Abstract
CHF1/Hey2 is a Notch-responsive basic helix–loop-helix transcription factor involved in cardiac development. Common variants in Hey2 are associated with Brugada syndrome. We hypothesized that absence of CHF1/Hey2 would result in abnormal cellular electrical activity, altered cardiac conduction system (CCS) development, and increased arrhythmogenesis. We isolated neonatal CHF/Hey2-knockout (KO) cardiac myocytes and measured action potentials and ion channel subunit gene expression. We also crossed myocardial-specific CHF1/Hey2-KO mice with cardiac conduction system LacZ reporter mice and stained for conduction system tissue. We also performed ambulatory ECG monitoring for arrhythmias and heart rate variability. Neonatal cardiomyocytes from CHF1/Hey2-KO mice demonstrate a 50% reduction in action potential dV/dT, a 50–75% reduction in SCN5A, KCNJ2, and CACNA1C ion channel subunit gene expression, and an increase in delayed afterdepolarizations from 0/min to 12/min. CHF1/Hey2 cKO CCS-lacZ mice have a ∼3-fold increase in amount of CCS tissue. Ambulatory ECG monitoring showed no difference in cardiac conduction, arrhythmias, or heart rate variability. Wild-type cells or animals were used in all experiments. CHF1/Hey2 may contribute to Brugada syndrome by influencing the expression of SCN5A and formation of the cardiac conduction system, but its absence does not cause baseline conduction defects or arrhythmias in the adult mouse.—Hartman, M. E., Liu, Y., Zhu, W.-Z., Chien, W.-M., Weldy, C. S., Fishman, G. I., Laflamme, M. A., Chin, M. T. Myocardial deletion of transcription factor CHF1/Hey2 results in altered myocyte action potential and mild conduction system expansion but does not alter conduction system function or promote spontaneous arrhythmias.
Keywords: Brugada syndrome, Notch signaling, electrophysiology, heart rate variability
Sudden cardiac death from arrhythmias is a significant public health concern. Structural heart disease and heart failure predispose to these arrhythmias, as do genetic conditions that alter ion channel function. Replacing diseased myocardium with healthy and viable tissue is the fundamental principle underlying cardiovascular regenerative medicine, and the ability of the implanted cells to differentiate fully into mature, electrically coupled, contractile cardiac myocytes is a critical component of this paradigm (1).
Cardiac conduction system (CCS) development is incompletely understood. Several experimental model systems have implicated the level of neuregulin/ErbB signaling in the differentiation of cardiac myocytes into either nodal or ventricular CCS phenotypes (2, 3). Moskowitz and coworkers (4, 5) have shown that the transcription factors Tbx5, Id2, and Nkx2-5 are important in the development of the CCS. Suppression of Notch signaling through the use of dominant-negative mastermind-like protein leads to abnormal atrioventricular (AV) conduction, while myocardial overexpression of the Notch1 intracellular domain (N1ICD) leads to accessory pathway formation, ventricular preexcitation, and atrial arrhythmias (6). Myocardial overexpression of N1ICD promotes the expression of various genes enriched in AV nodal and Purkinje fiber myocardium, as well as electrophysiological changes consistent with a conduction-like phenotype. These effects were also noted after transient overexpression of N1ICD in cultured neonatal cardiomyocytes (7).
CHF1/Hey2 is a Hey family transcription factor that functions downstream of Notch signaling and is involved in embryonic cardiac development (8). Moreover, common variants of Hey2 are associated with Brugada syndrome (9), a disorder characterized by abnormal cardiac action potentials, delayed conduction, and increased risk of ventricular tachyarrhythmias and sudden cardiac death. Up to 30% of patients with Brugada syndrome demonstrate mutations in the gene encoding SCN5A, a subunit of the major sodium channel in cardiac myocytes, but other genes have also been implicated (reviewed in ref. 10). The potential mechanisms by which Hey2 may contribute to Brugada syndrome are unclear, but may involve alterations in right ventricular outflow tract (RVOT) conduction (9). The phenotype of CHF1/Hey2-knockout (KO) mice, which die by postnatal d 7 (11), includes a thin-walled myocardium, decreased proliferation of the compact myocardium, ectopic expression of atrial and trabecular genes, valvular abnormalities, altered coronary artery development, and septation defects (11–15). Septation defects have been associated with altered CCS development, as has alteration in Tbx5 expression (4, 5, 16, 17). CHF1/Hey2, along with Hey1, also regulates Tbx2 expression and subsequently the development of the AV canal (18). Cardiac myocytes isolated from the RVOT of Hey2+/− mice show increased action potential upstroke velocity (9), which is consistent with the RVOT origin of arrhythmias in Brugada syndrome. CHF1/Hey2 myocardial-specific conditional-KO (cKO) mice, surprisingly, do not demonstrate congenital cardiac anomalies and survive to adulthood (19, 20). Functional analysis of isolated cardiac myocytes from these mice demonstrates decreased fractional shortening and calcium transients (20). These findings demonstrate that CHF1/Hey2 is required for proper cardiac morphogenesis, myocyte differentiation, and contractile function, but do not address how it affects the electrophysiological phenotype of myocytes and CCS development and do not fully explain how it may contribute to the development of Brugada syndrome.
Since CHF1/Hey2 is associated with Brugada syndrome and functions downstream of Notch, which regulates cellular electrophysiological phenotype and CCS development, we hypothesized that CHF1/Hey2 may affect the electrical phenotype of myocardial cells, as well as the development of the CCS. Here we report the effects of CHF1/Hey2 loss of function on cardiac myocyte action potential, conduction system development, spontaneous arrhythmogenesis, global electrophysiology, and heart rate variability.
