the growing energetic demands of the d 8 heart. In contrast, Ex5 mitochondria did not gain cross-sectional area, and the cross-sectional area was similar between d 2 and 8 (0.64±0.03 μm2 vs. 0.23±0.01 μm2, P<0.05; Figs. 6 and 7B), consistent with a failure to fuse and form larger mitochondria. The mitochondrial network was less well formed in Ex5 cardiomyocytes compared to WT. The mean number of intermitochondrial contacts in WT was 2.95 contacts/mitochondrion vs. 1.70 in Ex5 cardiomyocytes (P<0.0001). The failure of mitochondrial maturation seen in Ex5 cardiomyocytes was not evident in Ex14/18 cardiomyocytes, which appeared similar to WT controls (Supplemental Fig. S2). The mitochondrial network was imaged using TMRE and quantified. Mitochondria were longer and less branched in WT compared to Ex5 cardiomyocytes, consistent with a well-formed network in WT cardiomyocytes but not in the absence of Abcc9 (Supplemental Fig. S3). Mitochondrial DNA content was not different between WT and Ex5 cardiomyocytes, and the mitochondrial protein SDHA was also unchanged, consistent with a failure to grow the mitochondrial network (Supplemental Fig. S3).
Figure 6.
Ex5 mice have altered mitochondrial morphology. Electron microscopy of postnatal d 2 and 8 WT (top panels) and Ex5 hearts (middle panels). After postnatal d 2, the mitochondria in cardiomyocytes increase in size. In contrast, mitochondria in Ex5 retain the immature pattern with mitochondria with reduced cross sectional area. Bottom panels: magnified regions of WT d 2 (a), WT d 8 (b) Ex5 d 2 (c), and Ex5 d 8 (d) mitochondria.
Figure 7.
Failure to develop the mitochondrial network and decreased fatty acid oxidation by Abcc9 deleted hearts. A) Postnatal d 2 mitochondrial cross-sectional area was 0.27 ± 0.01 μm2 vs. 0.27 ± 0.01 μm2 (P>0.05). B) Postnatal d 8 cross-sectional area was 10.64 ± 0.03 μm2 vs. 0.23 ± 0.01 μm2. These findings are consistent with reduction of mitochondrial fusion. *P < 0.05. C) Mitofusin 2, a protein that mediates mitochondrial fusion, was reduced, while DRP1, a protein that mediates mitochondrial fission, was unchanged. D) Reduced fatty acid oxidation in Ex5 cardiomyocytes compared to WT. Fatty acid oxidation was measured on a Seahorse XF24 analyzer by adding palmitate.
We examined the expression of the mitochondrial fusion protein mitofusin 2, which regulates formation of larger mitochondrial networks (29). Mitofusin 2 was down-regulated in Ex5 cardiomyocytes (Fig. 7C). In contrast, DRP1, a regulator of mitochondrial fission, was unchanged between Ex5 and WT (Fig. 7C). RT-qPCR for mitofusin 2 was unchanged between Ex5 and WT cardiomyocytes, indicating that the reduction in mitofusin 2 was a post-transcriptional event (Supplemental Fig. S3). The reduced size and increased area between mitochondria are similar to what is found in postnatal d 2 cardiomyocytes and is consistent with failure to mature by increasing mitochondrial size and number in Ex5 cardiomyocytes.
Shortly after birth, the heart begins to use fatty acids as its primary fuel, and there is an increase in β-oxidation by mitochondria. The rate of fatty acid oxidation was measured by using palmitate as a metabolic substrate in neonatal cardiac myocytes. Ex5 cardiomyocytes displayed a reduced normalized increase in fatty acid oxidation rate after 45 min of 200 mM palmitate application (24.4±2.1 vs. 15.9±2.0%; Fig. 7D). This decrease in fatty acid oxidation was not due to decreased import, since palmitate uptake did not differ between Ex5 and WT cardiomyocytes. However, lipid droplet accumulation was detectable in Ex5 cardiomyocytes, seen as perilipin 5 staining throughout the cardiomyocytes (Supplemental Fig. S4 and ref. 30). Thus, metabolically, the loss of Abcc9 associated with morphological and functional defects in adapting to the mature myocardial metabolism.
