Abstract
Akt/PKB is a key master regulator of a wide range of physiological functions including metabolism, proliferation, survival, growth, angiogenesis and migration and invasion. The Akt protein kinase family comprises three highly related isoforms encoded by different genes. The initial observation that the Akt isoforms share upstream activators as well as several downstream effectors, together with the high sequence homology suggested that their functions were mostly redundant. By contrast, an increasing body of evidence has recently uncovered the concept of Akt isoform signaling specificity, supported by distinct phenotypes displayed by animal strains genetically modified for each of the three genes, as well as by the identification of isoform-specific substrates and association with discrete subcellular locations. Given that Akt is regarded as a promising therapeutic target in a number of pathologies, it is essential to dissect the relative contributions of each isoform, as well as the degree of compensation in pathophysiological function. Here we summarize our view of how Akt selectivity is achieved in the context of subcellular localization, isoform-specific substrate phosphorylation and context-dependent functions in normal and pathophysiological settings.
Introduction
Since its initial identification as a proto-oncogene, the Akt/PKB (Protein Kinase B) serine/threonine kinase has taken central stage as a major effector downstream of the PI 3-kinase (PI 3-K) pathway, with critical regulatory roles in key cellular functions such as cell cycle progression, proliferation and survival. Well over two decades after its initial discovery, work on Akt continues to attract considerable attention because of newly emerging roles, such as the regulation of cell metabolism and cancer cell migration and invasion.
Akt Primary Structure and Activation Mechanisms
Primary Structure
Akt belongs to the AGC family of protein kinases, and consists of three highly homologous isoforms: Akt1 (PKBα), Akt2 (PKBβ) and Akt3 (PKBγ), encoded by distinct genes located on different chromosomes (Figure 1) (Hemmings and Restuccia, 2012, Manning and Cantley, 2007, Toker, 2012). With more than 80% sequence identity, Akt isoforms share a common architecture, consisting of a catalytic domain flanked by an amino-terminal Pleckstrin Homology (PH) domain and a regulatory carboxyl-terminal domain (Calleja et al., 2012, Mahadevan et al., 2008). According to the crystal structure of the PH domain of Akt1 bound to the inositol head group of PtdIns(3,4,5)P3, (myo-inositol(1,3,4,5)P4), Akt possesses a group 3 PH domain, indicating that it recognizes PtdIns(3,4)P2 and PtdIns(3,4,5)P3 (Hanada et al., 2004). Intramolecular PH domain-kinase domain interactions are important to maintain Akt in an inactive state. It is noteworthy that a somatic activating mutation (Glu17into Lys17) has been reported in the PH domain of Akt, at a frequency of approximately 4–8% in breast cancers (Carpten et al., 2007). Importantly, the above mutants are not effectively inhibited by allosteric AKT inhibitors, which require an intact PH-kinase domain interface (Parikh et al., 2012). Between the PH domain and the catalytic kinase core there is a short α-helical linker region, whose function remains unclear, but as described below may afford Akt isoform signaling selectivity. Akt is co-translationally phosphorylated at Thr450 in the Turn motif by the serine-threonine protein kinase TORC2 associated with the endoplasmic reticulum. This phosphorylation stabilizes Akt and protects it from degradation, and has been proposed to be the first step in Akt activation (Chan and Tsichlis, 2001). The regulatory domain contains a carboxyl-terminal extension of approximately 40 amino acids, with a F-X-XF/Y-S/T-YF hydrophobic motif (F-P-Q-F-S-Y in Akt) characteristic of all AGC-family kinases (Peterson and Schreiber, 1999). Phosphorylation at a Ser or Thr residue within the hydrophobic motif is a requisite for full activation, such that point mutation of this motif completely abolishes enzymatic activity (Andjelkovic et al., 1997).
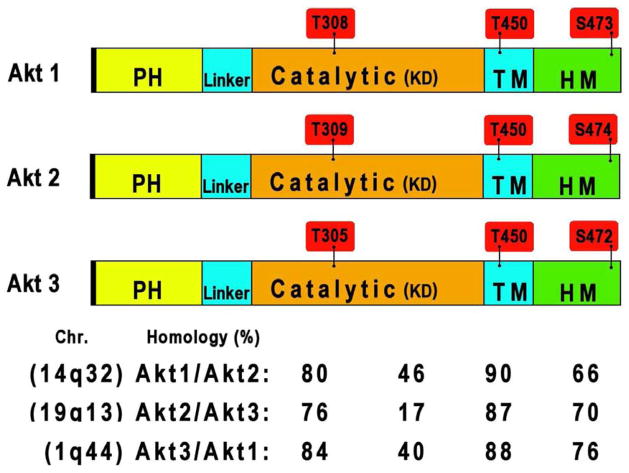
Figure 1.
Structure of Akt isoforms. All Akt isoforms possess a catalytic domain (KD), flanked by an amino-terminal PH domain and by a regulatory domain at the carboxyl-terminus. Phosphorylation sites in the activation loop of the catalytic domain and in the regulatory domain turn loop and hydrophobic motif are highlighted in red. The chromosome location of the genes coding for the Akt isozymes and the percentage of sequence homology are also indicated.