MATERIALS AND METHODS
Unless otherwise stated, materials were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Isolation of cardiac myocytes
Neonatal cardiac myocytes were harvested as described previously (21, 22). Briefly, ventricular tissue was isolated, minced, and incubated in trypsin/EDTA 0.25 mg/ml (5 ml for 8–10 ventricles; Cambrex, Charles City, IA, USA) with rotation at 4°C for 30 min. Cells were collected by centrifugation at 1800 rpm for 5 min, resuspended in Dulbecco's modified Eagle's medium (DMEM; with penicillin/streptomycin and amphotericin B; Life Technologies, Carlsbad, CA, USA) with 20% fetal calf serum (FCS; Life Technologies), centrifuged again, resuspended in 4 ml of collagenase type II solution (1 mg/ml) in Hank's balanced salt solution (Life Technologies) then transferred to a P60 dish at 37°C. Cells were pipetted every 10 min until dispersed (up to 30–40 min), then filtered with a 70-μm nylon cell strainer (BD Biosciences, San Jose, CA, USA) to remove debris. Collagenase was neutralized by adding DMEM with 20% FCS. Cells were collected at 800 rpm for 5 min, resuspended in 10 ml of DMEM with 20% FCS, and incubated on a P100 dish at 37°C for 1–2 h. The nonadherent cells (myocytes) were collected at 800 rpm for 5 min, resuspended in 10.5 ml of DMEM with 20% FCS, and quantified by Coulter counting. Cells were seeded onto fibronectin-coated P60 dishes at 3–5 × 106 cells/dish in medium with 20 μM Ara-C to inhibit proliferation of any contaminating fibroblasts.
Patch clamping of individual cardiac myocytes
Action potential recordings were obtained using patch-clamp techniques as described previously (23). Briefly, neonatal cardiac myocytes were transferred to polyethylenimine- and gelatin-coated glass-bottom dishes and maintained at 36–37°C. Spontaneous action potentials were then recorded using an EPC-10 amplifier (Heka, Bellmore, NY, USA) operated in current-clamp mode. We used patch pipettes with a resistance of 2–4 MΩ and filled with 135 mM KCl, 5 mM Na2-creatine phosphate, 5 mM MgATP, and 10 mM HEPES, adjusted to pH 7.20 with KOH. The bath medium was 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 0.33 mM NaH2PO4, 5 mM dextrose, and 10 mM HEPES, adjusted to pH 7.40 with NaOH. Action potential parameters were analyzed using Patchmaster (Heka) and IgorPro software (WaveMetrics, Lake Oswego, OR, USA). A total of 5 myocytes of each genotype were analyzed.
RNA isolation and quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from left ventricles by homogenization in Trizol reagent (Life Technologies), per the manufacturer's instructions. RNA was reverse transcribed into cDNA using Superscript III and oligo(dT)20 primers (Life Technologies). Real-time quantitative PCR was performed on cDNA samples using SYBR green fluorescent reagent and an Applied Biosystems 7500 Real-Time PCR System (Life Technologies) per standard protocols and the manufacturer's instructions. The GAPDH cDNA was used as a control. Primer sequences used: SCN5A, 5′-CTTCACCAACAGCTGGAACA-3′ and 5′-AGACGGATGACACGGAAGAG3′; KCNJ2, 5′-TTGCTTCGGCTCATTCTCTT-3′ and 5′-AGAGATGGATGCTTCCGAGA-3′; CACNA1C, 5′-TCCTGGTCTGAGGAGACGAC-3′ and 5′-GGTGGTGACCTCGATGAACT-3′; GAPDH, 5′-CCTTCATTGACCTCAACTAC-3′ and 5′-GGAAGGCCATGCCAGTGAGC-3′.
Data analyses were performed with the 2−ΔΔCt method, as described previously (24).
Generation of CCS-lacZ reporter and CHF1/Hey2 cKO mice
CCS-lacZ mice, maintained on a CD-1 background, were crossed with CHF1/Hey2flox/flox mice on a C57BL/6 background to generate CCS-lacZ+/− CHF1/Hey2flox/+ mice, which were further backcrossed with C57BL/6 CHF1/Hey2flox/flox mice for ≥5 more generations to produce incipient congenic strains. C57BL/6 CCS-lacZ+/− CHF1/Hey2flox/flox female and C57BL/6 CHF1/Hey2flox/flox α-MHC-Cre+/− male mice were mated to generate myocardial specific CHF1/Hey2-deficient (cKO) mice that express CCS-lacZ and control mice lacking α-MHC-Cre. We have previously demonstrated that the α-MHC-Cre line efficiently deletes the CHF1/Hey2flox/flox allele in the heart (20).
