Abstract
While rotator cuff repair is often successful at relieving pain, the repaired insertion site can fail. The repaired mechanical properties improved when the shoulder was immobilized in an animal model, but joint stiffness and range of motion were not evaluated. This study objective was to measure rotational mechanics before and after shoulders were immobilized following cuff injury/repair, not immobilized following cuff injury/repair, and immobilized without injury/repair. Humeral rotation was significantly less 4 and 8 weeks following injury/repair but did not decrease significantly when the injured/repaired shoulder was immobilized. Rotational stiffness increased significantly 4 and 8 weeks following injury/repair and was significantly greater at 4, but not 8, weeks when the injured/repaired shoulders were immobilized. This study demonstrates that the increase in joint stiffness caused by immobilizing an injured/repaired shoulder is transient and, therefore, does not outweigh the long-term benefits of immobilization on improved tendon to bone healing.
INTRODUCTION
A majority of the population over the age of 50 have tears in the rotator cuff tendons that are associated with pain and lost shoulder function (5). These tears typically involve the supraspinatus and are usually avulsions of the tendon from the bony insertion (7). Surgery is required to repair the tendon to bone, and while these surgeries are often successful at relieving pain, they continue to have a high rate of repair failure (20-50%, 94%) (2, 6). A variety of studies have identified age, tear size, and time from injury to repair as key factors in predicting the likelihood of repair failure, however, only recently has the effect of post-operative activity level on tendon to bone healing been investigated.
In a recent study in our laboratory, immobilization improved the biological and mechanical properties of the repaired supraspinatus insertion site in rats compared to either cage activity or exercise (18). This suggests that a healing insertion site benefits from the limited mechanical activity imposed by joint immobilization. This finding is indirectly supported by other tendon to bone healing animal studies in the shoulder (infraspinatus repair) and the knee (ACL reconstruction) in which reducing post-operative activity reduced the number of repair failures (4, 15). Thus, immobilization may benefit insertion site healing in the shoulder as it does bone healing following fracture or joint reconstruction. However, to date, it is unknown whether or not immobilization will also permanently increase joint stiffness following cuff repair.
Joint stiffness is of particular importance following surgical repair, since several human and animal studies in the hand have clearly shown that adhesions associated with repair surgery (typically flexor tendon laceration) increase joint stiffness when the hand is immobilized (3). There are, however, several significant differences between flexor and rotator cuff tendons, which impact how adhesions that form after injury and repair would affect joint mechanics, as well as how immobilization modulates this effect. First, the flexor tendons of the hand are surrounded in a synovial sheath but the cuff tendons of the shoulder are not. Flexor tendons produce primarily one degree of rotation (hinge joint), while the cuff tendons produce three degrees of rotation (ball and socket joint). Last, repairing most flexor tendon tears requires tendon to tendon healing, while repairing most rotator cuff tears requires tendon to bone healing. All of these differences warrant an investigation of immobilization on joint stiffness and range of motion in the shoulder following cuff repair. While several clinical studies prior to 1980, when immobilization was standard practice following cuff repair, have documented that the increase in shoulder stiffness was transient (12), (14), (9), (1), such studies made no attempt to quantify joint stiffness, nor has any study measured joint stiffness and range of motion following cuff repair surgery in a controlled animal model.
The objective of this study, therefore, was to determine if immobilization increased joint stiffness and reduced range of motion following cuff injury/repair surgery in a rat model. To meet this objective, we constructed a device capable of measuring passive shoulder mechanics in-vivo before and after one of the following three conditions: cuff injury/repair without immobilization, cuff injury/repair with immobilization, and immobilization without cuff injury/repair. We hypothesized that: 1) joint stiffness would increase and range of motion would decrease initially but return to uninjured values for all conditions and 2) joint stiffness would increase and range of motion would decrease the most for the group immobilized following cuff injury/repair at 4 weeks but would not be different at 8 weeks.
