Abstract
Atrial fibrillation (AF), the most common cardiac arrhythmia, is an electrocardiographic description of a condition with multiple and complex underlying mechanisms. Oxidative stress is an important driver of structural remodeling that creates a substrate for AF. Oxidant radicals may promote increase of atrial oxidative damage, electrical and structural remodeling, and atrial inflammation. AF and other cardiovascular morbidities activate angiotensin (Ang-II)-dependent and independent cascades. A key component of the renin–angiotensin-aldosterone system (RAAS) is the mineralocorticoid aldosterone. Recent studies provide evidence of myocardial aldosterone synthesis. Aldosterone promotes cardiac oxidative stress, inflammation and structural/electrical remodeling via multiple mechanisms. In HF patients, aldosterone production is enhanced. In patients and in experimental HF and AF models, aldosterone receptor antagonists have favorable influences on cardiac remodeling and oxidative stress. Therapeutic approaches that seek to reduce AF burden by modulating the aldosterone system are likely beneficial but underutilized.
Keywords: Aldosterone, Aldosterone antagonist, Atrial fibrillation, Oxidative stress
1. Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia, currently affects more than 3 million Americans, and more than 12 million Americans are projected to suffer from AF by 2050 [1]. Major complications associated with AF include thromboembolic events and impaired cardiac function, resulting in increased risk of heart failure (HF), stroke and mortality [2]. AF is frequently associated with co-morbid conditions such as hypertension (HTN), HF, valvular diseases, and cardiomyopathy [3].
The concept of substrates and triggers is useful to frame discussions of arrhythmic mechanisms and antiarrhythmic targets. An arrhythmia substrate constitutes a persistent change in atrial structure or function (e.g., myocyte hypertrophy, chamber dilatation, interstitial fibrosis and ion channel remodeling) that increases the persistence of arrhythmia episodes once they have been initiated; triggers are acute events that promote the initiation of arrhythmic episodes that may either spontaneously terminate, or persist, depending on the arrhythmia substrate. Triggers are spontaneous or evoked sources of depolarization, caused by exposure to high levels of catecholamines, spontaneous release of calcium from intracellular stores, or high rates of electrical activation.
Current pharmacologic strategies for controlling AF rely mainly on blocking cardiac ion channels either to slow electrical conduction (sodium channels) or prolong atrial refractoriness (potassium channels). These interventions have been largely unsuccessful, with most patients having recurrent AF within a year of treatment [4]. Thus, treatment efforts have increasingly focused on the use of anticoagulants to reduce risk of stroke, and invasive surgical (Maze) or endovascular ablation procedures to suppress AF [5]. The high rate of AF recurrence reflects in part our poor understanding of the mechanisms and causes of atrial arrhythmia substrates that initiate and maintain AF. The mechanisms underlying AF are complex and multiple, including electrical, structural, inflammatory, and metabolic factors [6] (Fig. 1).
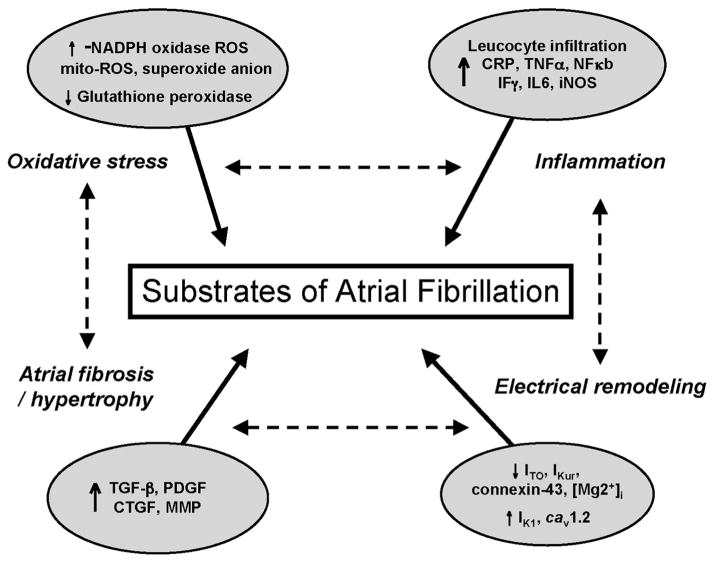
Fig. 1.
The substrates of atrial fibrillation. During AF, profound increases in expression of inflammatory proteins such as C reactive protein (CRP), tumor necrosis factor α (TNFα), nuclear factor κb (NFκb), interferon γ (Fγ), interleukin 6 (IL6), and inducible nitric oxide synthase (iNOS) occur. Generation of reactive oxygen species (ROS) is also increased in AF via activation of NADPH oxidase activity and expression, mitochondrial (mit) ROS generation, superoxide generation as well as reduction in antioxidants defenses such as glutathione peroxidase. Atrial fibrosis and hypertrophy also promotes atrial arrhythmogenesis through increased expression of profibrotic proteins such as tumor growth factor β (TGF β), platelet derived growth factor (PDGF), connective tissue growth factor (CTGF), and metalloproteinase (MMP). The decrease in outward (Ito) or rectifier (kur) K + current, the down regulation in connexin 43 (Cx 43) or intracellular Mg+, and the increase in native I (K1) current and L-type Ca + channel current (Cav1.2) promote atrial electrical remodeling leading to AF. These substrates are highly related where one substrate promotes generation of other substrates (AF begets AF).
In addition to generic cardiac ion channel blockade, some studies have considered development of new antiarrhythmic drugs and upstream therapies for AF that seek to be more effective and safer, particularly for patients with structural heart disease. These new drugs include selective atrial specific ion channel blockers and agents that target the underlying substrates that promote AF [7]. Among the latter category are upstream drugs that suppress activation of renin–angiotensin-aldosterone system (RAAS), which includes several known and novel therapeutic targets for AF [8].
The RAAS is involved in myocardial fibrosis, inflammation, oxidative stress and electrical abnormalities in HTN, HF, AF, myocardial infarction (MI), and cardiomyopathy [9]. Aldosterone, an adrenal hormone secreted after activation of the renin–angiotensin-aldosterone system (RAAS), is a critical regulator of blood pressure and electrolyte homeostasis [10,11]. HF is associated with increased production of aldosterone [12,13], and the use of an aldosterone receptor antagonist has been shown to attenuate atrial remodeling [14] and oxidative stress in HF [15]. Aldosterone receptor antagonists such as spironolactone and eplerenone have demonstrated several effects on cardiac diseases that are unrelated to their effects on blood pressure [16].
Aldosterone production is mainly regulated by the action of angiotensin II (Ang-II) on aldosterone producing cells of the adrenal cortex [17]. Evidence of myocardial aldosterone synthesis has led to a generation of new hypotheses regarding the physiological and the pathophysiologic significance of this hormone. Both cardiomyocytes and cardiac fibroblasts express mineralocorticoid receptors (MRs) that have a high affinity for aldosterone [18]. Genomic effects of aldosterone are mediated by its interaction with MRs [18]. Aldosterone also amplifies Ang-II signaling and induces expression of ventricular and vascular Angiotensin type-1 receptors (AT1R) [19,20] and angiotensin converting enzyme (ACE) [21]. Aldosterone also regulates vascular transcription of several pro-atherogenic and oxidant genes [22]. Aldosterone also induces rapid changes (nongenomic) [23] that are not prevented by MR antagonists, but which are likely mediated by still uncharacterized plasma membrane receptors [24].
In this review, we summarize the role of aldosterone on promoting substrates of AF with focus on oxidative stress and its relation to inflammation and structural/electrical remodeling. The potential use of aldosterone antagonists to prevent onset or progress of AF will also be discussed.
2. Oxidative stress and atrial fibrillation
There are multiple cardiac sources of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which contribute to the loss of redox homeostasis and oxidative stress [15]. Prominent sources of oxidant stress in the atria include the mitochondrial electron transport chain, nicotinamide adenine dinucleotide phosphate oxidases (NADPH oxidases), and xanthine oxidase, and uncoupled nitric oxide synthase (NOS) [15]. The body also has a system of antioxidant defense enzymes to protect from an excess of reactive species, including superoxide dismutases, glutathione reductase and thioredoxin reductase [15].