Histology of the CCS
Adult 8- to 16-wk old mice were euthanized, and hearts were harvested and stained for β-galactosidase activity as described previously (25). Briefly, hearts were fixed for 10 min at room temperature in PBS containing 2% formaldehyde, 0.2% glutaraldehyde, 0.02% Nonidet P-40 (NP-40), and 0.01% sodium deoxycholate. Hearts were bisected through the atria and ventricles, rinsed 3 times in PBS, and incubated overnight at 37°C in the dark in PBS containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 mg/ml 5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside (X-gal), 2 mM MgCl2, 0.02% NP-40, and 0.01% sodium deoxycholate. Hearts were rinsed twice with ice-cold PBS, then fixed overnight at 4°C in 4% paraformaldehyde (in PBS), rinsed once with PBS, and stored in the dark at 4°C in PBS. For sectioning and histological examination, β-galactosidase (LacZ)-stained whole hearts were processed and embedded in paraffin following standard procedures. Sections (7 μm thick) along the coronal plane of each heart were made and placed on glass slides. Sections were incubated at 60°C for 60 min, then deparaffinized by 3 × 5 min xylene rinses. Sections were hydrated by undergoing a series of rinses in 100, 95, and 70% ethanol, followed by water. Hydrated sections were counterstained with Nuclear Fast Red (Vector Laboratories, Burlingame, CA, USA), then dehydrated in ethanol and xylene, before mounting with Permount Mounting Medium (Fisher Scientific, Hampton, NH, USA). Images of sections were taken at ×4 and ×10. Quantification of LacZ-positive area was done by background subtraction using Image J (U.S. National Institutes of Health, Bethesda, MD, USA). The sum of CCS LacZ-positive pixel area across an entire myocardial section was recorded, and then averaged across each mouse. The average CCS LacZ-positive area per section for each genotype was expressed in square micrometers.
Implantation of ambulatory telemetry monitors
Ambulatory telemetry monitors from Data Sciences International (DSI, New Brighton, MN, USA) were implanted into 12- to 21-wk old male CHF1/Hey2flox/flox α-MHC-Cre+/− and CHF1/Hey2flox/flox mice weighing ≥20 g using sterile operative technique. Mice were placed on a 37°C warming pad during surgery. A 3.5-cm incision was made with microdissecting scissors (curved 4–1/16-inch-long delicate 25-mm blades) along the dorsal surface, followed by blunt dissection along the left lateral aspect to make a device pocket. Incisions (0.5 cm) were made over the right pectoral region and left upper abdominal quadrant. The white (negative) lead was passed from the device pocket to the pectoral site via blunt dissection and anchored to the pectoral muscle with one 6-0 prolene suture over the exposed wire and one over the insulation. The red (positive) lead was anchored in a similar fashion to the abdominal musculature. Incisions were kept moist with sterile saline. The skin was closed using 6-0 prolene sutures. Postoperative analgesia was administered for 48 h with buprenorphine (0.05 mg/kg s.c. BID).
Electrocardiogram (ECG) measurements
Mice recovered for a minimum of 5 d prior to ECG recording. ECGs from each mouse were recorded in continuous 24 h blocks using DSI Ponemah software, sampling at 2500 Hz. Heart rate, QRS duration, PR interval, and QT interval with preceding RR intervals were measured using the DSI Ponemah system. Linear regression was used to determine a best-fit line (26) allowing for comparison of QT-RR regression line slope and y intercept. All data used for these measurements was verified manually. Arrhythmias were evaluated by manual beat-by-beat analysis over two 15-min recordings, one during the light cycle and one during the dark cycle.
Heart rate variability
Heart rate variability was assessed as described previously (27), using DSI Ponemah software. Data points, excluding RR intervals <70 or >150 ms, were taken from continuous ECG recordings during the light and dark cycles. A minimum of 60,000 data points were used to determine the heart rate variability via the standard deviation of successive RR intervals (SDNN) and the root mean squared of successive differences (RMSSD). To examine parasympathetic and sympathetic contributions of the autonomic nervous system, animals were given intraperitoneal atropine (0.5 mg/kg), propranolol (1 mg/kg), or atropine + propranolol. After a 10-min drug equilibration period, continuous ambulatory telemetry was collected for 30 mi, and data points were collected and analyzed as above. Pharmacologic manipulations were performed on different days to allow pharmacologic washout of each drug.
Animals used
Seventy animals were used for the experiments.
Statistical analysis
All data are reported as means ± sem. Comparisons between groups were made using an unpaired Student's t test (2 groups). All analyses were performed using Excel software (Microsoft, Redmond, WA, USA). Values of P < 0.05 were taken as the minimal level of significance.
Heart imaging
Photographs of stained hearts were taken on an Olympus SZX12 stereoscope with an Olympus DP71 camera (Olympus, Tokyo, Japan). All settings, including exposure time, were set to be the same for pictures taken between control and cKO hearts.
RESULTS
CHF1/Hey2-deficient myocytes show altered action potentials, delayed afterdepolarizations, and altered ion channel gene expression
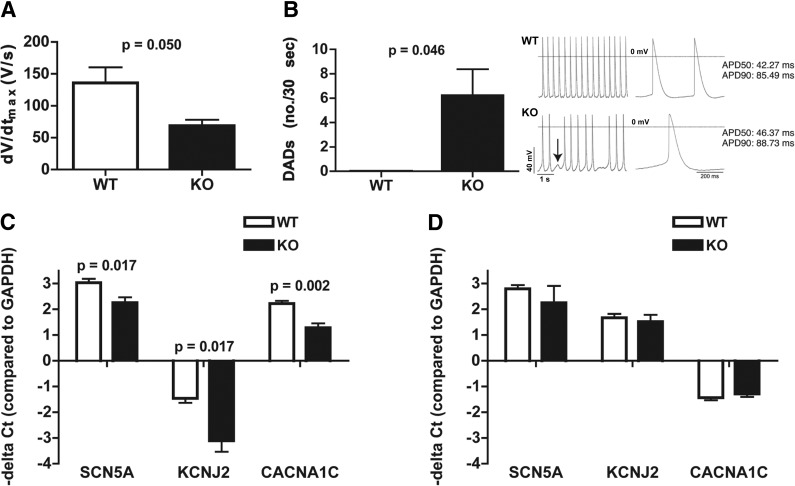
To investigate whether CHF1/Hey2 affects the electrical phenotype of individual cardiac myocytes, we performed patch-clamp experiments on neonatal ventricular myocytes from control and CHF1/Hey2-deficient mice. Neonatal myocytes from CHF1/Hey2-deficient mice have altered spontaneous action potentials, characterized by reduced dV/dt but normal action potential duration at 50 and 90% repolarization (APD50 and APD90, respectively) and a greater incidence of delayed afterdepolarizations (Fig. 1A, B and Table 1). Evaluation of mRNA expression for 3 major cardiac ion channel subunits responsible for sodium, potassium, and calcium currents (SCN5A, KCNJ2, and CACNA1C) showed that mRNA levels are decreased for each of these transcripts in the CHF1/Hey2-deficient neonatal hearts (Fig. 1C). Expression of these genes was not different in CHF1/Hey2-deficient adult hearts (Fig. 1D). These findings indicate that CHF1/Hey2 influences the development of a normal electrical phenotype in immature ventricular myocytes but may not significantly affect the mature myocytes.
