Abstract
The present study was conducted to investigate the feasibility and efficacy of a RSV F DNA vaccine incorporated with a mucosal adjuvant. Two DNA vaccine vectors (DRF-412 and DRF412-P) were developed containing residues 412-524 of the RSV F gene. These antigenic regions were cloned into the phCMV1 DNA vaccine vector. One of the DNA vaccine vectors, DRF-412, contained the ctxA2B region of the cholera toxin gene as a mucosal adjuvant. The in vitro expression of these DNA vectors were confirmed in Cos-7 cells by indirect immunofluorescence and western blot analyses. In vivo expression of the cloned gene was further confirmed in mouse muscle tissue by immunohistological analysis. The active transcription of the RSV F gene in mouse muscle cells was confirmed by RT-PCR. The purified DRF-412 and DRF412-P DNA vectors were used to immunize mice by intramuscular injections. Our results indicated that DRF-412 and DRF412-P vaccine vectors were as effective as live RSV in inducing neutralization antibody, systemic Ab (IgG, IgG1, IgG2a, and IgG2b) responses, and mucosal antibody responses (Ig A). The Th1 (TNF-α, IL-12p70, IFN-γ, IL-2) and Th2 (IL-10, IL-6) cytokine profiles were analyzed after stimulation of spleen cells from mice immunized with purified RF-412 protein. We observed that mice inoculated with vector DRF-412 induced a higher mixed Th1/Th2 cytokine immune response than DRF412-P. Reverse transcriptase and quantitative real-time PCR (qRT-PCR) revealed that mice immunized with the DRF-412 vector contained less viral RNA in lung tissue and the lung immunohistology study confirmed that mice immunized with DRF-412 had better protection than those immunized with the DRF412-P vector. These results indicate that the RSV DRF-412 vaccine vector, which contains the cholera toxin subunit ctxA2B as a mucosal adjuvant may provide a better DNA vaccination strategy against RSV.
Keywords: Fusion protein, DNA vaccine, cholera toxin, mucosal vaccine
1. Introduction
Respiratory syncytial virus (RSV) is a Pneumovirus of the family Paramyxoviridae. It is a major cause of bronchiolitis and pneumonia in infants and young children infecting almost all infants less than two years of age. The worldwide estimated RSV infection is around 64 million resulting in approximately 160,000 deaths (Girard et al., 2005). Natural RSV infection mediated immunity is incomplete and disease occurs throughout life. Despite numerous vaccine development strategies and efforts, there is no effective vaccine available against RSV (Girard et al., 2005). RSV vaccine development is hampered by several factors: a) lack of induction of viral neutralizing antibodies, b) incomplete immune response by RSV immunization, c) induction of inappropriate immune response as witnessed in Formalin Inactivated-RSV vaccine trial, d) lack of Th1 immunity induced by RSV antigens, and e) limited induction of CD8+ T-cells (Dudas et al., 1998).
Recently, DNA vaccines have emerged as potential vaccine candidates for RSV and other pathogens. The DNA vaccines induce a selective Th1 immune response when administered at an early age which is advantageous for an RSV vaccine (Martinez et al., 1997). DNA vaccines have the ability to induce both neutralization antibody and cell-mediated immune responses, including CTLs, and the possibility to modulate the pattern of immune responses by the route of DNA administration (Ulmer et al., 1996; Pertmer et al., 1996). Furthermore, DNA vaccines also provide long-term immunity against viruses, a highly desirable property for a practical RSV vaccine (Justewicz and Webster, 1996). DNA vaccines against RSV are under investigation and several of these induce protective immune responses (Li et al., 1998; Harcourt et al., 2004;Tree et al., 2004; Vaughan et al., 2005). DNA vaccines encoding RSV F and G genes provide protection in animals after RSV challenge (Bembridge et al., 2000). In addition, a Newcastle disease virus (NDV)-based vector with adjuvant properties, has been used to deliver the RSV F protein to induce a protective immune response (Martinez-Sobrido et al., 2006).
The RSV genomic RNA is made up of 15,222 nucleotides which encode for eleven proteins (Tripp et al., 2005). Due to the immunogenicity of RSV antigens, only the fusion (F) and attachment (G) glycoproteins are the prominent targets for vaccine development. The F glycoprotein induces high titers of neutralizing antibodies, RSV-specific cytotoxic and T helper lymphocytes and also provides significant protection against RSV infection(Connors et al., 1991; Walsh, 1994; Wertz et al., 1987). Since the RSV F protein is conserved among RSV A and B strains, it has the potential to provide cross protection (Munoz et al., 2003).
Most of the DNA vaccines have limited efficacy due to the lower level of target gene protein expression and a requirement for large dosages to induce modest cellular and humoral responses. Moreover, DNA vaccines fail to induce local immunity because of the route of delivery. Several investigators have utilized strategies to improve the delivery of DNA vaccines and their efficacy by using adjuvants. To induce mucosal immunity, RSV DNA vaccines encoding the M2 protein wer used in combination with chitosan to induce RSV-specific CTL responses (Iqbal et al., 2003). Some of the DNA vaccines have incorporated cytokines (Barouch et al., 2004), liposomes (Perrie et al., 2001), ISCOMs (Le et al., 2001) and CpG DNA motifs (Klinman, 2006) to improve antigen presentation and enhance the immune response.
Although lower respiratory tract (LRT) infection is responsible for severe disease, the upper respiratory mucosa is important in limiting RSV colonization and subsequent infection. Anti-RSV IgA antibodies have been shown to protect the epithelium of the nasal cavity and prevent RSV infection (Graham et al., 1991; Walsh and Falsey, 2004). Induction of mucosal antibodies has been the focus of several studies where adjuvants such as cholera toxin (CT) are used. CT is a well characterized adjuvant (Ogra et al., 2001) which has been shown to induce mucosal antibody responses against RSV when co-administered with antigens (Walsh, 1994; Godefroy et al., 2003; Singh et al., 2007a). The cholera holotoxin (CT) is an exotoxin produced by Vibrio cholera that causes severe diarrhea in humans. The cholera toxin is composed of one A subunit (CTA) and five identical B subunits (CTB). The CTB homopentamers are noncovalently associated to form a ring, which is associated with one A subunit (Sixma et al., 1992). CTA is a 28KDa protein while the CTB monomer is a 12 KDa protein (Hardy et al., 1988). The adjuvant effect of cholera toxin (CT) and cholera toxin B subunit (CTB) had also been demonstrated in a DNA vaccine. In a study, plasmids containing CT and CTB were used as DNA vaccines and a strong cellular immune response was reported (Arrington et al., 2002). A DNA vaccine with the CTB gene has been used as an adjuvant to enhance the immune response against HPV (Sanchez et al., 2004).