Metabolic consequences of Abcc9 deletion in neonatal hearts
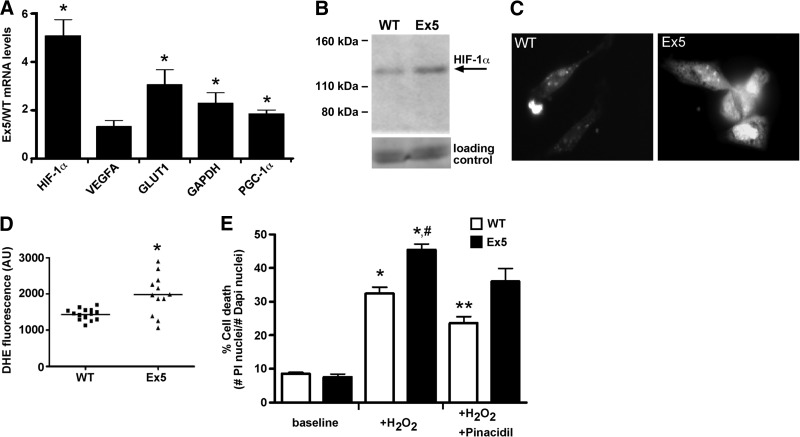
We examined mRNA transcript levels of stress and mitochondria pathway components using RT-qPCR. HIF-1α mRNA and its downstream target glucose transporter type 1 (GLUT1) were significantly up-regulated in Ex5 hearts, while another HIF-1α target, vascular endothelial growth factor A (VEGFA), was increased but not to significance (Fig. 8A). Since the Ex5 mitochondria were smaller with fewer contacts, the mitochondrial fusion protein mRNA, PGC-1α, was also examined. Ex5 hearts exhibited increased PGC-1α mRNA levels (Fig. 8A), but this was not accompanied by an increase in PGC-1α protein (Supplemental Fig. S4). In contrast, the increased HIF-1α mRNA levels correlated with increased HIF1-α protein levels in Ex5 hearts (Fig. 8B). Isolated Ex5 cardiomyocytes had increased dihydroethidium staining, consistent with increased reactive oxygen species compared to WT (Fig. 8C, D). Interestingly, Ex5 neonatal cardiomyocytes were more susceptible to cell death in response to H2O2-induced stress compared to WT (Fig. 8E). The KATP channel opener pinacidil was cardioprotective in WT neonatal cardiomyocytes but less so for Ex5, consistent with reduced response to potassium channel openers mediated by Abcc9 (Fig. 8E). Together, these data support the failure to transition normally to oxidative metabolism.
Figure 8.
Metabolic defects consistent with an inability to transition to oxidative metabolism. A) mRNA transcripts were probed with RT-qPCR. HIF-1α, GLUT1, GAPDH, and PGC-1α were up-regulated in Ex5 hearts. *P < 0.05. B) Total HIF-1α protein levels were elevated in Ex5 hearts. Together, these data support that Ex5 hearts are metabolically hypoxic. C, D) DHE staining (C) and analysis (D) showed that Ex5 cardiomyocytes had elevated levels of ROS. *P < 0.05. E) H2O2 induced stress in both WT and Ex5 cardiomyocytes with increased propidium iodide (PI)-positive cells. However, Ex5 cardiomyocytes are more susceptible to H2O2. Opening of KATP channels with pinacidil was cardioprotective for WT cardiomyocytes, but was less so for Ex5 cardiomyocytes (P=0.057). *P < 0.05 vs. WT baseline; **P < 0.05 vs. WT + H2O2; #P < 0.05 vs. Ex5 baseline.
DISCUSSION
Abcc9 regulates the formation of energetic microdomains in the newborn heart
As the neonatal heart adapts to the extrauterine environment and takes on the increased workload of the systemic circulation and neonatal growth, there is a transition from carbohydrate to fatty acid utilization. The energetic advantage from β-oxidation is thought to provide the necessary ATP to sustain the growing myocardium and organism (1, 31, 32). During early postnatal life, neonatal cardiac myocytes hypertrophy by increasing sarcomere content alongside mitochondrial content (33). Mitochondria are often concentrated in between rows of sarcomeres, and this pattern of mitochondrial distribution, where mitochondria interweave between sarcomeres, forms during the first few days of life in the mammalian heart (4). The positioning of mitochondria to regions of high energy use, namely actomyosin cross-bridge formation, is thought to create energetic microdomains that supply ATP near the sites of utilization. We now found that disruption of the major KATP channel produces neonatal lethality from cardiomyopathy and heart failure during this developmental metabolic transition.