Figure 1.
CHF1/Hey2-deficient myocytes show altered action potentials, delayed afterdepolarizations, and altered ion channel gene expression. Patch clamping was performed and spontaneous action potentials were measured on neonatal cardiac myocytes from wild-type (WT) and CHF1/Hey2-KO (KO) mice. A) Maximal upstroke velocity (dV/dtmax) in WT control (n=5) and KO myocytes (n=5). B) Left panel: number of delayed afterdepolarizations (DADs) per 30 s is shown for both WT (n=3) and KO (n=5) myocytes. Right panel: representative action potential recordings from WT and KO myocytes, with the KO showing a DAD (arrow). C) Expression of SCN5A, KCNJ2, and CACNA1C in WT (n=8) and KO (n=5) neonatal hearts by quantitative RT-PCR. D) Expression of SCN5A, KCNJ2, and CACNA1C in WT (n=3) and KO (n=3) adult hearts by quantitative RT-PCR. Averages ± sem are shown; P = nonsignificant if not displayed.
Table 1.
Cardiomyocyte action potential parameters
| Genotype | n | dV/dtmax (V/s) | Rate (bpm) | MDP (mV) | APA (mV) | APD50 (ms) | APD90 (ms) |
|---|---|---|---|---|---|---|---|
| WT | 5 | 135.8 ± 24.6 | 194.3 ± 44.1 | −64.2 ± 3.2 | 87.6 ± 3.9 | 42.3 ± 2.6 | 85.4 ± 6.7 |
| KO | 5 | 68.6 ± 9.6* | 87.1 ± 25.7 | −69.8 ± 3.3 | 86.6 ± 2.7 | 46.4 ± 8.0 | 88.7 ± 10.0 |
APA, action potential amplitude; APD50, action potential duration at 50% repolarization; APD90, action potential duration at 90% repolarization; dV/dtmax, maximum rate of action potential upstroke; MDP, maximum diastolic potential.
P = 0.05 vs. WT.
CHF1/Hey2 regulates CCS development
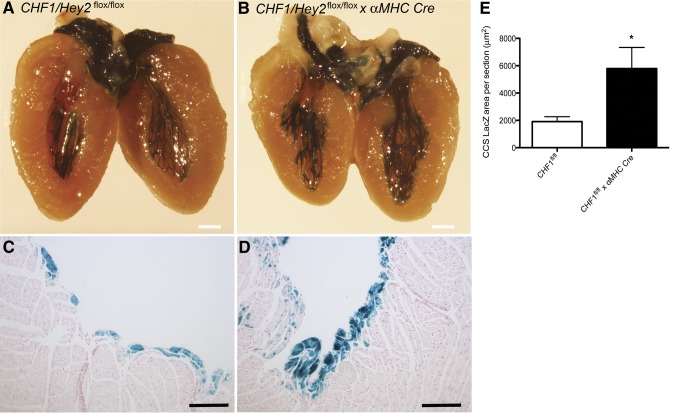
CCS specification is thought to arise from differentiation of ventricular myocyte precursors (28) and occurs primarily in the trabecular myocardium through the action of endothelially derived factors including neuregulin (3). Conduction myocardium has been challenging to study due to a lack of markers specific for cardiac conduction myocyte subtypes (29), but has been aided by the development of mice that express reporter genes specifically in CCS (24). To assess the role of CHF/Hey2 in CCS development, we crossed CHF1/Hey2 cKO mice with CCS-lacZ reporter mice, which display β-galactosidase activity throughout the CCS (25). Adult male CHF1/Hey2flox/flox α-MHC-Cre+/− CCS-lacZ+/− mice have an ∼3 fold increase in CCS tissue compared to CHF1/Hey2flox/flox CCS-lacZ+/− controls (Fig. 2). Qualitatively, the conduction tissue appears to penetrate more deeply into the myocardium (Fig. 2C, D). These findings suggest that CHF1/Hey2 regulates the formation of the CCS in a myocardium-specific manner.
Figure 2.
Myocardial deficiency of CHF1/Hey2 increases the formation of CCS myocardium. Hearts from adult (8- to 16-wk-old) male and female CHF1/Hey2flox/flox mice expressing β-galactosidase in the CCS and expressing αMHC-Cre (cKO) or not (control) were collected and stained for β-galactosidase activity using X-gal substrate. A) Representative image from control hearts (n=6). B) Representative image from cKO (n=7) hearts. C) Representative histological section (×100) from control heart showing LacZ-stained CCS tissue. D) Representative histological section from cKO heart showing LacZ-stained CCS tissue. E) Quantification of average LacZ-positive cross-sectional area per section in control (n=4) and cKO (n=3) mice. Averages ± sem are shown. Scale bars = 1 mm (A, B); 100 μm (C, D). *P < 0.05.
