Abstract
Placozoans are the simplest extant free-living animals consisting of only five cell types and lacking neurons and muscle cells. Their phylogenetic position implies they are important for uncovering the origins of metazoans. Although recent studies show multiple groups within the phylum, most placozoan research has been performed on laboratory-cultured clones deriving from a single specimen. Reports of placozoan discovery are concentrated in the tropic and subtropic seas, especially in the Mediterranean and the Caribbean. Here, I report the unexpected abundance of placozoans from the Japanese coast. They were found from all six studied sites, even during winter for two sites, suggesting that they are more tolerant to low temperatures than previously regarded. These results suggest an unknown abundance of placozoans in the Northern Pacific Ocean and further studies on these populations may be essential in solving important biological problems of the phylum.
Placozoans are flat amoeba-like marine animals about 1–2 mm, made up of only five cell types arranged in three layers1,2. They lack neurons and muscle cells, but are not sessile or parasitic, making them the simplest extant free-living animals. They are able to reproduce vegetatively in several ways, including budding in which floating spheres are formed3, but their sexual reproduction still remains a mystery. Although molecular signatures for sex have been reported4, all embryos found so far arrest development at the cleavage stage5. Placozoa is one of the four extant animal phyla not included within the Bilateria, together with the Ctenophora, Porifera, and Cnidaria. Their simple morphology and phylogenetic position make them important animals for uncovering the origins and early radiation of multicellular animals6,7,8, and they were one of the first invertebrate animals to have their genome sequenced9. However, due to their small size and lack of easily distinguishable traits, they are extremely difficult to find in the natural environment. The original report in 1883, in which the only species to have a scientific name assigned within the phylum, Trichoplax adhaerens, was described, is based on specimens found in an aquarium10. Over the years, many studies on the phylum Placozoa have been published, but research has been mainly performed on laboratory maintained cultures. Although new wild populations have been found since, morphological characters that can be used for species classification could not be found using light microscopy. Recent molecular phylogenetic analyses and ultrastructure observations using electron microscopy revealed 19 groups within the phylum, with genetic distances between the groups equaling to different families of cnidarians and sponges11,12,13,14,15. Much phylogeographical research has been done on the Mediterranean and Caribbean populations16,17,18,19, but data have been largely missing for the Pacific Ocean, including Japan.
Surprisingly, the second report ever for placozoans from a wild environment and not from an aquarium was from Japan in 1977, more than 30 years ago20. The animal was found from glass slides submerged in the sea at Shimoda. However, little research has been performed on Japanese placozoans since. Over the years, some research has been performed on the Shirahama population, with behavior21, seasonal variations in population size22, and mitochondrial genome23 being reported. There have also been a number of personal communications of placozoans being discovered in aquaria and tanks around the country. However, origins of these aquaria-found placozoans are almost impossible to clarify, with the possibility of the animal being introduced together with the seawater, other animals, or non-biological objects (e.g. rocks, sand) when they were placed in the tanks. This results in the wild habitat of aquaria-found placozoans being unidentified. In recent years, brief accounts of placozoan discovery from natural environments other than Shimoda and Shirahama have been reported from Japanese waters, (e.g. Okinawa, Iriomote, Oki)24,25,26,27 but there have been no attempts to elucidate the abundance of placozoans along the Japanese coast.
Here, I report the discovery of placozoan wild populations from multiple sites around Japan. Discoveries as far north as lat. 37°18′27″ N reveal that they may be abundant in the Northern Pacific Ocean. Research using placozoan populations from various natural environments is essential for uncovering the diversity within the phylum and shall be useful for future developmental and reproductive experiments.