We have recently shown that genetic fusion of antigenic regions of RSV F (412-524 residues) and ctxA2B genes induces strong mucosal immune response and provides significant protection against RSV infection (Singh et al., 2007a). Thus in the present study, we developed a DNA vaccine that consisted of residues 412-524 of the RSV F protein and the CTA2B unit of the cholera toxin. To study the effect of CTA2B, we developed another DNA vaccine vector lacking CTA2B. Our results indicate that vaccination of mice with these vectors induced virus-neutralizing antibodies and provided a better protection against viral challenge. To our knowledge, this is the first report that an RSV DNA vaccine with genetically modified cholera toxin can induce a protective immune response.
2. Materials and methods
2.1. Animals and RSV Stock Preparation
BALB/c female mice (4-6 wk old) were purchased from Charles River Laboratories (Wilmington, MA). The animals were housed in laminar flow racks under pathogen-free conditions with a cycle of 12 h of light and 12 h darkness. Care and handling of animals was followed according to Alabama State University Institutional Animal Care and Use Committee.
Human RSV Long strain was purchased from American Type Culture Collection (ATCC, Manassas, VA, #VR-26) and propagated in HEp-2 cells (ATCC#CCL-23). HEp-2 cells were grown in tissue culture flasks in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. RSV with multiplicity of infection (m.o.i.) of 4:1 was added to the flask and virus adsorption was carried out for 1 hr at 37°C in a humidified atmosphere with 5% CO2. MEM with 2% FBS was added to the flask and infection of cells was observed for an additional 3-4 days. RSV infected cells were collected and subjected to two freeze-thaw cycle and centrifuged at 3,000 × g at 4°C to remove cellular debris, aliquoted and stored at −70°C until they were used.
2.2. Bacterial strains and eukaryotic cells
The competent E. coli Novablue cells were purchased from EMD Biosciences (San Diego, CA), stored at −70°C and used for cloning of the DNA vectors. Transformation was performed following manufacturer's protocols. Cos-7 cell lines were purchased from ATCC (CRL-1651) and maintained in MEM containing 10% fetal bovine serum with antibiotics. Cos-7 cells were used for transient transfection using purified DNA vaccine vectors to analyze protein expression of genes of interest.
2.3. Plasmid construction
The phCMV1 vector was obtained from Genlantis (San Diego, CA). A plasmid vector, pASU-RSF described previously (Singh et al., 2007a) was used as template to generate a PCR fragment of 812 bp containing residues 412-524 of the RSV F gene and the ctxA2B gene of cholera toxin. The resulting PCR fragment was cloned into the phCMV1 vector between the XhoI and BamHI restriction sites and designated as pDRF-412. Similarly, another DNA vaccine vector containing only residues 412-524 (336 bp) of the RSV F gene (without ctxA2B gene) was generated and named pDRF412-P. The recombinant clones were selected and analyzed by restriction enzyme digestion and gene sequencing. The recombinant DNA vaccine vectors were purified using the Qiagen Endofree Plasmid Giga kit (Qiagen) and used for transient transfection and immunization experiments.
2.4. In vitro and in vivo expression of DNA vaccine vectors
For the transfection experiments, endofree plasmid DNA was used to transfect Cos-7 cells using the ExGen 500 in vitro transfection reagent from Fermentas Inc. (Hanover, MD). Cos-7 cells (100,000) were grown in a 12-well plate to 50-70% confluency in Minimal Essential Medium (MEM) containing 10% fetal bovine serum. Cells were transfected with 100 μl of a solution containing plasmid DNA-Transfection mixture in media. The transfection was carried out for 10 min and cells were incubated at 37°C (5% CO2). The expression of the RSV F gene was evaluated by indirect immunofluorescence after 24 h using anti-RSV F monoclonal antibody (BioDesign International, Saco, ME) for 1 h. Anti-mouse IgG conjugated with FITC (Southern Biotech, Birmingham, AL) was used as the secondary antibody. The expression was observed using an Olympus IX51 immunofluorescence microscope.
For in vivo expression, 50 μg of Endofree DNA vaccine vectors were injected intramuscularly into the thigh muscles of BALB/c female mice. One week after injection, the thigh muscles of mice were collected and used to prepare cryo-sections of 10 μm thickness. The gene expression was analyzed using immunofluorescence with the Monoclonal antibody against RSV described earlier. In addition, to confirm the expression of RSV F gene in mice, mRNA from muscle tissue was extracted using Trizol reagent (Qiagen, Valencia, CA) and RT-PCR was performed using RSV F specific primers.
2.5. Immunization of mice with DNA vaccines
BALB/c mice (4-6 week-old, female) were randomly separated into 5 groups (10/group). The vaccines included pDRF-412, pDRF412-P, live RSV, phCMV1 (blank vector) and PBS. Animals were immunized intramuscularly (i.m.) three times on day 1, day 14 and day 28 each with 50 μg of Endotoxin-free DNA in a volume of 50 μl of PBS. Live RSV (105 PFU) was used intranasally (i.n.) once on day 1. Sera samples were collected from the tail vein on days 0, 14, 28, and 49. Saliva was collected at the same intervals using pipettes fitted with plastic tips after i.p. injection of carbachol (0.5 μg) to stimulate salivation. Serum and saliva samples were stored at −20°C until they were analyzed. Spleens from three mice per group were collected at day 49 for cytokine analysis. The remaining 7 mice in each group were challenged with105 PFU live RSV i.n. 1 wk after the last immunization. Five days following challenge, mice were sacrificed and lungs were collected for immunoprotection studies.