Myocardial deficiency of CHF1/Hey2 does not alter the function of the CCS, produce arrhythmias, or change heart rate variability
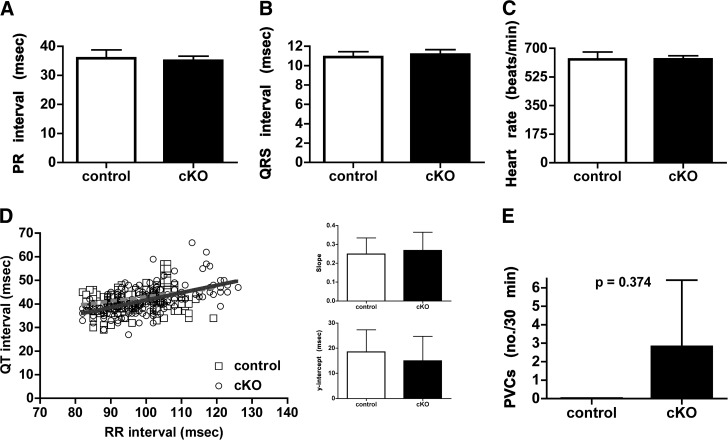
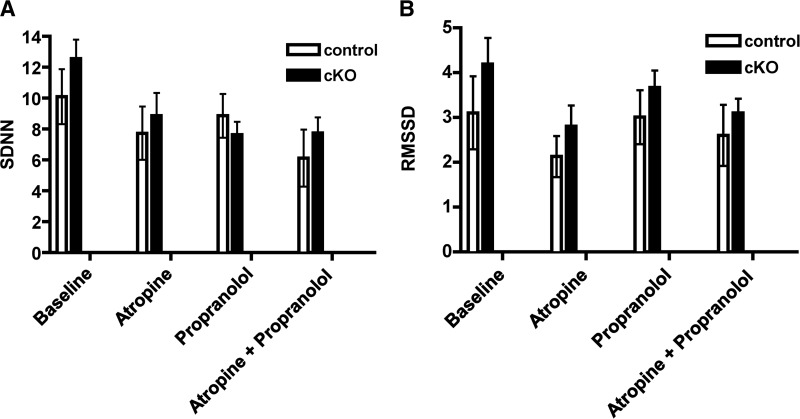
To assess whether CHF1/Hey2 cKO mice had alterations in in vivo cardiac electrophysiology, we implanted telemeters and obtained continuous ambulatory single lead (modified lead II) electrocardiography. We did not observe any differences in baseline electrocardiographic measures including PR duration, QRS duration, heart rate, QT duration as assessed by QT-RR regression, or heart rate variability (Figs. 3 and 4). We did observe intermittent AV dissociation; however, this was present in both CHF1/Hey2 cKO and control mice (data not shown). We did not observe any episodes of ventricular tachycardia, nor were there any differences in the frequency of single ventricular ectopic beats between control and CHF1/Hey2 cKO mice (Fig. 3E). We assessed the sympathetic and parasympathetic contributions to heart rate variability using atropine, propranolol, and combined atropine + propranolol without detecting a difference between CHF1/Hey2 cKO and control mice (Fig. 4). These data suggest that, while myocardial CHF1/Hey2 deficiency affects the development of the ventricular CCS, it does not alter global CCS function or its regulation by autonomic tone.
Figure 3.
Myocardial deficiency of CHF1/Hey2 does not alter the function of the CCS nor produce arrhythmias. Continuous ambulatory telemetry on adult (12- to 21-wk-old) male CHF1/Hey2flox/flox (control, n=3) and CHF1/Hey2flox/flox α-MHC-Cre+/− (cKO, n=5) mice. A–C) Twenty separate data points during sinus rhythm were used to determine the average PR interval, QRS duration, and heart rate. D) Left panel: linear regression generated from a minimum of 40 QT intervals, with the preceding RR intervals for each animal shown. Right panels: average linear regression line slopes and y intercepts. E) Ventricular ectopy is shown as number of premature ventricular contractions (PVCs) per 30 min of recording time. Averages ± sem are shown, P = nonsignificant if not displayed.
Figure 4.
Myocardial deficiency of CHF1/Hey2 does not affect heart rate variability. Continuous ambulatory telemetry on adult (12- to 21-wk-old) male CHF1/Hey2flox/flox (control, n=3) and CHF1/Hey2flox/flox α-MHC-Cre+/− (cKO, n=5) mice was performed at baseline and following treatment with atropine, propranolol, and atropine + propranolol. Standard deviation of successive RR intervals (SDNN) and troot mean squared of successive differences (RMSSD) are displayed on the left and right panels, respectively. Averages ± sem are shown, P = nonsignificant if not displayed.
DISCUSSION
Here we demonstrate that loss of CHF1/Hey2 results in abnormal action potentials in neonatal ventricular cardiomyocytes, altered expression of several ion channel subunits and expansion of the CCS, albeit with overall preservation of the general architecture of the ventricular CCS. We did not observe functional effects on global cardiac electrophysiology, nor did we observe changes in heart rate variability or spontaneous arrhythmia frequency.
