Abstract
To study the role of abscisic acid (ABA) and gibberellins (GA) in pre-maturity α-amylase (PMA) formation in developing wheat grain, two glasshouse experiments were conducted under controlled conditions in the highly PMA-susceptible genotype Rialto. The first, determined the relative efficacy of applying hormone solutions by injection into the peduncle compared to direct application to the intact grain. The second, examined the effects of each hormone, applied by either method, at mid-grain development on PMA in mature grains. In the first experiment, tritiated ABA (3H-ABA) and gibberellic acid (3H-GA3) were diluted with unlabelled ABA (100 µM) and GA3 (50 µM), respectively, and applied at mid-grain development using both methods. Spikes were harvested after 24, 48 and 72 h from application, and hormone taken up by grains was determined. After 72 h, the uptake per grain in terms of hormones applied was approximately 13% for ABA and 8% for GA3 when applied onto the grains, and approximately 17% for ABA and 5% for GA3 when applied by injection. In the second experiment, applied ABA reduced, whereas applied GA3 increased α-amylase activity. This confirmed that exogenously applied ABA and GA were absorbed in sufficient amounts to alter grain metabolism and impact on PMA.
Exogenous hormone application is an important experimental technique for helping to understand the hormonal control of plant growth and development. Different methods have been used to study the effects of exogenous hormones on grain development. A few studies have dealt with pre-anthesis hormone regulation of floret development31, whereas most studies investigated grain development after anthesis22. Generally in cereals, abscisic acid (ABA) and gibberellic acid (GA3) solutions have been applied separately either by spraying onto the leaves and spikes1,7,31,32 or by injecting into the leaf sheath31 to study their role in floret development, grain set and filling. Yang et al. (2004)32 applied ABA (20 µM) by spraying the leaves and panicles in two rice cultivars, Wuyujing 3 and Yangdao 4, daily for 7 days starting at 9 days after anthesis (DAA), and observed a 3- to 4-fold increase in ABA levels in grains at 12 and 20 DAA in ABA-treated plants compared to plants treated with water in both cultivars. Similar results were reported later by Yang et al. (2006)33, after spraying ABA (20 µM) solution onto the panicles of rice plants daily for 8 days starting at the initiation of heading, and ABA levels in grains were measured at 9 and 16 DAA. They observed that an increase in the endogenous ABA levels in grains was about 4-fold at 9 DAA and 2-fold at 16 DAA in ABA-treated plants compared to plants treated with water. In both studies, ABA was quantified by the enzyme-linked immunosorbent assay (ELISA) using mouse monoclonal antibodies against ABA. This shows that a substantial amount of applied ABA reaches the grain in a biological active form. The role of ABA and gibberellins (GA) in regulating α-amylase formation in germinating and pre-harvest sprouted wheat grains is well documented in the literature4,9,10,12,26. However, their role in regulating pre-maturity α-amylase (PMA) synthesis in intact, developing wheat grains is less well known. In order to determine the role of ABA and GA in α-amylase formation in developing grains, it is necessary to investigate the most effective method for in situ hormone application to ensure that the applied hormones are being taken up by the grains.
PMA, describes the pre-mature production of α-amylase in developing wheat grains. PMA is not associated with sprouting of the gain, but results in a loss of quality that represents a substantial problem for bread-wheat growers worldwide20. PMA production has been associated with events that occur during mid-grain developmental6,8,16,20,23,24. In particular, a cold-shock applied during a window of sensitivity, i.e. 26-30 DAA, can induce PMA6,8,23,24. It was proposed by Farrell and Kettlewell (2008)6 and Mares and Mrva (2008)20 that there may be a change in ABA- and/or GA-sensitivity at mid-grain development during PMA-induction. Our previous work showed that applied GA3 at mid-grain development increased PMA whereas applied ABA had little effect on PMA in susceptible wheat genotype16. In order to understand the relevance of this finding to plants in the field, it is necessary to quantify the production of PMA under the different hormone regimes.
In the first glasshouse experiment, we compared the uptake of ABA and GA3 by intact, developing wheat grains, using two methods of application: 1. application onto the grains and 2. injection into the peduncle of the spike. In the second experiment, the effect of these hormones on α-amylase activity in mature grains was determined.
Results
Comparison between the methods for hormone uptake
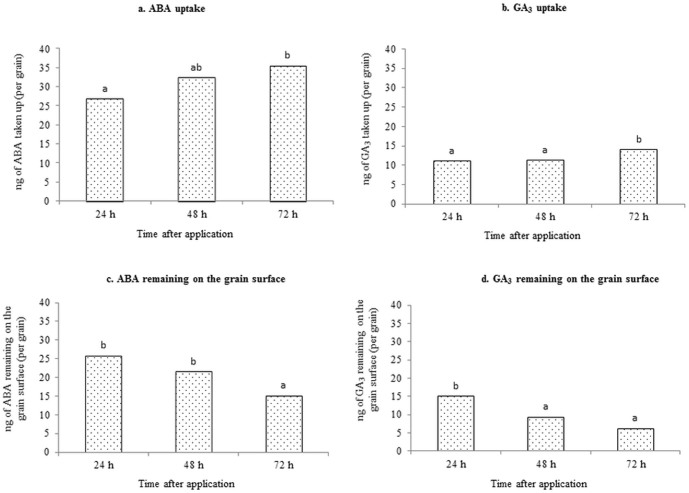
The volume of the ABA solution applied per grain was 10 µl, which contained 264 ng of unlabelled ABA (as the weight of 3H-ABA used was negligible). After 24 h, an average of 26.83 ng of ABA was taken up per grain, increasing to 32.31 ng after 48 h and 35.45 ng after 72 h. The increase in the uptake after 72 h was not substantial compared to 48 h, but it was significant compared to 24 h (P = 0.025; Fig. 1a). Thus, after 72 h of application, ABA uptake per grain as a percentage of that applied was approximately 13% in this method. The average amount of ABA remaining on the grain surface was 25.75 ng per grain after 24 h, 21.47 ng after 48 h and 14.99 ng after 72 h (Fig. 1c). The decrease in the amount of ABA remaining on the grain surface after 72 h was significant (P<0.001) compared to that at 24 h and 48 h, which were not considerably different from each other.
