Abstract
IMPORTANCE
Postoperative hospital-acquired infections (HAIs) may result from disruption of natural barrier sites. Recent studies have linked vitamin D status and barrier site integrity.
OBJECTIVE
To investigate the association between preoperative vitamin D status and the risk for HAIs.
DESIGN, SETTING, AND PARTICIPANTS
A retrospective analysis was performed using propensity score methods to construct a matched-pairs cohort to reduce baseline differences between patients with 25-hydroxyvitamin D (25[OH]D) levels less than 30 ng/mL vs 30 ng/mL or greater. Multivariable logistic regression analysis was then performed to examine the association between 25(OH)D levels and HAIs while adjusting for additional perioperative factors. Locally weighted scatterplot smoothing was used to depict the relationship between increasing 25(OH)D levels and the risk for HAIs. This study was conducted in a single, teaching hospital in Boston, Massachusetts, and involved 770 gastric bypass surgery patients between January 1, 2007, and December 31, 2011.
EXPOSURES
Preoperative 25(OH)D levels.
MAIN OUTCOMES AND MEASURES
Association between preoperative 25(OH)D levels and the risk for postoperative HAIs.
RESULTS
The risk for HAIs was 3-fold greater (adjusted odds ratio, 3.05; 95%CI, 1.34–6.94) in patients with 25(OH)D levels less than 30 ng/mL vs 30 ng/mL or greater. Further adjustment for additional perioperative factors did not materially change this association. Locally weighted scatterplot smoothing analysis depicted a near inverse linear relationship between vitamin D status and the risk for HAIs for 25(OH)D levels around 30 ng/mL.
CONCLUSIONS AND RELEVANCE
In our patient cohort, a significant inverse association was observed between preoperative 25(OH)D levels and the risk for HAIs. These results suggest that preoperative 25(OH)D levels may be a modifiable risk factor for postoperative nosocomial infections. Prospective studies must determine whether there is a potential benefit to preoperative optimization of vitamin D status.
Hospital-acquired infections (HAIs) are a major source of nosocomial morbidity and mortality.1 Approximately 10% to 13% of all hospitalized patients in the United States develop HAIs,2,3 which translates to an annual incidence of roughly 2 million new cases1,4 and almost 100 000 potentially avoidable deaths per year.5 Excess annual health care expenditures attributable to HAIs range from $28 billion to $45 billion,6 and the average hospital length of stay is prolonged by 5 to 9 days in patients who develop a nosocomial infection.7 While catheter-related urinary tract infections (CRUTIs) are the most common HAI in the United States, surgical site infections (SSIs), pneumonias, and bloodstream infections are all significant causes of HAIs.5
Despite a flurry of research on the prevention of nosocomial infections, current understanding of HAIs remains limited.1,8 Indeed, existing strategies have only resulted in modest reductions in postoperative infection rates.2,9–17 Temperature regulation,15,16 minimization of blood transfusions,13,14 and less time under general anesthesia17 appear to derive their benefit from attenuating surgical stress–induced suppression of the immune system. In essence, robust postoperative immune function is associated with a lower likelihood of HAIs.18–20 However, it is important to note that aside from the use of neuraxial anesthesia, all existing strategies to minimize HAIs look to modify factors external to the host. Moreover, to our knowledge, few studies have looked at methods of optimizing natural host responses to infection in the perioperative setting.18–20
Recently, the immune-modulating effects of vitamin D have received significant attention.21 Indeed, a role for vitamin D in the prevention of HAIs has been suggested.7 However, to date, the influence of vitamin D status on surgical outcomes is largely unknown.22 A limited number of reports suggests that the prevalence of preoperative vitamin D insufficiency (defined as 25-hydroxyvitamin D [25(OH)D]<30 ng/ mL) may be as high as 70% to 80% in bariatric surgery patients.23,24 At the same time, SSI rates in Roux-en-Y gastric bypass surgery patients range from 1% to 10% following laparoscopic procedures to 15% to 25% following open abdominal surgery.25,26 Furthermore, obesity is a well-known risk factor for postoperative urinary tract infections and pulmonary complications.27,28 As such, our goal was to determine whether preoperative serum 25(OH)D less than 30 ng/mL is associated with a higher risk for postoperative HAIs in patients who undergo Roux-en-Y gastric bypass surgery.
Methods
The need for informed patient consent was waived as the Partners Human Research Committee (institutional review board) granted an exempt status for the study. We performed a retrospective cohort analysis of all Roux-en-Y gastric bypass surgical procedures performed at Massachusetts General Hospital (MGH) between January 1, 2007, and December 31, 2011. Cases were identified through the MGH Research Patient Data Registry and validated through individual electronic medical record review and cross-referencing with the MGH Department of Surgery records.