MATERIALS AND METHODS
Animal study
A total of 15 Sprague-Dawley rats were used, and all procedures were approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee. Animals were randomly assigned to one of the following three groups (5 animals per condition), where a single shoulder was either not immobilized following injury and repair (REP-CTL), immobilized immediately after injury and repair (REP-IMM), or immobilized with no injury (IMM-CTL). Rotator cuff injury and repair was done using methods described previously (17). Briefly, the supraspinatus was detached from the bony insertion, the remaining fibro-cartilage was removed with a burr, and the tendon was repaired to the original insertion site with suture (5.0 proline) using a modified Mason-Allen technique passed through a transverse bone tunnel. Animals in the REP-CTL group were returned to caged activity immediately following surgery, while the repaired shoulders of animals in the REP-IMM group were immobilized in approximately 90 degrees of forward flexion and 90 degrees of abduction in a plaster cast for 4 weeks (17). The shoulder of animals in the IMM-CTL group was also immobilized with a cast in the same position for 4 weeks but was otherwise uninjured. For both REP-IMM and IMM-CTL groups, animals were allowed free caged activity once the cast was removed, and within a week of cast removal, there was no apparent difference in animal activity. Passive shoulder mechanics were measured in all animals before and 4 and 8 weeks following initiation of treatment, using a custom device described in the following section. It should be noted that for repaired and uninjured immobilized shoulders, 4 week measurements were taken immediately after the cast was removed.
Shoulder stiffness device
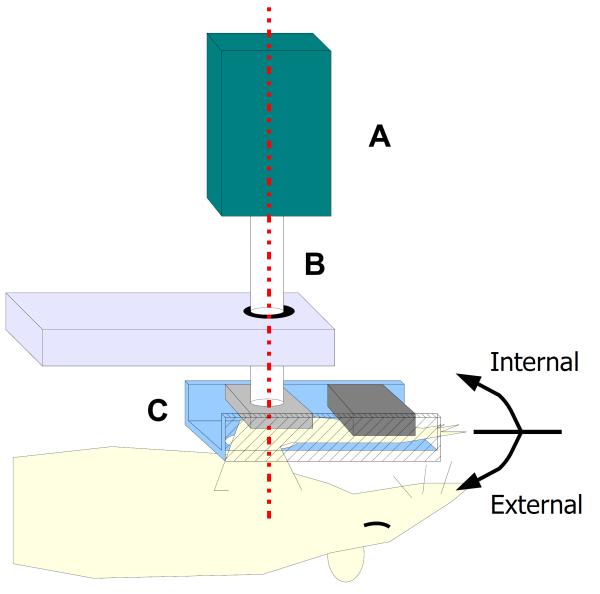
The shoulder stiffness device consisted of a sensor assembly, a pivoting axle, and an arm clamp (parts A-C in figure 1). The sensor assembly (part A in figure 1) contained a 3 degree of freedom orientation sensor (accuracy 0.01° Microstrain 3DM-DH) as well as a single degree of freedom miniature torque cell (accuracy ± 0.1 N-mm Futek T5175). The sensor assembly was secured to the pivoting axle (part B in figure 1) such that the sensing axis was aligned with the rotation axis. The axle passed through a low-friction ball-bearing joint and terminated in the arm clamp. This clamp held the animal’s arm such that the sensing axis coincided with the long axis of the animal’s humerus (dotted line in figure 1). In this way, rotation of the sensor assembly, restricted to a single degree of freedom through the ball-bearing joint, resulted in external/internal rotation of the animal’s shoulder.
1.
Rat passive shoulder mechanics device with the sensor assembly (A), pivoting axle (B) and arm clamp (C) as well as axis of rotation noted. (note: forward flexion in the rat is equivalent to scapular plane elevation in humans.)
Passive shoulder mechanical test
Under general anesthesia, using isoflurane administered through a nose-cone, the arm of the treated shoulder was placed into 90 degrees of forward flexion and secured into the arm clamp (figure 1). Due to the orientation of the scapula in the rat, forward flexion is equivalent to scapular plane elevation in humans. This position was chosen to minimize tension on the repaired supraspinatus insertion site during joint mechanical testing. The sensor assembly (part A in figure 1) was then rigidly secured to the pivoting axis (part B in figure 1), such that the orientation sensor’s local coordinate system coincided with the humeral coordinate system and the torque cell sensing axis aligned with the long axis of the humerus. A user then cycled the sensor assembly between 20 N-mm of positive and negative torque 3 times, while torque and orientation data were displayed and recorded for subsequent analysis through custom software written in LABVIEW (v6.0 National Instruments). The torque and angular data , which included both glenohumeral and scapulothracic motion, were subsequently analyzed as humeral torque and rotation. In a preliminary unpublished study, blinded users applied a torque until he or she felt significant resistance; the average across three trials and two users (20 N-mm) was used as the peak torque for this study. In addition, a one-way anova of data prior to treatment showed no differences between users (13)..