2.6. Analysis of antibody response
Antibody titer analysis and isotyping of all sera and saliva samples were conducted following published protocols (Singh et al., 2006; Tebbey et al., 2000). ELISA plates were coated with 100 μl RF-412(1μg/ml), 100 μl RF412-P (1μg/ml) or RSV respectively; RF-412 and RF-412-P were the F proteins expressed in an E.coli system and purified previously in our lab(Singh et al, 2007a). ELISA plates were blocked with 3% BSA in PBS for 4 h and then ten-fold serial dilutions of serum and saliva samples were added to designated wells, and the plates were incubated at room temperature (RT) overnight. The plates were washed, followed by addition of HRP-conjugated goat anti-mouse Ig (100μl, 1:2000) (Southern Biotech Associates Inc.{SBA}, Birmingham, AL). The enzymatic reaction was developed by adding 50 μl of the TMB substrate for 15 min, the reaction was stopped by adding 50 μl of the stop solution (Kirkegard and Perry Laboratories, KPL, Gaithersberg, MD) and the absorbance was read at 450nm using a Tecan ELISA plate reader (Tecan, Research Triangle Park, NC). Isotype determination kits (Southern Biotech Associates (SBA, Birmingham, AL) were used for antibody isotyping (IgA, IgG,IgG1, IgG2a, and IgG2b) as described previously (Singh et al., 2007b).
2.7. Virus neutralization assay
Sera, saliva, nasal wash and BAL were analyzed for RSV-specific percentage neutralization titers according to a previous report (Anderson et al., 1985). Briefly, serum or a monoclonal antibody which had been heat inactivated at 56°C for 30min, was added to the duplicate wells, and serial dilutions were performed in the microtiter plates. All dilutions were made using MEM plus 2% FBS, and the final volume was 75 μl per well. 100PFU of RSV in 25μl were added to each well, and the mixture was incubated at 4 °C for 2h, following which, approximately 15,000 HEp-2 cells in 100μl of MEM plus 5%FBS were added to each well, and plates were incubated at 37°C in a CO2 incubator for 3 days. The plates were fixed by aspirating the contents of the wells, washing three times with PBS at pH 7.2 with 0.5% Tween 20, adding 75μl of an 80% (vol/vol) solution of acetone-PBS, and incubating at 4°C for 15 min. After incubation, the contents were aspirated, and the plates were air dried. ELISA was performed on the same plates with mouse anti-RSV F primary antibody (1:500 dilution), and Goat-anti-Mouse Ig-HRP (1:2000 dilution). OD values were read at 450nm. The RSV-specific percentage neutralization titer was defined as follows: % RSV neutralization titer = (1-sample O.D450/RSV control O.D.450) X 100%. The neutrilization assay was performed in duplicate and data expressed as the means of two determinations.
2.8. Lung Immunohistology Studies
1 wk after viral challenge, lungs from immunized mice were aseptically removed and preserved in 30% sucrose made in PBS. Lungs were then sectioned into 10μm thick slices and placed on glass slides. Individual slides were evaluated by indirect immunofluorescence analysis. Briefly, tissues were fixed for 10 min with cold acetone and allowed to dry for 3 min. Slides were then washed 3 times in 1X PBS, blocked with 0.1% FBS in PBS for 30 min, and washed 3 times in 1X PBS, followed by addition of diluted primary antibodies in 0.1% FBS in PBS, and incubated overnight at 4°C or at RT for 1h. The wash steps were repeated, and then diluted secondary antibodies in 0.1% FBS in PBS were added, and slides were incubated in the dark room at RT for 1-4 h, washed and observed under the fluorescent microscope.
2.9. Reverse transcription and quantitative PCR (qRT-PCR)
RNA from lung tissue was extracted by using Trizol (Invitrogen, Carlsbad, CA) following manufacturer's instructions and treated with DNase I (Promega) to remove residual genomic DNA. One to 200ng of isolated RNA was reverse transcribed using SuperScript II (Invitrogen) in a 25μl mix using random primers. 2 μl of the cDNA mix were used in each quantitative PCR (Applied Biosystems, Foster city, CA) with forward primer GTC GTT TCG AGC CCA TTG TGT CAT GCT ATG GC and reverse primer AAG TAA GTC GAC TTG TGG TGG ATT TAC CAG C following manufacturer's protocols. Results were analyzed using Applied Biosystems software to obtain Ct values, where Ct values are threshold cycles at which a statistically significant increase in detection of SYBR green emission intensity occurs. Then, these Ct values were normalized to a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control to account for variability in RNA concentrations between samples to obtain net Ct values.
2.10. Cytokine Response Analysis
Spleen cells from each group of mice (3 per group) were pooled and cells were isolated using 40-mesh stainless steel screens (Sigma). Spleen cells (3.0×106/ml) were cultured in vitro in 24-well plates (Costar, Cambridge, MA) in the presence of RF-412 purified antigen at 1, 0.5 and 0.25 μg/ml concentrations. The concentration of antigen eliciting the maximal response was used for data analysis. Some wells received 5 μg/ml of Concanavalin A (Con A; Sigma) as a nonspecific control. The control supernatant was generated by culturing cells in medium alone. Culture medium consisted of 45% RPMI 1640, 45% Iscove's modified Dulbecco's medium (Gibco-Invitrogen), 10% heat-inactivated FBS (JRH Biosciences, Inc., Lenexa, KS), 1 mM Hepes (Gibco), 0.5 mM sodium pyruvate (Sigma), 2 mM L-glutamine, 0.05 mM mercaptoethanol (Sigma), and 1 μg/ml gentamicin sulfate (Gibco-Invitrogen) (complete medium). Cultures were incubated at 37°C in a humidified atmosphere with 5% CO2. Cell free-supernatants were harvested at 24, 48 and 72 h and stored at −70°C until they were used. ELISA kits were used to assess the concentration of IFN-α (Interferon alpha multi subtype ELISA kit, PBL Biomedical Laboratories, Piscataway, NJ) and IFN-β (Invitrogen) according to the manufacturer's instructions. All other cytokines (IL-2, IL-6, IL-10, IL-12 and TNFα) were detected with a Luminex multiplex bead assay (Linco Research, St. Charles, MO) according to the manufacturer's instructions.
2.11. Statistical analysis
Comparisons of antibody concentrations and isotypes between various groups of mice at different time intervals were performed by ANOVA and two-tailed unpaired Student's t-test for samples with unequal variances. P<0.05 was considered significant.