Mitochondrial content was comparable between WT and Abcc9-deleted hearts immediately after birth. However, over the period between postnatal d 2 and 8, Abcc9-deleted hearts failed to develop adequate mitochondrial networks to support cardiac growth. Consistent with these morphological observations, oxygen consumption and fatty acid oxidation were reduced. The structural and functional mitochondrial defects, especially reduced oxygen consumption, suggest that Ex5 cardiomyocytes are in a hypoxic state from their inability to transition to fatty acid oxidation. The observed increase in HIF1α is also consistent with a hypoxic state. The down-regulation of mitofusin 2 suggests that Ex5 mitochondria have a fusion defect that may contribute to the lower cellular respiration (34, 35). It has been previously shown that forced overexpression of PGC-1α is sufficient to promote cardiomyocyte mitochondrial biogenesis (36). The absence of compensation by PGC-1α in Abcc9-deleted hearts may suggest that these mitochondrial defects are upstream from PGC-1α.
Abcc9 and mitochondrial function
Abcc9 produces 2 gene products, a long form that encodes SUR2 and a smaller SUR2-55 form that enriches in mitochondria (18). Both proteins are capable of associating with potassium channels to form an ATP-responsive potassium channel, but the long and short SUR2 proteins differ in their capacity to bind sulfonylurea agents. The small SUR2-55 protein localizes to mitochondria and forms sulfonylurea-insensitive KATP currents when coexpressed with Kir6.2 (17). We observed a reduced KATP current and mitochondrial response to diazoxide in Ex5 hearts consistent with the loss of both mitochondria- and sarcolemma-associated KATP channel activity. There is a small residual KATP current in Ex5 cardiomyocytes that may derive from SUR1-containing KATP channels (37, 38) or components like ROMK (28). We previously generated mice with deleted Abcc9 exons 14–18, and these mice survive to adulthood with a phenotype of vascular spasm and hypertension (20). The phenotype in Abcc9 Ex14/18 mice is very similar to mice with deleted KCNJ8, the gene encoding Kir6.1 (39). The phenotype of Abcc9 Ex14/18 mice and KCNJ8 mice, and notably their survival beyond the neonatal window, ascribes a distinct role to the KATP channel containing a full-length, sulfonylurea-responsive regulatory subunit. Interestingly, the Ex14/18 mice display enhanced cardioprotection from ischemia and ischemic preconditioning (17, 40). In Ex14/18 hearts, the repetitive transient ischemic episodes produced from vascular spasm allow the heart to adapt to ischemia, and this adaptation arises independent of full-length SUR2 protein and may employ SUR2-55. It is plausible that other ABC transporters compete for binding to Kir6.2 to aid in both its normal intracellular trafficking and membrane-associated function. The small SUR2-55 protein binds to Kir6.2, but the association of SUR2-55 with Kir6.2 does not exclude the possibility that other pore components may contribute to this complex. The striking phenotypic difference between Ex14/18 mice, which live to adulthood, and Ex5 mice, which die in the newborn period, indicates that SUR2-55 has a function independent of the full-length SUR2. Our results support the hypothesis that Abcc9-encoded SUR2-55 plays a vital role in mitochondrial function, consistent with its role as a functional mitochondrial KATP channel.
Cardiomyopathy and heart failure are an increasing medical problem. The causes of heart failure include ischemic and nonischemic causes, and many nonischemic causes have a genetic component. Mutations in ABCC9 cause an adult onset dilated cardiomyopathy that is prone to ventricular arrhythmias (41), and this gene is part of multigene panel genetic testing for inherited cardiomyopathy. Recently, dominant mutations in ABCC9 were also described in Cantu syndrome, a developmental disorder that also includes cardiac hypertrophy (42–44). The position of mutations along the ABCC9 gene suggests that cardiac hypertrophy- and cardiomyopathy-associated mutations may affect both full-length SUR2 and SUR2-55. Therefore, both plasma membrane and mitochondrial KATP channels may be important for substrate utilization, and the balance between these two forms may also be critical for managing energy production in the cardiomyocyte.