Rotational data analysis
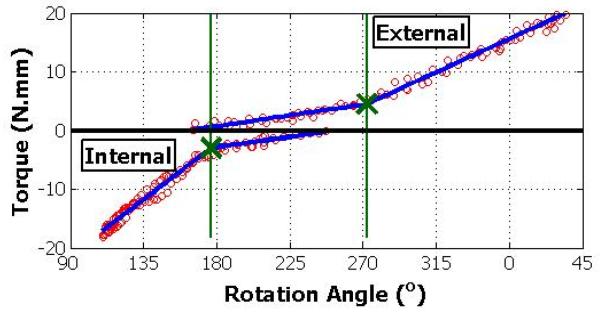
The loading portions of the non-linear humeral torque-rotation data (figure 2) were pooled across cycles for subsequent analysis. The total range of motion was taken as the difference between the minimum and maximum angles. Rotational stiffness was calculated for negative and positive loading data using a bilinear fit through custom software written in MATLAB (v7.2 Mathworks Inc). The bilinear fit used a non-linear least squares optimization (Levenberg-Marquardt algorithm) to determine the break point and slopes, which produced the minimum error. The slopes past the break-point near the end range of negative and positive rotation appeared linear and were, therefore, used to describe rotation stiffness in internal and external rotation, respectively. Data in the low stiffness regions (generally 1/8 to ¼ the stiffness in the other regions) appeared to be non-linear, and therefore, the slopes were not considered to be representative of the data. It should be noted that the humeral rotation measured by our device is a combination of glenohumeral and scapulothoracic motion. Furthermore, since the physiologically neutral angle was not recorded, we could not determine the range of internal or external rotation but rather the total range. Stiffness, on the other hand, was computed at the extremes of motion. We, therefore, distinguish between internal or external stiffness.
2.
Example positive and negative loading torque versus rotation angle data (open circle) of 3 cycles and corresponding dual bi-linear fits (thick lines), with the break-points noted (X). Note: the curves do not intersect due to hysteresis between loading cycles.
Statistical Analysis
To test our study hypotheses, all post-treatment data (total range of motion and internal/external stiffness) were analyzed as the difference from the pre-treatment value using a two-way ANOVA with repeated measures in SAS (version 9.0) to determine if either treatment or time had a significant effect (p<0.05). If the effect of time was significant, we used paired t-tests to determine if, for each treatment, pre-treatment data were different from data 4 weeks after treatment but not different from 8 weeks after treatment, as hypothesized. This was done using the Bonferroni method to account for multiple comparisons (p<0.05/6 = 0.0083). In addition, if the effect of treatment was significant, we used unpaired t-tests to determine if repaired-immobilized data were different than repaired-control at 4 and 8 weeks as hypothesized, again using the Bonferroni method to correct for multiple comparisons (p<0.05/4 = 0.0125).
RESULTS
Post-treatment – Total Range of Motion
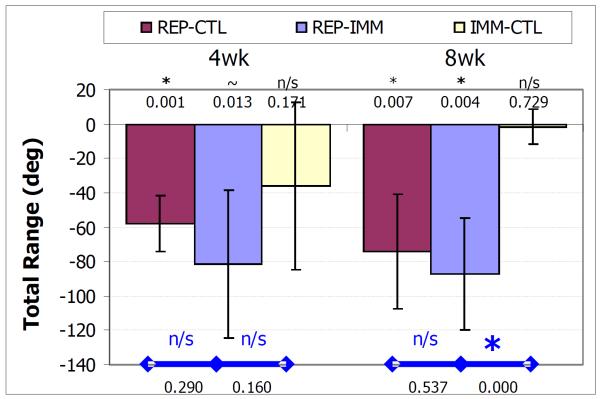
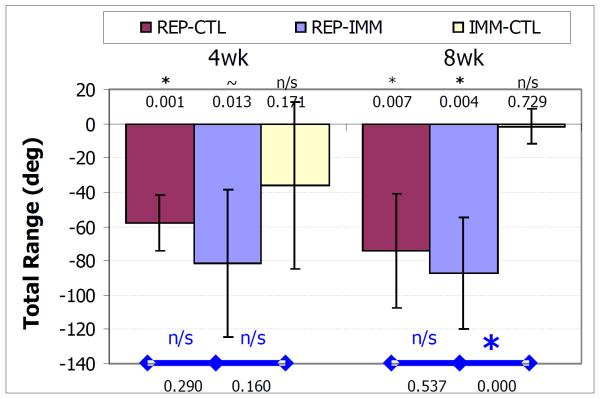
The total range of motion decreased in the conditions studied. For the REP-CTL group this decrease was significant at both 4 and 8 weeks (p = 0.001 and p = 0.008 respectively). For the REP-IMM group, this decrease was a trend at 4 weeks and significant at 8 weeks (p = 0.013 and p = 0.004, respectively). For the IMM-CTL group, the decrease was not significant at either time point (p = 0.171 and p = 0.729 respectively). There were no differences between the REP-IMM and REP-CTL groups at either time point (p = 0.290 and p = 0.537 respectively), but the loss in total range was significantly greater for the REP-IMM than the IMM-CTL group at 8 but not 4 weeks (p < 0.001 and p = 0.160 respectively).