Primary Exposure and Outcomes
Serum 25(OH)D levels are routinely measured in individuals scheduled to undergo Roux-en-Y gastric bypass surgery at MGH as part of the preoperative nutrition assessment. Only patients with 25(OH)D levels within 30 days of surgery were considered for study inclusion. Hospital-acquired infection was defined as a SSI, a CRUTI, pneumonia, or bacteremia more than 48 hours after hospital admission and within 30 days of surgery. Surgical site infection rates in this population are validated and reported to the American College of Surgeons National Quality Improvement Project.29 Only SSI cases that were validated for inclusion in the American College of Surgeons National Quality Improvement Project database were considered in our analysis. A diagnosis of CRUTI was based on published guidelines and was defined as the presence of at least 3 of the following criteria more than 48 hours after surgery or during a readmission within 30 days after surgery: (1) leukocytosis; (2) fever (defined as temperature >101.5°F); (3) positive urinalysis results; or (4) positive urine culture results.30 Similarly, a diagnosis of pneumonia was based on previously published guidelines and was defined as the presence of at least 3 of the following criteria during the index admission or during a readmission within 30 days after surgery: (1) leukocytosis; (2) fever; (3) new infiltrate on chest x-ray; (4) positive sputum Gram stain finding; or (5) positive sputum culture finding.31 As defined in the existing literature, bacteremia required documented evidence of microbial growth from 2 peripheral blood cultures.32
Covariates
Each patient’s electronic medical record was reviewed to collect information related to the following covariates: (1) age; (2) sex; (3) race/ethnicity; (4) body mass index (calculated as weight in kilograms divided by height in meters squared); (5) American Society of Anesthesiologists physical status; (6) medical comorbidities (hypertension, diabetes mellitus, obstructive sleep apnea, and chronic obstructive pulmonary disease); (7) date of admission (coded as seasons: winter = December–February, spring = March–May, summer = June–August, fall = September–November); (8) type of surgery (open vs laparoscopic); (9) use of neuraxial anesthesia; (10) timely administration of prophylactic antibiotics (within 60 minutes before surgical incision); (11) duration of general anesthesia; (12) intraoperative fluid balance; (13) intraoperative temperature nadir; (14) intraoperative fraction of inspired oxygen concentration (<0.8 vs ≥0.8); (15) perioperative blood transfusions; (16) preoperative levels of nutritional markers (hemoglobin A1c, iron, ferritin, hemoglobin, albumin, thiamine, parathyroid hormone, and calcium); and(17)preoperative daily vitamin D supplement (none, ≤1000 IU, or >1000 IU).
Statistical Analysis
Bivariate tests were used to compare the characteristics of patients who did or did not develop HAIs and, more specifically, SSIs (t test, Mann-Whitney U test, χ2 test, and Fisher exact test). Propensity score–matched-pairs analyses were used to determine the adjusted association of 25(OH)D level (dichotomized to <30 ng/mL vs ≥30 ng/mL)with the primary outcomes (HAI and SSI). The rationale and methods underlying the use of propensity score analysis for proposed causal exposure variables have been previously described.33,34 A non-parsimonious multivariable logistic regression model was developed to estimate propensity scores for the preoperative dichotomized 25(OH)D level, irrespective of outcome. Clinical considerations guided the initial choice of covariates in these models to include: (1) age; (2) sex; (3) race/ethnicity; (4) body mass index; (5) American Society of Anesthesiologists physical status score; (6) medical comorbidities; (7) season; (8) preoperative hemoglobin level; and (9) preoperative vitamin D supplementation status. A structured iterative approach was used to refine the model, with the goal of achieving covariate balance within the matched pairs. Covariate balance was measured using the standardized difference where an absolute standardized difference greater than 10% represented meaningful covariate imbalance.33,34 Patients with 25 (OH)D levels less than 30ng/mL were matched to patients with vitamin D levels 30 ng/mL or greater using a greedy matching algorithm35 with a caliper width of 0.2 SDs of the log odds of the estimated propensity score. This method involved sampling without replacement.
A multivariable logistic regression model was then constructed to evaluate the association of the dichotomized 25 (OH)D level with HAIs and SSIs using the matched-pairs data, while controlling for other preoperative and intraoperative variables that might be associated with nosocomial infections based on clinical considerations. These variables included (1) type of surgery; (2) duration of general anesthesia; (3) intraoperative fluid balance; (4) intraoperative temperature nadir; (5) intraoperative fraction of inspired oxygen concentration; and (6) preoperative albumin level.