Results
Placozoan populations in Shimoda
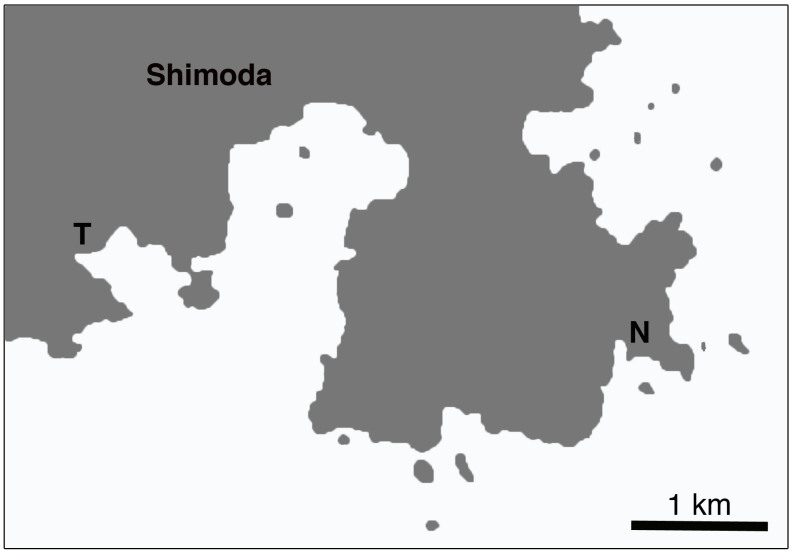
Sudzuki reported that he was able to find Trichoplax at Marine Biological Station, Nihon University, but was unable to find any at Shimoda Marine Research Center (SMRC), University of Tsukuba20, less than five kilometers apart (Fig. 1). To confirm this, I conducted a search of placozoans in five locations within and around SMRC (Table 1). At SMRC, seawater is constantly pumped up from a depth of 3 meters. Outdoor Tank 1 is where the pumped natural seawater first enters SMRC, and can be considered as most similar to a natural environment of the four tanks in terms of fresh seawater. In Outdoor Tank 2 and the two indoor tanks, seawater that has circulated through a lengthy tubing system are distributed into the tanks. A metal shelf containing three plastic racks was also placed in the sea near SMRC at a depth of about 3 meters. Each rack contained ten glass plates for placozoan collections (for details, see Methods). The racks were elevated about 100 cm, 65 cm, and 30 cm respectively from the stony sea bottom. Placozoans were found from all the searched locations; two outdoor tanks, two indoor tanks, and one station in the natural sea (Table 1). They were found throughout the year, even in the winter when the average sea temperature can be low as 13 degrees28 (Fig. 2). There were no clear trends concerning seasonal fluctuations, with instances such as in Outdoor Tank 1, 255 specimens being found in August 2011, but only 39 in August 2012, or 274 specimens found in December 1, 2011 but only 15 animals just 25 days later. Statistical analyses revealed no significant differences between seasons as well (e.g. t-test between the number of collected specimens in the summer months (June to August) and winter months (December to January) had a p-value of 0.908). In the natural sea, 7 animals were found from the top rack, 10 from the middle rack, and 0 from the bottom rack. Overall, placozoans were more abundant in the outdoor tanks than in indoor tanks (t-test, p-value<0.01), and more animals could be collected by slide sampling than by substrate sampling (t-test, p-value<0.01).
Figure 1. Sites in Shimoda where placozoans were collected.
T: Shimoda Marine Research Center, University of Tsukuba: N: Marine Biological Station, Nihon University. Generated using Adobe Photoshop CS5 based on blank map publicly available from the Geospatial Information Authority of Japan39.
Table 1. Placozoans around Shimoda Marine Research Center.
| Location | Method | Days in seawater | Date retrieved/collected | Number collected |
|---|---|---|---|---|
| Outdoor Tank 1 | slide | 222 | 2011 4/25 | 107 |
| 35 | 6/1 | 20 | ||
| 26 | 6/27 | 6 | ||
| 32 | 8/29 | 255 | ||
| 62 | 12/1 | 274 | ||
| 24 | 12/26 | 15 | ||
| 52 | 2012 1/23 | 219 | ||
| 76 | 2/10 | 38 | ||
| 41 | 5/8 | 4 | ||
| 83 | 7/3 | 158 | ||
| 104 | 8/7 | 39 | ||
| 84 | 9/26 | 84 | ||
| Outdoor Tank 2 | slide | 30 | 2010 10/14 | 241 |
| 34 | 2011 5/23 | 16 | ||
| 27 | 6/20 | 12 | ||
| 34 | 7/25 | 14 | ||
| 28 | 8/23 | 3 | ||
| 57 | 11/24 | 4 | ||
| substrate | - | 2011 7/1 | 12 | |
| - | 2012 1/16 | 3 | ||
| Indoor Tank 1 | slide | 202 | 2011 4/4 | 1 |
| Indoor Tank 2 | slide | 34 | 2011 9/13 | 3 |
| 171 | 2012 4/26 | 67 | ||
| 24 | 11/5 | 10 | ||
| Sea (Nabeta Bay) | slide | 15 | 2010 10/27 | 17 |
| substrate | - | 2011 8/16 | 32 | |
| - | 2012 1/5 | 5 |
There were instances not shown when no placozoans were found.