3. Results
3.1. Construction of plasmid vectors (DRF-412 & DRF412-P)
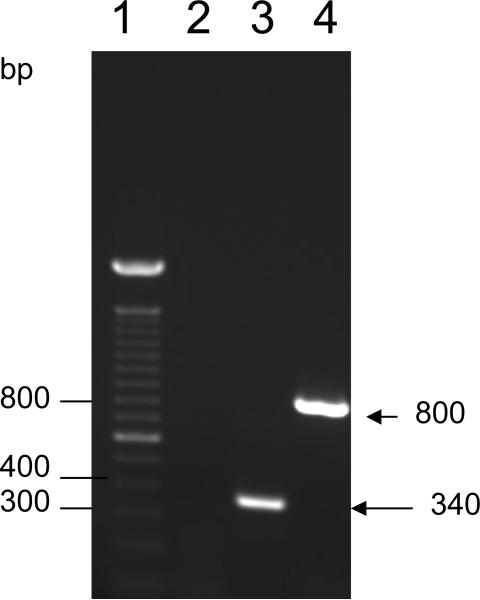
To determine whether better protective immunity against RSV could be elicited by these two DNA vectors and compare the effect of adding the genetically modified CTB gene into the vector, we developed two plasmid constructs expressing the RSV F gene under the control of the immediate-early promoter and intron A sequences of human CMV. Plasmid DRF-412 contains not only the same region as DRF412-P but also has a CTB gene, plasmid DRF412-P encode regions 412-524 residues of RSV F protein, Clones with the correct insert and plasmid size were selected and confirmed by agarose gel electrophoresis after restriction enzyme digestion. Sequence of the junction regions and RSV F genes were also confirmed. These plasmids were then tested for expression of the encoded gene both in vitro and in vivo. Expression of the encoded proteins of the plasmid DRF-412 and DRF412-P in Cos-7 cells was revealed by Indirect Immunofluorescence Assay (IFA) (Data not shown) and western blot (Fig. 1). Protein expression in thigh muscles collected at 1 week after injection of these two plasmids was detected by IFA with a monoclonal antibody against RSV (Fig. 2). The active transcription of the F gene in muscle cells was confirmed by RT-PCR (Fig.3).
Fig. 1.
Western blot analysis of protein expressed in Cos-7 cells. Lane 1, DRF-412; lane 2, DRF412-P; lane 3, MagicMark XP western protein standard (Invitrogen, Calrsbad, CA). Anti-RSV F monoclonal antibody (BioDesign International, Saco, ME) used as primary antibody and HRP conjugated anti-mouse IgG (Southern Biotech, Birmingham, AL) was used as the secondary antibody.
Fig. 2.
Expression of F gene in thigh muscles of 4-week-old mice. (A) Expression of F gene and CTB gene in thigh muscles; (B) Expression of F gene in thigh muscles (C) mock-transfected thigh muscles. Anti-RSV F monoclonal antibody (BioDesign International, Saco, ME) was used as the primary antibody and HRP conjugated anti-mouse IgG (Southern Biotech, Birmingham, AL) was used as the secondary antibody.
Fig. 3.
Detection of active transcription of RSV F gene in mice muscles using RT-PCR. Lane 1, 100 bp DNA molecular marker (Invitrogen, Carlsbad, CA); lane 2, RNA extracted from normal mice ; lane 3, DRF412-P; lane 4, DRF-412.
3.2 Mouse anti-F antibody responses elicited by DRF412-P and DRF-412
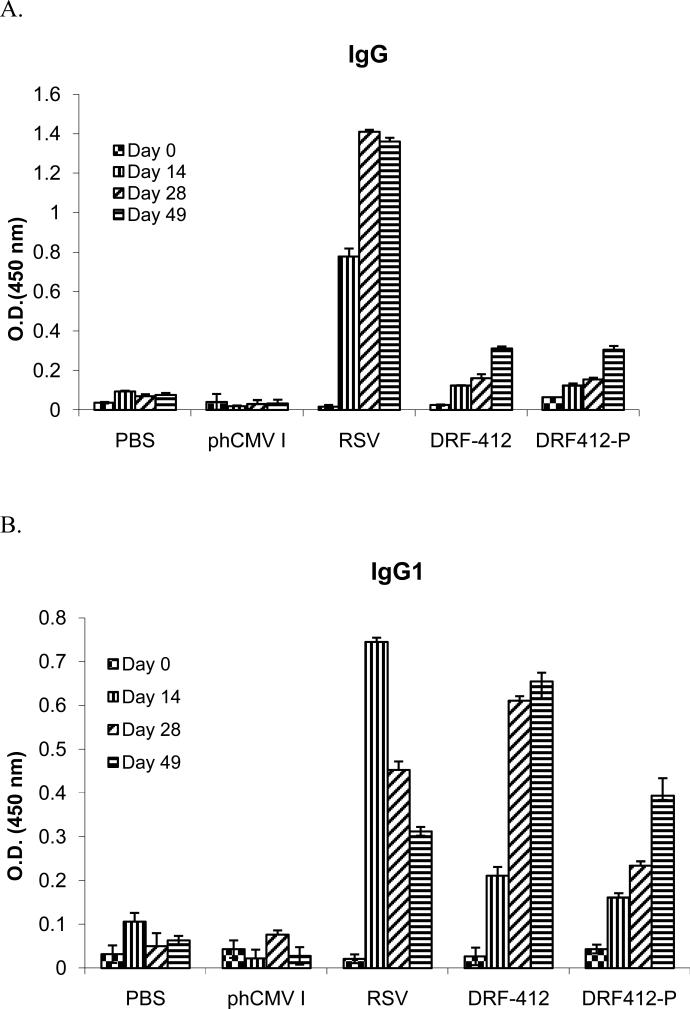
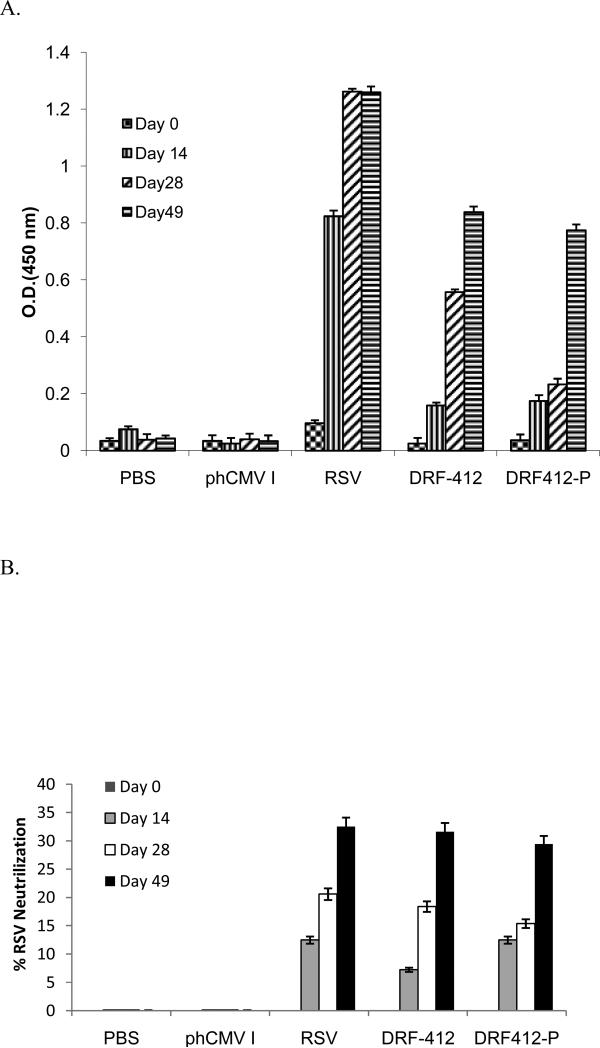
Groups of 10 BALB/c mice received intramuscular injections of 50 μg of each individual plasmid, followed by two boosters at 2 wk intervals. Blood and saliva samples were collected at two wk intervals following each immunization; 100 μl PBS nasal wash and 100 μl PBS of BAL samples were collected at day 28 and BAL samples were collected 1wk after live RSV challengefor antibody measurement. These samples were analyzed by using ELISA with recombinant proteins RF-412 and RF412-P. DRF412-P and DRF-412 induced strong humoral responses both in serum and saliva as compared with the PBS and phCMV1 control groups (Fig.4A & Fig.5A.). Initial responses were first detected at day 14 (after the first immunization) and these responses continued to increase after the second immunization. Although there was no significant difference between anti-F IgG antibody levels elicited by DRF412-P and DRF-412 vaccine vectors, further Ab isotyping revealed that DRF-412 induced better IgG1(Fig 4B & 5B), IgG2a(Fig. 4C & 5C) and IgA (Fig 4E & 5E) antibodies than DRF412-P; whereas DRF412-P induced better IgG 2b antibodies (Fig. 4D and 5D) than DRF-412. Substantial RSV neutralization in the serum neutralization assay confirmed that these anti-F antibodies (Fig 7A & 8A) had strong virus-neutralizing activity; moreover, DRF-412 induced better neutralization against RSV than DRF412-P (Fig 7B & Fig 8B)