Post-treatment - Rotational Stiffness
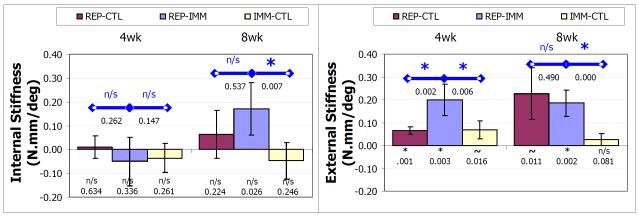
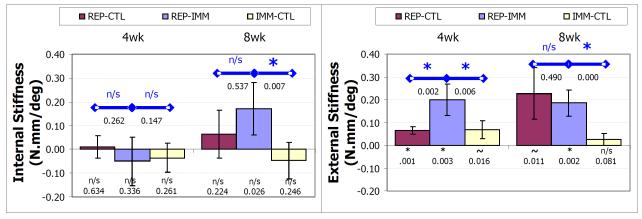
Following treatment, shoulders became stiffer in external, but not the internal rotation. Specifically, the increase in external rotational stiffness was significant (p < 0.0083) for the REPCTL group at 4, but not 8, weeks (p = 0.001 and p = 0.011 respectively), was significant at both 4 and 8 weeks for the REP-IMM group (p = 0.003 and p = 0.002 respectively), and a trend at 4, but not significant, at 8 weeks for the IMM-CTL group (p = 0.016 and p = 0.081 respectively) (panel B figure Krot). Internal rotational stiffness was not significantly increased or decreased relative to the uninjured values at either 4 or 8 weeks (p>0.026) (panel B figure Krot).
In addition, the increase in external rotational stiffness of the REP-IMM group was significantly (p < .0125) greater than the REP-CTL at 4, but not 8, weeks (p = 0.002 and p = 0.490 respectively) and significantly greater than the IMM-CTL group at both 4 and 8 weeks post-treatment (p = 0.002 and p < 0.001 respectively). The change in internal rotational stiffness of the REP-IMM group was not significantly different than the REP-CTL group at either 4 or 8 weeks post-treatment (p = 0.262 and p = 0.147 respectively) but was significantly different than IMM-CTL at 8, but not 4, weeks post-treatment (p = 0.007 and p = 0.788 respectively).
DISCUSSION
Consistent with our first hypothesis, rotator cuff repair surgery without immobilization increased shoulder stiffness and reduced the range of motion. Since the animal was returned to caged activity following the repair, the most likely source of this increased stiffness (58% externally) and reduced motion (19% total range) is scar forming within the subacromial space in response to the repair surgery, which has been observed in other animal (18)as well as clinical studies (10). Although muscle fibrosis and atrophy, capsular tightening, and other changes are likely to contribute to joint stiffness, such contributions would likely be negligible after 8 weeks of caged activity, whereas the scar which forms at the insertion site is likely to be present for several months. Scar formation would also explain the lack of improvement in immobilized and non-immobilized repaired shoulders during 4 weeks of caged activity. Interestingly, joint stiffness increased during external, but not internal rotation, which suggests that post-surgical scar formed such that it restricted external more than internal rotation. Unfortunately, it was not possible to separate glenohumeral and scapulothoracic motion in our study, so we could not determine if the range of external glenohumeral rotation (compared to internal) decreased, as one would expect with an increase in external rotational stiffness.