Sensitivity analyses for the robustness of the propensity score matching was performed using multivariable logistic regression on the entire patient sample (N = 770) to determine the association of preoperative 25(OH)D level with HAIs and SSIs while adjusting for (1) age; (2) sex; (3) race/ethnicity; (4) body mass index; (5) American Society of Anesthesiologists physical status score; (6) medical comorbidities; (7) season; (8) preoperative hemoglobin level; and (9) preoperative vitamin D supplementation status. Sensitivity analysis was repeated on the patients remaining in the fully adjusted model to verify the robustness of the final results of the association of preoperative 25(OH)D level with HAIs and SSIs.
Locally weighted scatter plot smoothing (LOWESS) was used to graphically represent the relationship between preoperative 25(OH)D level and the risk for HAIs and SSIs. Locally weighted scatter plot smoothing is a type of nonparametric regression, which summarizes a relationship between 2 variables in a fashion that initially relies on limited assumptions about the form or strength of the relationship.36 The rationale and methods underlying the use of LOWESS for depicting the local relationship between measurements of interest across parts of their ranges have previously been described.37
All analyses were performed using Stata version 10 (Stata-Corp LP). All P values were 2-tailed, with statistical significance defined by P < .05.
Results
Of an identified 802 patients, 770 met the inclusion criteria. The 32 excluded cases comprised 3 patients with missing baseline 25(OH)D levels and 29 patients with levels that were obtained more than 30 days before surgery. The overall HAI rate in the study cohort was 5.3%(n = 41) and the SSI rate was 2.6% (n = 20). The overall prevalence of preoperative 25(OH)D less than 30 ng/mL in the study cohort was 58%.
A comparison of baseline characteristics between HAI and non-HAI patients demonstrated no substantial differences between groups except for a difference in preoperative 25(OH)D levels and a slightly higher rate of open Rouxen-Y gastric bypass surgical procedures in the HAI group compared with the non-HAI group (Table). Similar comparison of baseline characteristics between SSI and non-SSI patients demonstrated no substantial differences between groups except for a difference in preoperative 25(OH)D levels, a slightly higher rate of chronic obstructive pulmonary disease in the SSI group, and a statistically (but not clinically) significant difference in preoperative albumin levels (Table).
Table.
Baseline Characteristics of 770 Patients Who Did and Did Not Develop a HAI or SSI
| Variable | Non-HAI (n = 729) |
HAI (n = 41) |
P Value | Non-SSI (n = 750) |
SSI (n = 20) |
P Value |
|---|---|---|---|---|---|---|
| Age, y | 46 (12) | 47 (12) | .65 | 46 (12) | 50 (9) | .15 |
| Female, % | 70 | 73 | .73 | 70 | 70 | >.99 |
| Race/ethnicity, % | ||||||
| Non-Hispanic white | 84 | 78 | .55 | 84 | 70 | .18 |
| Non-Hispanic black | 6 | 10 | 6 | 15 | ||
| Hispanic | 8 | 10 | 8 | 10 | ||
| Other | 2 | 2 | 2 | 5 | ||
| BMI | 48 (9) | 46 (8) | .17 | 48 (9) | 47 (8) | .48 |
| ASA physical status, % | ||||||
| 1 | 1 | 0 | .82 | 1 | 0 | .36 |
| 2 | 44 | 42 | 44 | 30 | ||
| 3 | 55 | 58 | 55 | 70 | ||
| Comorbidities, % | ||||||
| Diabetes mellitus | 38 | 49 | .19 | 38 | 55 | .16 |
| COPD | 4 | 10 | .09 | 4 | 15 | .05 |
| OSA | 46 | 44 | .87 | 46 | 45 | >.99 |
| HTN | 61 | 51 | .25 | 61 | 50 | .36 |
| Home oxygen | 2 | 2 | .59 | 2 | 0 | >.99 |
| Season of surgery, % | ||||||
| Winter | 21 | 27 | .57 | 22 | 15 | .94 |
| Spring | 26 | 17 | 25 | 25 | ||
| Summer | 26 | 27 | 26 | 30 | ||
| Fall | 27 | 29 | 27 | 30 | ||