Figure 1.
The amount of (a) ABA and (b) GA3 taken up per grain with the time after application onto the grains. The amount of (c) ABA and (d) GA3 remaining on the grain surface (per grain) with the time after application onto the grains.
The volume of the GA3 solution applied per grain was 10 µl, which contained 173 ng of unlabelled GA3 (as the weight of 3H-GA3 used was negligible). After 24 h of application, 10.95 ng of GA3 was taken up per grain. This uptake increased to 11.23 ng after 48 h and 13.86 ng after 72 h (Fig. 1b). The increase in uptake after 72 h was significant (P = 0.022) compared to that at 24 h and 48 h, which were not considerably different. Thus, after 72 h of application, GA3 uptake per grain as a percentage of that applied was approximately 8% in this method. The amount of GA3 remaining on the grain surface per grain was 14.96 ng after 24 h. This amount was significantly (P<0.001) reduced to 9.21 ng after 48 h and 6.12 ng after 72 h, although the difference between these last values was not substantial (Fig. 1d).
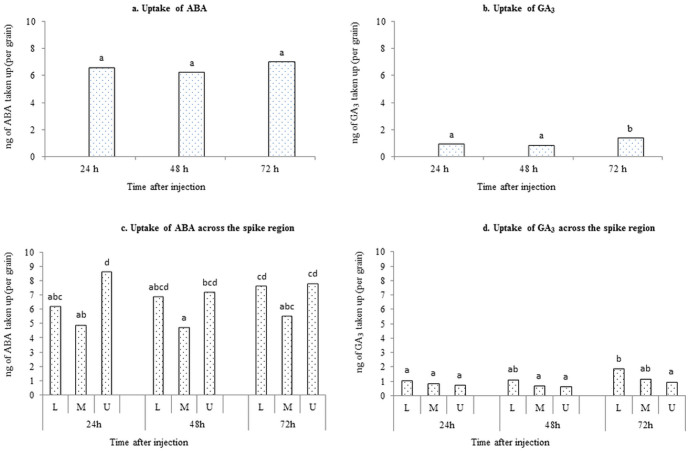
The total amount of ABA injected into the peduncle of each spike was 2640 ng. On average, 66 grains were present per spike (data not shown). Thus, of the total amount of ABA injected, the amount injected per individual grain is approximately 40 ng. The uptake of ABA per grain was 6.57 ng for 24 h, 6.23 ng for 48 h and 6.97 ng for 72 h. Thus, the uptake of ABA did not increase significantly from 24 h to 72 h (P = 0.540; Fig. 2a). After 72 h of application, ABA uptake per grain as a percentage of that applied was approximately 17% in this method.
Figure 2.
The uptake of (a) ABA and (b) GA3 per grain with respect to the time after injection into the peduncle. The uptake of (c) ABA and (d) GA3 per grain for the L, M and U region of the spike with respect to the time after injection into the peduncle.
Out of 66 grains present per spike for the plants used for ABA injection, on average 24 grains were present in the lower (L) or middle (M) region whereas 18 grains were present in the upper (U) region. At 24 h following ABA injection, grains in the U region of the spike had taken up considerably more ABA (8.61 ng/grain) compared to grains in the L region (6.21 ng/grain) and M region (4.92 ng/grain) (P<0.001). Amounts of ABA taken up per grain in the L, M and U regions after 48 h were similar to those at 24 h. These amounts were 7.2 ng/grain in the U region, 6.9 ng/grain in the L region and 4.73 ng/grain in the M region. After 72 h, the amounts of ABA taken up by grain in different regions increased slightly compared to 48 h. The U region had taken up 7.8 ng/grain, followed by the L region (i.e. 7.6 ng/grain) and the M region (i.e. 5.5 ng/grain) (Fig. 2c). The uptake of ABA per grain in the spike regions did not significantly increase from 24 h to 72 h (P = 0.496). There was no significant interaction (P = 0.581) between the spike regions (L, M, U) and time after application (24 h, 48 h, 72h ).
The total amount of GA3 injected into the peduncle of each spike was 1730 ng. On average, 65 grains were present per spike (data not shown). Thus, of the total amount of GA3 injected, the amount injected per grain is approximately 27 ng. The uptake of GA3 taken up per grain was 0.91 ng after 24 h, 0.84 ng after 48 h and 1.36 ng after 72 h. The GA3 uptake per grain increased significantly after 72 h compared to 24 h and 48 h (P = 0.005; Fig. 2b). Thus, after 72 h of application, GA3 uptake per grain as a percentage of that applied was approximately 5% in this method.