The development of the CCS occurs in parallel with septation and coronary vascular development (25, 30). This close temporal association may reflect a mechanistic linkage between CCS development and normal cardiac structural development. Alternatively, the role of CHF1/Hey2 in CCS development may reflect an intrinsic myocardial cell autonomous process. CHF1/Hey2 global deletion affects septation, regulation of Tbx genes, expression of ion channel proteins, and cardiac conduction velocity in the RVOT (9). However, CHF1/Hey2 myocardial cKO mice do not develop structural cardiac defects (20) and consequently are well suited to address whether CCS development as regulated by CHF1/Hey2 includes a myocardial cell autonomous contribution. By combining myocardial deletion of CHF1/Hey2 and the CCS-lacZ reporter, we were able to distinguish between direct effects of myocardial CHF1/Hey2 vs. indirect effects resulting from congenital structural defects. Our results demonstrate that the pathway involving CHF1/Hey2 in the patterning of the CCS during development appears distinct from the processes required for septation and coronary vascular development in the heart. Our findings are consistent with a model where CHF1/Hey2 loss of function increases the sensitivity of myocytes to endothelial-derived factors such as neuregulin, thereby allowing the threshold for conversion of working ventricular myocytes to Purkinje-like cells to extend further from the endocardial surface.
While loss of function of CHF1/Hey2 resulted in expansion of the CCS, this did not affect global cardiac electrophysiology nor did it confer a spontaneous arrhythmic phenotype, as measured by continuous telemetric monitoring. However with the increased delayed afterdepolarizations observed in isolated neonatal ventricular myocytes, one would expect an increase in arrhythmias. One possible explanation is that the properties of neonatal ventricular myocytes from CHF1/Hey2-KO mice reflect a delay rather than a block in maturation such that arrhythmias would occur in the neonatal but not the adult mouse heart. Our finding that adult hearts have normal ion channel subunit expression is consistent with this possible explanation. Whether increased arrhythmia susceptibility could be elicited by provocative maneuvers, such as programmed electrical stimulation, remains to be determined.
As described above, alteration in myocardial Notch signaling results in AV conduction abnormalities and can promote the expression of conduction system-related genes and a conduction-like electrical phenotype in neonatal cardiac myocytes (6, 7). Surprisingly, the phenotypes observed in our study are distinct from those observed for Notch. CHF1/Hey2 has been described as a downstream effector of Notch; however, in some contexts, its expression is independent of canonical Notch signaling (31, 32). It has also been shown to inhibit activation of Notch-dependent promoters (33, 34), suggesting that it acts as a negative feedback inhibitor of Notch signaling. Our findings indicate that the effects of CHF1/Hey2 on cardiac myocyte electrical properties and on CCS development may be working through Notch-independent pathways. Also, loss of CHF1/Hey2 may potentiate Notch-signaling by eliminating a negative feedback loop, thereby promoting further differentiation to conduction myocardium. Alternatively, the effects of Notch signaling may be time and context dependent, involving combinatorial factors such that its behavior in the developing compact vs. trabecular myocardium may influence target gene expression.
Our observation that complete loss of CHF1/Hey2 leads to altered action potentials and reduced dV/dt is incongruous with a report that myocytes isolated from the RVOT of CHF1/Hey2+/− mice show increased action potential upstroke velocity and that conduction velocity is increased in the RVOT but not in the right ventricular (RV) or left ventricular (LV) free walls (9). Interestingly, these researchers assert that the increased upstroke velocity is consistent with Brugada syndrome, but Brugada syndrome is associated with decreased conduction and, in many cases, reduced function of SCN5A. Their findings are also surprising because previous reports studying alterations in cardiac gene expression in CHF1/Hey2-KO or myocardial-KO mice showed effects primarily in the LV, not the RV (19, 35). Possible sources of discrepancy include the use of unselected populations of myocytes in our study, the use of neonatal myocytes in our study and the use of KO myocytes in place of heterozygous myocytes in our study. In addition, there may be gene dosage effects in heterozygous mice and background strain related effects that contribute to the discordant results of these studies. Finally, it is possible that compensatory effects of other Hey genes (Hey1 or HeyL) may limit the effects of CHF1/Hey2 deletion in terms of conduction abnormalities or arrhythmogenesis, although these genes do not compensate for effects of CHF1/Hey2 deletion on development of heart failure (20).
Our study raises the interesting possibility that CHF1/Hey2 influences the development of Brugada syndrome through an effect on SCN5A expression and on conduction system development. In further support of this hypothesis, we have found that CHF1/Hey2 loss of function in mouse embryonic stem cell-derived cardiomyocytes have altered expression of ANF and CX43, both of which are expressed highly in the CCS (36). The lack of obvious conduction abnormalities in our mice may possibly be attributed to the inherent difficulties in measuring subtle changes in conduction in rodent systems, due to their smaller size in relation to humans. Even in patients, sometimes these abnormalities are not apparent in the absence of challenge with pharmacologic agents that block sodium channels, such as flecainide. A future study on mice involving flecainide challenge may be informative. It is also possible that increased arrhythmia susceptibility may be unmasked by pathogenic stimuli, such as myocardial injury. Anecdotally, we have seen increased death in CHF1/Hey2 myocardial cKO mice after aortic banding (unpublished results), but determining whether the increased mortality is the result of arrhythmogenic death requires further study.
Currently, the processes that regulate the development of the CCS and the electrical maturation of cardiac myocytes are not fully understood. Understanding these processes will help develop better therapies for sudden cardiac death and is relevant to the field of cardiac regenerative medicine. Our findings suggest that CHF1/Hey2 is involved in these processes during the patterning of the CCS but does not affect the function of the adult CCS or the electrical function of the adult myocardium in mice. The molecular basis for its contribution to the pathophysiology of Brugada syndrome requires further investigation.