ROS generation modulates the redox signaling of molecules such as NF-κB. NF-κB signaling stimulates many downstream inflammatory responses, including upregulation of tumor necrosis factor-alpha (TNF-α), C reactive protein (CRP), interleukin-6 (IL-6) and a reduction in endothelial NOS (eNOS) activity and NO production [25].
Several studies have documented evidence of increased oxidative stress in the atria of AF patients [26,27]. Rapid atrial pacing is also associated with increased nitrotyrosine abundance and decreased Ca2+ current [28], suggesting an important role of ROS in AF pathogenesis. Use of glutathione [28] and ascorbic acid [29], a water-soluble antioxidant, attenuated acute atrial electrical remodeling. Studies have also documented increased oxidative damage and ROS production in AF. Enhanced ROS generation via NADPH oxidase (NOX) is also associated with the development of post operative AF (POAF) [30]. In addition, increased NOX activity promotes cardiac remodeling and dysfunction [31].
NOS uncoupling promotes ROS generation [15]. NOS 1 and 3 forms are expressed under normal conditions. However, under pathological conditions associated with inflammation such as in HF, an induction of the inducible form of NOS (NOS 2, iNOS) occurs, which can become uncoupled, shifting the balance from a predominant synthesis of nitric oxide to that of superoxide anion [15].
ROS are also produced by the mitochondria, and AF is associated with mitochondrial injury and dysfunction [32]. Myocardial injury promotes the production of inflammatory cytokines including TNF-α. TNF-α is associated with mitochondrial dysfunction, apoptosis, and oxidative stress [33]. Similarly, in a goat model of AF, atrial tissues showed signs of mitochondrial dysfunction [32], decreased oxidative phosphorylation, and increased proton leak [34]. This was associated with increased ROS from the mitochondria. In addition to uncoupling of electron transport, NADPH oxidase 4 (NOX4) is thought to be localized to the mitochondria and may contribute to the production of H2O2 and under conditions of aging [35]. Oxidative stress can promote a cycle of oxidative damage in which the mitochondrial dysfunction promotes impaired energy production, which can impair contractile and electrical activity and promote cell death.
3. Aldosterone and oxidative stress
The RAAS is a primary modulator of cardiac oxidative damage [25]. AngII increases the generation of ROS in the vascular system via activation of the membrane-bound NADPH oxidase enzymes, endoplasmic reticulum stress and mitochondrial oxidative stress [25]. Several studies suggested that MR activation might potentiate the pro-inflammatory/fibrotic effects of AngII-AT1R signaling by increasing cardiac oxidative stress [19,36,37]. Aldosterone infusion in rats resulted in impaired endothelial relaxation, and increased oxidative stress in the vessel [36,38]. In the former study, use of eplerenone improved endothelial function in ischemic rat by reducing NADPH oxidase subunits, and increasing protein expression of eNOS and angiogenic factors such as VEGF and angiopoietin-1&2 [36]. Aldosterone antagonist treatments increased the levels of NO and prostacyclin, and reduced the levels of Ang-II, aldosterone, glutathione [39], and superoxide [40]. Spironolactone reduces oxidative cardiac damage and remodeling via generation of Ang-I, the less active form of angiotensin, in macrophages of HF patients. Aldosterone also induced oxidative stress independent of Ang via ET-1 signaling [41] and the Rho kinase pathway [42].
Interestingly, low dose spironolactone treatment (1 mg/kg for 3 weeks) suppressed diastolic dysfunction, oxidative stress and fibrosis without affecting blood pressure [43,44] suggesting that the pleiotropic effects of aldosterone antagonists are independent of blood pressure lowering.
In addition to its effect on NADPH oxidase activity and NO levels, aldosterone increased the levels of the NOX subunit p22phox, urinary 8-isoprostane levels (a marker of renal oxidative stress), superoxide dismutase (SOD) mRNA levels, and plasma oxidants markers such as malonyl dialdehyde (MDA) and hydrogen peroxide (H2O2) [45–48]. Interestingly, eplerenone only partially attenuated these effects, suggesting that additional mechanisms are involved in aldosterone effects that may be mediated by receptors other than the MRs [45]. Interestingly, apocynin, a superoxide production inhibitor, appeared to reverse the effects of aldosterone on protein expression of selective components of the NADPH oxidase complexes and cardiac remodeling, suggesting that therapeutic targeting of NOX isoforms might present an alternative approach to attenuate the oxidant-stress associated with aldosterone generation [47,49].
Mitochondrial dysfunction appears to be a critical factor in the development of several cardiovascular conditions such as arrhythmia [50] and HF [51]. Aldosterone increases mitochondrial-ROS generation independent of NADPH oxidase via the ERK1/2 pathway [52]. In the isolated mitochondria and in cardiac myocytes, Ca2+ serves as pro-oxidant while Zn2+ serves as antioxidant. Aldosterone causes dyshomeostasis of intracellular Ca2+ and Zn2+ via respective induction of oxidative stress and generation of antioxidant defenses [53].
Loss of intracellular Mg2+ has been associated with risk of cardiovascular diseases and arrhythmia [54]. Aldosterone was reported to cause a reduction in the cytosolic free Mg2+ concentration in rats resulting in intramural coronary artery remodeling accompanied by increased markers of cardiac and plasma oxidative stress, arterial inflammation and evidence of perivascular fibrosis [55]. Intriguingly, spironolactone has been shown to prevent AF in HF patients by modulating Mg2+ homeostasis [56].
The RAAS is implicated as a primary pathway underlying cardiorenal syndrome, and the use of aldosterone antagonists has been shown to reduce kidney oxidative damage, and also to prevent cardiac hypertrophy and oxidative stress in renal failure [46–48,57].
4. Aldosterone and inflammation
Inflammation is mechanistically related to oxidative stress. A role for inflammation in AF can be inferred from the common association of AF with inflammatory conditions like pericarditis and myocarditis [58]. Impaired atrial contractility promotes pro-inflammatory changes such as platelet adhesion, cytokine and neurohormone production. Monocytes and leukocytes are abundant in atria specimens from AF patients [59]. Increased levels of CRP-complement 4B complex have also been associated with increased risk of POAF, and the time of peak elevation of CRP 2–3 days post-surgery coincides with the peak incidence of POAF [60]. Elevated levels of IL-6 have been identified as an independent predictor of stroke and death in high risk AF patients, linking abnormal inflammation with risk of thromboembolism [61]. Elevated plasma IL-6 levels have also been identified as a risk marker for AF [62], and polymorphisms in both IL6 [62] and the IL-6 receptor [63] have been associated with AF risk. Interestingly, aldosterone has been suggested as a mediator of the increased levels of IL-6 in the DOCA-salt model of hypertension [64].
4.1. Role of aldosterone and inflammation in the development of fibrosis
Aldosterone promotes tissue inflammation leading to fibrosis and remodeling in the heart, vascular system, and the kidney [65]. Several studies suggested cell-specific effects of MR activation on inflammatory cell adhesion and infiltration. Aldosterone promotes kidney macrophage infiltration, complement C3 deposition and formation of nitrotyrosine, collagens, chemokines, adhesion molecules and profibrotic cytokines. These effects were all reduced by use of spironolactone [66]. Aldosterone also induces several inflammatory processes in adipocytes and contributes to insulin resistance by promoting oxidative stress, suggesting that it may also contribute to development of diabetes mellitus [25].
In rat heart, aldosterone treatment increased perivascular levels of the inflammatory mediators cyclooxygenase-2 and osteopontin, leading to perivascular fibrosis [67]. These changes were attenuated by eplerenone treatment. In rat aortic tissues, aldosterone induced Ang-II expression and expression of several pro-inflammatory genes such as TNF-α, monocyte chemotactic protein-1 (MCP-1) and NADPH oxidase [68]. In addition, aldosterone induced expression of adhesion molecules such as soluble intercellular and vascular adhesion molecule-1 (ICAM-1 and VCAM-1), matrix metalloproteinase-2 (MMP-2), platelet derived growth factor A (PDGF) and plasminogen activator inhibitor (PAI) [69]. Interestingly, use of eplerenone suppressed all of these effects, while candesartan, an angiotensin-II receptor blocker, and tempol, a mimetic for superoxide dismutase only partially suppressed these effects, suggesting a more important role for aldosterone in promoting inflammation and oxidative stress [69]. Intriguingly, tempol has been shown to significantly attenuate cardiac oxidative stress and transforming growth factor (TGF) beta expression [70].