Akt Activation
Akt isoforms share a common, multi-step mechanism of activation downstream of class IA PI 3-K (Yuan and Cantley, 2008). Upon activation of receptor tyrosine kinases that respond to a variety of growth factors such as insulin, the lipid kinase PI 3-K phosphorylates the 3′-position of the inositol ring of phosphoinositides. In turn, the 3′ polyphosphoinositides PtdIns(3,4,5)P3 and PtdIns(3,4)P2 are generated, and engage the Akt PH domain. This binding effectively relocalizes Akt from the cytoplasm to the plasma membrane and furthermore promotes a conformational change that unmasks the catalytic kinase core. This relocalization primes Akt for phosphorylation at two regulatory sites: Thr308 within the catalytic domain, and Ser473, in the hydrophobic motif (Andjelkovic, Alessi, 1997) (Figure 2). Phosphorylation of Thr308 is also required for Akt activation in a manner dependent on PtdIns(3,4,5)P3 or PtdIns(3,4)P2, and is mediated by phosphoinositide-dependent kinase 1 (PDK-1) (Alessi et al., 1997). Similar to Akt, PDK-1 possesses a carboxyl-terminal PH domain which facilitates its binding to the plasma membrane, a necessary step to allow proximity to Akt and Thr308 phosphorylation.
Figure 2.

Regulation of Akt phosphorylation. PI 3-K activated downstream of RTKs phosphorylates PIP2 generating PIP3. PDK1 and Akt are transiently docked to the membrane by their PIP3-binding module, the PH domain, thereby exposing their catalytic domain. By this mechanism, PDK1 and Akt relocate in close proximity to one another and PDK1 phosphorylates Akt at Thr308. Phosphorylation at Ser473 is carried out by mTORC2, leading to full activation of Akt which then redistributes to discrete subcellular locations where it phosphorylates target proteins containing the RxRxxS/T motif. Dephosphorylation of PIP3 by PTEN, and direct dephosphorylation of Akt by PP2A at Thr308 and PHLPP at Ser473 all contribute to terminate Akt signaling. Abbreviations: mTORC2, mammalian target of rapamycin complex 2; PDK-1, phosphoinositide-dependent kinase 1; PH, pleckstrin homology; PHLPP, PH domain leucine-rich repeat protein phosphatase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5- trisphosphate; PP2A, protein phosphatase 2A; PTEN, phosphatase and tensin homolog deleted on chromosome ten; RTK, receptor tyrosine kinase.
Conversely, the hydrophobic motif kinase Ser473 kinase, initially referred to as PDK2, has been the subject of considerable debate. Depending on the context, phosphorylation of Ser473 has been attributed to a number of different kinases. Most of these are anecdotal, are likely to be cell type-dependent and the physiological significance for Akt signaling remains to be rigorously established. By general consensus, phosphorylation of Akt at Ser473 in response to growth factor signaling is attributed to the Rictor-mTOR complex mTORC2 (Sarbassov et al., 2005). The target of rapamycin (TOR) was originally discovered in budding yeast, as the mediator of the toxic effects of the bacterial macrolide rapamycin (Kunz et al., 1993), and later the mammalian counterpart, mTOR, was identified (Sabatini et al., 1994). The mTOR protein is a 289-kDa serine/threonine kinase that belongs to the PI 3-K-related kinase family. By differential interaction with an array of adaptors, mTOR operates as the catalytic subunit of two distinct complexes, namely mTORC1 and mTORC2, which differ greatly in terms of substrate specificity, functions and drug sensitivity (Figure 3). When complexed with Raptor (regulatory associated protein of mTOR), mLST8 (mammalian lethal with Sec13 protein 8) also known as GβL, PRAS40 (proline-rich rich Akt substrate 40kDa) and Deptor (DEP-domain-containing mTOR interacting protein), mTOR constitutes the rapamycin-sensitive mTORC1 complex. Alternatively, mTOR can assemble with mLST8 and Deptor (also common to mTORC1), mSIN1 (mammalian stress-activated protein kinase interacting protein), Protor-1 (protein observed with Rictor-1) and Rictor (rapamycin insensitive-companion of mTOR), producing the rapamycin-insensitive complex mTORC2 (Laplante and Sabatini, 2012).
Figure 3.

Components of the two mTOR complexes. mTORC1 is composed of mTOR, mLST8, raptor and PRAS40, and is sensitive to rapamycin. Besides mLST8, the rapamycin-insensitive mTORC2 complex contains mTOR in association with rictor, mSIN1 and PRR5.
Relatively little is known regarding the regulation of mTORC2. Its activity is controlled primarily by PI 3-K, and unlike mTORC1 is largely insensitive to nutrients or energy conditions (Alessi et al., 2009). Moreover, it is known that in vitro Rictor is required for mTORC2 to function as the Ser473 kinase (Sabatini, 2006). Very recently two mechanisms have been uncovered that may regulate mTORC2 activity: first, phosphorylation of SIN1 at Ser260 by mTOR itself prevents SIN1 lysosomal degradation and in turn preserves mTORC2 complex integrity (Chen et al., 2013); and second, GSK-3β-mediated rictor Ser1235 phosphorylation results in mTORC2 inactivation by interfering with substrate binding ability (Chen et al., 2011). Interestingly, Akt Ser473 phosphorylation in response to genotoxic stimuli has been studied in considerable detail, and has been attributed to a member of the PI 3-K-like family of kinases, the DNA-dependent protein kinase DNA-PK. In this context, DNA damage recruits DNA-PK directly to sites of double strand breaks and triggers phosphorylation of Akt at Ser473 (Bozulic et al., 2008, Feng et al., 2004). Regardless of the precise mechanism, once fully phosphorylated Akt loses the lipid-binding requirement, is locked into the catalytically-competent conformation and may translocate to discrete intracellular locations where specific substrates are located. By candidate screening approaches or by whole phosphoproteomic mass spectrometry sequencing technologies, well over 200 Akt substrates have been identified to date, that contain the optimal Akt consensus motif RxxRxS/T (where R is Arg, S is Ser, T is Thr and x is any amino acid), often followed by a +1 hydrophobic residue, and by a +2 Pro residue that provides an optimal consensus for binding to 14-3-3 proteins (Manning and Cantley, 2002, Moritz et al., 2010).