Out of 65 grains present per spike for the plants used for GA3 injection, on average 22 grains were present in the L region, 23 in the M region and 20 were present in the U region. For all three time points, GA3 uptake showed a different distribution among the spike regions compared to the ABA uptake. After 24 h of GA3 injection, the uptake of GA3 did not differ between the L region (1.1 ng/grain), the M region (0.9 ng/grain) and the U region (0.8 ng/grain). After 48 h, the uptake of GA3 per grain in the L, M and U region was similar to that after 24 h and the GA3 taken up in each region did not differ considerably. These amounts were 1.2 ng/grain in the L region, 0.72 ng/grain in the M region and 0.7 ng/grain in the U region. After 72 h, the uptake of GA3 per grain in the L region was significantly higher (P<0.001) compared to 24 h, but not compared to 48 h. The L region had taken up 1.9 ng/grain, followed by the M region (1.2 ng/grain) and the U region (1.0 ng/grain) (Fig. 2d). However, the interaction between the spike regions (L, M, U) and time after application (24 h, 48 h, 72 h) was not significant (P = 0.365).
Comparison between the methods with respect to α-amylase activity
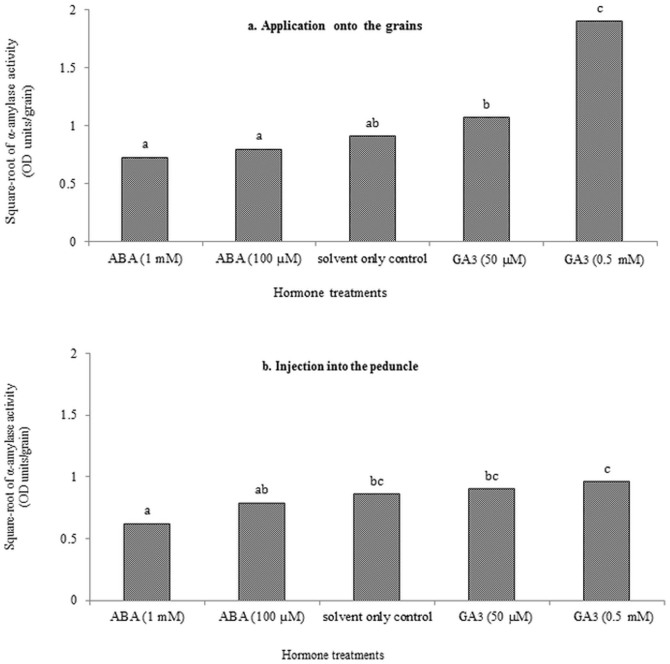
ABA solution applied to intact, developing grains around mid-grain development at both concentrations (i.e. 100 µM and 1 mM) produced no substantial effect on α-amylase activity in mature grains compared to the solvent-only control. Applied GA3 at 50 µM concentration resulted in a small but not substantial increase in grain α-amylase at maturity, while the higher concentration of GA3 (i.e. 0.5 mM) resulted in a highly significant increase (P<0.001) compared to the solvent-only control (n = 10, SEM = 0.20, d.f. = 45, CV = 19%) (Fig. 3a). ABA solution injected at the lower concentration (i.e. 100 µM) into the peduncle around mid-grain development produced no substantial effect on α-amylase activity in mature grains, but ABA solution injected at the higher concentration (i.e. 1 mM) showed a substantial reduction in α-amylase activity compared to the solvent-only control (P<0.001). Neither concentration of GA3 solution (50 µM or 0.5 mM) injected into the peduncle of a wheat spike produced a substantial effect on α-amylase activity in mature grains compared to the solvent-only control (n = 15, SEM = 0.17, d.f. = 70, CV = 20.2%) (Fig. 3b).
Figure 3. Effects of applied hormones (ABA [100 µM and 1 mM] and GA3 [50 µM and 0.5 mM]) at 30 DAA on grain α-amylase at maturity (a) when applied onto the grains and (b) when injected into the peduncle of the spike.
Discussion
When applied onto the grains, the decrease in the amount of ABA remaining on the grain surface over time (Fig. 1c) clearly suggested that some part of this amount was taken up by the grains and as a result, the uptake of ABA was increased considerably from 24 h to 72 h (Fig. 1a). Of the total amount of ABA applied per grain (i.e. 264 ng), only 19% was either taken up by the grain or remained on the grain surface. The remaining 81% is presumed to have been lost to the surrounding tissues such as the lemma, palea and glume. Since the solvent from applied ABA solution evaporated after 24 h, the mechanism responsible for the increased uptake after 24 h is unknown. One possibility is that the high moisture content of grains at about 580 degree DAA (which was approximately 55% [wet basis]13) might have allowed the ABA transfer. Another possibility is the occurrence of condensation on the grains in the glasshouse due to low temperature during the night when stomata on grain surfaces were open. Either one or both mechanisms could have enabled a part of the ABA remaining on the grain surface to be taken up over time. The current study showed that the uptake of ABA by intact grains increased over time points (24, 48 and 72 h) following its exogenous application at mid-grain development (26–29 DAA). Moreover, exogenously applied ABA at mid-grain development was able to reduce α-amylase activity in mature grains (60 DAA) compared to solvent-only treated grains. These findings indicate at least some part of the exogenously applied ABA remains in a biological active form, as seen in other studies by Yang et al. (2004; 2006)32,33. Although the studies with exogenously applied ABA show the stability of ABA, this gives little indication of the extent of ABA metabolism. Moreover, there is limited literature available on GA3 metabolism in plants when applied exogenously (although it is also known that GA3 does not subject to 2-beta-hydroxylation, which would occur with GA1 or GA4) (Hedden, Pers. Comm.)
As the [3H]-ABA was available as a racemic mixture, it was necessary to apply ABA in this form. ABA occurs naturally as the (+)-enantiomer, although the (−)-enantiomer has some biological activity. Several studies have investigated the relative biological effectiveness of these enantiomers. During the inhibition of germination30 or inhibition of GA-stimulated synthesis and release of α-amylase in barley half seeds29, both isomers were found to have equivalent biological activity. In contrast, (+)-ABA is found to be more effective than (−)-ABA in the stomatal response19 or inhibition of root growth29. These studies suggested the existence of more than one ABA receptor and/or multiple signal transduction pathways. Few studies have reported the rates of uptake and metabolism of ABA isomers. In sunflower embryos, the rates of uptake for both isomers were found to be equivalent3, but differences in uptake have been observed with bean root segments21. Moreover, the metabolism of (+)-ABA occurs more rapidly than with (−)-ABA and yields different products3.