Fig. 4.
Systemic antibody response to DRF-412 & DRF412-P. Serum samples were collected at two weeks interval, with PBS, phCMV I and RSV as controls. RSV was administrated via i.n. route. RSV specific antibody isotypes IgG(A), IgG1(B), IgG2a(C), IgG2b(D), and IgA (E) were determined by ELISA, using RSV as coating antigen. Results are presented as an average of data from three groups in triplicate; error bars represent standard deviation.
Fig. 5.
Isotyping of mucosal antibody. Saliva samples were collected at two weeks interval, set PBS, phCMV I and RSV as control , RSV was administrated via i.n. route.RSV specific antibody isotypes IgG(A), IgG1(B), IgG2a(C), IgG2b(D), and IgA(E) were determined by ELISA using the anti-RSV F recombinant protein as the coating antigen. Results are presented as an average of data from three groups in triplicate; error bars represent standard deviation.
Fig. 7.
Detection of the specific mucosal antibody response. The mice were immunized i.m. respectively, with 50μg of DRF-412 or DRF412-P, and boosted twice at 2 weeks intervals, while saliva samples were collected from the mice at day 0, 14, 28, and 49 post-immunization. A. RSV-F specific antibodies in the saliva. B. The RSV % neutralization titers in sera. Results are presented as an average of data from three groups in triplicate; error bars represent standard deviation.
Fig. 8.
Analysis of neutralizing antibody response in nasal wash and BAL samples. The mice were immunized i.m. with either 50 μg of DRF-412 or DRF412-P, nasal washes were collected at day 28 and BAL samples were collected 1wk after live RSV challenge. The % RSV neutralization titer in the sera was measured by a neutralization assay. Results are presented as an average of data from three groups in triplicate; error bars represent standard deviation.
3.3. Protection efficacy against challenge with RSV
To assess the protective ability of these two vectors, DNA-immunized mice were challenged intranasally with RSV on day 49 after the booster and lungs were recovered 4 days later for measurement of viral mRNA quantities using qRT-PCR. Immuno-histopathology was also used to assess the virus distribution in lung tissue. qRT-PCR revealed that mice immunized with DRF-412 and DRF412-P have a higher average Ct value compared with PBS and phCMV I group (P<0.001), which means that they have less viral mRNA in the lungindicating better protection, but there was no statistically significant difference between DRF-412 and DRF412-P(Table 1). To assess the degree of lung histopathology induced by these two vectors, the virus distribution in lung tissue after live RSV challenge was analyzed using a modified immunofluorescence assay. Mice immunized with DRF-412 had less fluorescence than the DRF412-P group. Cytokine analysis revealed that DRF-412 is capable of inducing a higher Th1/Th2 mixed immune response than DRF412-P (Table 2).
Table 1. Average Ct values detected by qRT-PCRa.