Figure 2. Average surface seawater temperatures during 2011.
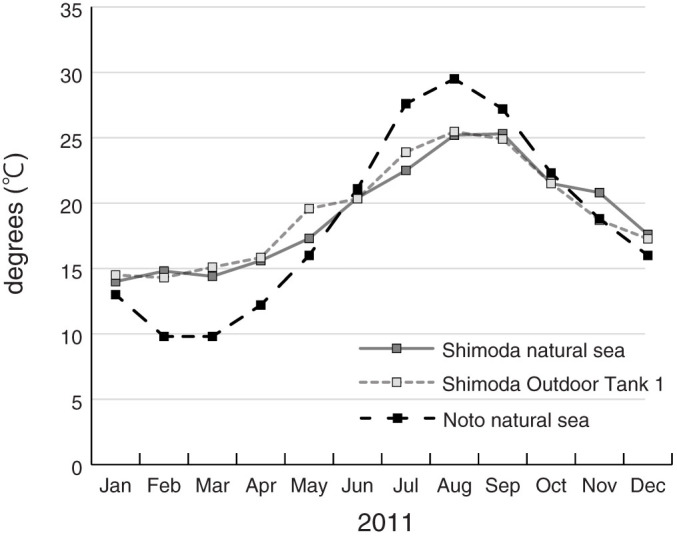
Temperatures for Shimoda natural sea were measured at Nabeta Bay, just outside Shimoda Marine Research Center and adjacent to where placozoans were collected (Based on Shimoda Marine Research Center28). Shimoda Outdoor Tank 1 measurements were performed by the author. Temperatures for Noto natural sea is based on Noto Marine Center36, using data for Tsukumo Bay just outside Noto Marine Laboratory, near where placozoans were collected.
Placozoan populations around Japan
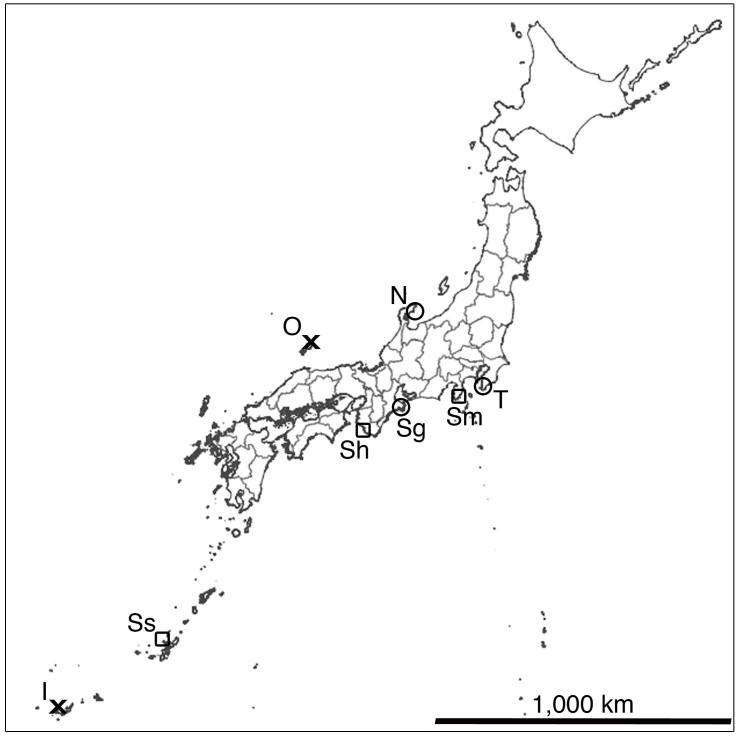
To further extend the research of the phylum Placozoa, I performed a search of the animal at five different sites around Japan (Fig. 3). At Noto Marine Laboratory, Ishikawa (Fig. 3; N), two racks were hung in the sea from a pier at a depth of about 50 cm. Substrate mainly consisting of stones and pebbles were collected from tide pools at a stony beach near the Laboratory. At Marine and Costal Research Center, Tateyama, Chiba (Fig. 3; T), one rack was placed in an open outdoor tank with running natural seawater, and another was placed in a closed outdoor tank with running seawater. Substrate sampling was performed at a shore near the Center where coastal revetments are present, and therefore pieces of concrete were present in the collected samples. Two racks were placed in an outdoor tank with running seawater, and substrate were collected from a nearby stony beach at Sugashima Marine Biological Laboratory, Mie (Fig. 3; Sg). At Seto Marine Biological Laboratory, Shirahama, Wakayama (Fig. 3; Sh), one rack was placed in an indoor aquarium with running seawater, and another was hung in the sea from a pier at a depth of about 100 cm. Substrate was collected from a rocky shore near the Laboratory. At Tropical Biosphere Research Center Sesoko Station, Okinawa (Fig. 3; Ss), two racks were placed in an outdoor tank with running seawater, and substrate were collected from a rocky limestone shore near the Station. Substrate at Sesoko was unique in that coral skeletons consisted a large portion, whereas they were absent in the four other sites, as well as Shimoda. Of the five sites, placozoans have been previously reported from Shirahama21,22,23 and Sesoko24,25,26,27.