Also consistent with our first hypothesis, immobilizing the shoulder following rotator cuff repair resulted in a significant increase in shoulder stiffness and reduced total range of motion. The increase in rotational stiffness occurred when the shoulder was rotated externally, but not internally, for both immobilized and non-immobilized repaired shoulders. Furthermore, the increase in rotational stiffness following cuff repair was larger when the shoulder was immobilized for 4 weeks but was not significantly different from the non-immobilized repaired shoulder 4 weeks after cast removal (8 weeks post-treatment).. The fact that stiffness was significantly greater when the shoulder was immobilized suggests that, as has been found following tendon repair in the hand, immobilization increases joint stiffness following cuff repair in the shoulder. Surprisingly, this increase in external rotational stiffness did not result in significantly less total motion nor was the increase in stiffness due to immobilization permanent. Since we have previously shown that immobilization improves the mechanical properties of the repaired insertion site (18), and repair failure remains a common problem clinically, future studies are required to determine if passive motion following cuff repair can minimize this transient increase in shoulder stiffness, while not adversely affecting the mechanical properties of the repaired insertion site.
Inconsistent with our first hypothesis, immobilization alone did not have a significant effect on either total range or rotational stiffness in either internal or external rotation, although the increase in joint stiffness during external rotation at 4 weeks was a trend (p = 0.016). Since the increase in rotational stiffness was large (>50%), the lack of statistical significance is likely due to the conservative nature of the Bonferroni correction combined with the small number of animals. This was seen in a post-hoc power analysis, which showed that, for the rather large effect of immobilization alone for 4 weeks on external rotation stiffness (2.33), the Bonferroni correction dropped the statistical power from 90% to 60%. Given the conservative nature of this correction, this would also suggest that the effect of immobilization alone, while large, was smaller than the effect of the repair. This is further supported by the return of all parameters to pre-treatment levels 4 weeks after the cast was removed and would also support the observation that the effect of immobilization on joint mechanics is joint specific. For example, 3 weeks of immobilization has been shown to cause a significant reduction in motion of dog distal interphalangeal joints (16), and the elbow is generally not immobilized in adults, since it is known to become stiff rapidly following trauma (11). Although muscle is just one contributing factor to joint stiffness following immobilization, these observations are consistent with muscle literature which has shown that atrophy induced by immobilization is also joint specific, where muscles that have predominantly slow fibers and span a single joint are the most sensitive to immobilization (8). Caution must, therefore, be used when extrapolating the effect of immobilization or other procedures from one joint onto another.
As with any study, there are several limitations which must be considered when interpreting the clinical meaning of our results. First, as stated in our methods section, the current device is not capable of separating glenhumeral and scapulo-thoracic motion, thus the loss in rotation reported in this study is a combination of a loss in glehonumeral as well as scapulothoracic motion. It is conceivable that immobilization would affect the scapulothoracic more than the glenohumeral joint; however, it is unlikely that the repair would affect anything other than the glenohumeral joint. Since the effect of immobilization alone was not significant and smaller than the effect of the repair-alone, our results suggest that the changes in shoulder mechanics were primarily the result of scar forming at the glenohumeral joint rather than stiffening at the scapulothoracic joint. Future studies could use fluoroscopy to determine the relative contributions of glenohumeral and scapulothoracic motion to shoulder rotation measured by our device to clarify this issue better. Furthermore, the current apparatus measures rotational stiffness only; passive mechanical behavior of the joint in other rotational and translational degrees of freedom would require the construction of a much more complicated device. Although this may provide useful information, the current device obtains passive mechanical data in an orientation and about an axis which is used clinically to assess shoulder function following cuff repair and, therefore, may provide the basis from which a human equivalent could be built and used during clinical examination of the shoulder. Last, while several methods exist to immobilize the glenohumeral joint, we chose plaster casts for simplicity, since it is not possible to place rat limbs in slings. While animals tolerated cast immobilization well, a few managed to remove their casts for short periods of time, which may have contributed to variability in the immobilized groups. Alternate immobilization methods are currently being evaluated.
In summary, the transient increase in shoulder stiffness found in this study and the large number of case reports demonstrating that the increase in joint stiffness following immobilization was transient, combined with the high rate of repair failure clinically and the results of our previous study showing that immobilization improved tendon to bone healing, support the need to investigate immobilization further as a post-operative treatment following rotator cuff repair.
3.