Determination of viral RNA in lungs by qPCR. Groups of 10 BALB/c mice received intramuscular injections of 50 μg of each individual plasmid, followed by two boosters at 2 wk intervals. Mice were challenged with105 PFU live RSV i.n. 1 wk after last immunization. Five days following challenge, mice were sacrificed and lungs were collected for immunoprotection studies, and RNA from lung was extracted by using Trizol, Extracted RNA was further used to amplify the F gene, by qPCR performed in Applied Biosystems to obtain Ct values, where Ct values are threshold cycles at which a statistically significant increase in detection of SYBR green emission intensity occurs. These Ct values were normalized to a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control to account for variability in RNA concentrations between samples to obtain net Ct values. Average Ct values represent 3 replicates with Standard Errors. Lower Ct value represents higher copies of the F gene.
| Groups | Average Ct |
|---|---|
| PBS | 11.3± 0.01 |
| phCMV I | 11.7±0.02 |
| RSV | 21.5±0.01 |
| DRF-412 | 19.63±0.041 |
| DRF412-P | 19.43±0.08 |
RNA from lung was used to amplify the F gene, qPCR was performed in Applied Biosystems to obtain Ct values, where Ct values are threshold cycles at which a statistically significant increase in detection of SYBR green emission intensity occurs. These Ct values were normalized to a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control to account for variability in RNA concentrations between samples to obtain net Ct values. Average Ct values represent 3 replicates with Standard Errors. Lower Ct value represents higher copies of the F gene.
Table 2. Concentrations of cytokines produced by mice inoculated with DRF-412, DRF412-P or RSVa.
Concentrations of cytokines produced by mice inoculated with DRF-412, DRF412-P or RSV. Mice were inoculated with PBS, DRF-412, DRF412-P or RSV. Samples from 3 donors were taken at various times post-inoculation and cytokine concentrations were assayed by a luminex bead assay (or by ELISA for IFNγ). Shown here are maximum median values irrespective of time. Cytokine concentrations in clarified supernatants, expressed as pg/ml (or IU/ml for IFNγ) and, in parentheses, as fold-difference compared to mice inoculated with PBS, unless the value for PBS group was “0”
| Cytokine | PBSb | DRF-412b | DRF412-Pb | RSVb | |
|---|---|---|---|---|---|
| Interferons | IFNγ | 0 | 50 | 30 | 81 |
| Interleukins | IL-2 | 6 | 25 (4) | 18 (3) | 30 (5) |
| IL-6 | 10 | 40 (4) | 20 (2) | 61 (6) | |
| IL-10 | 8 | 40 (5) | 33 (4) | 70 (8) | |
| IL-12 | 11 | 34 (3) | 23 (2) | 77 (7) | |
| TNF family | TNFα | 7 | 49 (7) | 22 (3) | 84 (12) |
Mice were inoculated with PBS, DRF-412, DRF412-P or RSV. Samples from 3 donors were taken at various times post-inoculation and cytokine concentrations were assayed by a luminex bead assay (or by ELISA for IFNγ). Shown here are maximum median values irrespective of time.
Cytokine concentration in clarified supernatants, expressed as pg/ml (or IU/ml for IFNγ) and, in parentheses, as fold-difference compared to mice inoculated with PBS, unless the value for PBS group was “0”
4. Discussion
Despite the advancement of DNA vaccination technology, the protection efficacy against various infectious agents is not always satisfactory. Only a few reports have shown full protection (Bahloul et al., 1998; Bray, 1989). Development of a vaccine against RSV has been a challenge due to detrimental effects of the formalin inactivated RSV vaccine. An effective RSV vaccine should be safe for neonates and should protect against both RSV-A and –B. Based on the evidence from human and animal trials with other RSV vaccines, secretory IgA and serum antibodies appear to protect from infection of the upper and lower respiratory tracts, respectively. An effective RSV vaccine should mimic the stimulation of the immune system induced by natural infection and thus induce a balanced immune response, with induction of RSV-specific neutralizing antibodies, CD8 T cells, Th1/Th2 CD4 T-cells and preferably secretory IgA antibodies (Hurk et al., 2007).
In the present study, we developed two DNA vaccine vectors, DRF-412 contained the RSV F gene and the ctxA2B gene, whereas DRF412-P contained only the RSV F gene. The results from our study clearly demonstrated that DRF-412 could induce a higher mixed Th1 and Th2 immune response than DRF412-P in immunized mice. Expression of the F gene and ctxA2B gene could improve the mucosal immune response and solve the problem of the low efficiency of DNA vaccine. The genetic manipulation is fairly simple and this approach has been utilized for design and development of mucosal vaccines against various pathogens (Singh et al., 2006). Analysis of serum and salivary antibody responses from DNA vaccine immunized mice revealed that both DRF-412 and DRF412-P are capable of inducing robust systemic and mucosal immune responses. In general, antibody responses in serum were higher compared to the salivary antibody responses. This is consistent with similar studies which also demonstrated that serum antibody responses are higher than other mucosal antibody responses (Walsh et al., 1993; Tebby et al., 2000). In our studies, mice immunized with RSV showed lower levels of all antibody isotypes; this may be due to our experimental design, which was targeted to test for only F protein specific antibody responses rather than total antibody responses to all RSV antigens. In addition, mice were immunized only once with 105 PFU of live RSV to simulate natural infection.
The DNA vaccine-specific IgA response in serum and saliva was significantly high. Several studies (Tebby et al., 2000) including our previous studies (Singh et al, 2007a) support the notion that the RSV-F protein can induce significant mucosal IgA responses only when used in combination with an adjuvant. Interestingly most of these studies employed either CTB or CT as an adjuvant. In our present study, we selected the modified ctxA2B as a co-expressed gene in the DNA vaccine vector, so that when the F gene was expressed in tissues, the ctxA2B protein would also be expressed and act as adjuvant to improve mucosal immunity. Our results clearly demonstrate that the genetically linked RSV-F gene with ctxA2B can be successfully expressed both in vitro and in vivo.
We found a Th1/Th2 mixed immune response when the DNA vaccine was administered to mice and DRF-412 induced higher cytokine concentrations than DRF412-P. This may be due to the presence of more than one antigenic site in the RSV-F immunogen and the ctxA2B gene. The role of the modified CT (ctxA2B), which was part of the recombinant antigen, is a strong possibility for improvement of mucosal immunity. CT and other enterotoxin based adjuvants have been shown to induce a Th1/Th2 mixed immune response in combination with RSV antigens (Lycke, 1997; Simmons et al., 2001).
To our knowledge this is the very first report demonstrating that a genetically linked RSV-F gene with ctxA2B can be successfully expressed in a DNA vaccine vector. When the fused antigen is used to immunize mice intramuscularly it can induce a strong mucosal as well as systemic immune responses and protect animals from RSV challenge.