NF-κB signaling is associated with both inflammatory and pro-fibrotic responses during AF [71]. In a Dahl salt-sensitive rat model of HF, eplerenone reduced iNOS and activated NF-κB [72]. Activation of NF-κB in vascular SMCs involves complex interaction between the AT1-R and MR [25].
In human HF, aldosterone levels were closely correlated with levels of pro-inflammatory markers such as 8-isoprostaglandin F-2alpha (8-iso-PGF), I-CAM and tissue inhibitor metalloproteinase-1 (TIMP-1) [73]. Aldosterone and Ang-II infusion increased levels of human IL-6 but not CRP, and use of spironolactone attenuated these effects suggesting that Ang-II induces IL-6 expression through a MR-dependent pathway [74]. Similar to IL-6, IL-18 also promotes myocardial hypertrophy, impaired contractility and apoptosis. Aldosterone promotes IL-18 expression in cardiac myocytes via parallel activation of Ang-II, ET-1, Rho/Rho kinase and peroxisome proliferator activated receptors (PPARs) [75].
5. Aldosterone and cardiac fibrotic remodeling
Fibrosis is a hallmark of atrial structural remodeling and a common feature of clinical AF [10]. Increased interstitial fibrosis can physically separate myocytes, decreasing myocyte electrical coupling and creating a barrier to rapid impulse propagation [10]. AF is associated with marked structural changes in atrial tissues that, to a large extent, depend on increased activity of the RAAS [71]. In a HF model, development of atrial fibrosis was associated with increased atrial Ang-II levels [13]. Increased Ang-II production in transgenic mice with cardiac-restricted ACE over expression causes marked atrial dilation with focal fibrosis and AF [76]. In this experimental system, treatment with ACE inhibitors improved left ventricular function and attenuated the development cardiac fibrosis [13,77]. In the RALES clinical trial [78], use of spironolactone led to a significant improvement of LV function, and a reduction in cardiac fibrosis and sudden death in patients with severe HF.
Elevated plasma and myocardial aldosterone levels are reported in HF patients and are associated with development of cardiac fibrosis [12,13]. An increase in plasma and myocardial aldosterone has been shown also to cause cardiac fibrosis [11,79,80]. Interestingly, in normal rat hearts, atrial aldosterone levels are higher than those in the ventricles, suggesting a differential role of aldosterone in the atria [81]. The antioxidant effects of spironolactone decreased MMP-2 [69], and improved vascular fibrosis found in rat models of HTN and HF [40,72]. Cardiac fibrosis induced by aldosterone and a high salt diet was exaggerated in AT1-R knockout mice [42]. This effect was attenuated by antioxidants effects of eplerenone [42]. Eplerenone also attenuated the transition from ventricular hypertrophy to failure associated with chronic pressure overload [37]. Intriguingly, mineralocorticoid receptor antagonism has been shown to prevent cardiac remodeling associated with hypertension, but not with aging [82], suggesting a more dominant role of other profibrotic neurohormones that may increase with aging.
Cardiac apoptosis is a complex process that is mediated in part by redox signaling [83]. Spironolactone prevented cardiac apoptosis and remodeling in aldosterone infused rats [83]. Although aldosterone has been shown to cause cardiac interstitial fibrosis, it is still unclear whether the mechanism is solely related to specific cardiac effects of aldosterone, to the combined effects of aldosterone with that of other local mediators (e.g., angiotensin-II, endothelin-1 or oxidant stress), or if it is also related to extra-cardiac effects, such as those on the kidney. Interestingly, in a cardiac-specific conditional MR knock-down model, mice developed severe HF and cardiac fibrosis in the absence of HTN or hyperaldosteronemia. These changes were fully reversed when MR antisense mRNA was suppressed [84]. Intriguingly, use of spironolactone potentiates cardiac fibrosis in this model, raising questions about the physiologic/pathophysiologic role of MR in the heart, and the potential role of receptors other than MR.
It is important to know whether Ang-II or aldosterone effects predominate. In a large study using rats, Milliez et al. [14] evaluated the role spironolactone, lisinopril (ACE inhibitor), and atenolol (β blocker) or their combination on the development of atrial fibrosis in their HF model. Only spironolactone attenuated atrial fibrosis. This result was further validated in a study by Yang et al. [85], where spironolactone also attenuated atrial fibrosis in a canine HF model. These observations raise the interesting possibility of using an aldosterone/MR antagonist to reverse atrial fibrosis.
The RAAS activates key downstream profibrotic mediators like transforming growth factor (TGF)-β [9]. Another potential factor is PDGF, which has recently become of great interest [9,86,87]. MR antagonists attenuate Ang-II induced cardiac hypertrophy and fibrosis [88,89]. Nishioka et al. [90], reported that myocardial fibrosis promoted by AngII induced PDGF-A, B and PDGFR-α, was ameliorated by use of eplerenone. Similarly, aldosterone increases PDGF B protein [91] in hepatocytes, and use of a MR antagonist reduced PDGF and TGF-β induced cell proliferation and migration [92,93].
We and others have shown that connective tissue growth factor (CTGF) is increased in the atria of patients with AF [94,95]. Interestingly, CTGF has been shown to promote atrial remodeling via Ang-II induced NADPH oxidase/Rac-1 dependent pathway [96]. Aldosterone also induces CTGF expression by activation of the NF-κB [97].
6. Aldosterone and electrical remodeling
Many studies suggest that improper Ca2+ handling is an important arrhythmogenic factor and a candidate mechanism to underlie AF-generating ectopic foci.
During AF, profound changes in Ca2+ cycling occur in the atria. At the single channel level, increased single L-type Ca2+ channel (Cav1.2) activity due to an increase of channel open probability has been observed in human AF [98]. In human and animal models of AF [99,100], whole cell L-type Ca2+ current densities are decreased by 60–70%, possibly as an adaptive response to arrhythmia-induced Ca2+ overload.
In rat ventricular myocytes, aldosterone treatment increased the density of L- and T-type Ca2+ currents, and mRNA expression of alpha1C and beta2 subunits of cardiac Cav1.2 channels [101,102]. Use of spironolactone reduced Ca2+ current density. In rabbits, eplerenone also attenuated the decrease in atrial Ca2+ current following atrial tachypacing [103]. Eplerenone suppressed AngII induced-inward Ca2+ current, and this effect was reversed by an addition of aldosterone, suggesting that the intracellular effects of AngII on Ca2+ current of cardiac mycocytes are mediated through the MR [104].
AF is associated with cellular changes of several potassium currents [105]. Aldosterone decreases the transient outward K+ current, Ito1 density secondary to the rise in Ca2+ current [106]. Both spironolactone and canrenoic acid (active metabolite of spironolactone) directly blocked Kv1.5, Kv4.3 and Kv7.1 channels that generate the human IKur, Ito1 and IKs [106]. In a rat model of inducible AF, aldosterone promotes AF via both increased structural remodeling and shortening of action potential via alteration of K+ currents [107]. These data suggest that aldosterone antagonists may be helpful in treatment of supraventricular arrhythmias via modulation of both K+ and Ca2+ channels.
Cardiac myocytes contain inositol 1,4,5 trisphosphate receptors (IP3R), which also contribute to Ca2+ release from intracellular stores when activated by IP3. Enhanced IP3 signaling increases the likelihood of spontaneous Ca2+ release/overload. In addition, Ca2+ release through plasmalemmal IP3Rs may sensitize RyRs. Interestingly, in human mononuclear leukocytes, aldosterone caused a concentration-dependent increase in IP3 levels through sodium hydrogen antiporter (Na+/H) [108,109]. In addition, aldosterone increased generation of diacylglycerol (DAG) and IP3 via increase activity of phospholipase C (PLC) [110]. These studies suggest that aldosterone may mediate rapid effects by membrane receptors similar to GPCRs.