| Open gastric bypass surgery, % | 2 | 7 | .05 | 2 | 10 | .06 |
| Timely antibiotics, % | 100 | 100 | >.99 | 100 | 100 | >.99 |
| Duration of general anesthesia, mean (SD), min | 233 (62) | 232 (59) | .71 | 233 (61) | 235 (67) | .77 |
| Intraoperative fluid balance, mean (SD), mL | 3008 (1053) | 2883 (1089) | .76 | 3008 (1054) | 2751 (1069) | .30 |
| Temperature nadir, mean (SD), °C | 36.0 (0.4) | 35.9 (0.4) | .21 | 36.0 (0.4) | 35.8 (0.3) | .06 |
| Intraoperative FiO2>0.8, % | ||||||
| <30 min | 72 | 63 | .28 | 72 | 65 | .67 |
| 30–60 min | 14 | 15 | 14 | 15 | ||
| >60 min | 14 | 22 | 14 | 20 | ||
| Intraoperative PRBC transfusion, % | 0 | 0 | >.99 | 1 | 0 | >.99 |
| Preoperative laboratory values | ||||||
| Hemoglobin A1c, % | 6.5 (1.4) | 6.8 (1.5) | .24 | 6.5 (1.4) | 7.0 (1.5) | .11 |
| Albumin, g/dL | 4.3 (0.3) | 4.2 (0.3) | .10 | 4.3 (0.3) | 4.2 (0.3) | .02 |
| Iron, µg/dL | 71 (30) | 68 (27) | .31 | 71 (30) | 66 (24) | .32 |
| Ferritin, ng/mL | 118 (115) | 156 (215) | .65 | 119 (120) | 156 (204) | .31 |
| Thiamine, nmol/L | 99 (77) | 126 (204) | .97 | 99 (77) | 181 (322) | .76 |
| Hemoglobin, % | 13.5 (1.3) | 13.5 (1.4) | .82 | 13.5 (1.3) | 13.8 (1.5) | .55 |
| 25(OH)D, ng/mL | 28 (12) | 22 (11) | .002 | 28 (12) | 20 (11) | .003 |
| Calcium, mg/dL | 9.5 (0.4) | 9.5 (0.4) | .93 | 9.5 (0.4) | 9.4 (0.3) | .15 |
| PTH, pg/mL | 59 (42) | 63 (36) | .91 | 59 (42) | 64 (38) | .60 |
| Preoperative vitamin D supplementation, % | ||||||
| None | 37 | 49 | .33 | 37 | 55 | .20 |
| ≤1000 IU daily | 56 | 46 | 56 | 45 | ||
| >1000 IU daily | 7 | 5 | 7 | 5 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; FiO2, fraction of inspired oxygen concentration; HAI, hospital-acquired infection; HTN, hypertension; 25(OH)D, 25-hydroxyvitamin D; OSA, obstructive sleep apnea; PRBC, packed red blood cell; PTH, parathyroid hormone; SSI, surgical site infection.
SI conversion factors: To convert albumin to g/L, multiply by 10; calcium to mmol/L, multiply by 0.25; ferritin to pmol/L, multiply by 2.247; 25-hydroxyvitamin D to nmol/L, multiply by 2.496; PTH to ng/L, multiply by 1.
Propensity score matching, based on the selection of clinically relevant variables outlined in the Methods section, resulted in a reduction of the sample size from 770 to 544 patients. Compared with patients with baseline 25(OH)D levels less than 30 ng/mL, patients with 25(OH)D levels 30 ng/mL or greater had an adjusted odds ratio (OR) of 3.05 (95% CI, 1.34–6.94) for HAIs and of 4.14 (95%CI, 1.16–14.83) for SSIs. In a sensitivity analysis, we compared patients with baseline 25(OH)D levels less than 30 ng/mL to patients with 25(OH)D levels 30 ng/mL or greater in the entire study cohort (n = 770) and found that those with lower 25(OH)D had an adjusted OR of 3.00 (95% CI, 1.31–6.89) for HAIs and an adjusted OR of 3.93 (95% CI, 1.08–14.19) for SSIs.
Multivariable logistic regression analyses to examine the relationship between preoperative 25(OH)D level and HAI/SSI using the propensity score–matched sample together with other potential perioperative confounders, as described in the Methods section, resulted in a further reduction in sample size to 502 patients. Comparing patients with baseline 25(OH)D levels less than 30 ng/mL to patients with 25(OH)D levels 30 ng/mL or greater in this fully adjusted model resulted in an OR of 2.91 (95% CI, 1.25–6.76) for HAIs and an OR of 4.32 (95% CI, 1.16–16.17) for SSIs. Repeated sensitivity analysis, as described in the Methods section, and comparing patients with baseline 25(OH)D levels less than 30 ng/mL to patients with 25(OH)D levels 30 ng/mL or greater in the final study cohort (n = 502) resulted in an adjusted OR of 2.92 (95% CI, 1.23–6.96) for HAIs and an adjusted OR of 4.37 (95% CI, 1.12–16.51) for SSIs.