Analysis of antibody responses in serum and saliva in conjunction with analysis of Th1–Th2 cytokine profiles generated from stimulated spleen cells indicate a Th1/Th2 mixed immune response, with DRF-412 producing a much better response than DRF412-P. The RSV lung titer, qRT-PCR results and serum antibody neutralization data support this conclusion. Therefore, we conclude that the RSV DNA vaccine with genetically detoxified CTB gene is able to induce a mucosal immune response resulting in protection of mice against subsequent virus challenge. Consequently, this vaccination strategy may provide a safe and efficacious mechanism for the immunization of humans against RSV infection, and DRF-412 may be a potential vaccine candidate against RSV.
Fig. 6.
Evaluation of the specific antibody response. The mice were immunized i.m. respectively, with 50 μg of DRF-412 or DRF412-P, and boosted twice at 2 weeks intervals, while serum samples were collected from the mice at day 0, 14, 28, and 49 post-immunization. A. RSV-F specific antibodies in serum detected by ELISA. B. The RSV % neutralization titer in sera. Results are presented as an average of data from three groups in triplicate; error bars represent standard deviation.
Fig. 9.
Evaluation of RSV distribution in lungs after live RSV challenge. Mice were immunized 3 times with either DRF-412 or DRF412-P and challenged on day 49 with live RSV via i.n. route; lungs were removed 4 d later and fixed with 30% sucrose-PBS. Sections were treated with monoclonal antibodies against RSV as primary antibodies (1:500), and then reacted with anti-mouse-FITC (1:50). (A) DRF-412; (B) DRF412-P; (C) PBS control; (D) RSV
Acknowledgments
This project was supported by National Institutes of Health Grant (2S06 GM008219-200012) and National Science Foundation-CREST grant (#HRD-0734232).The authors thank Praseetha Subbarayan and Komal Vig for their help with animal experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson L, Hierholzer JC, Bingham PG, Stone YO. Microneutralization test for respiratory syncytial virus based on an enzyme immunoassay. J Clin. Microbiol. 1985;22:1050–1052. doi: 10.1128/jcm.22.6.1050-1052.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrington J, Braun RP, Dong L, Fuller DH, Macklin MD, Umlauf SW, Wagner SJ, Wu MS, Payne LG, Haynes JR. Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvant for DNA vaccines. J Virol. 2002;76(9):4536–4546. doi: 10.1128/JVI.76.9.4536-4546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahloul C, Jacob Y, Tordo N, Perin P. DNA based immunization for exploring the enlargement of immunological cross-reactivity against the lyssaviruses. Vaccine. 1998;16:417–425. doi: 10.1016/s0264-410x(97)00204-1. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Letvin NL, Seder RA. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunol Rev. 2004;202:266–274. doi: 10.1111/j.0105-2896.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- Bembridge GP, Rodriguez N, Garcia-Beato R, Nicolson C, Melero JA, Taylor G. DNA encoding the attachment (G) or fusion (F) protein of respiratory syncytial virus induces protection in the absence of pulmonary inflammation. J Gen Virol. 2000;81:2519–2523. doi: 10.1099/0022-1317-81-10-2519. [DOI] [PubMed] [Google Scholar]
- Bleek GM, Poelen MC, Most RV, Brugghe HF, Timmermans HA, Boog CJ, Hoogerhout P, Otten HG, Els CA. Identification of immunodominant epitopes derived from the respiratory syncytial virus fusion protein that are recognized by human CD4 T cells. J Virol. 2003;77(2):980–988. doi: 10.1128/JVI.77.2.980-988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray P. Evaluation of tick-borne encephalitis DNA vaccines in monkeys. Virology. 1989;263:166–174. doi: 10.1006/viro.1999.9918. [DOI] [PubMed] [Google Scholar]
- Connors M, Collins PL, Firestone C-Y, Murphy BR. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistnace to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudas RA, Karron RA. Respiratory syncytial virus vaccine Clin. Micro. Rev. 1998;11(3):430–439. doi: 10.1128/cmr.11.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapamo F, Dennis VA, Philipp MT. Differential acquired immune responsiveness to bacterial lipoproteins in Lyme disease-resistant and -susceptible mouse strains. Eur J Immunol. 2003;33:1934–1940. doi: 10.1002/eji.200323655. [DOI] [PubMed] [Google Scholar]
- Girard MP, Cherian T, Pervikov Y, Kieny MP. A review of vaccine research and development: human acute respiratory infections. Vaccine. 2005;23:5708–5724. doi: 10.1016/j.vaccine.2005.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy S, Goestch L, Plotnicky-Gilquin H, Ngueyen T, Schmitt D, Staquet M-J, Corvaia N. Immunization onto shaved skin with a bacterial enterotoxin adjuvant protects mice against Respiratory Syncytial Virus (RSV). Vaccine. 2003;21:1665–1671. doi: 10.1016/s0264-410x(02)00733-8. [DOI] [PubMed] [Google Scholar]
- Graham BS, Bunton LA, Wright PF, Karzon DT. Reinfection of mice with respiratory syncytial virus. J Med Virol. 1991;34:7–13. doi: 10.1002/jmv.1890340103. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Hollingshead SK, Koga T, Russell MW. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–4332. [PubMed] [Google Scholar]
- Harcourt JL, Anderson LJ, Sullender W, Tripp RA. Pulmonary delivery of respiratory syncytial virus DNA vaccines using macroaggregated albumin particles. Vaccine. 2004;22(17-18):2248–2260. doi: 10.1016/j.vaccine.2003.11.050. [DOI] [PubMed] [Google Scholar]
- Hardy SJS, Holmgren J, Johansson S, Sanchez J. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 1988;85:7109–7113. doi: 10.1073/pnas.85.19.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurk SV, Mapletoft JW, Arsic N, Kovacs-Nolan J. Immunopathology of RSV infection: prospects for developing vaccines without this complication. Reviews in Medical Virology. 2007;17(1):5–34. doi: 10.1002/rmv.518. [DOI] [PubMed] [Google Scholar]