Figure 3. Japanese distribution of Placozoa.
 : three sites where placozoans were newly discovered in this study; Noto (N), Tateyama (T), Sugashima (Sg).
: three sites where placozoans were newly discovered in this study; Noto (N), Tateyama (T), Sugashima (Sg).  : three sites where previous reports of placozoans are present, and where I was able to collect the animals in this study; Shimoda (Sm), Shirahama (Sh), Sesoko (Ss). X: two sites where brief reports of placozoan collection are present and not studied in this study; Oki (O) Iriomote (I). Generated using Adobe Photoshop CS5 based on blank map publicly available from the Geospatial Information Authority of Japan39.
: three sites where previous reports of placozoans are present, and where I was able to collect the animals in this study; Shimoda (Sm), Shirahama (Sh), Sesoko (Ss). X: two sites where brief reports of placozoan collection are present and not studied in this study; Oki (O) Iriomote (I). Generated using Adobe Photoshop CS5 based on blank map publicly available from the Geospatial Information Authority of Japan39.
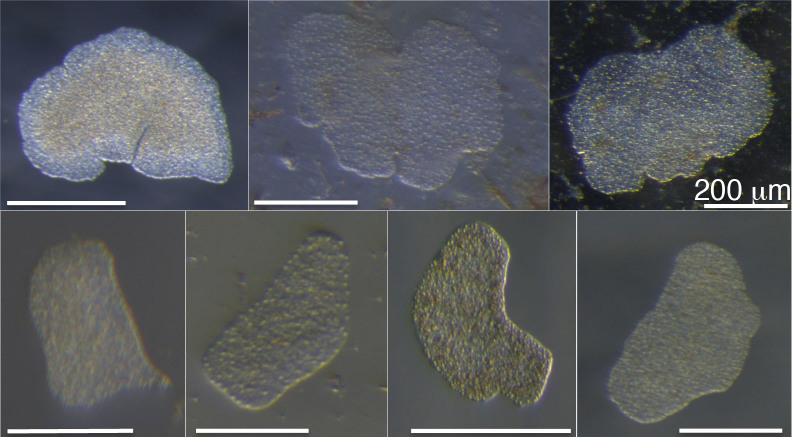
Although the number of collected placozoans differed greatly from one site to another (only one at Sesoko, but more than 1,000 at Sugashima), I succeeded in collecting placozoans in all five sites (Table 2). At Noto and Sesoko, no placozoans were collected by slide sampling, whereas only slide sampling was successful at Tateyama, Sugashima, and Shirahama. At Tateyama, one animal each was found from the rack placed in an open tank and the closed tank. At Shirahama, all 48 placozoans were collected from the rack hung in the sea from a pier, and none were found from the rack in the indoor tank. No conspicuous differences in morphology or behavior were observed between the collected sites and months (Fig. 4).