Gap junctions underlie impulse propagation in cardiac myocytes. Spironolactone prevented gap junction remodeling and reversed the down regulation of connexin-43 (Cx-43), phosphorylated Cx-43 expression, and the progressive slowing of impulse propagation in a rat model of HF [111].
7. Aldosterone system expression in atrial fibrillation
Patients with primary aldosteronism have a 12-fold higher risk of developing AF when compared to blood pressure-matched controls [14], suggesting that aldosterone strongly contributes to AF development. Additional evidence documenting a causal impact of aldosterone on AF substrates has been recently reported [112]. Aldosterone contributes to a substrate for AF by promoting atrial fibrosis/hypertrophy, and by causing conduction disturbances without affecting ventricular hemodynamics [112].
Elevated plasma and myocardial aldosterone levels are routinely reported in HF and HTN patients [21,12,13]. Low plasma aldosterone post-MI is an independent predictor of survival and reduction in hospitalization in HF patients [113]. In addition, plasma aldosterone is independently associated with LV structure and hypertrophy in patients with cardiovascular disease risk and preserved ejection fraction [114]. Changes in mRNA expression of elements of the aldosterone system have been also shown in AF. Both the MR and 11beta-hydroxysteroid dehydrogenase type 2 (11betaHSD2) proteins are increased in the cytoplasm of atrial myocytes from patients with AF [115]. In a canine model of AF, atrial tachypacing increased plasma and atrial aldosterone levels [116]. Use of spironolactone or perindopril (an ACE inhibitor) attenuated atrial remodeling and improved atrial function by reducing plasma and atrial aldosterone levels, suggesting a critical role of aldosterone in AF pathogenesis and progression [116].
The aldosterone synthase (CYP11B2) T-344C gene polymorphism, which is associated with increased aldosterone activity, is also associated with AF in HF patients [117].
In patients with established AF, AF at long-term follow-up visit was associated with elevated plasma aldosterone levels [118]. Electrical cardioversion of atrial arrhythmia significantly lowers plasma levels of aldosterone in patients with persistent AF [119]. Similarly, the decrease in plasma aldosterone is associated with maintenance of sinus rhythm following cardioversion of AF [120]. These data suggests that aldosterone may be involved in the development of AF in patients, and that modulation of aldosterone levels may modify the atrial substrates for AF.
Surprisingly, data from the Framingham cohort study revealed that plasma aldosterone was not associated with AF incidence [121]. Further studies are required to explain the discrepancy in findings.
8. Aldosterone antagonists and atrial fibrillation
Accumulating evidence supports a critical pathophysiologic role of aldosterone in AF and has led to increased interest in the development of aldosterone/MR antagonists to treat or prevent the progression of cardiovascular diseases including HF, MI and AF.
In an experimental canine model of persistent AF, spironolactone treatment markedly prevented AF-related changes in atrial structure and function [122]. Spironolactone maintained left atrial ejection fraction and attenuated apoptosis, myolysis, fibrotic pathways, and mitochondrial swelling [122]. In a canine HF model, eplerenone attenuated the inducibility of sustained atrial tachyarrhythmias, prolonged atrial effective refractory periods, and attenuated LV remodeling [123].
In human studies, spironolactone therapy was associated with a reduction in the burden of AF and a reduction in hospitalizations for AF direct current cardioversion [124]. A recent study showed that eplerenone markedly improved maintenance of sinus rhythm after catheter ablation in patients with long term persistent AF [125]. Aldosterone antagonists also prevented onset of AF and flutter in patients with systolic HF [126]. In the RALES study [78], use of spironolactone led to a marked improvement of LV function and a reduction in cardiac fibrosis and sudden death in patients with severe HF. This may be due to the reduction in norepinephrine levels and the increase in the threshold of ventricular fibrillation [127]. In addition, 6 months use of spironolactone markedly decreased the 24-hour mean heart rate, the frequency of atrial and ventricular premature beats, and the risk of AF/flutter in patients with HF, in part by maintaining magnesium homeostasis [56]. The mechanism may due also to improving atrial conduction and remodeling in patients with HF [128]. These data suggest that aldosterone/MR antagonists may provide a preferential effect on the development of AF in the setting of HF.
Close monitoring of serum potassium and creatinine is important in patients when administering aldosterone antagonists, especially in the presence of comorbid conditions associated with impaired kidney function. Eplerenone, the newer selective aldosterone antagonist, has fewer side effects than spironolactone and holds greater promise and safer therapeutic target in patients with HF and other comorbidities [41]. Aldosterone synthase inhibition may also serve as potential future drug therapy similar to MR antagonists. Use of aldosterone synthase inhibitor (FAD286) improves left ventricular (LV) hemodynamics, function and remodeling similar to spironolactone, but only FAD286 persistently reduced LV oxidative stress [129]. Another recent novel MR antagonist with fewer side effects has also been tested and may have promise as a future drug [130].
Several studies suggested that MR antagonists provide additional antiarrhythmic effects to Ang-II or catecholamines, and it may be helpful to add an aldosterone/MR antagonist to ACEi/ARBs or beta blockers in patients. In human MI patients, MR antagonists combined with ACE inhibitor prevented post-infarct LV remodeling more effectively than ACE inhibitor alone [131]. In human AF patients, 12 month combination of spironolactone and beta-blocker prevented AF episodes in patients with normal LV function and history of recurrent paroxysmal AF [132]. Interestingly, beta-blocker alone or in combination with enalapril was significantly less effective in preventing AF relative to a combination that included spironolactone [132]. Combination of spironolactone and atenolol also attenuated atrial and ventricular remodeling in patients with permanent AF [133]. Addition of an aldosterone antagonist to optimal medical therapy reduces morbidity and mortality in patients with acute MI and HF [78,134]. This practice is important because it’s unclear if routine use of statins, ACEi, or ARBs does improve the outcome of AF ablation [135]. Surprisingly, in a retrospective study of human HF, use of aldosterone antagonists did not appear to prevent onset of AF, unlike ACEi and ARBs [136]. These results suggest that aldosterone antagonists may be more helpful in preventing the progression of AF than in suppressing the onset of AF. This suggests that aldosterone has a greater effect on AF substrate than triggers. However, further studies are required to investigate this hypothesis.
Several ongoing clinical trials are designed to evaluate the efficacy of aldosterone antagonists in AF and other cardiovascular disease such as HF. The treatment of preserved cardiac function heart failure with an aldosterone antagonist trial (TOPCAT) is designed to evaluate the effect of spironolactone on morbidity, mortality, and quality of life in patients with HF and preserved ejection fraction [137]. On the other hand, the Prospective Appraisal of the Prevalence of Primary aldosteronism in Hypertensive patients presenting with atrial flutter or fibrillation study (PAPPHY) will prospectively study prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation [138]. Results from these ongoing clinical trials should provide further insights into the impact of aldosterone antagonists on the prevention of atrial arrhythmias.
9. Conclusions
Strong clinical and preclinical evidence suggest an important role for aldosterone in the setting of cardiovascular pathology. Elevated plasma aldosterone levels in patients with HF and AF suggest a role of aldosterone in the etiology of these diseases. Further studies evaluating the biosynthesis and localization of aldosterone enzymes and receptors would provide improved insights into the functional integration of this system in the heart.
Aldosterone seems especially likely to contribute to AF development in the setting of HF. Activation of the aldosterone system has an important role in atrial oxidative stress and structural remodeling. Aldosterone antagonists appear to be effective in reducing circulating levels of oxidants, inflammatory, fibrotic and neurohormonal factors that influence atrial molecular biology (Fig. 2). The clinical impact of aldosterone antagonists has not yet been adequately studied in AF patients after onset of AF. Further studies to evaluate the impact aldosterone/MR antagonists on AF patients or patients with risk of AF are warranted.
Fig. 2.