Signal Termination
Negative regulation of Akt is primarily accomplished by protein phosphatases.
PP2A
Protein phosphatase 2, PP2A, is known to dephosphorylate Thr308. It comprises a large family of phosphatases with a highly regulated, well-conserved catalytic subunit and two additional subunits: one operates as a scaffold, while the other which comprises many family members, confers substrate specificity, subcellular localization and catalytic activity of the holoenzymes (Lambrecht et al., 2013) (Figure 2).
PHLPP
The enzyme responsible for dephosphorylation of Ser473 is attributed to a family of novel protein phosphatases, namely PHLPP for PH domain and leucine-rich repeat protein phosphatase (Gao et al., 2005) (Figure 2). Importantly, it has been shown that PHLPP differentially terminates Akt signaling by regulating distinct Akt isoforms. PHLPP2 dephosphorylates Akt1 and Akt3, whereas PHLPP1 is specific for Akt2 and Akt3 (Brognard et al., 2007). PHLPP has been demonstrated to dephosphorylate only the hydrophobic motif of AGC kinases Akt, PKC and S6K, tough it is possible that many more substrates exist. Notably, PHLPP1 can be phosphorylated by GSK-3β. In its phosphorylated form, it is recruited by the E3 ligase βTrCP, and undergoes ubiquitination and degradation. Because GSK-3β activity is inhibited by Akt, Akt can upregulate PHLPP levels by suppressing GSK-3β-dependent degradation (Warfel et al., 2011). Thus, Akt can mitigate its own activity through this feedback loop. Moreover, the mTORC1 target S6K1 upregulates PHLPP expression (Newton and Trotman, 2014). Since the PI3K/Akt/mTOR pathway harbors genes that are frequently mutated in many human solid and liquid malignancies, numerous small molecule inhibitors targeting enzymes in this pathway are undergoing pre-clinical and clinical evaluation. Therefore, these feedback loops should be taken into account to avoid mitigating drug efficacy.
PTEN
Akt activity is also regarded as a physiological, albeit indirect, target of the dual lipid and protein phosphatase and Tensin homologue (PTEN). PTEN specifically dephosphorylates the 3′-position of the inositol ring in PtdIns-3,4,5-P3, thereby resulting in PtdIns-4,5-P2. Decreased PtdIns-3,4,5-P3 ultimately results in inhibition of Akt activity (Barata, 2011, Leslie et al., 2009). Importantly, inactivating mutations or loss of PTEN expression leads to a constitutive activation of Akt signaling, characterized by increased cell proliferation and resistance to apoptosis. In addition, codeletion of PHLPP1 and PTEN is strongly associated with metastatic prostate cancer (Newton and Trotman, 2014).
Akt signaling specificity
In spite of our detailed understanding of the mechanisms by which Akt is regulated and the large body of evidence on the diverse functions of Akt, our understanding of how Akt activity drives discrete intracellular responses remains incomplete. Selectivity might be reached, at least in part, through tissue-specific expression, spatial segregation at distinct subcellular locations, and phosphorylation of isoform-specific substrates. Given that Akt is regarded as a promising therapeutic target in a number of pathologies, it is essential to dissect the relative contribution of each isoform to pathophysiological function.
Selectivity by location
Since its discovery, it has been known that upon activation at the plasma membrane Akt redistributes throughout many cellular compartments, and association of Akt isoforms with specific organelles or compartments has been described. In particular, both the presence and the activity of Akt in the nucleus has been widely documented (Martelli et al., 2012). A genetically-encoded fluorescent reporter for Akt activity allowing for real-time imaging of phosphorylation catalyzed by Akt has been described (Kunkel et al., 2005). In this study Akt signaling in the nucleus was found to be less rapid but more sustained compared with Akt activity in the cytosol, suggesting that Akt signaling in these two compartments is regulated by different mechanisms. Whether or not Akt needs to be phosphorylated for nucleus translocation is still debated. Expression of an unphosphorylatable Akt T308A/S473A mutant has been suggested to impair nuclear relocation in PC12 cells exposed to Nerve Growth Factor (NGF), indicating that Akt phosphorylation is a pre-requisite for translocation (Xuan Nguyen et al., 2006). Conversely, the same mutants are localized to nuclei of HEK293 cells (Saji et al., 2005). Predictably, these discrepancies are most likely cell type and/or stimulus-dependent, but raise the rather controversial issue of whether Akt can be phosphorylated within the nucleus. Phosphoinositide signaling has been demonstrated to occur in the nucleus (Keune et al., 2011), where all the molecular components necessary for Akt phosphorylation at Thr 308 and at Ser 473, including PI 3-K (Bavelloni et al., 1999), PtdIns-4,5-P2 (Follo et al., 2014, Follo et al., 2013), PtdIns-3,4,5-P3 (Neri et al., 2002), PDK1 (Scheid et al., 2005) and mTORC2 (Rosner and Hengstschlager, 2012) have been identified. Such a mechanism may therefore sustain Akt phosphorylation in the nucleus. Further evidence for such a mechanism comes from studies in cardiomyocytes (Shiraishi et al., 2004). However, separate studies indicate that Akt translocates to the nucleus subsequent to phosphorylation at the plasma membrane (Andjelkovic, Alessi, 1997) and that nuclear PDK1 is dispensable for Akt phosphorylation at Thr 308. Rather PDK1 nuclear translocation is suggested to be a mechanism to sequester PDK1 from the cytoplasm.