Table 2. Number of collected placozoan specimens around Japan.
| Site | Previous reports | Method | Days in seawater | Date retrieved/collected (2012) | Number collected |
|---|---|---|---|---|---|
| Noto | none | slide | 54 | 10/17 | 0 |
| substrate | - | 10/19 | 3 | ||
| Tateyama | none | slide | 63 | 11/12 | 2 |
| substrate | - | 11/14-15 | 0 | ||
| Sugashima | none | slide | 64 | 11/19 | >1,000 |
| substrate | - | 11/21 | 0 | ||
| Shirahama | Reference 21,22,23 | slide | 66 | 12/18 | 48 |
| substrate | - | 12/20 | 0 | ||
| Sesoko | Reference 24,25,26,27 | slide | 26 | 6/23 | 0 |
| substrate | - | 6/24-26 | 1 |
Figure 4. Placozoans collected around Japan.
Top from left; Placozoans from Shimoda collected in May, September, and December. Bottom from left; Placozoans from Noto collected in October, Tateyama in November, Sugashima in November, and Shirahama in December. All pictures were taken by the author (H.N.). All scale bars represent 200 μm.
Discussions
Placozoan research is carried out in a number of laboratories worldwide, with almost all studies using the Grell clone established from a single specimen caught from the Red Sea in 196929,30,31,32. Studies on the Grell clone have uncovered many interesting features of this simple animal. But recent molecular phylogenetics and ultrastructural studies have shown that there are close to 20 groups within the phylum14,15. Studies on groups other than the Grell clone, especially multiple wild populations, are essential to reveal the true biological nature of the phylum Placozoa.
There have been sporadic reports of placozoans from Japan20,21,22,23,24,25,26,27, with many of them just mentioned briefly in conference proceedings or as personal communications in scientific papers. They have also been reported from the Russian coast of the Sea of Japan33, but they were found in aquaria in Moscow containing animals from the Sea of Japan and not from the Sea of Japan per se. My study here is the first to elucidate the placozoan population in the Northern Pacific Ocean using standardized methods in multiple collections sites, with special care to exclude the possibility of contamination between different sites. I was able to find placozoans in all six sites where they were searched, including three sites (Noto, Tateyama, and Sugashima) where placozoans were previously unknown. This shows that placozoans are present around the Japanese coast both on the Pacific coast and the Sea of Japan coast, and are probably found in wide areas in the Northern Pacific Ocean.
Surprisingly, only one specimen was found at Sesoko, Okinawa, the southern most site in this study (Fig. 3; Ss). This is most probably due to a typhoon that landed at Okinawa two days before the collections. As placozoans are known to be intolerant to low salinities26, most specimens may have died owning to the heavy rain brought by the typhoon. The lack of clear trends concerning seasonal fluctuations in the number of collected placozoans at SMRC (Table 1) also show that the effects of weather on the day or days preceding the collection is much greater than that of seasonal climate. There were also instances in which placozoans were absent from glass plates and substrate just 5 meters apart from the substrate where they were found. This is most possibly due to local environment such as currents and light levels. These observations show that success of placozoan collection is largely dependent on the preceding weather and the micro-environment of the collection site. Hence, studies at multiple locations within an area spanning several days may reveal placozoan populations in places where they were reported as absent previously, such as the case of SMRC in this study.
The two performed collection methods yielded different results. Slide sampling was successful in Tateyama, Sugashima, and Shirahama, whereas substrate sampling was successful in Noto and Sesoko. Placozoans were collected with both methods at Shimoda, but slide sampling yielded more animals (Table 1; t-test, p-value<0.01). Usually, more animals were collected by slide sampling, with over 1,000 found at Sugashima. However, I do not suggest slide sampling as the superior of the two methods, nor do I suggest that slide sampling is suited at Tateyama, Sugashima, and Shirahama, and substrate sampling should be performed at the other two sites. I hypothesize that the difference in successful methods between the sites has more to do with the weather prior to the collections and the microenvironment as discussed above. These conditions are difficult to comprehend beforehand, and hence it is desirable to perform both methods when collecting placozoans.
One trend evidently observed in Shimoda was that more placozoans could be collected from outdoor tanks than from indoor tanks (Table 1; t-test, p-value<0.01). The seawater in Outdoor Tank 2 and the two indoor tanks are similar in composition, and there were no large differences in water temperatures. I hypothesize that the largest difference is the light levels, with the outdoor tanks receiving sunlight during daytime whereas indoor tanks received artificial light from fluorescent lamps only when people were present in the rooms. This possibly resulted in the clear difference of density of algae found on the glass plates, further leading to a larger number of animals, both in terms of species and specimens, being found on the glass plates in the outdoor tanks. As placozoans are known to feed on algae6,26,34,35, and since they are not easily preyed upon by other animals26, they were able to flourish in the outdoor tanks.