The primary intracellular pathways activated downstream of aldosterone. Several stimuli increase aldosterone expression and release. Aldosterone acts through the mineralocorticoid receptor (MR) and a putative unknown membrane receptor (membrane R??). Aldosterone diffuses through the plasma membrane to the cytosol where it binds the MR and induces MR homodimerization, activation, and translocation to the nucleus. Activated MR promotes gene transcription of fibrotic and hypertrophic proteins (PDGF, CTGF, TGF β), and inflammatory cytokines and chemokines. Aldosterone acts through the MR and membrane Rs and promotes ROS generation via NADPH oxidase (NOX) and the mitochondria. ROS generation and inflammatory molecules activate the iNOS and NFκb signaling. Activated NFκb translocates to the nucleus promoting transcription of genes involved in atrial oxidative damage and inflammation. Aldosterone rapid signaling on membrane receptors also leads to phospholipase C (PLC) activation and other cellular signal-transduction cascades such as those mediated by inositol 1,4,5 trisphosphate (IP3), protein kinase C (PKC). Phosphorylation of membrane Na+/H+ antiporters increases intracellular pH and modulates cardiac myocyte myofilament Ca2+ sensitivity. Influx of Na+ can activate reverse mode activity of the Na+/Ca2+ exchanger (NCX), resulting in elevated intracellular Ca2+, positive inotropy, and arrhythmogenesis. Hydrolysis of phosphatidylinositol 4,5-bis-phosphate (PIP2) increases intracellular IP3, which subsequently promotes Ca2+ release through IP3 receptors (IP3Rs) that are located under the plasma-lemma adjacent to ryanodine receptors (RyRs). Ca2+ release through the IP3Rs may also contribute to the inotropic and arrhythmogenic activity of aldosterone, by sensitizing RyRs located there. Gene transcription pathways are also activated resulting in atrial apoptosis and connexin remodeling. During atrial fibrillation, aldosterone signaling may be enhanced, promoting oxidative damage, increased intracellular Ca2+and hypertrophic/fibrotic remodeling.
References
- 1.Rennison JH, Van Wagoner DR. Impact of dietary fatty acids on cardiac arrhythmogenesis. Circ Arrhythm Electrophysiol. 2009;2:460–9. doi: 10.1161/CIRCEP.109.880773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goette A, Ittenson A, Hoffmanns P, et al. Increased expression of P-selectin in patients with chronic atrial fibrillation. Pacing Clin Electrophysiol. 2000;23:1872–5. doi: 10.1111/j.1540-8159.2000.tb07041.x. [DOI] [PubMed] [Google Scholar]
- 3.Kozlowski D, Budrejko S, Lip GY, et al. Lone atrial fibrillation: what do we know? Heart. 2009;96:498–503. doi: 10.1136/hrt.2009.176321. [DOI] [PubMed] [Google Scholar]
- 4.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 5.Crandall MA, Bradley DJ, Packer DL, Asirvatham SJ. Contemporary management of atrial fibrillation: update on anticoagulation and invasive management strategies. Mayo Clin Proc. 2009;84:643–62. doi: 10.1016/S0025-6196(11)60754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Wagoner DR. Basic mechanisms of atrial fibrillation. Cleve Clin J Med. 2003;70(Suppl 3):S2–5. doi: 10.3949/ccjm.70.suppl_3.s2. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein RN, Stambler BS. New antiarrhythmic drugs for prevention of atrial fibrillation. Prog Cardiovasc Dis. 2005;48:193–208. doi: 10.1016/j.pcad.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Raschi E, Boriani G, De Ponti F. Targeting the arrhythmogenic substrate in atrial fibrillation: focus on structural remodeling. Curr Drug Targets. 2011;12:263–86. doi: 10.2174/138945011794182728. [DOI] [PubMed] [Google Scholar]
- 9.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 10.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–9. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 11.Delcayre C, Swynghedauw B. Molecular mechanisms of myocardial remodeling. The role of aldosterone. J Mol Cell Cardiol. 2002;34:1577–84. doi: 10.1006/jmcc.2002.2088. [DOI] [PubMed] [Google Scholar]
- 12.Ohtani T, Ohta M, Yamamoto K, et al. Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: beneficial effects of mineralocorticoid receptor blocker. Am J Physiol Regul Integr Comp Physiol. 2007;292:R946–54. doi: 10.1152/ajpregu.00402.2006. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Shinagawa K, Pang L, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–14. doi: 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 14.Milliez P, Deangelis N, Rucker-Martin C, et al. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J. 2005;26:2193–9. doi: 10.1093/eurheartj/ehi478. [DOI] [PubMed] [Google Scholar]
- 15.Bonilla IM, Sridhar A, Gyorke S, Cardounel AJ, Carnes CA. Nitric oxide synthases and atrial fibrillation. Front Physiol. 2012;3:105. doi: 10.3389/fphys.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambroisine ML, Milliez P, Nehme J, et al. Aldosterone and anti-aldosterone effects in cardiovascular diseases and diabetic nephropathy. Diabetes Metab. 2004;30:311–8. doi: 10.1016/s1262-3636(07)70122-2. [DOI] [PubMed] [Google Scholar]
- 17.Nattel S. Aldosterone antagonism and atrial fibrillation: time for clinical assessment? Eur Heart J. 2005;26:2079–80. doi: 10.1093/eurheartj/ehi477. [DOI] [PubMed] [Google Scholar]
- 18.Funder JW. Mineralocorticoid receptors: distribution and activation. Heart Fail Rev. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- 19.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–8. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 20.Ullian ME, Schelling JR, Linas SL. Aldosterone enhances angiotensin II receptor binding and inositol phosphate responses. Hypertension. 1992;20:67–73. doi: 10.1161/01.hyp.20.1.67. [DOI] [PubMed] [Google Scholar]
- 21.Harada E, Yoshimura M, Yasue H, et al. Aldosterone induces angiotensin-converting-enzyme gene expression in cultured neonatal rat cardiocytes. Circulation. 2001;104:137–9. doi: 10.1161/01.cir.104.2.137. [DOI] [PubMed] [Google Scholar]
- 22.Newfell BG, Iyer LK, Mohammad NN, et al. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol. 2011;31:1871–80. doi: 10.1161/ATVBAHA.111.229070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihailidou AS, Mardini M, Funder JW. Rapid, nongenomic effects of aldosterone in the heart mediated by epsilon protein kinase C. Endocrinology. 2004;145:773–80. doi: 10.1210/en.2003-1137. [DOI] [PubMed] [Google Scholar]
- 24.Williams JS. Evolving research in nongenomic actions of aldosterone. Curr Opin Endocrinol Diabetes Obes. 2013;20:198–203. doi: 10.1097/MED.0b013e328360c200. [DOI] [PubMed] [Google Scholar]
- 25.Cooper SA, Whaley-Connell A, Habibi J, et al. Renin–angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293:H2009–23. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 26.Mihm MJ, Coyle CM, Schanbacher BL, Weinstein DM, Bauer JA. Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc Res. 2001;49:798–807. doi: 10.1016/s0008-6363(00)00307-2. [DOI] [PubMed] [Google Scholar]
- 27.Kim YM, Guzik TJ, Zhang YH, et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–36. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 28.Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol. 2007;43:215–22. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carnes CA, Chung MK, Nakayama T, et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 30.Briggs LE, Takeda M, Cuadra AE, et al. Perinatal loss of Nkx2-5 results in rapid conduction and contraction defects. Circ Res. 2008;103:580–90. doi: 10.1161/CIRCRESAHA.108.171835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grieve DJ, Byrne JA, Siva A, et al. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol. 2006;47:817–26. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 32.Bukowska A, Rocken C, Erxleben M, et al. Atrial expression of endothelial nitric oxide synthase in patients with and without atrial fibrillation. Cardiovasc Pathol. 2010;19:e51–60. doi: 10.1016/j.carpath.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Kaduce TL, Fang X, Harmon SD, et al. 20-Hydroxyeicosatetraenoic acid (20-HETE) metabolism in coronary endothelial cells. J Biol Chem. 2004;279:2648–56. doi: 10.1074/jbc.M306849200. [DOI] [PubMed] [Google Scholar]