The molecular determinants required for Akt translocation remain obscure. Association to specific binding partners or scaffolding proteins may be crucial for intracellular redistribution. Work by our laboratories and others has demonstrated that in response to specific signals, Akt is targeted to the cytoskeleton by direct interaction of its amino-terminal PH domain with actin, and that this process is regulated by the small GTP-binding proteins Cdc42 and Rac (Cenni et al., 2003). This mechanism is suggested to contribute to Akt redistribution to diverse subcellular locations, such as the nucleus. A distinct mechanism that mediates Akt nuclear translocation has been described in human mature T-cell leukemia: the product of TCL1 gene, Tcl1, interacts with the PH domain of phosphorylated Akt, thus driving Akt to the nucleus (Pekarsky et al., 2000). In this context, Tcl1 may act to directly translocate Akt or may contribute to the formation of a complex that promotes the transport of active Akt to the nucleus.
Lamins are type V intermediate filaments proteins found in the nucleus of higher eukaryotes where, together with lamin-binding proteins, form the lamina at the nuclear envelope and provide mechanical stability for the nuclear membrane. Lamins belong to two major groups, the B and the A type lamins, which differ in their primary amino acid sequence (Dechat et al., 2009, Marmiroli et al., 2009). While lamin B appears to play an housekeeping role, lamin A is mostly expressed in differentiated cells, suggesting involvement in tissue-specific differentiation and/or function (Maraldi et al., 2010). Lamins are also found in stable complexes in the nuclear matrix where they are involved in numerous functions, including DNA replication, RNA polymerase II-dependent transcription, chromatin organization, spacing of nuclear pores and possibly RNA splicing. Our work has shown that Akt1 phosphorylates lamin A/C at S404, in a canonical Akt consensus motif (Cenni et al., 2008). Remarkably, a somatic mutation of an Arg at-3 in the Akt consensus motif has been described in Emery Dreifuss muscular dystrophy syndrome (R401C, AD-EDMD) (Mall et al., 2012, Morris, 2001). By using an anti-pSer404 antibody, we showed that mutation of this residue is sufficient to abrogate lamin A Ser404 phosphorylation in primary cells from AD-EDMD-patients harboring this mutation as well as in normal primary fibroblasts expressing Arg401Cys lamin A. Moreover, expression of a non–phosphorylatable mutant lamin Ser404Ala in cultured fibroblasts is sufficient to induce the AD-EDMD phenotype, characterized by fragile, misshapen nuclei, with blebs and loss of peripheral heterochromatin. Further studies showed that Ser404 phosphorylation of the precursor prelamin A peaks at the G2/M phase of the cell cycle, concomitantly with the peak of Akt activity. This observation is particularly interesting when considering that Akt activation is essential to overcome the G2/M checkpoint (Kandel et al., 2002) and that lamin phosphorylation is a key event in the mitotic breakdown of the nuclear lamina (Eriksson et al., 2009). Additional studies established that pSer404-lamin A undergoes rapid lysosomal degradation, leading to the conclusion that Akt-dependent phosphorylation of prelamin A triggers its degradation in cells undergoing division or approaching mitosis when the nuclear lamina disassembles (Bertacchini et al., 2013). Unexpectedly, Akt not only prompts lamin A degradation, but also modulates its expression. Indeed, Akt1 silencing by specific shRNAs leads to a marked increase in Lmna expression. Thus, Akt1 balances prelamin A content at G2/M through increased degradation as well as transcription repression (Bertacchini, Beretti, 2013).
Selectivity by isoform-substrate specificity
Akt isoforms were initially assumed to have fully redundant functions. Although it is clear that they can compensate for one other to some extent, in vivo studies with single isoform knock-out mice have assigned non-overlapping roles to specific Akt family members (Bae et al., 2003, Cho et al., 2001b). Specifically, Akt1 null mice are characterized by growth retardation and perinatal lethality (Chen et al., 2001, Cho, Thorvaldsen, 2001b). Akt2 knockout mice develop an insulin-resistant diabetes-like syndrome (Cho et al., 2001a, Garofalo et al., 2003). Akt3 null mice display a decrease in brain size (Dummler et al., 2006, Tschopp et al., 2005). Further insight has come came from mice with combined disruption of Akt isoforms. For example, mice with combined disruption of Akt1 and Akt2 die shortly after birth and are characterized by weakened skin and bone development, severe skeletal muscle atrophy and reduced adipogenesis (Dummler, Tschopp, 2006). Conversely, Akt1/Akt3 double-knockout induces embryonic lethality caused by severe developmental defects in the cardiovascular and nervous systems (Dummler, Tschopp, 2006).