Placozoan populations are present at Shimoda through the winter (Table 1), and numerous specimens were found at Shirahama in December (Table 2). These results show that some groups within the phylum are able to endure low temperatures and lower light levels of winter, and are persistent year around at some areas of the Japanese coast. It still remains to be seen if they can survive the winter at Noto (Fig. 3; N), the northern most site where placozoans were collected in this study (roughly lat. 37°18′27″ N), where average winter temperatures can reach as low as 9 degrees36. It is possible that the Noto population die out every winter, and new animals are recruited every spring by the Tsushima Current coming from the south. This might be clarified by comparing the Noto population genetic structure between autumn and spring, or between different years.
My study here uncovers a previously unknown abundance of placozoans in the Northern Pacific Ocean, especially around the Japanese coast. The northern limit of placozoans in the Northern West Pacific may be extended through further studies along the northern Japanese coast, where research was not conducted in this study. Moreover, although they are regarded as tropical-subtropical animals, and are presumably absent from the Antarctic coast37, there are reports from Roscoff, Plymouth, and Woods Hole15,38, and further research may uncover large placozoan populations in temperate and cold water seas worldwide. Studies on these populations may be useful to uncover important biological problems that still remain unsolved for the phylum, such as sexual reproduction, development, evolutionary history, and phylogenetic position.
Methods
Placozoans were collected using the two sampling methods explained in Maruyama 200422, namely, slide sampling and substrate sampling, with certain modifications. In slide sampling, glass plates larger than typical glass slides, measuring 107 mm × 82 mm, were used. Ten glass plates were placed in each plastic rack (320 mm × 110 mm × 90 mm), and the racks were placed in each study site. For the five collection sites other than Shimoda (Table 2), two racks were placed in each location. Rather than placing the racks directly on the substrate, they were suspended in the water column where possible, since this was reported to be more efficient26. The racks and the glass plates were always handled in seawater before and during the checking process, so as not to harm the placozoans. On the day of retrieval, the racks were carried to the laboratory and both sides of the glass plates were observed under a streo microscope. In substrate sampling, substrate measuring up to 6 cm in length were placed in 50 ml tubes together with ambient seawater. The majority of samples were stones and pebbles, but molluscan shells, echinoderm skeletons, and pieces of wood were also collected. For the checking process, the tubes were shaken for 5–10 seconds and both the seawater and the substrate were decanted into glass dishes or plastic petri dishes. The dishes were examined everyday under stereo microscopes. Some of the substrate were checked at the marine stations near the collection site, while others were brought back to Shimoda Marine Research Center and checked. Substrate brought back to Shimoda were treated only with ambient seawater collected together with the substrate, and at no time did the samples touch the seawater of Shimoda, or equipment that were used at Shimoda.
Due to technical and administrative difficulties, it was impossible to directly hang racks in the sea in some sites. In these sites, racks were placed only in tanks that follow these rules: Only seawater, organisms, and substrate (e.g. sand, rock, shells, pebbles) from the surrounding areas have been put in the tank since being bought or since the latest cleaning in which the tank was emptied and dried. Furthermore, no artificial objects (e.g. nets, baskets, pipes) used in other areas or used in tanks that contain seawater, organisms, and substrate from other areas have been put in the tank since being bought or since the latest cleaning.
To fully clarify the origin of the identified placozoan specimens, new glass plates, plastic racks, tubes, containers, tips, tubes, dishes were used for each collection site.
Unpaired, two-tailed Student's t-test analyses were conducted to reveal differences in the efficiency of collection methods, between locations, and seasons at Shimoda Marine Research Center. Instances not shown in Table 1, when no placozoans were found, were included in these analyses.
Author Contributions
H.N. designed the study, performed the experiments, prepared the figures, and wrote the manuscript.