- Iqbal A, Lin W, Jabbal-Gill I, Davis SS, Steward MW, Illum L. Nasal delivery of chitosan-DNA plasmid expressing epitopes of respiratory syncytial virus (RSV) induces protective CTL responses in BALB/c mice. Vaccine. 2003;21:1478–1485. doi: 10.1016/s0264-410x(02)00662-x. [DOI] [PubMed] [Google Scholar]
- Justewicz D, Webster RG. Long term maintenance of B cell immunity to influenza virus hemagglutinin in mice following DNA-based immunization. Virology. 1996;224:10–17. doi: 10.1006/viro.1996.0501. [DOI] [PubMed] [Google Scholar]
- Klinman DM. Adjuvant activity of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25:135–154. doi: 10.1080/08830180600743057. [DOI] [PubMed] [Google Scholar]
- Le TT, Drane D, Malliaros J, Cox JC, Rothel L, Pearse M, Woodberry T, Gardner J, Suhrbier A. Cytotoxic T cell polyepitope vaccines delivered by ISCOMs. Vaccine. 2001;19:4669–4675. doi: 10.1016/s0264-410x(01)00243-2. [DOI] [PubMed] [Google Scholar]
- Li X, Sambhara S, Li CX, Ewasyshyn M, Parrington M, Caterini J, James O, Cates G, Du R-P, Klein M. Protection against respiratory syncytial virus infection by DNA immunization. J Exp Med. 1998;188:681–688. doi: 10.1084/jem.188.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N. The mechanism of cholera toxin adjuvanticity. Res Immunol. 1997;148:504–20. doi: 10.1016/s0923-2494(98)80144-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, Cros J, Mertz SE, Jewell NA, Hammond SA, Flano E, Durbin RK, Garcia-Sastre A, Durbin JE. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol. 2006;80(3):1130–1139. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert PH, Siegrist C-A. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte response in neonates and during ealry life. Proc Natl Acad Sci USA. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz FM, Piedra PA, Glenzen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;24:3465–3467. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]
- Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001;14:430–445. doi: 10.1128/CMR.14.2.430-445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie Y, Frederik PM, Gregoriadis G. Liposome-mediated DNA vaccination:the effect of vesicle composition. Vaccine. 2001;19:3301–3310. doi: 10.1016/s0264-410x(00)00432-1. [DOI] [PubMed] [Google Scholar]
- Pertmer TM, Roberts TR, Haynes JR. Influenza virus nucleoprotein specific immunoglobulinG subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AE, Aquino G, Ostoa-Saloma P, Laclette JP, Rocha-Zavaleta L. Cholera toxin B-subunit gene enhances mucosal immunoglobulin A, Th1-type, and CD8+ cytotoxic responses when coadministered intradermally with a DNA vaccine. Clin Diagn Lab Immunol. 2004;11:711–719. doi: 10.1128/CDLI.11.4.711-719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C, Hussell T, Sparer T, Walzl G, Openshaw P, Dougan G. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic and immunoregulatory antiviral CD8+ T cell responses. J Immunol. 2001;166(2):1106–13. doi: 10.4049/jimmunol.166.2.1106. [DOI] [PubMed] [Google Scholar]
- Singh SR, Dennis VA, Carter CL, Pillai SR, Jefferson A, Sahi SV, Moore EG. Immunogenicity and efficacy of recombinant RSV-F vaccine in a mouse model. Vaccine. 2007a;25:6211–6223. doi: 10.1016/j.vaccine.2007.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Dennis VA, Carter CL, Pillai SR, Moore EG. Respiratory Syncytial Virus Recombinant F protein (residues 255-278) induces a helper T cell type 1 immune response in mice. Viral Immunol. 2007b;20(2):261–275. doi: 10.1089/vim.2007.0008. [DOI] [PubMed] [Google Scholar]
- Singh SR, Hulett K, Pillai SR, Dennis VA, Oh MK, Scissum-Gunn K. Mucosal immunization with recombinant MOMP genetically linked with modified cholera toxin confers protection against Chlamydia trachomatis infection. Vaccine. 2006;24:1213–1224. doi: 10.1016/j.vaccine.2005.08.097. [DOI] [PubMed] [Google Scholar]
- Sixma TK, Pronk SE, Kalk KH, Van BA, Berghuis AM, Hol WG. Lactose binding to heat-labile enterotoxin revealed by X-ray crystallography. Nature. 1992;355(6360):561–564. doi: 10.1038/355561a0. [DOI] [PubMed] [Google Scholar]
- Tebbey PW, Scheuer CA, Peek JA, Zhu D, LaPierre NA, Green BA, Phillips ED, Ibraghimov AR, Eldridge JH, Hancock GE. Effective mucosal immunization against respiratory syncytial virus using purified F protein and a genetically detoxified cholera holotoxin, CT-E29H. Vaccine. 2000;18:2723–2734. doi: 10.1016/s0264-410x(00)00058-x. [DOI] [PubMed] [Google Scholar]
- Tree JA, Bembridge GP, Hou S, Taylor G, Fashola-Stone E, Melero J, Carnage MP. An assessment of different DNA delivery systems for protection against respiratory syncytial virus infection in the murine model: gene-gun delivery induces IgG in the lung. Vaccine. 2004;22:2438–2443. doi: 10.1016/j.vaccine.2003.11.069. [DOI] [PubMed] [Google Scholar]
- Tripp RA, Oshansky C, Alvarez R. Cytokines and respiratory syncytial virus infection. Proc Am Thorac Soc. 2005;2:147–149. doi: 10.1513/pats.200502-014AW. [DOI] [PubMed] [Google Scholar]
- Ulmer JB, Sadoff JC, Liu MA. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- Vaughan K, Rhodes GH, Gershwin LJ. DNA immunization against respiratory syncytial virus (RSV) in infant rhesus monkeys. Vaccine. 2005;23:2928–2942. doi: 10.1016/j.vaccine.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Walsh EE. Humoral, mucosal and cellular immune response to topical immunization with a subunit respiratory syncytial virus vaccine. J Infect Dis. 1994;170:345–350. doi: 10.1093/infdis/170.2.345. [DOI] [PubMed] [Google Scholar]
- Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- Wertz GW, Stott EJ, Young KKY, Anderson K, Ball LA. Expression of the fusion protein of human respiratory syncytial virus from recombinant vaccinia virus vectors and protection of vaccinated mice. J Virol. 1987;61:293–301. doi: 10.1128/jvi.61.2.293-301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]