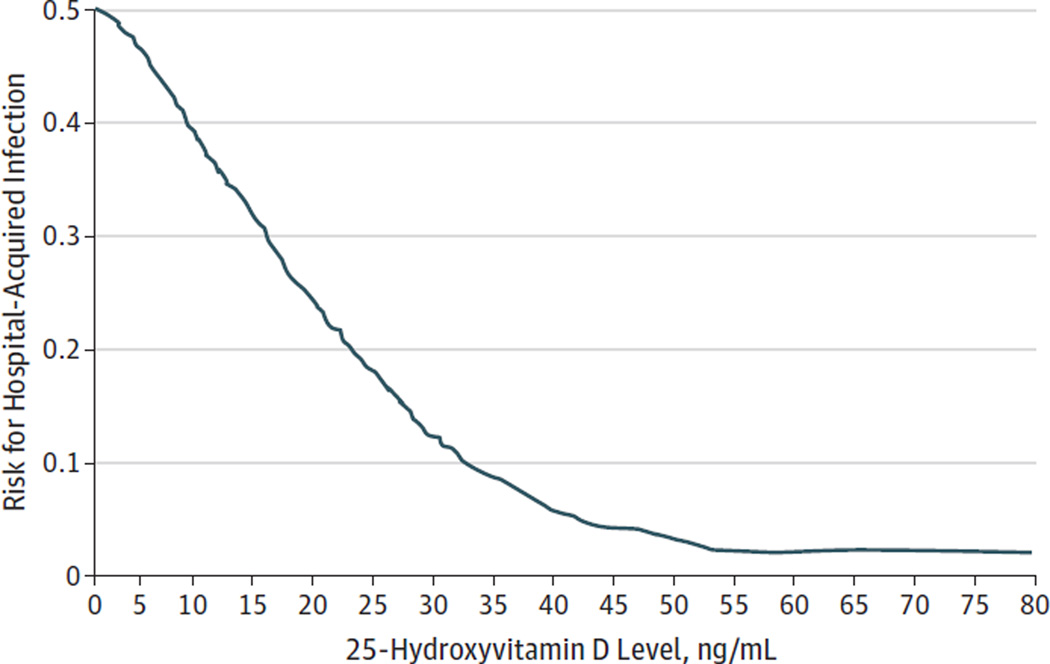
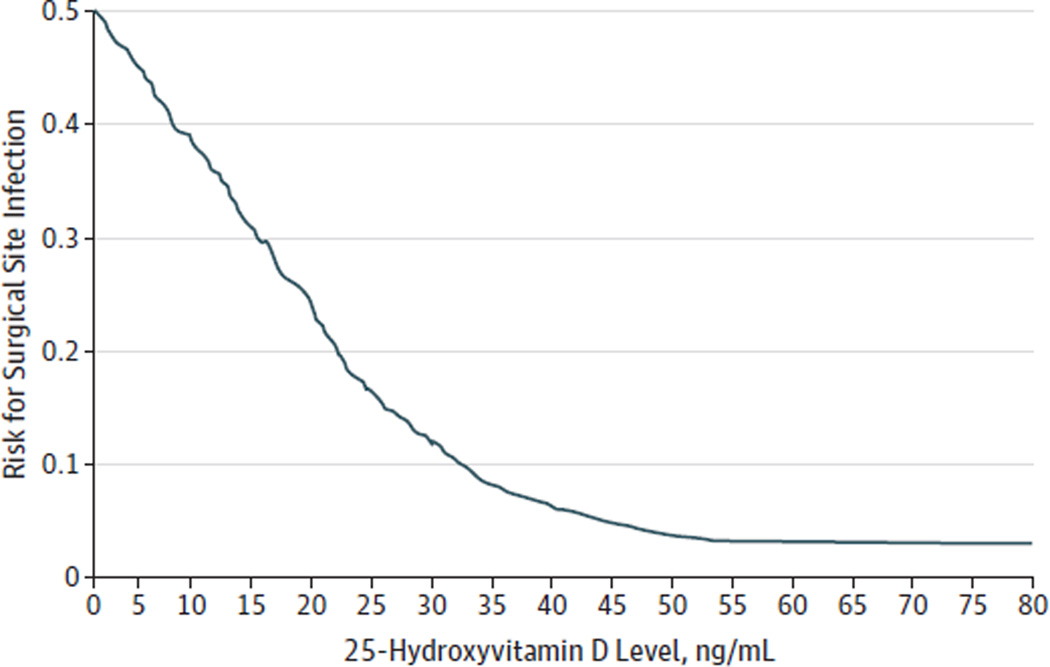
Locally weighted scatter plot smoothing plots (Figure 1 and Figure 2) showed a near inverse relationship between 25 (OH)D level and the risk for nosocomial infection for 25(OH)D levels around 30 ng/mL. Between 25(OH)D levels of 30 ng/mL and 50 ng/mL, there was a progressive flattening of the curve. Beyond 50 ng/mL, the curve appeared flat.
Figure 1.
Vitamin D Status vs Risk for Hospital-Acquired Infections
Between 25-hydroxyvitamin D levels of 0 and 30 ng/mL, there is an almost linear reduction in the risk for infection.
Figure 2.