Acknowledgments
I am grateful to the following colleagues and collaborators for help in placozoan sampling: Yasunori Saito, Takeo Horie, Yasutaka Tsuchiya, Toshihiko Sato, Hideo Shinagawa, Yutaro Yamada, and the staff of Shimoda Marine Research Center, University of Tsukuba, Toshio Sekiguchi, Masahiro Matada, and the staff of Noto Marine Laboratory, Kanazawa University, Mamiko Hirose, Masato Kiyomoto, Mamoru Yamaguchi, and the staff of Marine and Costal Research Center, Ochanomizu University, Masashi Fukuoka and the staff of Sugashima Marine Biological Laboratory, Nagoya University, Masanori Okanishi and the staff of Seto Marine Biological Laboratory, Kyoto University, and Masaya Morita and the staff of Tropical Biosphere Research Center Sesoko Station, University of Ryukyus. I also thank Masa-aki Yoshida and Hideyuki Miyazawa for helpful comments in planning the experiments. This research was financially supported by the Sasakawa Scientific Research Grant from The Japan Science Society, JSPS Grant-in-Aid for Research Activity Start-up (22870003), and for Young Scientists (B)(70586403).
References
- Schierwater B. My favorite animal, Trichoplax adhaerens. Bioessays 27, 1294–1302 (2005). [DOI] [PubMed] [Google Scholar]
- Schierwater B. et al. Trichoplax and Placozoa: one of the crucial keys to understanding metazoan evolution. Key Transitions in Animal Evolution (DeSalle, R. & Schierwater, B. (eds.)) 289–326 (CRC Press, 2010). [Google Scholar]
- Thiemann M. & Ruthmann A. Alternative modes of asexual reproduction in Trichoplax adhaerens (Placozoa). Zoomorphology 110, 165–174 (1991). [Google Scholar]
- Signorovitch A. Y., Dellaporta S. L. & Buss L. W. Molecular signatures for sex in the Placozoa. Proc. Natl. Acad. Sci. USA 102, 15518–15522 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel M., Guidi L., Hadrys H., Balsamo M. & Schierwater B. New insights into Placozoa sexual reproduction and development. PLoS ONE 6, e19639 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed T. & Schierwater B. The evolution of the Placozoa: A new morphological model. Senck. leth. 82, 315–324 (2002). [Google Scholar]
- Syed T. & Schierwater B. Trichoplax adhaerens: discovered as a missing link, forgotten as a hydrozoan, re-discovered as a key to metazoan evolution. Vie Milieu 52, 177–187 (2002). [Google Scholar]
- Schierwater B., de Jong D. & DeSalle R. Placozoa and the evolution of Metazoa and intrasomatic cell differentiation. Int. J. Biochem. Cell Biol. 41, 370–379 (2009). [DOI] [PubMed] [Google Scholar]
- Srivastava M. et al. The Trichoplax genome and the nature of placozoans. Nature 454, 955–961 (2008). [DOI] [PubMed] [Google Scholar]
- Schulze F. E. Trichoplax adhaerens, nov. gen., nov. spec. Zool. Anz. 6, 92–97 (1883). [Google Scholar]
- Voigt O. et al. Placozoa – no longer a phylum of one. Curr. Biol. 14, R944–945 (2004). [DOI] [PubMed] [Google Scholar]
- Signorovitch A. Y., Buss L. W. & Dellaporta S. L. Comparative genomics of large mitochondria in placozoans. PLoS Genet. 3, e13 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel M. & Schierwater B. The phylogeography of the Placozoa suggests a taxon rich phylum in tropical and subtropical waters. Mol. Ecol. 19, 2315–2327 (2010). [DOI] [PubMed] [Google Scholar]
- Guidi L., Eitel M., Cesarini E., Schierwater B. & Balsamo M. Ultrastructural analyses support different morphological lineages in the Placozoa, Grell 1971. J. Morphol. 272, 371–378 (2011). [DOI] [PubMed] [Google Scholar]
- Eitel M., Osigus H., DeSalle R. & Schierwater B. Global Diversity of the Placozoa. PLoS ONE 8, e57131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassetti P. et al. Placozoans (Trichoplax adhaerens Schulze, 1883) in the Mediterranean Sea. Meiofauna Marina 14, 5–7 (2005). [Google Scholar]