Some part of the GA3 remaining on the grain surface appeared to be taken up by developing grains over the time points and this is likely the reason why GA3 uptake increased considerably over the time points (Fig. 1b, Fig. 1d). Of the total amount of GA3 applied per grain (i.e. 173 ng), only 13% of the radioactivity was either taken up per grain or remained on the surface of a single grain, whereas the remaining 87% might have either been taken up or remained on the surface of surrounding tissues. The ABA uptake per grain was approximately 2.5-fold higher compared to GA3 uptake. This could be partially explained by the amount of ABA applied (i.e. 264 ng per grain) being approximately 1.5 fold higher than the amount of GA3 applied (i.e. 173 ng per grain), but is also likely to be due to differences in their chemical properties and rates of metabolism, which may influence their rate of uptake into developing wheat grains.
When injected into the peduncle, the ABA uptake (which was approximately 6 ng per grain) across the entire spike (Fig. 2a) was nearly 6-fold higher than the GA3 uptake (which was approximately 1 ng per grain) (Fig. 2b). This difference in ABA and GA3 uptake may be due to the amount of ABA applied, (i.e. 2640 ng) was approximately 1.5 fold higher compared to the amount of GA3 applied (i.e. 1730 ng), or to differences in the movement of the two hormones within the vascular system.
Dewdney and McWha (1978)5 observed when ABA (14C-labelled) was injected into the flag leaf blade at 30 days after emergence (which corresponded with the cessation of dry matter accumulation), about two thirds of radioactivity was found in the stems and rachis, whereas the remaining one third of radioactivity was found in the spikelets. Further, the radioactivity actually reaching the grains should be less than that in the spikelet as a whole as some of the activity would be lost to other tissues in the spikelets. This observation by Dewdney and McWha (1978)5 may partly explain why only small amount of the ABA (17%) and GA3 (5%) applied was taken up by grains. Thus, both methods are viable for ABA and GA3 application. Therefore, either method could be useful in future to investigate various mechanisms of hormonal control during grain development. When injected into the peduncle, it was clear that ABA and GA3 were able to reach all grains in a spike. Therefore, the method of injecting into the peduncle over the method of application onto the grains will surely save time in future work if it is necessary to apply ABA and GA3 to all grains in a spike.
Goldbach and Goldbach (1977)11 showed that ABA applied (14C-labelled) to wheat leaves was readily transported to the spike and grain. Later, King (1979)14 found that in stems of detached wheat spikes in a culture solution with ABA for 2 to 3 days, the ABA content in grains relative to endogenous ABA was increased by approximately 8-fold (i.e. from 0.31 ng/grain to 2.56 ng/grain). These findings by Goldbach and Goldbach (1977)11 and by King (1979)14 combined, with the success of the method of injecting into the peduncle, suggested that wheat grains around the mid-grain developmental phase are able to receive ABA and GA signals from the rest of the plant. This finding has important implications for understanding the regulation of grain development.
Considering the results of ABA uptake from experiment 1 for the application onto the grains method, when 10 µl of 100 µM ABA was applied onto the grains, approximately 36 ng of ABA was taken up per grain after 72 h of application. It is expected that the higher amount of ABA would have been taken up per grain when 1 mM ABA was applied compared to when 100 µM ABA was applied. However, both concentrations of ABA (i.e. 100 µM and 1 mM) produced a small, but non-substantial decrease in grain α-amylase at maturity. Previous work has also suggested that PMA shows little or no response to exogenously applied ABA14,15,16,17.
In contrast, considering the results of GA3 uptake from experiment 1, when 10 µl of 50 µM GA3 was applied onto the grains approximately 14 ng of GA3 was taken up per grain after 72 h of application. It is expected that the amount of GA3 taken up per grain would be higher when 0.5 mM GA3 was applied. GA3 application at 50 µM concentration produced a small, but non-substantial increase in grain α-amylase, whereas GA3 application at 0.5 mM concentration produced a considerable increase in grain α-amylase at maturity. Thus, relatively high concentrations of GA are required to increase α-amylase. This is in agreement with previous work, where GA was applied at 50 µM concentrations to developing grains and no substantial effect on α-amylase activity was observed in mature grains15,16,17,18. Nishikawa and Watanabe (1988)25 observed that the aleurone tissue of ripening grains acquires GA sensitivity at approx. 30 to 40 DAA and not in early developmental stages. Earlier, Jacobsen (1973)13 observed that the lower concentration of GA3 (i.e. 2.5 × 10−8 M) applied to barley aleurone tissue produced a low level of α-amylase, which increased considerably with higher concentrations of GA3 (i.e. 1 × 10−7 M and 1 × 10−6 M). It is also possible that GA3 itself would become a limiting factor at low GA3 concentration (i.e. 50 µM in our experiment) and as a result, GA3 would be unable to saturate the GA receptors in the aleurone tissue. Furthermore, Ritchie et al. (1999)28 studied the sensitivity of barley aleurone tissue and found that only a few cells in the aleurone exhibited GA-response at a low GA concentration, whereas most cells exhibited GA-response at increased GA concentration. This may also explain why GA3 application at 50 µM concentration produced a small, but non-substantial increase in grain α-amylase, whereas GA3 application at 0.5 mM concentration produced a substantial increase in grain α-amylase at maturity. Thus, the method of application onto the grains was more efficient for GA uptake and it appeared to be associated with a larger response in terms of α-amylase activity.