Non-redundant Akt functions have also been explored also in vitro, with the combined use of short hairpin interfering RNAs (shRNA) and isoform-specific inhibitors, allowing for the identification of proteins phosphorylated only a specific isoform. In an effort to shed additional light into Akt-mediated signaling in muscle differentiation, regeneration and hypertrophy, Cenni and colleagues have shown that Ankrd2 is an Akt2-specific substrate (Cenni et al., 2011). Ankrd2 is a member of the Muscle Ankyrin Repeat Protein family, expressed both in skeletal and cardiac muscle fibers, with regulatory and structural roles in muscle stress response pathways. In addition to a well-documented role in mechanostransduction where Ankrd2 participates in the machinery that regulates the protection of the fibers after muscle injury during muscle differentiation, Akt-phosphorylated Ankrd2 contributes to the regulation of the balance between proliferation and apoptosis of differentiating myoblasts and stabilizes forming myotubes (Cenni, Bavelloni, 2011).
Akt isoform specificity in breast cancer signaling and progression
Much information on Akt isoform signaling specificity has been defined by the role of Akt1, Akt2 and Akt3 in breast cancer phenotypes. Initial studies using antibody micro-injection revealed that Akt1, but not Akt2, plays an essential role in the G1/S cell cycle transition, leading to proliferation (Heron-Milhavet et al., 2006). This finding was later confirmed by shRNA targeting specific isoforms(Vandromme et al., 2001). However, these studies are likely to be entirely context dependent, and so it is predicted that the specific role of a given Akt isoform in cell growth and proliferation will depend on the expression pattern, localization and genetic background. Nonetheless, at least in the contact of breast cancer, a clear picture has emerged on the specific role of Akt1 and Akt2 in modulating phenotypes associated with cell migration leading to metastatic dissemination. The notion that Akt1 may act as a migration and metastasis suppressor was first recognized by the Muller laboratory who found that in transgenic mice harboring a mammary-specific activated Akt1 transgene, tumorigenesis is accelerated, whereas metastatic dissemination is actually decreased compared to control mice (Hutchinson et al., 2004). Similar findings have since been reported by many groups, using either distinct Akt1 and Akt2 transgenes, or Akt1 and Akt2 knockout mice, or even mice xenografted with breast cancer cell lines harboring Akt1 and Akt2 specific shRNA (Chin and Toker, 2009, Toker, 2012). One of the clearest indications of Akt1 and Akt2 specificity in breast cancer progression was revealed by the Tsichlis group who showed using Akt1 and Akt2 null mice that Akt1 promotes, whereas Akt2 inhibits, mammary tumor induction and growth (Maroulakou et al., 2007). Conversely, Akt2 has been shown to function primarily in metastatic dissemination (Dillon et al., 2009). Although a role for Akt3 in breast cancer progression has not been thoroughly evaluated, a recent study from our group showed that Akt3 is amplified in basal-like, triple negative breast cancer cell lines, and that Akt3 is required for TNBC growth in vitro and in vivo, whereas Akt1 and Akt2 are dispensable (Chin et al., 2014).
The process of metastatic dissemination is dependent on multiple genetic and epigenetic alterations, as well as induction of specific signaling events that culminate in the modulation of phenotypes that result in invasion to distant organs. One of these in the Epithelial to Mesenchymal transition (EMT), a phenotype that is intimately associated with changes in epithelial and mesenchymal markers such as E-cadherin. Moreover, EMT is also associated with profound changes in the migratory capacity of tumor cells, and Akt isoforms have been shown modulate both cell migration and EMT. For example, levels of phosphorylated, hence activated Akt are highest in metastatic breast cancer cells (Qiao et al., 2007). Akt1 has been show to suppress migration, whereas Akt2 can enhance the same phenotype in multiple breast cancer cell lines in vitro (Irie et al., 2005, Liu et al., 2006, Yoeli-Lerner et al., 2005). These data are consistent with in vivo findings that have highlighted an anti-metastatic function for Akt1 and a pro-metastatic role for Akt2. The specific mechanism that account for the non-redundant role of Akt1 and Akt2 in modulating cell, migration and EMT are likely to be many-fold, but a number of examples are noteworthy. For example, the miR-200 family of micro-RNAs has been shown to be regulated by the balance of Akt1 and Akt2 expression and in turn modulate EMT in breast epithelial cells (Iliopoulos et al., 2009). Moreover, our laboratory has provided evidence for Akt1-isoform specific substrates that account for the anti-migratory activity of this isoform. Akt1 specifically leads to the degradation of the NFAT transcription factor, which control the transcriptional induction of genes that enhances cell migration in breast cancer cells (Yoeli-Lerner, Yiu, 2005). Moreover, the actin regulatory protein palladin is an Akt1-specific substrate whose phosphorylation by Akt1 modulates its actin bundling activity leading to inhibition of cell migration (Chin and Toker, 2010). Conversely, upregulation of integrin beta subunits by Akt2 has been proposed to account for its pro-migratory activity (Arboleda et al., 2003). There likely exist many other Akt1- and Akt2-specific substrates that account for the specific role of Akt isoforms in modulating breast cancer progression. Indeed, a number of Akt1- and Akt2-specific substrates have been identified in a number of settings, although it is noteworthy that to no Akt3-soecific substrates have been identified. Such information will likely be gained from global phosphoproteomic screens using cell lines in which individual Akt isoforms have been knocked out using homologous recombination strategies, or inducible shRNA targeting. Indeed, one such approach recently made use of MEFs null for either Akt1, Akt2 or Akt3, and described the discovery of IWS1, an RNA processing regulator that is phosphorylated by Akt1 and Akt3 (Sanidas et al., 2014).