Supplementary Material
Acknowledgments
This study was supported by U.S. National Institutes of Health/National Heart, Lung, and Blood Institute grants R01-HL078926 (E.M.M), R01-HL57414 (J.C.M.), R21-HL93626 (N.-Q.S.), K08-HL098565, (G.K.), F32-HL097587 (J.P.F.), and U54 AR052646. N.-Q.S. is also supported by American Heart Association National Center grants 11GRNT7600070 and 0630268N.
The authors thank Stephen Archer and Glenn Marsboom for helpful conversations.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ABC
- ATP-binding cassette
- DRP1
- dynamin-related protein 1
- ESC
- embryonic stem cell
- Ex5
- exon 5 deletion
- Ex14-18
- exon 14–18 deletion
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GLUT1
- glucose transporter type 1
- HIF-1α
- hypoxia-inducible factor 1α
- KATP
- ATP-sensitive potassium
- Kir
- inwardly rectifying potassium
- PCR
- polymerase chain reaction
- PGC-1α
- peroxisome proliferator-activated receptor γ coactivator 1-α
- ROMK
- renal outer medullary potassium
- RT-PCR
- reverse transcription polymerase chain reaction
- RT-qPCR
- quantitative reverse transcription polymerase chain reaction
- SDHA
- succinate dehydrogenase A
- SUR
- sulfonylurea receptor
- SUR2-55
- 55-kDa sulfonylurea receptor 2
- TMD
- transmembrane domain
- TMRE
- tetramethylrhodamine ethyl ester perchlorate
- VEGFA
- vascular endothelial growth factor A
- WT
- wild type
REFERENCES
- 1. Finck B. N., Lehman J. J., Barger P. M., Kelly D. P. (2002) Regulatory networks controlling mitochondrial energy production in the developing, hypertrophied, and diabetic heart. Cold Spring Harb. Symp. Quant. Biol. 67, 371–382 [DOI] [PubMed] [Google Scholar]
- 2. Warshaw J. B. (1972) Cellular energy metabolism during fetal development. IV. Fatty acid activation, acyl transfer and fatty acid oxidation during development of the chick and rat. Dev. Biol. 28, 537–544 [DOI] [PubMed] [Google Scholar]
- 3. Leu M., Ehler E., Perriard J. C. (2001) Characterisation of postnatal growth of the murine heart. Anat. Embryol. (Berl.) 204, 217–224 [DOI] [PubMed] [Google Scholar]
- 4. Piquereau J., Novotova M., Fortin D., Garnier A., Ventura-Clapier R., Veksler V., Joubert F. (2010) Postnatal development of mouse heart: formation of energetic microdomains. J. Physiol. 588, 2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flagg T. P., Enkvetchakul D., Koster J. C., Nichols C. G. (2010) Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol. Rev. 90, 799–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang H., Flagg T. P., Nichols C. G. (2010) Cardiac sarcolemmal KATP channels: latest twists in a questing tale! J. Mol. Cell. Cardiol. 48, 71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lascano E. C., Negroni J. A., del Valle H. F. (2002) Ischemic shortening of action potential duration as a result of KATP channel opening attenuates myocardial stunning by reducing calcium influx. Mol. Cell. Biochem. 236, 53–61 [DOI] [PubMed] [Google Scholar]
- 8. Crawford R. M., Ranki H. J., Botting C. H., Budas G. R., Jovanovic A. (2002) Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J. 16, 102–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhar-Chowdhury P., Harrell M. D., Han S. Y., Jankowska D., Parachuru L., Morrissey A., Srivastava S., Liu W., Malester B., Yoshida H., Coetzee W. A. (2005) The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function. J. Biol. Chem. 280, 38464–38470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong M., Kefaloyianni E., Bao L., Malester B., Delaroche D., Neubert T. A., Coetzee W. A. (2011) Cardiac ATP-sensitive K+ channel associates with the glycolytic enzyme complex. FASEB J. 25, 2456–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ardehali H., O'Rourke B. (2005) Mitochondrial K(ATP) channels in cell survival and death. J. Mol. Cell. Cardiol. 39, 7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown D. A., O'Rourke B. (2010) Cardiac mitochondria and arrhythmias. Cardiovasc. Res. 88, 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garlid K. D., Paucek P., Yarov-Yarovoy V., Murray H. N., Darbenzio R. B., D'Alonzo A. J., Lodge N. J., Smith M. A., Grover G. J. (1997) Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ. Res. 81, 1072–1082 [DOI] [PubMed] [Google Scholar]