Considering the results of GA3 uptake from injection into the peduncle in experiment 1, the uptake per grain was approximately 1.5 ng for the 50 µM GA3 application and it would be expected to be higher when 0.5 mM GA3was applied. It is possible that due to the lower GA3 uptake efficiency using this method (5% compared to 8% for direct application), even after applying GA3 at 0.5 mM it was below the threshold needed to induce PMA. The ultimate production of α-amylase in response to applied ABA or GA will not only depend on the concentration applied but also on the hormone sensitivity and endogenous levels of developing grains at the time of application. At a higher sensitivity, the lower concentration of GA3 is required to increase α-amylase in grains. The injection method (17%) was more efficient for ABA uptake compared to direct application (13%), and this appeared to be associated with a larger response in α-amylase activity. Although the efficiency of ABA taken up per grain may be higher after injection, the amount that is actually recovered in the grain after 72 h is much higher by direct application (36 ng/grain) compared to injection method (6 ng/grain). Perhaps more ABA reaches the appropriate tissue (in this case, the aleurone cells) with the injection method, whereas much of the ABA applied onto the grain surface may be sequestered in the outer layers (e.g. pericarp or testa). These suggestions may have contributed to the observation that applied ABA (1 mM) considerably reduced α-amylase activity in injection method. In the injection method, the pattern of GA3 uptake by grains across the L, M and U region of the spike was in the order: L > M > U (Fig. 2d). For ABA, the pattern was in the order: U ≥ L > M (Fig. 2c). Gale et al. (1987)7 observed that in the spikes of Spica and Huntsman (PMA-constitutive genotypes) grains in the lower central region contained most PMA activity whereas the grains in the spikelets of the uppermost region showed least PMA activity. Thus, the pattern of ABA and GA uptake in the current study are in agreement with this study, suggesting the association between GA delivered via the vascular system and PMA in the grains within the spike.
The transcriptional change of ABA and GA related genes during PMA formation and also during exogenous GA application to mature grains was recently investigated by Barrero et al. (2013)2. Barrero et al. (2013)2 studied the expression of GA-responsive genes such as GAMYB (a transcription regulator), β-glucanase (a cell wall-degrading enzyme) and triticain-α and triticain-γ (proteases) and ABA metabolic genes such as NCED1 and NCED2 (biosynthesis genes), ABA8'OH-1 and ABA8'OH-2 (catabolic genes) during grain development from PMA and non-PMA genotypes and also in mature grains in response to exogenous GA. In developing grains, the expression of GA or ABA related genes did not change during grain development. The expression levels of these genes between PMA and non-PMA genotypes were very similar in developing grains. In mature grains, the expression of GA-related genes was substantially induced when treated with GA, whereas in developing grains the expression of these genes was not affected. Moreover, the expression of NCED1 and ABA8'OH-2 was not substantially affected by GA in mature grains, but that of ABA8'OH-1 was induced approximately 4-fold by GA. Further, Barrero et al. (2013)2 observed that ABA levels were nearly two-fold higher in PMA genotypes compared to non-PMA genotypes at 23 DAA. The level of GA19 in developing grains was also considerably higher in PMA genotypes compared to non-PMA genotypes at 23 DAA. Thus, Barrero et al. (2013)2 found changes in GA and ABA levels during the PMA occurrence, even though there was little detected difference in gene expression, and further suggested that the differences in hormone contents are more likely to have originated from differences existing in the whole tissue and not just from the small number of aleurone cells that produce LMA. Although the transcriptional changes in ABA and GA related genes during PMA formation and exogenous GA application to mature wheat grains was recently investigated, a more detailed study of the changes in hormone related genes during development of grains after ABA and GA application in PMA and non-PMA genotypes would be of interest.
In this work it was found that ABA was taken up more efficiently in intact developing wheat grains than GA3 when the respective solutions were applied onto the grains or injected into the peduncle. In terms of the proportion of applied hormone taken up, the direct application method was more efficient for GA, whereas the injection method was more efficient for ABA. In both methods, the majority of hormone uptake occurred in the first 24 h, but further uptake did occur after 48 and 72 h. Thus, both methods are viable for ABA and GA3 application. Where the aim is to expose all grain in the spike to exogenous hormones, the injection method is more efficient. Conversely, the direct application method allows for manipulation of individual grain within the same spike. Overall, applying ABA using either application method resulted in reduced α-amylase activity in mature grains and applying GA3 resulted in increased α-amylase activity, once a threshold concentration was passed. The method of injecting into the peduncle shows that developing grains around mid-grain development are able to receive ABA and GA signals from the rest of the plant and suggests these are important determinants of PMA.
Methods
Experiment 1
A 100 μM solution (7 ml) was prepared by combining (+/−)-[G-3H]ABA (Amersham Radiochemicals; 127 kBq) and unlabelled (+/−)-ABA (Sigma; 70 μl from a 10 mM stock solution). Similarly, [G-3H]GA3 (prepared by Amersham Radiochemicals by catalytic exchange of GA3 with carrier-free tritium); 140 kBq) and GA3 (Sigma; 70 μl from a 5 mM stock solution) were diluted with water to give a 50 μM solution (7 ml). The current study was conducted with Rialto (a highly PMA-susceptible wheat genotype) as our earlier studies have shown that Spark (less PMA-susceptible wheat genotype) showed no response to exogenous ABA and GA3 when hormones were applied using similar methods16,17. Seeds were sown in a glasshouse early in April 2011 at Rothamsted Research (Harpenden, Hertfordshire, UK) as described previously4,14. Plants were vernalized for 8 weeks at 4°C and transplanted to pots (pot dimensions: 11 × 12 × 18 cm) in a glasshouse. In this experiment, spikes were tagged with coloured tape at early anthesis (Zadoks growth stage 61 [ZGS61])34, which occurred between late July and early August 2011.