Concluding Remarks
Akt isoform-specific functions are predicted to be entirely context dependent. This is perhaps best illustrated by the finding that while Akt1 suppresses breast cancer cell migration and Akt2 enhances, in fibroblasts the exact opposite is observed, whereby Akt1 enhances fibroblast cell migration whereas Akt2 suppresses (Zhou et al., 2006). The precise mechanism by which Akt isoforms differentially regulate substrate phosphorylation in a context-dependent manner remains to be fully elucidated, but some observation are noteworthy. First, the localization of distinct Akt isoforms in a given cell and tissue is likely to be completely different. Akt isoforms have been localized in their activated state to virtually all cellular compartments, including the cytoplasm, the nucleus, the plasma membrane the Golgi and even mitochondria. This promiscuous localization will profoundly affect the accessibility of a specific substrate to a specific Akt isoform, and thus account for substrate phosphorylation selectivity. At the same time, intermolecular determinants on Akt isoforms may also afford specificity. For example, the PH and linker regions of Akt12 and Akt2 have been shown to dictate both phenotypic specificity as well as substrate phosphorylation, a finding illustrated by the use of chimeras of Akt1 and Akt2 domains in in vitro studies (Chin and Toker, 2010, Wang and Basson, 2008). However, very little is known concerning docking motifs or scaffolding proteins that may determine Akt specificity at the levels of localization and substrate access. This is an area of study that requires considerable more investigation, and with the advent of highly specific tools for Akt isoforms, such as specific antibodies and phosphorylation state specific antibodies, specific shRNAs, and knockout cells, much progress is anticipated in this field.
Acknowledgments
The authors would like to thank all the members of their respective laboratories, past and present, for their contributions. Work in the Toker laboratory is funded in part by the National Institutes of Health, the National Cancer Institute, the Department of Defense Breast Cancer Research Program and the Susan G. Komen Breast Cancer Foundation. Work in the Marmiroli laboratory is funded by Istituto Superiore Sanita’ oncoproteome network, prot. 2011-527TR1 and by MIUR PRIN.
Footnotes
Conflict of Interest
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Pearce LR, Garcia-Martinez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2:pe27. doi: 10.1126/scisignal.267pe27. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, et al. Role of translocation in the activation and function of protein kinase B. The Journal of biological chemistry. 1997;272:31515–24. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, et al. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer research. 2003;63:196–206. [PubMed] [Google Scholar]
- Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. The Journal of biological chemistry. 2003;278:49530–6. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- Barata JT. The impact of PTEN regulation by CK2 on PI3K-dependent signaling and leukemia cell survival. Advances in enzyme regulation. 2011;51:37–49. doi: 10.1016/j.advenzreg.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Bavelloni A, Santi S, Sirri A, Riccio M, Faenza I, Zini N, et al. Phosphatidylinositol 3-kinase translocation to the nucleus is induced by interleukin 1 and prevented by mutation of interleukin 1 receptor in human osteosarcoma Saos-2 cells. Journal of cell science. 1999;112 (Pt 5):631–40. doi: 10.1242/jcs.112.5.631. [DOI] [PubMed] [Google Scholar]
- Bertacchini J, Beretti F, Cenni V, Guida M, Gibellini F, Mediani L, et al. The protein kinase Akt/PKB regulates both prelamin A degradation and Lmna gene expression. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2013;27:2145–55. doi: 10.1096/fj.12-218214. [DOI] [PubMed] [Google Scholar]
- Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBa/Akt1 Acts Downstream of DNA-PK in the DNA Double-Strand Break Response and Promotes Survival. Molecular cell. 2008 doi: 10.1016/j.molcel.2008.02.024. In Press. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Molecular cell. 2007;25:917–31. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Calleja V, Laguerre M, Larijani B. Role of the C-terminal regulatory domain in the allosteric inhibition of PKB/Akt. Advances in biological regulation. 2012;52:46–57. doi: 10.1016/j.advenzreg.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- Cenni V, Bavelloni A, Beretti F, Tagliavini F, Manzoli L, Lattanzi G, et al. Ankrd2/ARPP is a novel Akt2 specific substrate and regulates myogenic differentiation upon cellular exposure to H(2)O(2) Molecular biology of the cell. 2011;22:2946–56. doi: 10.1091/mbc.E10-11-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenni V, Bertacchini J, Beretti F, Lattanzi G, Bavelloni A, Riccio M, et al. Lamin A Ser404 is a nuclear target of Akt phosphorylation in C2C12 cells. J Proteome Res. 2008;7:4727–35. doi: 10.1021/pr800262g. [DOI] [PubMed] [Google Scholar]
- Cenni V, Sirri A, Riccio M, Lattanzi G, Santi S, de Pol A, et al. Targeting of the Akt/PKB kinase to the actin skeleton. Cell Mol Life Sci. 2003;60:2710–20. doi: 10.1007/s00018-003-3349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TO, Tsichlis PN. PDK2: a complex tail in one Akt. Science’s STKE: signal transduction knowledge environment. 2001;2001:pe1. doi: 10.1126/stke.2001.66.pe1. [DOI] [PubMed] [Google Scholar]