Acknowledgments
This work was supported by a grant from the John L. Locke Foundation to M.T.C. M.E.H. was supported by U.S. National Institutes of Health (NIH) training grant T32 HL07828. C.S.W. was supported by NIH training grant T32 HL007312. W.Z.Z. and M.A.L. were supported by NIH grant PO1-HL094374. G.I.F. was supported by NIH grant R01HL105983. M.A.L. is a cofounder of and has equity in BEAT Biotechnology.
Footnotes
- AV
- atrioventricular
- CCS
- cardiac conduction system
- cKO
- conditional knockout
- DMEM
- Dulbecco's modified Eagle's medium
- ECG
- electrocardiogram
- FCS
- fetal calf serum
- KO
- knockout
- LacZ
- β-galactosidase
- LV
- left ventricular
- N1ICD
- Notch1 intracellular domain
- NP-40
- Nonidet P-40
- RT-PCR
- reverse transcription-polymerase chain reaction
- RV
- right ventricular
- RVOT
- right ventricular outflow tract
- WT
- wild-type
- X-gal
- 5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside
REFERENCES
- 1. Shiba Y., Hauch K. D., Laflamme M. A. (2009) Cardiac applications for human pluripotent stem cells. Curr. Pharm. Des. 15, 2791–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu W. Z., Xie Y., Moyes K. W., Gold J. D., Askari B., Laflamme M. A. (2010) Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ. Res. 107, 776–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rentschler S., Zander J., Meyers K., France D., Levine R., Porter G., Rivkees S. A., Morley G. E., Fishman G. I. (2002) Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc. Natl. Acad. Sci. U.S.A. 99, 10464–10469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moskowitz I. P., Kim J. B., Moore M. L., Wolf C. M., Peterson M. A., Shendure J., Nobrega M. A., Yokota Y., Berul C., Izumo S., Seidman J. G., Seidman C. E. (2007) A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell 129, 1365–1376 [DOI] [PubMed] [Google Scholar]
- 5. Moskowitz I. P., Pizard A., Patel V. V., Bruneau B. G., Kim J. B., Kupershmidt S., Roden D., Berul C. I., Seidman C. E., Seidman J. G. (2004) The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development 131, 4107–4116 [DOI] [PubMed] [Google Scholar]
- 6. Rentschler S., Harris B. S., Kuznekoff L., Jain R., Manderfield L., Lu M. M., Morley G. E., Patel V. V., Epstein J. A. (2011) Notch signaling regulates murine atrioventricular conduction and the formation of accessory pathways. J. Clin. Invest. 121, 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rentschler S., Yen A. H., Lu J., Petrenko N. B., Lu M. M., Manderfield L. J., Patel V. V., Fishman G. I., Epstein J. A. (2012) Myocardial Notch signaling reprograms cardiomyocytes to a conduction-like phenotype. Circulation 126, 1058–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fischer A., Steidl C., Wagner T. U., Lang E., Jakob P. M., Friedl P., Knobeloch K. P., Gessler M. (2007) Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circ. Res. 100, 856–863 [DOI] [PubMed] [Google Scholar]
- 9. Bezzina C. R., Barc J., Mizusawa Y., Remme C. A., Gourraud J. B., Simonet F., Verkerk A. O., Schwartz P. J., Crotti L., Dagradi F., Guicheney P., Fressart V., Leenhardt A., Antzelevitch C., Bartkowiak S., Borggrefe M., Schimpf R., Schulze-Bahr E., Zumhagen S., Behr E. R., Bastiaenen R., Tfelt-Hansen J., Olesen M. S., Kaab S., Beckmann B. M., Weeke P., Watanabe H., Endo N., Minamino T., Horie M., Ohno S., Hasegawa K., Makita N., Nogami A., Shimizu W., Aiba T., Froguel P., Balkau B., Lantieri O., Torchio M., Wiese C., Weber D., Wolswinkel R., Coronel R., Boukens B. J., Bezieau S., Charpentier E., Chatel S., Despres A., Gros F., Kyndt F., Lecointe S., Lindenbaum P., Portero V., Violleau J., Gessler M., Tan H. L., Roden D. M., Christoffels V. M., Le Marec H., Wilde A. A., Probst V., Schott J. J., Dina C., Redon R. (2013) Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 45, 1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizusawa Y., Wilde A. A. (2012) Brugada syndrome. Circ. Arrhythm. Electrophysiol. 5, 606–616 [DOI] [PubMed] [Google Scholar]
- 11. Sakata Y., Kamei C. N., Nakagami H., Bronson R., Liao J. K., Chin M. T. (2002) Ventricular septal defect and cardiomyopathy in mice lacking the transcription factor CHF1/Hey2. Proc. Natl. Acad. Sci. U.S.A. 99, 16197–16202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kokubo H., Miyagawa-Tomita S., Tomimatsu H., Nakashima Y., Nakazawa M., Saga Y., Johnson R. L. (2004) Targeted disruption of hesr2 results in atrioventricular valve anomalies that lead to heart dysfunction. Circ. Res. 95, 540–547 [DOI] [PubMed] [Google Scholar]
- 13. Reamon-Buettner S. M., Borlak J. (2006) HEY2 mutations in malformed hearts. Hum. Mutat. 27, 118. [DOI] [PubMed] [Google Scholar]
- 14. Watanabe T., Koibuchi N., Chin M. T. (2010) Transcription factor CHF1/Hey2 regulates coronary vascular maturation. Mech. Dev. 127, 418–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakata Y., Koibuchi N., Xiang F., Youngblood J. M., Kamei C. N., Chin M. T. (2006) The spectrum of cardiovascular anomalies in CHF1/Hey2 deficient mice reveals roles in endocardial cushion, myocardial and vascular maturation. J. Mol. Cell. Cardiol. 40, 267–273 [DOI] [PubMed] [Google Scholar]