In the application onto the grains method, the two outermost grains from spikelets 5, 7, 9, 11 and 13 (counting acropetally) to which hormones were to be applied were marked by a red marker pen. The crease regions of developing grains at 26 DAA were made visible by pulling back the lemma with the help of forceps. Ten microliters of the 3H-ABA (100 μM) or 3H-GA3 (50 μl) solutions were applied in situ directly with a pipette onto the crease region of ten intact grains (per spike) (Fig. 4a, b). Spikes containing hormone-treated grains were harvested in polythene bags at 24, 48 and 72 h following the hormone application.
Figure 4. The image shows (a) the two outermost grains from spikelets 7 and 9 marked by a red marker pen, (b) the method of hormone application onto the grains with a pipette, and (c) the method of hormone injection into the peduncle with a syringe.
Ten hormone-treated grains per spike were pooled and washed twice by briefly immersing in 5 ml of 10% ethanol to remove any substrate on the grain surface. The washing solvent was transferred into a new 15 ml conical tube (Sarstedt, UK). Grains were wiped with absorbent paper and transferred to a new 15 ml Falcon tube. All tubes with grains were then kept on ice. Grains were homogenised in 5 ml of absolute methanol using a mortar and pestle. The homogenised grains were then transferred into a 50 ml conical tube (Sarstedt, UK), which was vortexed, and kept on ice for 1 h. The tubes were then centrifuged (Fisher Scientific, Eppendorf, Centrifuge 5810, UK) at 3500 rpm (1647 × g) for 5 min. The total volume of the supernatant was measured and recorded. The supernatant was transferred to a new 15 ml conical tube (referred to as the 1st extraction). The pellet from the 1st extraction was re-suspended in 10 ml of absolute methanol, vortexed and tubes were kept on ice for 1 h. Tubes were then centrifuged at 3500 rpm (1647 × g) for 5 min. The total volume of the supernatant was measured and recorded. The supernatant was transferred to a new 15 ml conical tube (referred to as the 2nd extraction). Aliquots of each supernatant (1 ml) were analysed for the 3H content by liquid scintillation counting (Liquid Scintillation Counter, TriCarb 2500TR, PerkinElmer, UK) after addition of Ultima GoldTM liquid scintillation cocktail (PerkinElmer, USA) (3 ml). Quenching was corrected for using a quench correction curve.
The ABA working solution (10 µl) contained 180 Bq of 3H-ABA and 264 ng unlabelled ABA. In this method, ten grains were each treated with 10 µl of the ABA working solution. Therefore, the total quantity of ABA applied was 100 µl, which contained 1800 Bq. The total amount of 3H-ABA taken up per 10 grains is the addition of the total radioactivity determined from the 1st and 2nd extractions.
Radioactivity for the 1st extraction (X Bq): Activity for 1 ml x Volume of supernatant from the 1st extraction.
Radioactivity for the 2nd extraction (Y Bq): Activity for 1 ml x Volume of supernatant from the 2nd extraction.
Thus, the total amount of 3H-ABA taken up per 10 grains = (X+Y Bq).
The total amount of 3H-ABA taken up per grain (Z Bq) = (X+Y)/10.
Therefore, the total amount of unlabelled ABA taken up per grain = (Z/180) x 264 ng.
The GA3 working solution (10 µl) contained 266.6 Bq of 3H-GA3 and 173 ng unlabelled GA3. The rest of the calculations were done according to the method described for ABA.
Data were analysed by general analysis of variance (ANOVA) with time after application as a factor using Genstat 13th edition (VSN International Ltd., Hemel Hempstead, UK) with Tukey's multiple comparison test (P<0.05). Data represented means of 10 spikes per hormone application with 10 hormone-treated grains from the middle region of each replicate spike used to quantify the uptake of ABA and GA3 (i.e. n = 10).
In injection into the peduncle method, 100 µl of the 3H-ABA solution (100 µM) or 3H-GA3 solution (50 µM) was injected at 30 DAA into the peduncle of each spike (Fig. 4c) with a 50 µl fixed needle syringe (SGE Analytical Science, Australia). Two doses of 50 µl each were injected in such a way that the first dose was given 1 cm below the 1st spikelet and the second dose was given halfway along the length of peduncle by opening the flag sheath gently with forceps. A similar approach for the ABA application was used earlier by Zeng et al. (1985)35 in wheat and by Oliver et al. (2007)27 in rice, where ABA solution was injected with a syringe into the cavity between the leaf sheath and unemerged spike. Entire spikes were harvested in separate bags at 24, 48 and 72 h following injection. In this method, the total volume of ABA or GA3 solution injected into the peduncle of each spike was 100 µl. All grains from each spike were pooled from three regions, lower (L), middle (M) and upper (U) for the measurement of ABA and GA3 uptake. Measurement of ABA and GA3 in all the grains and the calculations for the uptake per grain used the same method as described above, except all the grains in the whole spike were accounted in the calculations. Data were analysed either with one way or two way ANOVA using Genstat with Tukey's multiple comparison test (P<0.05) depending on the aims of analysis. Data represented means of 7 spikes per hormone application with all grains quantified from each replicate spike (i.e. n = 7).