Vitamin D Status vs Risk for Surgical Site Infections
Between 25-hydroxyvitamin D levels of 0 and 30 ng/mL, there is an almost linear reduction in the risk for infection.
Discussion
While it has been hypothesized that vitamin D sufficiency may play an important protective role against nosocomial infections,7 our work presents important evidence in support of such theoretical assertions. Our data suggests that in obese patients who have preoperative 25(OH)D levels less than 30 ng/mL, there is a 3-fold increase in the risk for postoperative HAIs and a 4-fold increase in the risk for SSIs after Rouxen-Y gastric bypass surgery when compared with similar patients with 25(OH)D levels 30 ng/mL or greater. Furthermore, our results suggest that reductions in the risk for HAIs and SSIs through improvements in preoperative vitamin D status plateau beyond 25(OH)D levels of 50 ng/mL in our patient cohort. Taken together, these findings reinforce a growing recognition that 25(OH)D levels between 30 and 50 ng/mL may offer distinct biological advantages.22
To control for potential confounding in this observational study, we used propensity score matching based on a set of conservative a priori parameters. This resulted in the exclusion of 226 patients from the original patient cohort. Sensitivity analysis confirmed that the observed association between 25(OH)D levels and the risk for HAIs (and SSIs) was consistent whether multivariable logistic regression analysis was performed using the propensity score–matched cohort or the entire study cohort. Adjustment of the propensity score– matched cohort for additional perioperative factors resulted in a further modest reduction in sample size. However, repeated sensitivity analysis confirmed the validity of the fully adjusted model and its ability to represent the associations between vitamin D status and the risk for nosocomial infections in the overall study cohort.
The decision to dichotomize the 25(OH)D level at less than 30 ng/mL and 30 ng/mL or greater for the matched propensity score analysis was a priori and based on several factors. From a clinical perspective, vitamin D insufficiency is defined as a 25(OH)D level less than 30 ng/mL.38 Although this traditional delineation is based on bone health–related studies demonstrating maximal parathyroid hormone suppression at 25(OH)D levels around 30 ng/mL,39 some immunomodulatory effects of vitamin D are also maximized at this threshold.40,41 Moreover, to address any possible concerns about our chosen threshold, we also set thresholds at less than 20 ng/mL and less than 10 ng/mL, and the results were not materially different from what we reported for levels less than 30 ng/mL (data not shown). This relationship was further confirmed on the LOWESS analysis, which graphically represented a well-defined change in the relationship between vitamin D status and the risk for HAIs or SSIs at 25(OH)D levels less than 30 ng/mL vs 30 ng/mL or greater.
Recent studies have demonstrated that cells of the innate and adaptive immune system express the vitamin D receptor and respond to stimulation by 1,25-dihydroxyvitamin D, the hormonally active vitamin D metabolite.42,43 Also, 1,25-dihydroxyvitamin D is important for the interferon-γ–dependent T-cell response to infection.44 Moreover, 25(OH)D links toll-like receptor activation and innate immunity, upregulating expression of the antimicrobial peptides cathelicidin (LL-37) and β-defensin.42 In humans, LL-37 has been shown to have potent activity against bacteria, viruses, fungi, andmycobacteria,45 and it is highly expressed at barrier sites. As such, LL-37 may provide important first-line defense mechanisms for the innate immune system.46 Prospective studies by members of our research group and others have shown maximal LL-37 expression to occur at 25(OH)D levels between 30 and 35 ng/mL,47,48 thereby strengthening the biological basis for the association between higher 25(OH)D levels and optimal immune function.
While our results support emerging insights on the pathophysiology of postoperative infections, it is important to discuss a number of possible study limitations. First, almost all patients underwent a laparoscopic procedure and thus had a significantly lower overall risk for SSIs; however, this was balanced with a relatively high prevalence of vitamin D insufficiency in the study cohort. Indeed, the overall SSI rate in our patient sample (2.6%) is in line with published reports. On the other hand, our rate of HAIs (5.3%) is slightly lower than published reports (7%–8%) and likely reflects some of the advantages of a minimally invasive approach to abdominal surgical procedures. Second, the study cohort was confined to a very specific patient population (obese adults undergoing gastric bypass surgery) rather than a random sample of patients following various surgical procedures. The reason we focused on this group was that perioperative data related to 25(OH)D levels and nosocomial infections are well documented in these patients at our institution. Although this may limit the generalizability of our findings to all surgical procedures, it significantly minimizes the potential for confounding related to the implicit and explicit differences between surgical procedures. Third, unlike our analysis related to SSIs, we did not specifically explore the association between 25(OH)D and other HAIs such as CRUTI, pneumonia, and bacteremia. The incidence of these nosocomial infections was too low to support an adequately powered analysis. Lastly, we conducted a retrospective study of patients from a single institution, which may also limit the generalizability of our findings. Future studies should aim to pool data from multiple institutions to yield study populations with adequate statistical power to study the relationship between 25(OH)D levels and the risk for postoperative infections after a variety of surgical procedures.