- 14. Jaburek M., Yarov-Yarovoy V., Paucek P., Garlid K. D. (1998) State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J. Biol. Chem. 273, 13578–13582 [PubMed] [Google Scholar]
- 15. Hanley P. J., Daut J. (2005) K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J. Mol. Cell. Cardiol. 39, 17–50 [DOI] [PubMed] [Google Scholar]
- 16. Rodrigo G. C., Davies N. W., Standen N. B. (2004) Diazoxide causes early activation of cardiac sarcolemmal KATP channels during metabolic inhibition by an indirect mechanism. Cardiovasc. Res. 61, 570–579 [DOI] [PubMed] [Google Scholar]
- 17. Ye B., Kroboth S. L., Pu J. L., Sims J. J., Aggarwal N. T., McNally E. M., Makielski J. C., Shi N. Q. (2009) Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ. Res. 105, 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pu J. L., Ye B., Kroboth S. L., McNally E. M., Makielski J. C., Shi N. Q. (2008) Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive KATP activity. J. Mol. Cell. Cardiol. 44, 188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chutkow W. A., Samuel V., Hansen P. A., Pu J., Valdivia C. R., Makielski J. C., Burant C. F. (2001) Disruption of Sur2-containing K(ATP) channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 98, 11760–11764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chutkow W. A., Pu J., Wheeler M. T., Wada T., Makielski J. C., Burant C. F., McNally E. M. (2002) Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J. Clin. Invest. 110, 203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pilbrow A. P., Ellmers L. J., Black M. A., Moravec C. S., Sweet W. E., Troughton R. W., Richards A. M., Frampton C. M., Cameron V. A. (2008) Genomic selection of reference genes for real-time PCR in human myocardium. BMC Med. Genomics 1, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kakkar R., Ye B., Stoller D. A., Smelley M., Shi N. Q., Galles K., Hadhazy M., Makielski J. C., McNally E. M. (2006) Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ. Res. 98, 682–689 [DOI] [PubMed] [Google Scholar]
- 23. Stoller D. A., Fahrenbach J. P., Chalupsky K., Tan B. H., Aggarwal N., Metcalfe J., Hadhazy M., Shi N. Q., Makielski J. C., McNally E. M. (2010) Cardiomyocyte sulfonylurea receptor 2-KATP channel mediates cardioprotection and ST segment elevation. Am. J. Physiol. Heart Circ. Physiol. 299, H1100–H1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hill B. G., Dranka B. P., Zou L., Chatham J. C., Darley-Usmar V. M. (2009) Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 424, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pike L. S., Smift A. L., Croteau N. J., Ferrick D. A., Wu M. (2011) Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta 1807, 726–734 [DOI] [PubMed] [Google Scholar]
- 26. Stoller D., Pytel P., Katz S., Earley J. U., Collins K., Metcalfe J., Lang R. M., McNally E. M. (2009) Impaired exercise tolerance and skeletal muscle myopathy in sulfonylurea receptor-2 mutant mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1144–R1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Welling P. A., Ho K. (2009) A comprehensive guide to the ROMK potassium channel: form and function in health and disease. Am. J. Physiol. Renal Physiol. 297, F849–F863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foster D. B., Ho A. S., Rucker J., Garlid A. O., Chen L., Sidor A., Garlid K. D., O'Rourke B. (2012) Mitochondrial ROMK channel is a molecular component of mitoK(ATP). Circ. Res. 111, 446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zorzano A., Liesa M., Sebastian D., Segales J., Palacin M. (2010) Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Semin. Cell. Dev. Biol. 21, 566–574 [DOI] [PubMed] [Google Scholar]