Experiment 2
In this experiment, spikes were tagged with coloured tape at early anthesis, which occurred between late March and early April 2012. Unlabelled ABA or GA3 was applied to intact, developing grains at 30 DAA by the two methods, i.e. application onto the grains and injection into the peduncle, as for experiment 1, except that only the two outermost grains from spikelets 7, 8 and 9 were treated with hormone solution when it was applied directly onto the grains. Moreover in this experiment, two separate concentrations of working solutions were used: ABA (100 µM and 1 mM) and GA3 (50 µM and 0.5 mM). For control plants, grains were treated with the same volume of 10% ethanol as used for the hormone treatments. Spikes (only the main spike per replicate plant) were harvested at maturity (i.e. 60 DAA).
In the application onto the grains method, six hormone-treated grains per spike from the harvested spikes were transferred to a 96-well block with one grain per well. Individual grains were ground against the force exerted by the stainless steel balls (one ball per well) (Appleton Woods, UK) using a TissueLyser II (Qiagen, UK) at 2 × 30 Hz for 4 min. Following grinding, the stainless steel balls were removed and α-amylase activity in flour was measured by the Megazyme assay. Activity was expressed as OD units/grain (as described in Kondhare et al. [2013; 2014]16,17), which was calculated as the mean of six hormone-treated grains from each main spike of ten replicate plants (n = 10).
In the injection into the peduncle method, each harvested spike was divided into three regions (L, M and U) with each region having nearly the same number of spikelets. Individual grains from each region of the spike were pooled and counted. Eight grains with uniform size from each region of the spike were randomly chosen for the measurement of α-amylase activity. Grains were ground as described above and α-amylase activity, measured by the Megazyme assay (as described in Kondhare et al. [2013; 2014]15,16), was expressed as OD units/grain. The mean α-amylase activity was calculated from 8 individual grains for each spike region of ten replicate plants (n = 10).
In both methods, α-amylase activity values were square-root transformed prior to ANOVA. Data from application onto the grains were analysed by one way ANOVA using Genstat 13th edition with Tukey's multiple comparison test (P<0.05). However, data from the injection into the peduncle method were analysed by two way ANOVA with the spike regions and three time points as factors using Genstat 13th edition with Tukey's multiple comparison test (P<0.05) for comparison of α-amylase activity across three regions of a spike.
Author Contributions
K.K., A.F., P.K., J.M. and P.H. designed the experiments. Experimental work was carried out by K.K. with the guidance and suggestions from P.K., A.F., J.M. and P.H. P.H. germinated the seeds and looked after the plants until anthesis in experiment 1 at Rothamsted Research. After anthesis, K.K. conducted the hormone application work, harvested and processed the samples under the supervision of P.H. K.K. conducted experiment 2 at Harper Adams University under the supervision of P.K. and suggestions from P.H., A.F. and J.M. K.K. carried out data extraction and statistical analysis with the suggestions from all the authors. K.K. wrote the manuscript with the useful comments/suggestions from all the authors.
Acknowledgments
The authors are equally grateful to the staff in the Crop and Environment Research Centre and Princess Margaret Laboratories for their kind support. Thanks to Rothamsted Research for providing glasshouse and lab facilities for this work and for the training to work with radiolabeled ABA and GA3. Work at Rothamsted Research is supported via the 20:20 Wheat® Programme by the UK Biotechnology and Biological Sciences Research Council. We also acknowledge advice from John Lenton and Tony Evers regarding methods of hormone application. This work was funded by Harper Adams University (Grant number: PHD059) and Home-Grown Cereals Authority (HGCA; Grant number: RD-2009-3623).
References
- Bano A. & Yasmeen S. Role of phytohormones under induced drought stress in wheat. Pakistan J. Bot. 42, 2579–2587 (2010). [Google Scholar]
- Barrero J. M. et al. Genetic, hormonal and physiological analysis of late maturity a-amylase (LMA) in wheat. Plant Physiol. 161, 1265–1277; 10.1104/pp.112.209502 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthe P. & Le Page-Degivry M. T. The resolution by HPLC of (R, S)-abscisic acid methyl ester and the metabolism of (−)-R- and (+)-S-abscisic acid by sunflower embryos. [68] (The 14th International Conference on Plant Growth Substances Amsterdam., 1991).