Decrease in the total range of motion 4 and 8 weeks following treatment (REP-IMM, REPCTL and IMM-CTL) where * denotes p<.05/6 and ~ denotes p<0.011/6, symbols within the bar denote differences relative to uninjured, and symbols above with bars denote differences between groups. At 4 weeks post-treatment, the decrease in the total range was a trend and significant for the REP-IMM and REP-CTL groups and at 8 weeks the decrease was significant for both groups. The decrease was not significant at either time point for the IMM-CTL group and at 8 weeks the decrease in the REP-IMM group was significantly less than the near zero decrease of the IMM-CTL group.
4.
Rotational stiffness as the difference from pre-treatment value in the linear regions during internal (A) and external (B) rotation 4 and 8 weeks following treatment (REP-IMM, REP-CTL and IMM-CTL) where * denotes p<.05/6 and ~ denotes p<0.10/6, symbols within the bar denote differences relative to uninjured, and symbols above with bars denote differences between groups. Stiffness during internal rotation (A) was not significantly different than uninjured for any treatment group at any time. Stiffness was greater for all groups at all time points, but was significant for the REP-IMM group at 4 and 8 weeks, for the REP-CTL group at 4 weeks and not significant for the IMM-CTL group. In addition, stiffness of the REP-IMM group was significantly greater than both the REP-CTL and IMM-CTL groups at 4 weeks, but only the IMM-CTL group at 8 weeks.
Acknowledgments
FUNDING: National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS) of the National Institutes of Health (NIH), National Science Foundation (NSF)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Debeyre J, Patte D, Elmelik E. Repair Of Ruptures Of The Rotator Cuff Of The Shoulder. J Bone Joint Surg Br. 1965;47:36–42. [PubMed] [Google Scholar]
- 2.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219–24. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg [Am] 1982;7(2):170–5. doi: 10.1016/s0363-5023(82)80083-x. [DOI] [PubMed] [Google Scholar]
- 4.Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281–90. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Gomoll AH, Katz JN, Warner JJ, Millett PJ. Rotator cuff disorders: recognition and management among patients with shoulder pain. Arthritis Rheum. 2004;50(12):3751–61. doi: 10.1002/art.20668. [DOI] [PubMed] [Google Scholar]
- 6.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982–9. [PubMed] [Google Scholar]
- 7.Iannotti JP, Naranja RJ, Gartsman GM. Surgical treatment of the intact cuff and repairable cuff defect: Arthroscopic and open techniques. In: N TR, editor. Orthopaedic Knowledge Update: Shoulder and Elbow. American Academy of Orthopaedic Surgeons; Rosemont IL: 1994. pp. 151–156. [Google Scholar]
- 8.Lieber RL, Friden JO, Hargens AR, Danzig LA, Gershuni DH. Differential response of the dog quadriceps muscle to external skeletal fixation of the knee. Muscle Nerve. 1988;11(3):193–201. doi: 10.1002/mus.880110302. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin HL. Lesions of the musculotendinous cuff of the shoulder. The Journal of Bone and Joint Surgery. 1944;26(1):31–51. [PubMed] [Google Scholar]
- 10.Mormino MA, Gross RM, McCarthy JA. Captured shoulder: a complication of rotator cuff surgery. Arthroscopy. 1996;12(4):457–61. doi: 10.1016/s0749-8063(96)90040-7. [DOI] [PubMed] [Google Scholar]
- 11.Morrey BF. The posttraumatic stiff elbow. Clin Orthop Relat Res. 2005;(431):26–35. [PubMed] [Google Scholar]
- 12.Nixon JE, DiStefano V. Ruptures of the rotator cuff. Orthop Clin North Am. 1975;6(2):423–47. [PubMed] [Google Scholar]
- 13.Peltz CM, Sarver JJ, Williams GR, Soslowsky LJ. A NOVEL TECHNIQUE FOR MEASURING PASSIVE SHOULDER MECHANICS IN A RAT. ASME Summer Bioegineering Conference; 2006; Amelia Island, Florida. ASME; 2006. p. 155007. [Google Scholar]
- 14.Post M. Injuries to the Rotator Cuff. In: Post M, editor. The shoulder: surgical and nonsurgical management. Lea and Febiger; Philadelphia PA: 1978. pp. 304–328. [Google Scholar]
- 15.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795–803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Rothkopf DM, Webb S, Szabo RM, Gelberman RH, May JW., Jr An experimental model for the study of canine flexor tendon adhesions. J Hand Surg [Am] 1991;16(4):694–700. doi: 10.1016/0363-5023(91)90196-i. [DOI] [PubMed] [Google Scholar]
- 17.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20(3):454–63. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 18.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125(1):106–13. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]