- Chen CH, Kiyan V, Zhylkibayev AA, Kazyken D, Bulgakova O, Page KE, et al. Autoregulation of the mechanistic target of rapamycin (mTOR) complex 2 integrity is controlled by an ATP-dependent mechanism. The Journal of biological chemistry. 2013;288:27019–30. doi: 10.1074/jbc.M113.498055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Shaikenov T, Peterson TR, Aimbetov R, Bissenbaev AK, Lee SW, et al. ER stress inhibits mTORC2 and Akt signaling through GSK-3beta-mediated phosphorylation of rictor. Sci Signal. 2011;4:ra10. doi: 10.1126/scisignal.2001731. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes & development. 2001;15:2203–8. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cellular signalling. 2009;21:470–6. doi: 10.1016/j.cellsig.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Toker A. The Actin-Bundling Protein Palladin Is an Akt1-Specific Substrate that Regulates Breast Cancer Cell Migration. Molecular cell. 2010;38:333–44. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Yoshida T, Marusyk A, Beck AH, Polyak K, Toker A. Targeting Akt3 signaling in triple-negative breast cancer. Cancer research. 2014;74:964–73. doi: 10.1158/0008-5472.CAN-13-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001a;292:1728–31. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. The Journal of biological chemistry. 2001b;276:38349–52. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Dechat T, Adam SA, Goldman RD. Nuclear lamins and chromatin: when structure meets function. Advances in enzyme regulation. 2009;49:157–66. doi: 10.1016/j.advenzreg.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer research. 2009;69:5057–64. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Molecular and cellular biology. 2006;26:8042–51. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, et al. Introducing intermediate filaments: from discovery to disease. The Journal of clinical investigation. 2009;119:1763–71. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. The Journal of biological chemistry. 2004;279:41189–96. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- Follo MY, Faenza I, Piazzi M, Blalock WL, Manzoli L, McCubrey JA, et al. Nuclear PI-PLCbeta1: an appraisal on targets and pathology. Advances in biological regulation. 2014;54:2–11. doi: 10.1016/j.jbior.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Follo MY, Marmiroli S, Faenza I, Fiume R, Ramazzotti G, Martelli AM, et al. Nuclear phospholipase C beta1 signaling, epigenetics and treatments in MDS. Advances in biological regulation. 2013;53:2–7. doi: 10.1016/j.jbior.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Molecular cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. The Journal of clinical investigation. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochimica et biophysica acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harbor perspectives in biology. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron-Milhavet L, Franckhauser C, Rana V, Berthenet C, Fisher D, Hemmings BA, et al. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Molecular and cellular biology. 2006;26:8267–80. doi: 10.1128/MCB.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer research. 2004;64:3171–8. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K, et al. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. The Journal of cell biology. 2005;171:1023–34. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, et al. Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Molecular and cellular biology. 2002;22:7831–41. doi: 10.1128/MCB.22.22.7831-7841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keune W, Bultsma Y, Sommer L, Jones D, Divecha N. Phosphoinositide signalling in the nucleus. Advances in enzyme regulation. 2011;51:91–9. doi: 10.1016/j.advenzreg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Kunkel MT, Ni Q, Tsien RY, Zhang J, Newton AC. Spatio-temporal dynamics of protein kinase B/Akt signaling revealed by a genetically encoded fluorescent reporter. The Journal of biological chemistry. 2005;280:5581–7. doi: 10.1074/jbc.M411534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J, Henriquez R, Schneider U, Deuter RM, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73:585–96. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Lambrecht C, Haesen D, Sents W, Ivanova E, Janssens V. Structure, regulation, and pharmacological modulation of PP2A phosphatases. Methods Mol Biol. 2013;1053:283–305. doi: 10.1007/978-1-62703-562-0_17. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie NR, Maccario H, Spinelli L, Davidson L. The significance of PTEN’s protein phosphatase activity. Advances in enzyme regulation. 2009;49:190–6. doi: 10.1016/j.advenzreg.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA, et al. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4134–9. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan D, Powis G, Mash EA, George B, Gokhale VM, Zhang S, et al. Discovery of a novel class of AKT pleckstrin homology domain inhibitors. Mol Cancer Ther. 2008;7:2621–32. doi: 10.1158/1535-7163.MCT-07-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Walter T, Gorjanacz M, Davidson IF, Nga Ly-Hartig TB, Ellenberg J, et al. Mitotic lamin disassembly is triggered by lipid-mediated signaling. The Journal of cell biology. 2012;198:981–90. doi: 10.1083/jcb.201205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Hitting the target: emerging technologies in the search for kinase substrates. Science’s STKE: signal transduction knowledge environment. 2002;2002:PE49. doi: 10.1126/stke.2002.162.pe49. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraldi NM, Lattanzi G, Cenni V, Bavelloni A, Marmiroli S, Manzoli FA. Laminopathies and A-type lamin-associated signalling pathways. Advances in enzyme regulation. 2010;50:248–61. doi: 10.1016/j.advenzreg.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Marmiroli S, Bertacchini J, Beretti F, Cenni V, Guida M, De Pol A, et al. A-type lamins and signaling: the PI 3-kinase/Akt pathway moves forward. Journal of cellular physiology. 2009;220:553–61. doi: 10.1002/jcp.21807. [DOI] [PubMed] [Google Scholar]
- Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer research. 2007;67:167–77. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Tabellini G, Bressanin D, Ognibene A, Goto K, Cocco L, et al. The emerging multiple roles of nuclear Akt. Biochimica et biophysica acta. 2012;1823:2168–78. doi: 10.1016/j.bbamcr.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Moritz A, Li Y, Guo A, Villen J, Wang Y, MacNeill J, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Science signaling. 2010;3:ra64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GE. The role of the nuclear envelope in Emery-Dreifuss muscular dystrophy. Trends in molecular medicine. 2001;7:572–7. doi: 10.1016/s1471-4914(01)02128-1. [DOI] [PubMed] [Google Scholar]
- Neri LM, Bortul R, Tabellini G, Borgatti P, Baldini G, Celeghini C, et al. Erythropoietin-induced erythroid differentiation of K562 cells is accompanied by the nuclear translocation of phosphatidylinositol 3-kinase and intranuclear generation of phosphatidylinositol (3,4,5) trisphosphate. Cellular signalling. 2002;14:21–9. doi: 10.1016/s0898-6568(01)00224-8. [DOI] [PubMed] [Google Scholar]
- Newton AC, Trotman LC. Turning off AKT: PHLPP as a drug target. Annual review of pharmacology and toxicology. 2014;54:537–58. doi: 10.1146/annurev-pharmtox-011112-140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh C, Janakiraman V, Wu WI, Foo CK, Kljavin NM, Chaudhuri S, et al. Disruption of PH-kinase domain interactions leads to oncogenic activation of AKT in human cancers. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19368–73. doi: 10.1073/pnas.1204384109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Koval A, Hallas C, Bichi R, Tresini M, Malstrom S, et al. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3028–33. doi: 10.1073/pnas.040557697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RT, Schreiber SL. Kinase phosphorylation: Keeping it all in the family. Curr Biol. 1999;9:R521–4. doi: 10.1016/s0960-9822(99)80326-1. [DOI] [PubMed] [Google Scholar]
- Qiao M, Iglehart JD, Pardee AB. Metastatic potential of 21T human breast cancer cells depends on Akt/protein kinase B activation. Cancer research. 2007;67:5293–9. doi: 10.1158/0008-5472.CAN-07-0877. [DOI] [PubMed] [Google Scholar]
- Rosner M, Hengstschlager M. Detection of cytoplasmic and nuclear functions of mTOR by fractionation. Methods Mol Biol. 2012;821:105–24. doi: 10.1007/978-1-61779-430-8_8. [DOI] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Saji M, Vasko V, Kada F, Allbritton EH, Burman KD, Ringel MD. Akt1 contains a functional leucine-rich nuclear export sequence. Biochemical and biophysical research communications. 2005;332:167–73. doi: 10.1016/j.bbrc.2005.04.109. [DOI] [PubMed] [Google Scholar]
- Sanidas I, Polytarchou C, Hatziapostolou M, Ezell SA, Kottakis F, Hu L, et al. Phosphoproteomics screen reveals akt isoform-specific signals linking RNA processing to lung cancer. Molecular cell. 2014;53:577–90. doi: 10.1016/j.molcel.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Parsons M, Woodgett JR. Phosphoinositide-dependent phosphorylation of PDK1 regulates nuclear translocation. Molecular and cellular biology. 2005;25:2347–63. doi: 10.1128/MCB.25.6.2347-2363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, et al. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circulation research. 2004;94:884–91. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- Toker A. Achieving specificity in Akt signaling in cancer. Advances in biological regulation. 2012;52:78–87. doi: 10.1016/j.advenzreg.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, et al. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–54. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- Vandromme M, Rochat A, Meier R, Carnac G, Besser D, Hemmings BA, et al. Protein kinase B beta/Akt2 plays a specific role in muscle differentiation. The Journal of biological chemistry. 2001;276:8173–9. doi: 10.1074/jbc.M005587200. [DOI] [PubMed] [Google Scholar]
- Wang S, Basson MD. Identification of functional domains in AKT responsible for distinct roles of AKT isoforms in pressure-stimulated cancer cell adhesion. Experimental cell research. 2008;314:286–96. doi: 10.1016/j.yexcr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel NA, Niederst M, Stevens MW, Brennan PM, Frame MC, Newton AC. Mislocalization of the E3 ligase, beta-transducin repeat-containing protein 1 (beta-TrCP1), in glioblastoma uncouples negative feedback between the pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1) and Akt. The Journal of biological chemistry. 2011;286:19777–88. doi: 10.1074/jbc.M111.237081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Nguyen TL, Choi JW, Lee SB, Ye K, Woo SD, Lee KH, et al. Akt phosphorylation is essential for nuclear translocation and retention in NGF-stimulated PC12 cells. Biochemical and biophysical research communications. 2006;349:789–98. doi: 10.1016/j.bbrc.2006.08.120. [DOI] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Molecular cell. 2005;20:539–50. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. The Journal of biological chemistry. 2006;281:36443–53. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]