- 34.Anmann T, Eimre M, Kuznetsov AV, et al. Calcium-induced contraction of sarcomeres changes the regulation of mitochondrial respiration in permeabilized cardiac cells. FEBS J. 2005;272:3145–61. doi: 10.1111/j.1742-4658.2005.04734.x. [DOI] [PubMed] [Google Scholar]
- 35.Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging (Albany NY) 2010;2:1012–6. doi: 10.18632/aging.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi N, Fukushima H, Takeshima H, et al. Effect of eplerenone on endothelial progenitor cells and oxidative stress in ischemic hindlimb. Am J Hypertens. 2010;23:1007–13. doi: 10.1038/ajh.2010.91. [DOI] [PubMed] [Google Scholar]
- 37.Kuster GM, Kotlyar E, Rude MK, et al. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation. 2005;111:420–7. doi: 10.1161/01.CIR.0000153800.09920.40. [DOI] [PubMed] [Google Scholar]
- 38.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112:375–84. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- 39.Bayorh MA, Mann G, Walton M, Eatman D. Effects of enalapril, tempol, and eplerenone on salt-induced hypertension in Dahl salt-sensitive rats. Clin Exp Hypertens. 2006;28:121–32. doi: 10.1080/10641960500468276. [DOI] [PubMed] [Google Scholar]
- 40.Ceron CS, Castro MM, Rizzi E, et al. Spironolactone and hydrochlorothiazide exert antioxidant effects and reduce vascular matrix metalloproteinase-2 activity and expression in a model of renovascular hypertension. Br J Pharmacol. 2010;160:77–87. doi: 10.1111/j.1476-5381.2010.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauersachs J, Fraccarollo D. Aldosterone antagonism in addition to angiotensin-converting enzyme inhibitors in heart failure. Minerva Cardioangiol. 2003;51:155–64. [PubMed] [Google Scholar]
- 42.Kagiyama S, Matsumura K, Goto K, Otsubo T, Iida M. Role of Rho kinase and oxidative stress in cardiac fibrosis induced by aldosterone and salt in angiotensin type 1a receptor knockout mice. Regul Pept. 2010;160:133–9. doi: 10.1016/j.regpep.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Habibi J, DeMarco VG, Ma L, et al. Mineralocorticoid receptor blockade improves diastolic function independent of blood pressure reduction in a transgenic model of RAAS overexpression. Am J Physiol Heart Circ Physiol. 2011;300:H1484–91. doi: 10.1152/ajpheart.01000.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lastra G, Whaley-Connell A, Manrique C, et al. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab. 2008;295:E110–6. doi: 10.1152/ajpendo.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gromotowicz A, Szemraj J, Stankiewicz A, et al. Study of the mechanisms of aldosterone prothrombotic effect in rats. J Renin Angiotensin Aldosterone Syst. 2011;12:430–9. doi: 10.1177/1470320310397405. [DOI] [PubMed] [Google Scholar]
- 46.Michea L, Villagran A, Urzua A, et al. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and prevents oxidative stress in uremic rats. Hypertension. 2008;52:295–300. doi: 10.1161/HYPERTENSIONAHA.107.109645. [DOI] [PubMed] [Google Scholar]
- 47.Bayorh MA, Rollins-Hairston A, Adiyiah J, Lyn D, Eatman D. Eplerenone suppresses aldosterone/salt-induced expression of NOX-4. J Renin Angiotensin Aldosterone Syst. 2011;12:195–201. doi: 10.1177/1470320310391330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawarazaki H, Ando K, Fujita M, et al. Mineralocorticoid receptor activation: a major contributor to salt-induced renal injury and hypertension in young rats. Am J Physiol Renal Physiol. 2011;300:F1402–9. doi: 10.1152/ajprenal.00691.2010. [DOI] [PubMed] [Google Scholar]
- 49.Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun. 2004;313:812–7. doi: 10.1016/j.bbrc.2003.11.173. [DOI] [PubMed] [Google Scholar]
- 50.Jeong EM, Liu M, Sturdy M, et al. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012;52:454–63. doi: 10.1016/j.yjmcc.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pei DA, Li L, Xu ZY, et al. Expression of mineralocorticoid receptor and 11-beta-hydroxysteroid dehydrogenase type 2 in human atria during chronic atrial fibrillation: study of 25 cases. Zhonghua Yi Xue Za Zhi. 2007;87:816–9. [PubMed] [Google Scholar]
- 53.Kamalov G, Deshmukh PA, Baburyan NY, et al. Coupled calcium and zinc dyshomeostasis and oxidative stress in cardiac myocytes and mitochondria of rats with chronic aldosteronism. J Cardiovasc Pharmacol. 2009;53:414–23. doi: 10.1097/FJC.0b013e3181a15e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seelig MS. Consequences of magnesium deficiency on the enhancement of stress reactions; preventive and therapeutic implications (a review) J Am Coll Nutr. 1994;13:429–46. doi: 10.1080/07315724.1994.10718432. [DOI] [PubMed] [Google Scholar]
- 55.Weber KT, Gerling IC, Kiani MF, et al. Aldosteronism in heart failure: a proinflammatory/fibrogenic cardiac phenotype. Search for biomarkers and potential drug targets. Curr Drug Targets. 2003;4:505–16. doi: 10.2174/1389450033490948. [DOI] [PubMed] [Google Scholar]
- 56.Gao X, Peng L, Adhikari CM, Lin J, Zuo Z. Spironolactone reduced arrhythmia and maintained magnesium homeostasis in patients with congestive heart failure. J Card Fail. 2007;13:170–7. doi: 10.1016/j.cardfail.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 57.Whaley-Connell A, Sowers JR. Oxidative stress in the cardiorenal metabolic syndrome. Curr Hypertens Rep. 2012;14:360–5. doi: 10.1007/s11906-012-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgera T, Di Lenarda A, Dreas L, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. 1992;124:455–67. doi: 10.1016/0002-8703(92)90613-z. [DOI] [PubMed] [Google Scholar]
- 59.Abdelhadi RH, Chung MK, Van Wagoner DR. New hope for the prevention of recurrent atrial fibrillation. Eur Heart J. 2004;25:1089–90. doi: 10.1016/j.ehj.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 60.Bruins P, HV, Yazdanbakhsh AP, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–8. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 61.Conway DS, Buggins P, Hughes E, Lip GY. Prognostic significance of raised plasma levels of interleukin-6 and C-reactive protein in atrial fibrillation. Am Heart J. 2004;148:462–6. doi: 10.1016/j.ahj.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 62.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155:303–9. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schnabel RB, Kerr KF, Lubitz SA, et al. Large-scale candidate gene analysis in whites and African Americans identifies IL6R polymorphism in relation to atrial fibrillation: the National Heart, Lung, and Blood Institute’s Candidate Gene Association Resource (CARe) project. Circ Cardiovasc Genet. 2011;4:557–64. doi: 10.1161/CIRCGENETICS.110.959197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sturgis LC, Cannon JG, Schreihofer DA, Brands MW. The role of aldosterone in mediating the dependence of angiotensin hypertension on IL-6. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1742–8. doi: 10.1152/ajpregu.90995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilbert KC, Brown NJ. Aldosterone and inflammation. Curr Opin Endocrinol Diabetes Obes. 2010;17:199–204. doi: 10.1097/med.0b013e3283391989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klanke B, Cordasic N, Hartner A, Schmieder RE, Veelken R, Hilgers KF. Blood pressure versus direct mineralocorticoid effects on kidney inflammation and fibrosis in DOCA-salt hypertension. Nephrol Dial Transplant. 2008;23:3456–63. doi: 10.1093/ndt/gfn301. [DOI] [PubMed] [Google Scholar]
- 67.Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology. 2002;143:4828–36. doi: 10.1210/en.2002-220120. [DOI] [PubMed] [Google Scholar]