Conclusions
Our results suggest that low preoperative 25(OH)D levels are associated with a higher risk for postoperative infections. We hypothesize that higher levels of vitamin D sufficiency are associated with optimal expression of endogenous antimicrobial peptides and that this may attenuate the effect of barrier site disruptions that are characteristic of postoperative HAIs. Prospective studies are needed to validate our findings, assess the potential benefit of optimizing preoperative vitamin D status, and identify the mechanism by which vitamin D sufficiency may confer protection against nosocomial infections.
Acknowledgments
Funding/Support: Dr Quraishi received support from the National Institutes of Health grants 5T32GM007592-33 and UL1 RR025758. Dr Camargo received support from the National Institutes of Health grants R01 AI093723 and U01 AI087881.
Role of the Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Quraishi and Ms Blum had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Quraishi, Bittner, Blum, Camargo.
Acquisition of data: Quraishi, Blum.
Analysis and interpretation of data: Quraishi, Bittner, Hutter, Camargo.
Drafting of the manuscript: Quraishi, Bittner.
Critical revision of the manuscript for important intellectual content: Bittner, Blum, Hutter, Camargo.
Statistical analysis: Bittner.
Administrative, technical, or material support: Blum, Hutter.
Study supervision: Quraishi, Camargo.
Conflict of Interest Disclosures: Dr Quraishi serves on the board of directors of the Vitamin D Council in an advisory capacity. No other disclosures were reported.
REFERENCES
- 1.Ranji SR, Shetty K, Posley KA, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol 6: Prevention of Healthcare-Associated Infections) Rockville, MD: Agency for Healthcare Research and Quality; 2007. [PubMed] [Google Scholar]
- 2.Guggenbichler JP, Assadian O, Boeswald M, Kramer A. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials: catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg Interdiszip. 2011;6(1):doc18. doi: 10.3205/dgkh000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts RR, Scott RD, II, Hota B, et al. Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Med Care. 2010;48(11):1026–1035. doi: 10.1097/MLR.0b013e3181ef60a2. [DOI] [PubMed] [Google Scholar]
- 4.Welsh CA, Flanagan ME, Hoke SC, Doebbeling BN, Herwaldt L. Agency for Healthcare Research and Quality Hospital-Acquired Infections Collaborative. Reducing health care-associated infections (HAIs): lessons learned from a national collaborative of regional HAI programs. Am J Infect Control. 2012;40(1):29–34. doi: 10.1016/j.ajic.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott RD. The Direct Medical Costs of Healthcare-Associated Infections in US Hospitals and the Benefits of Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 7.Youssef DA, Ranasinghe T, Grant WB, Peiris AN. Vitamin D’s potential to reduce the risk of hospital-acquired infections. Dermatoendocrinol. 2012;4(2):167–175. doi: 10.4161/derm.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martone WJ, Nichols RL. Recognition, prevention, surveillance, and management of surgical site infections: introduction to the problem and symposium overview. Clin Infect Dis. 2001;33(suppl 2):S67–S68. doi: 10.1086/321859. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DJ. Surgical site infections. Infect Dis Clin North Am. 2011;25(1):135–153. doi: 10.1016/j.idc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Salkind AR, Rao KC. Antiobiotic prophylaxis to prevent surgical site infections. Am Fam Physician. 2011;83(5):585–590. [PubMed] [Google Scholar]
- 11.Qadan M, Akça O, Mahid SS, Hornung CA, Polk HC Perioperative supplemental oxygen therapy and surgical site infection: ameta-analysis of randomized controlled trials. Arch Surg. 2009;144(4):359–366. doi: 10.1001/archsurg.2009.1. discussion 366–367. [DOI] [PubMed] [Google Scholar]
- 12.Ata A, Lee J, Bestle SL, Desemone J, Stain SC. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145(9):858–864. doi: 10.1001/archsurg.2010.179. [DOI] [PubMed] [Google Scholar]
- 13.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256(2):235–244. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 14.Shokoohi A, Stanworth S, Mistry D, Lamb S, Staves J, Murphy MF. The risks of red cell transfusion for hip fracture surgery in the elderly. Vox Sang. 2012;103(3):223–230. doi: 10.1111/j.1423-0410.2012.01606.x. [DOI] [PubMed] [Google Scholar]
- 15.Beltramini AM, Salata RA, Ray AJ. Thermoregulation and risk of surgical site infection. Infect Control Hosp Epidemiol. 2011;32(6):603–610. doi: 10.1086/660017. [DOI] [PubMed] [Google Scholar]