- 30. Kuramoto K., Okamura T., Yamaguchi T., Nakamura T. Y., Wakabayashi S., Morinaga H., Nomura M., Yanase T., Otsu K., Usuda N., Matsumura S., Inoue K., Fushiki T., Kojima Y., Hashimoto T., Sakai F., Hirose F., Osumi T. (2012) Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J. Biol. Chem. 287, 23852–23863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopaschuk G. D., Collins-Nakai R. L., Itoi T. (1992) Developmental changes in energy substrate use by the heart. Cardiovasc. Res. 26, 1172–1180 [DOI] [PubMed] [Google Scholar]
- 32. Makinde A. O., Kantor P. F., Lopaschuk G. D. (1998) Maturation of fatty acid and carbohydrate metabolism in the newborn heart. Mol. Cell. Biochem. 188, 49–56 [PubMed] [Google Scholar]
- 33. Brook W. H., Connell S., Cannata J., Maloney J. E., Walker A. M. (1983) Ultrastructure of the myocardium during development from early fetal life to adult life in sheep. J. Anat. 137, 729–741 [PMC free article] [PubMed] [Google Scholar]
- 34. Bach D., Pich S., Soriano F. X., Vega N., Baumgartner B., Oriola J., Daugaard J. R., Lloberas J., Camps M., Zierath J. R., Rabasa-Lhoret R., Wallberg-Henriksson H., Laville M., Palacin M., Vidal H., Rivera F., Brand M., Zorzano A. (2003) Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 278, 17190–17197 [DOI] [PubMed] [Google Scholar]
- 35. Chen H., Chomyn A., Chan D. C. (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280, 26185–26192 [DOI] [PubMed] [Google Scholar]
- 36. Russell L. K., Mansfield C. M., Lehman J. J., Kovacs A., Courtois M., Saffitz J. E., Medeiros D. M., Valencik M. L., McDonald J. A., Kelly D. P. (2004) Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ. Res. 94, 525–533 [DOI] [PubMed] [Google Scholar]
- 37. Elrod J. W., Harrell M., Flagg T. P., Gundewar S., Magnuson M. A., Nichols C. G., Coetzee W. A., Lefer D. J. (2008) Role of sulfonylurea receptor type 1 subunits of ATP-sensitive potassium channels in myocardial ischemia/reperfusion injury. Circulation 117, 1405–1413 [DOI] [PubMed] [Google Scholar]
- 38. Flagg T. P., Kurata H. T., Masia R., Caputa G., Magnuson M. A., Lefer D. J., Coetzee W. A., Nichols C. G. (2008) Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ. Res. 103, 1458–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miki T., Suzuki M., Shibasaki T., Uemura H., Sato T., Yamaguchi K., Koseki H., Iwanaga T., Nakaya H., Seino S. (2002) Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat. Med. 8, 466–472 [DOI] [PubMed] [Google Scholar]
- 40. Stoller D., Kakkar R., Smelley M., Chalupsky K., Earley J. U., Shi N. Q., Makielski J. C., McNally E. M. (2007) Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress. J. Mol. Cell. Cardiol. 43, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bienengraeber M., Olson T. M., Selivanov V. A., Kathmann E. C., O'Cochlain F., Gao F., Karger A. B., Ballew J. D., Hodgson D. M., Zingman L. V., Pang Y. P., Alekseev A. E., Terzic A. (2004) ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat. Genet. 36, 382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harakalova M., van Harssel J. J., Terhal P. A., van Lieshout S., Duran K., Renkens I., Amor D. J., Wilson L. C., Kirk E. P., Turner C. L., Shears D., Garcia-Minaur S., Lees M. M., Ross A., Venselaar H., Vriend G., Takanari H., Rook M. B., van der Heyden M. A., Asselbergs F. W., Breur H. M., Swinkels M. E., Scurr I. J., Smithson S. F., Knoers N. V., van der Smagt J. J., Nijman I. J., Kloosterman W. P., van Haelst M. M., van Haaften G., Cuppen E. (2012) Dominant missense mutations in ABCC9 cause Cantu syndrome. Nat. Genet. 44, 793–796 [DOI] [PubMed] [Google Scholar]
- 43. Van Bon B. W., Gilissen C., Grange D. K., Hennekam R. C., Kayserili H., Engels H., Reutter H., Ostergaard J. R., Morava E., Tsiakas K., Isidor B., Le Merrer M., Eser M., Wieskamp N., de Vries P., Steehouwer M., Veltman J. A., Robertson S. P., Brunner H. G., de Vries B. B., Hoischen A. (2012) Cantu syndrome is caused by mutations in ABCC9. Am. J. Hum. Genet. 90, 1094–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grange D. K., Lorch S. M., Cole P. L., Singh G. K. (2006) Cantu syndrome in a woman and her two daughters: Further confirmation of autosomal dominant inheritance and review of the cardiac manifestations. Am. J. Med. Genet. A 140, 1673–1680 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.