- 68.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002;161:1773–81. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirono Y, Yoshimoto T, Suzuki N, et al. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology. 2007;148:1688–96. doi: 10.1210/en.2006-1157. [DOI] [PubMed] [Google Scholar]
- 70.Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem. 2008;317:43–50. doi: 10.1007/s11010-008-9803-8. [DOI] [PubMed] [Google Scholar]
- 71.Lendeckel UDD, Goette A. Aldosterone-receptor antagonism as a potential therapeutic option for atrial fibrillation. Br J Pharmacol. 2010;159:1581–3. doi: 10.1111/j.1476-5381.2010.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi N, Yoshida K, Nakano S, et al. Cardioprotective mechanisms of eplerenone on cardiac performance and remodeling in failing rat hearts. Hypertension. 2006;47:671–9. doi: 10.1161/01.HYP.0000203148.42892.7a. [DOI] [PubMed] [Google Scholar]
- 73.Kotlyar E, Vita JA, Winter MR, et al. The relationship between aldosterone, oxidative stress, and inflammation in chronic, stable human heart failure. J Card Fail. 2006;12:122–7. doi: 10.1016/j.cardfail.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Luther JM, Gainer JV, Murphey LJ, et al. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension. 2006;48:1050–7. doi: 10.1161/01.HYP.0000248135.97380.76. [DOI] [PubMed] [Google Scholar]
- 75.Doi T, Sakoda T, Akagami T, et al. Aldosterone induces interleukin-18 through endothelin-1, angiotensin II, Rho/Rho-kinase, and PPARs in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1279–87. doi: 10.1152/ajpheart.00148.2008. [DOI] [PubMed] [Google Scholar]
- 76.Xiao HD, Fuchs S, Campbell DJ, et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–32. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–93. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 78.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 79.Ramires FJ, Sun Y, Weber KT. Myocardial fibrosis associated with aldosterone or angiotensin II administration: attenuation by calcium channel blockade. J Mol Cell Cardiol. 1998;30:475–83. doi: 10.1006/jmcc.1997.0612. [DOI] [PubMed] [Google Scholar]
- 80.Sun Y, Ramires FJ, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 1997;35:138–47. doi: 10.1016/s0008-6363(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 81.Thum T, Borlak J. Gene expression in distinct regions of the heart. Lancet. 2000;355:979–83. doi: 10.1016/S0140-6736(00)99016-0. [DOI] [PubMed] [Google Scholar]
- 82.Hwang HS, Cirrincione G, Thomas DP, McCormick RJ, Boluyt MO. Aldosterone antagonism fails to attenuate age-associated left ventricular fibrosis. J Gerontol A Biol Sci Med Sci. 2007;62:382–8. doi: 10.1093/gerona/62.4.382. [DOI] [PubMed] [Google Scholar]
- 83.Burniston JG, Saini A, Tan LB, Goldspink DF. Aldosterone induces myocyte apoptosis in the heart and skeletal muscles of rats in vivo. J Mol Cell Cardiol. 2005;39:395–9. doi: 10.1016/j.yjmcc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Beggah AT, Escoubet B, Puttini S, et al. Reversible cardiac fibrosis and heart failure induced by conditional expression of an antisense mRNA of the mineralocorticoid receptor in cardiomyocytes. Proc Natl Acad Sci U S A. 2002;99:7160–5. doi: 10.1073/pnas.102673599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang SS, Han W, Zhou HY, et al. Effects of spironolactone on electrical and structural remodeling of atrium in congestive heart failure dogs. Chin Med J (Engl) 2008;121:38–42. [PubMed] [Google Scholar]
- 86.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–41. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 87.Sanchez-Guerrero E, Midgley VC, Khachigian LM. Angiotensin II induction of PDGF-C expression is mediated by AT1 receptor-dependent Egr-1 transactivation. Nucleic Acids Res. 2008;36:1941–51. doi: 10.1093/nar/gkm923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neves MF, Amiri F, Virdis A, Diep QN, Schiffrin EL. Role of aldosterone in angiotensin II-induced cardiac and aortic inflammation, fibrosis, and hypertrophy. Can J Physiol Pharmacol. 2005;83:999–1006. doi: 10.1139/y05-068. [DOI] [PubMed] [Google Scholar]
- 89.Fiebeler A, Schmidt F, Muller DN, et al. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension. 2001;37:787–93. doi: 10.1161/01.hyp.37.2.787. [DOI] [PubMed] [Google Scholar]
- 90.Nishioka T, Suzuki M, Onishi K, et al. Eplerenone attenuates myocardial fibrosis in the angiotensin II-induced hypertensive mouse: involvement of tenascin-C induced by aldosterone-mediated inflammation. J Cardiovasc Pharmacol. 2007;49:261–8. doi: 10.1097/FJC.0b013e318033dfd4. [DOI] [PubMed] [Google Scholar]
- 91.Li X, Meng Y, Yang XS, Wu PS, Zhang ZS. Aldosterone stimulating PDGF-B expression in HSC via activation of EGR-1. Zhonghua Gan Zang Bing Za Zhi. 2005;13:567–70. [PubMed] [Google Scholar]
- 92.Caligiuri A, De Franco RM, Romanelli RG, et al. Antifibrogenic effects of canrenone, an antialdosteronic drug, on human hepatic stellate cells. Gastroenterology. 2003;124:504–20. doi: 10.1053/gast.2003.50058. [DOI] [PubMed] [Google Scholar]
- 93.Macunluoglu B, Arikan H, Atakan A, et al. Effects of spironolactone in an experimental model of chronic cyclosporine nephrotoxicity. Transplant Proc. 2008;40:273–8. doi: 10.1016/j.transproceed.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 94.Mayyas F, Niebauer M, Zurick A, et al. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol. 2010;3:369–79. doi: 10.1161/CIRCEP.109.924985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adam O, Lavall D, Theobald K, et al. Rac1-induced connective tissue growth factor regulates connexin 43 and N-cadherin expression in atrial fibrillation. J Am Coll Cardiol. 2010;55:469–80. doi: 10.1016/j.jacc.2009.08.064. [DOI] [PubMed] [Google Scholar]
- 96.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 97.Terada Y, Ueda S, Hamada K, et al. Aldosterone stimulates nuclear factor-kappa B activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum- and glucocorticoid-inducible protein kinase-1. Clin Exp Nephrol. 2012;16:81–8. doi: 10.1007/s10157-011-0498-x. [DOI] [PubMed] [Google Scholar]
- 98.Asher CR, Klein AL. Transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation: ACUTE trial update. Card Electrophysiol Rev. 2003;7:387–91. doi: 10.1023/B:CEPR.0000023146.37030.8c. [DOI] [PubMed] [Google Scholar]
- 99.Cheng Y, Mowrey KA, Van Wagoner DR, Tchou PJ, Efimov IR. Virtual electrode-induced reexcitation: a mechanism of defibrillation. Circ Res. 1999;85:1056–66. doi: 10.1161/01.res.85.11.1056. [DOI] [PubMed] [Google Scholar]
- 100.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–25. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 101.Benitah JP, Vassort G. Aldosterone upregulates Ca(2+) current in adult rat cardiomyocytes. Circ Res. 1999;85:1139–45. doi: 10.1161/01.res.85.12.1139. [DOI] [PubMed] [Google Scholar]
- 102.Lalevee N, Rebsamen MC, Barrere-Lemaire S, et al. Aldosterone increases T-type calcium channel expression and in vitro beating frequency in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;67:216–24. doi: 10.1016/j.cardiores.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 103.Laszlo R, Bentz K, Konior A, et al. Effects of selective mineralocorticoid receptor antagonism on atrial ion currents and early ionic tachycardia-induced electrical remodelling in rabbits. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:347–56. doi: 10.1007/s00210-010-0553-2. [DOI] [PubMed] [Google Scholar]
- 104.De Mello WC. Intracellular and extracellular renin have opposite effects on the regulation of heart cell volume. Implications for myocardial ischaemia . J Renin Angiotensin Aldosterone Syst. 2008;9:112–8. doi: 10.3317/jraas.2008.014. [DOI] [PubMed] [Google Scholar]
- 105.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–31. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 106.Benitah JP, Perrier E, Gomez AM, Vassort G. Effects of aldosterone on transient outward K+ current density in rat ventricular myocytes. J Physiol. 2001;537:151–60. doi: 10.1111/j.1469-7793.2001.0151k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lammers C, Dartsch T, Brandt MC, et al. Spironolactone prevents aldosterone induced increased duration of atrial fibrillation in rat. Cell Physiol Biochem. 2012;29:833–40. doi: 10.1159/000178483. [DOI] [PubMed] [Google Scholar]