- 16.Qadan M, Gardner SA, Vitale DS, Lominadze D, Joshua IG, Polk HC Hypothermia and surgery: immunologic mechanisms for current practice. Ann Surg. 2009;250(1):134–140. doi: 10.1097/SLA.0b013e3181ad85f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CC, Lin HC, Lin HW, Lin HC. Anesthetic management and surgical site infections in total hip or knee replacement: a population-based study. Anesthesiology. 2010;113(2):279–284. doi: 10.1097/ALN.0b013e3181e2c1c3. [DOI] [PubMed] [Google Scholar]
- 18.Cardinale F, Mastrototaro MF, Cappiello A, et al. Immunological modifications induced from products used during the perioperative period. Int J Immunopathol Pharmacol. 2011;24(3) suppl:s13–s20. doi: 10.1177/03946320110240s303. [DOI] [PubMed] [Google Scholar]
- 19.von Dossow V, Sander M, MacGill M, Spies C. Perioperative cell-mediated immune response. Front Biosci. 2008;13:3676–3684. doi: 10.2741/2958. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki T, Sata T. Perioperative innate immunity and its modulation. J UOEH. 2011;33(2):123–137. doi: 10.7888/juoeh.33.123. [DOI] [PubMed] [Google Scholar]
- 21.Youssef DA, Miller CW, El-Abbassi AM, et al. Antimicrobial implications of vitamin D. Dermatoendocrinol. 2011;3(4):220–229. doi: 10.4161/derm.3.4.15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quraishi SA, Camargo CA Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care. 2012;15(6):625–634. doi: 10.1097/MCO.0b013e328358fc2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fish E, Beverstein G, Olson D, Reinhardt S, GarrenM, Gould J. Vitamin D status of morbidly obese bariatric surgery patients. J Surg Res. 2010;164(2):198–202. doi: 10.1016/j.jss.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Flancbaum L, Belsley S, Drake V, Colarusso T, Tayler E. Preoperative nutritional status of patients undergoing Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2006;10(7):1033–1037. doi: 10.1016/j.gassur.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Christou NV, Jarand J, Sylvestre J-L. McLean APH. Analysis of the incidence and risk factors for wound infections in open bariatric surgery. Obes Surg. 2004;14(1):16–22. doi: 10.1381/096089204772787239. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen NT, PayaM, Stevens CM, Mavandadi S, Zainabadi K, Wilson SE. The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Ann Surg. 2004;240(4):586–593. doi: 10.1097/01.sla.0000140752.74893.24. discussion 593–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013;37(3):333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 28.Staehr AK, Meyhoff CS, Rasmussen LS PROXI Trial Group. Inspiratory oxygen fraction and postoperative complications in obese patients: a subgroup analysis of the PROXI trial. Anesthesiology. 2011;114(6):1313–1319. doi: 10.1097/ALN.0b013e31821bdb82. [DOI] [PubMed] [Google Scholar]
- 29.Nandipati K, Lin E, Husain F, et al. Factors predicting the increased risk for return to the operating room in bariatric patients: a NSQIP database study. Surg Endosc. 2013;27(4):1172–1177. doi: 10.1007/s00464-012-2571-2. [DOI] [PubMed] [Google Scholar]
- 30.Hooton TM, Bradley SF, Cardenas DD, et al. Infectious Diseases Society of America. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 31.American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 32.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Steiner PM, Guo S, Fraser MW. Propensity score analysis: statistical methods and applications. Psychometrika. 2010;75(4):775–777. [Google Scholar]
- 34.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8, pt 2):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 35.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):69–80. doi: 10.1002/pds.3263. [DOI] [PubMed] [Google Scholar]
- 36.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836. [Google Scholar]
- 37.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 38.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 39.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 40.Quraishi S, Camargo C Vitamin D and major chronic illness. J Restor Med. 2012;1(1):9–23. doi: 10.14200/jrm.2012.1.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 43.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.FabriM, Stenger S, Shin DM, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104):104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dürr UHN, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 46.Tollin M, Bergman P, Svenberg T, Jörnvall H, Gudmundsson GH, Agerberth B. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides. 2003;24(4):523–530. doi: 10.1016/s0196-9781(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 47.Bhan I, Camargo CA, Jr, Wenger J, et al. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol. 2011;127(5)(e1):1302–1304 . doi: 10.1016/j.jaci.2010.12.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixon BM, Barker T, McKinnon T, et al. Positive correlation between circulating cathelicidin antimicrobial peptide (hCAP18/LL-37) and 25-hydroxyvitamin D levels in healthy adults. BMC Res Notes. 2012;5(1):575. doi: 10.1186/1756-0500-5-575. [DOI] [PMC free article] [PubMed] [Google Scholar]