- 16. Zhao Y., Ransom J. F., Li A., Vedantham V., von Drehle M., Muth A. N., Tsuchihashi T., McManus M. T., Schwartz R. J., Srivastava D. (2007) Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1–2. Cell 129, 303–317 [DOI] [PubMed] [Google Scholar]
- 17. Bruneau B. G., Nemer G., Schmitt J. P., Charron F., Robitaille L., Caron S., Conner D. A., Gessler M., Nemer M., Seidman C. E., Seidman J. G. (2001) A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106, 709–721 [DOI] [PubMed] [Google Scholar]
- 18. Kokubo H., Tomita-Miyagawa S., Hamada Y., Saga Y. (2007) Hesr1 and Hesr2 regulate atrioventricular boundary formation in the developing heart through the repression of Tbx2. Development 134, 747–755 [DOI] [PubMed] [Google Scholar]
- 19. Xin M., Small E. M., van Rooij E., Qi X., Richardson J. A., Srivastava D., Nakagawa O., Olson E. N. (2007) Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc. Natl. Acad. Sci. U.S.A. 104, 7975–7980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y., Korte F. S., Moussavi-Harami F., Yu M., Razumova M., Regnier M., Chin M. T. (2012) Transcription factor CHF1/Hey2 regulates EC coupling and heart failure in mice through regulation of FKBP12.6. Am. J. Physiol. Heart Circ. Physiol. 302, H1860–H1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Springhorn J. P., Claycomb W. C. (1989) Preproenkephalin mRNA expression in developing rat heart and in cultured ventricular cardiac muscle cells. Biochem. J. 258, 73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiang F., Sakata Y., Cui L., Youngblood J. M., Nakagami H., Liao J. K., Liao R., Chin M. T. (2006) Transcription factor CHF1/Hey2 suppresses cardiac hypertrophy through an inhibitory interaction with GATA4. Am. J. Physiol. Heart Circ. Physiol. 290, H1997–H2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu W. Z., Santana L. F., Laflamme M. A. (2009) Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLoS One 4, e5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 25. Rentschler S., Vaidya D. M., Tamaddon H., Degenhardt K., Sassoon D., Morley G. E., Jalife J., Fishman G. I. (2001) Visualization and functional characterization of the developing murine cardiac conduction system. Development 128, 1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drici M. D., Arrighi I., Chouabe C., Mann J. R., Lazdunski M., Romey G., Barhanin J. (1998) Involvement of IsK-associated K+ channel in heart rate control of repolarization in a murine engineered model of Jervell and Lange-Nielsen syndrome. Circ. Res. 83, 95–102 [DOI] [PubMed] [Google Scholar]
- 27. Berul C. I., McConnell B. K., Wakimoto H., Moskowitz I. P., Maguire C. T., Semsarian C., Vargas M. M., Gehrmann J., Seidman C. E., Seidman J. G. (2001) Ventricular arrhythmia vulnerability in cardiomyopathic mice with homozygous mutant Myosin-binding protein C gene. Circulation 104, 2734–2739 [DOI] [PubMed] [Google Scholar]
- 28. Gourdie R. G., Mima T., Thompson R. P., Mikawa T. (1995) Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development 121, 1423–1431 [DOI] [PubMed] [Google Scholar]
- 29. Fijnvandraat A. C., Lekanne Deprez R. H., Moorman A. F. (2003) Development of heart muscle-cell diversity: a help or a hindrance for phenotyping embryonic stem cell-derived cardiomyocytes. Cardiovasc. Res. 58, 303–312 [DOI] [PubMed] [Google Scholar]
- 30. Rentschler S., Morley G. E., Fishman G. I. (2003) Patterning of the mouse conduction system. Novartis Found. Symp. 250, 194–205; discussion 205–209, 276–279 [PubMed] [Google Scholar]
- 31. High F. A., Epstein J. A. (2008) The multifaceted role of Notch in cardiac development and disease. Nat. Rev. Genet. 9, 49–61 [DOI] [PubMed] [Google Scholar]
- 32. Timmerman L. A., Grego-Bessa J., Raya A., Bertran E., Perez-Pomares J. M., Diez J., Aranda S., Palomo S., McCormick F., Izpisua-Belmonte J. C., de la Pompa J. L. (2004) Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 18, 99–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. King I. N., Kathiriya I. S., Murakami M., Nakagawa M., Gardner K. A., Srivastava D., Nakagawa O. (2006) Hrt and Hes negatively regulate Notch signaling through interactions with RBP-Jkappa. Biochem. Biophys. Res. Commun. 345, 446–452 [DOI] [PubMed] [Google Scholar]
- 34. Nakagawa O., McFadden D. G., Nakagawa M., Yanagisawa H., Hu T. H., Srivastava D., Olson E. N. (2000) Members of the HRT family of basic helix-loop-helix proteins act as transcriptional repressors downstream of Notch signaling. Proc. Natl. Acad. Sci. U.S.A. 97, 13655–13660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koibuchi N., Chin M. T. (2007) CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ. Res. 100, 850–855 [DOI] [PubMed] [Google Scholar]
- 36. Hartman M. E., Librande J. R., Medvedev I. O., Ahmad R. N., Moussavi-Harami F., Gupta P. P., Chien W. M., Chin M. T. (2014) An optimized and simplified system of mouse embryonic stem cell cardiac differentiation for the assessment of differentiation modifiers. PLoS One 9, e93033. [DOI] [PMC free article] [PubMed] [Google Scholar]