- 108.Christ M, Eisen C, Aktas J, Theisen K, Wehling M. The inositol-1,4,5-trisphosphate system is involved in rapid effects of aldosterone in human mononuclear leukocytes. J Clin Endocrinol Metab. 1993;77:1452–7. doi: 10.1210/jcem.77.6.8263127. [DOI] [PubMed] [Google Scholar]
- 109.Liu F, Levin MD, Petrenko NB, et al. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol. 2008;45:715–23. doi: 10.1016/j.yjmcc.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Christ M, Meyer C, Sippel K, Wehling M. Rapid aldosterone signaling in vascular smooth muscle cells: involvement of phospholipase C, diacylglycerol and protein kinase C alpha. Biochem Biophys Res Commun. 1995;213:123–9. doi: 10.1006/bbrc.1995.2106. [DOI] [PubMed] [Google Scholar]
- 111.Qu J, Volpicelli FM, Garcia LI, et al. Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied heart. Circ Res. 2009;104:365–71. doi: 10.1161/CIRCRESAHA.108.184044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reil JC, Hohl M, Selejan S, et al. Aldosterone promotes atrial fibrillation. Eur Heart J. 2011;33:2098–108. doi: 10.1093/eurheartj/ehr266. [DOI] [PubMed] [Google Scholar]
- 113.Palmer BR, Pilbrow AP, Frampton CM, et al. Plasma aldosterone levels during hospitalization are predictive of survival post-myocardial infarction. Eur Heart J. 2008;29(20):2489–96. doi: 10.1093/eurheartj/ehn383. [DOI] [PubMed] [Google Scholar]
- 114.Edelmann F, Tomaschitz A, Wachter R, et al. Serum aldosterone and its relationship to left ventricular structure and geometry in patients with preserved left ventricular ejection fraction. Eur Heart J. 2012;33:203–12. doi: 10.1093/eurheartj/ehr292. [DOI] [PubMed] [Google Scholar]
- 115.De-An P, Li L, Zhi-Yun X, et al. Increased expression of mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type 2 in human atria during atrial fibrillation. Clin Cardiol. 2010;33:23–9. doi: 10.1002/clc.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luo TY, Liu XH, Du X, et al. Effects of perindopril and spirolactone on plasma aldosterone and left atrial remodeling in a canine model of atrial fibrillation. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37:626–9. [PubMed] [Google Scholar]
- 117.Amir O, Amir RE, Paz H, Mor R, Sagiv M, Lewis BS. Aldosterone synthase gene polymorphism as a determinant of atrial fibrillation in patients with heart failure. Am J Cardiol. 2008;102:326–9. doi: 10.1016/j.amjcard.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 118.Dixen U, Ravn L, Soeby-Rasmussen C, et al. Raised plasma aldosterone and natriuretic peptides in atrial fibrillation. Cardiology. 2007;108:35–9. doi: 10.1159/000095671. [DOI] [PubMed] [Google Scholar]
- 119.Goette A, Hoffmanns P, Enayati W, Meltendorf U, Geller JC, Klein HU. Effect of successful electrical cardioversion on serum aldosterone in patients with persistent atrial fibrillation. Am J Cardiol. 2001;88:906–9. A8. doi: 10.1016/s0002-9149(01)01905-1. [DOI] [PubMed] [Google Scholar]
- 120.Wozakowska-Kaplon BBR, Janiszewska G. A decrease in serum aldosterone level is associated with maintenance of sinus rhythm after successful cardioversion of atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:561–5. doi: 10.1111/j.1540-8159.2009.02673.x. [DOI] [PubMed] [Google Scholar]
- 121.Schnabel RB, Larson MG, Yamamoto JF, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–7. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao J, Li J, Li W, et al. Effects of spironolactone on atrial structural remodelling in a canine model of atrial fibrillation produced by prolonged atrial pacing. Br J Pharmacol. 2010;159:1584–94. doi: 10.1111/j.1476-5381.2009.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shroff SC, Ryu K, Martovitz NL, Hoit BD, Stambler BS. Selective aldosterone blockade suppresses atrial tachyarrhythmias in heart failure. J Cardiovasc Electrophysiol. 2006;17:534–41. doi: 10.1111/j.1540-8167.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 124.Bafford R, Sui XX, Park M, et al. Mineralocorticoid receptor expression in human venous smooth muscle cells: a potential role for aldosterone signaling in vein graft arterialization. Am J Physiol Heart Circ Physiol. 2011;301:H41–7. doi: 10.1152/ajpheart.00637.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ito Y, Yamasaki H, Naruse Y, et al. Effect of eplerenone on maintenance of sinus rhythm after catheter ablation in patients with long-standing persistent atrial fibrillation. Am J Cardiol. 2013;111:1012–8. doi: 10.1016/j.amjcard.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 126.Swedberg K, Zannad F, McMurray JJ, et al. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 127.Cittadini A, Monti MG, Isgaard J, et al. Aldosterone receptor blockade improves left ventricular remodeling and increases ventricular fibrillation threshold in experimental heart failure. Cardiovasc Res. 2003;58:555–64. doi: 10.1016/s0008-6363(03)00251-7. [DOI] [PubMed] [Google Scholar]
- 128.Kimura M, Ogawa H, Wakeyama T, et al. Effects of mineralocorticoid receptor antagonist spironolactone on atrial conduction and remodeling in patients with heart failure. J Cardiol. 2011;57:208–14. doi: 10.1016/j.jjcc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 129.Mulder P, Mellin V, Favre J, et al. Aldosterone synthase inhibition improves cardiovascular function and structure in rats with heart failure: a comparison with spironolactone. Eur Heart J. 2008;29:2171–9. doi: 10.1093/eurheartj/ehn277. [DOI] [PubMed] [Google Scholar]
- 130.Nariai T, Fujita K, Mori M, Katayama S, Hori S, Matsui K. SM-368229, a novel promising mineralocorticoid receptor antagonist, shows antihypertensive efficacy with minimal effect on serum potassium level in rats. J Cardiovasc Pharmacol. 2012;59:458–64. doi: 10.1097/FJC.0b013e3182495543. [DOI] [PubMed] [Google Scholar]
- 131.Hayashi M, Tsutamoto T, Wada A, et al. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post-infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation. 2003;107:2559–65. doi: 10.1161/01.CIR.0000068340.96506.0F. [DOI] [PubMed] [Google Scholar]
- 132.Dabrowski R, Borowiec A, Smolis-Bak E, et al. Effect of combined spironolactone-beta-blocker ± enalapril treatment on occurrence of symptomatic atrial fibrillation episodes in patients with a history of paroxysmal atrial fibrillation (SPIR-AF study) Am J Cardiol. 2010;106:1609–14. doi: 10.1016/j.amjcard.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 133.Dabrowski R, Sosnowski C, Michalak E, Szwed H. A beneficial effect of 3-year spironolactone therapy supplementing atenolol therapy on the remodeling of atria and ventricles in a patient with permanent atrial fibrillation. Intern Emerg Med. 2009;4:171–3. doi: 10.1007/s11739-009-0229-4. [DOI] [PubMed] [Google Scholar]
- 134.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 135.Richter B, Derntl M, Marx M, Lercher P, Gossinger HD. Therapy with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and statins: no effect on ablation outcome after ablation of atrial fibrillation. Am Heart J. 2007;153:113–9. doi: 10.1016/j.ahj.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 136.Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2012;165(1):17–24. doi: 10.1016/j.ijcard.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 137.Desai AS, Lewis EF, Li R, et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–72. e10. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 138.Rossi GP, Seccia TM, Gallina V, et al. Prospective appraisal of the prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation (PAPPHY Study): rationale and study design. J Hum Hypertens. 2012;27(3):158–63. doi: 10.1038/jhh.2012.21. [DOI] [PubMed] [Google Scholar]