- Chandler P. M. et al. The effects of gibberellins acid and abscisic acid on α-amylase mRNA levels in barley aleurone layers: studies using an α-amylase cDNA clone. Plant Mol. Biol. 3, 407–418; 10.1007/BF00033389 (1984). [DOI] [PubMed] [Google Scholar]
- Dewdney S. J. & McWha J. A. The metabolism and transport of abscisic acid during grain fill in wheat. J. Exp. Bot. 29, 1299–1308; 10.1093/jxb/29.6.1299 (1975). [Google Scholar]
- Farrell A. D. & Kettlewell P. S. The effect of temperature shock and grain morphology on α-amylase in developing wheat grain. Ann. Bot. 102, 287–293; 10.1093/aob/mcn091 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell A. D. et al. The effect of gibberellic acid on the response of leaf extension to low temperature. Plant Cell Environ. 29, 1329–1337; 10.1111/j.1365-3040.2006.01513.x (2006). [DOI] [PubMed] [Google Scholar]
- Farrell A. D. et al. Control of late maturity alpha-amylase in wheat by the dwarfing gene Rht-D1b and genes on the 1B/1R translocation. Mol. Breed. 32, 425–436; 10.1007/s11032-013-9881-5 (2013). [Google Scholar]
- Gale M. D. et al. [The induction of germination alpha-amylase during wheat grain development in unfavourable weather conditions. [Mares, D. J. (Ed.)] [273–282] (Fourth International Symposium on Pre-Harvest Sprouting in Cereals. Westview Press Inc., Boulder, Co., USA, 1987). [Google Scholar]
- Gold C. M. Pre-harvest sprouting in wheat. PhD thesis, University of Edinburgh (1991). [Google Scholar]
- Goldbach H. & Goldbach E. Abscisic acid transport and the influence of water stress on grain abscisic acid content. J. Exp. Bot. 28, 1342–1350 (1977). [Google Scholar]
- Hader A. et al. Characteristics of α-amylase induced in distal half-grains of wheat. Breed. Sci. 53, 119–124 (2003). [Google Scholar]
- Jacobsen J. V. Interactions between gibberellic acid, ethylene, and abscisic acid in control of amylase synthesis in barley aleurone layers. Plant Physiol. 51, 198–202; http://dx.doi.org/10.1104/pp.51.1.198 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. Abscisic acid synthesis and metabolism in wheat ears. Funct. Plant Biol. 6, 99–108; 10.1071/PP9790099 (1979). [Google Scholar]
- Kondhare K. R. The role of abscisic acid and gibberellins in pre-maturity α-amylase formation in wheat grain. PhD Thesis. Harper Adams University (2013). [Google Scholar]
- Kondhare K. R. et al. Effects of exogenous abscisic acid and gibberellic acid on pre-maturity α-amylase formation in wheat grains. Euphytica 188, 51–60; 10.1007/s10681-012-0706-0 (2012). [Google Scholar]
- Kondhare K. R. et al. The role of sensitivity to abscisic acid and gibberellin in pre-maturity α-amylase formation in wheat. J. Cereal Sci. 58, 472–478; http://dx.doi.org/10.1016/j.jcs.2013.09.009 (2013). [Google Scholar]
- Kondhare K. R. et al. Use of the hormone-biosynthesis inhibitors fluridone and paclobutrazol to determine the effects of altered abscisic acid and gibberellin levels on pre-maturity α-amylase formation in wheat grains. J. Cereal Sci. 10.1016/j.jcs.2014.03.001 (2014). [Google Scholar]
- Kriedemann P. E. et al. Abscisic acid and stomatal regulation. Plant Physiol. 49, 842–847 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares D. J. & Mrva K. Late-maturity α-amylase: Low falling number in wheat in the absence of preharvest sprouting. J. Cereal Sci. 47, 6–17; http://dx.doi.org/10.1016/j.jcs.2007.01.005 (2008). [Google Scholar]
- Milborrow B. V. & Rubery P. H. The specificity of the carrier mediated uptake of ABA by root segments of Phaseolus coccineus L. J. Exp. Bot. 36, 807–822; 10.1093/jxb/36.5.807 (1985). [Google Scholar]
- Miralles J. D. et al. Floret development in near isogenic wheat lines differing in plant height. Field Crops Res. 59, 21–30; http://dx.doi.org/10.1016/S0378-4290(98)00103-8 (1998). [Google Scholar]
- Mrva K. & Mares D. J. Induction of late maturity α-amylase in wheat by cool temperature. Aust. J. Agric. Res. 52, 477–484; 10.1071/AR00097 (2001). [Google Scholar]
- Mrva K. & Mares D. J. Influence of temperature on the expression of late maturity α-amylase. [Blanchard, C. L., Solah, V. A. & Crosbie, G. B. (Eds.)] [21–24] (Proceedings of the 56th Australian Cereal Chemistry Conference and Indian Ocean Rim Symposium. 2006). [Google Scholar]
- Nishikawa K. & Watanabe Y. Change in activity of alpha-amylase in developing and germinating wheat seed. [Miller, T. E. & Koebner, R. M. D. (Eds.)] [597–602] (Proceedings of the Seventh International Wheat Genetics Symposium. Cambridge, England, 1988). [Google Scholar]
- Nyachiro J. M. et al. The effects of cis-trans ABA on embryo germination and seed dormancy in wheat. Euphytica 126, 129–133; 10.1023/A,1019684025082 (2002). [Google Scholar]
- Oliver S. N. et al. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol. 48, 1319–1330; 10.1093/pcp/pcm100 (2007). [DOI] [PubMed] [Google Scholar]
- Ritchie S. et al. The sensitivity of barley aleurone tissue to gibberellin is heterogeneous and may be spatially determined. Plant Physiol. 120, 361–370; http://dx.doi.org/10.1104/pp.120.2.361 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer E. et al. Asymmetry, its importance to the action and metabolism of abscisic acid. Science 174, 829–831; 10.1126/science.174.4011.829 (1971). [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M. K. et al. Optically pure abscisic acid analogs. Tools for relating germination inhibition and gene expression in wheat embryos. Plant Physiol. 99, 501–507; http://dx.doi.org/10.1104/pp.99.2.501 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. et al. Effects of exogenous hormones on floret development and grain set in wheat. Plant Growth Regul. 35, 225–231; 10.1023/A,1014442006862 (2002). [Google Scholar]
- Yang J. et al. Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol. 135, 1621–1629; http://dx.doi.org/10.1104/pp.104.041038 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. et al. Post-anthesis development of inferior and superior spikelets in rice in relation to abscisic acid and ethylene. J. Exp. Bot. 57, 149–160; 10.1093/jxb/erj018 (2006). [DOI] [PubMed] [Google Scholar]
- Zadoks J. C. et al. A decimal code for the growth stages of cereals. Weed Res. 14, 415–421; 10.1111/j.1365-3180.1974.tb01084.x (1974). [Google Scholar]
- Zeng Z. R. et al. Regulation of grain number in wheat: genotype differences and responses to applied abscisic acid and to high temperatures. Aust. J. Physiother. 12, 609–619; 10.1071/PP9850609 (1985). [Google Scholar]