- Ocana A. & Ibanez A. A new record of Placozoa from the Mediterranean Sea. Belg. J. Zool. 136, 255–256 (2006). [Google Scholar]
- Grell K. C. & Lopez-Ochoterena E. A new record of Trichoplax adhaerens F. E. Schulze (Phylum Placozoa) in the Mexican Caribbean Sea. An. Inst. Cienc. Mar Limnol. 14, 255–256 (1987). [Google Scholar]
- Signorovitch A. Y., Dellaporta S. L. & Buss L. W. Caribbean placozoan phylogeography. Biol. Bull. 211, 149–156 (2006). [DOI] [PubMed] [Google Scholar]
- Sudzuki M. Microscopical marine animals scarcely known from Japan II. Occurrence of Trichoplax (Placozoa) in Shimoda. Proc. Jap. Soc. Syst. Zool. 13, 1–4 (1977). [Google Scholar]
- Ueda T., Koya S. & Maruyama Y. K. Dynamic patterns in the locomotion and feeding behaviors by the placozoan. Trichoplax adhaerence. Biosystems 54, 65–70 (1999). [DOI] [PubMed] [Google Scholar]
- Maruyama Y. K. Occurrence in the field of a long-term, year-round stable population of Placozoans. Biol. Bull. 206, 55–60 (2004). [DOI] [PubMed] [Google Scholar]
- Miyazawa H., Yoshida M., Tsuneki K. & Furuya H. Mitochondrial genome of a Japanese Placozoan. Zool. Sci. 29, 223–228 (2012). [DOI] [PubMed] [Google Scholar]
- Uehara T., Pearse V. B. & Yamazato K. Birefringent particles observed in Trichoplax adhaerens (Placozoa), the simplest metazoan. Zool. Sci. 6, 1209 (1989). [Google Scholar]
- Pearse V. B., Uehara T. & Miller R. L. Birefringent granules in placozoans (Trichoplax adhaerens). Trans. Am. Microsc. Soc. 113, 385–389 (1994). [Google Scholar]
- Pearse V. B. & Voigt O. Field biology of placozoans (Trichoplax): distribution, diversity, biotic interactions. Integr. Comp. Biol. 47, 677–692 (2007). [DOI] [PubMed] [Google Scholar]
- Pearse V. B. & Voigt O. Field Biology of Placozoans (Trichoplax): Distribution, Diversity, Biotic Interactions. Key Transitions in Animal Evolution (DeSalle, R. & Schierwater, B. (eds.)) 259–288 (CRC Press, 2010). [DOI] [PubMed] [Google Scholar]
- Shimoda Marine Research Center, University of Tsukuba. http://www.shimoda.tsukuba.ac.jp/ (2012).
- Grell K. G. Trichoplax adhaerens und die Entstehung der Metazoen. Naturw. Rundsch. 24, 160–161 (1971). [Google Scholar]
- Grell K. G. Embryonalentwicklung bei Trichoplax adhaerens F. E. Schulze. Naturwiss. 58, 570 (1971). [Google Scholar]
- Grell K. G. Uber den Ursprung der Metazoan. Mikrokosmos. 60, 97–102 (1971). [Google Scholar]
- Grell K. G. Eibildung und Furchung von Trichoplax adhaerens F. E. Schulze (Placozoa). Z. Morph. Tiere. 73, 297–314 (1972). [Google Scholar]
- Ivanov D. L., Malakhov V. V. & Tzetlin A. B. Discovery of a primitive multicellular organism Trichoplax sp. Zool. Zh. 59, 1735–1739 (1980). [Google Scholar]
- Ruthmann A., Behrendt G. & Wahl R. The ventral epithelium of Trichoplax adhaerens (Placozoa). Zoomorphology 106, 115–122 (1986). [Google Scholar]
- Grell K. G. & Ruthmann A. Placozoa. Microscopic Anatomy of Invertebrates, Placozoa, Porifera, Cnidaria, and Ctenophora (Harrison, F. W. & Westfall, J. A. (eds.)) 13–28 (Wiley- Liss, 1991). [Google Scholar]
- Noto Marine Center. Annual report of weather and water quality around Tsukumo Bay for 2011. Rep. Noto Mar. Center 18, 50–54 (2013). [Google Scholar]
- Pearse V. B. & Pearse J. S. Year-long settling plate study yields no Antarctic placozoans, and surprisingly little else. Antarct. J. US. 26, 149–150 (1991). [Google Scholar]
- von der Chevallerie K., Eitel M. & Schierwater B. Focus on an unexpected discovery in Roscoff - a warm water species of the phylum Placozoa. Cah. Biol. Mar. 51, 212–213 (2010). [Google Scholar]
- Geospatial Information Authority of Japan. GSI Maps. http://portal.cyberjapan.jp/index.html (2013).