Abstract
Immunologic memory is the adaptive immune system's powerful ability to remember a previous antigen encounter and react with accelerated vigor upon antigen re-exposure. It provides durable protection against reinfection with pathogens and is the foundation for vaccine-induced immunity. Unlike the relatively restricted immunologic purview of memory B cells and CD8 T cells, the field of CD4 T-cell memory must account for multiple distinct lineages with diverse effector functions, the issue of lineage commitment and plasticity, and the variable distribution of memory cells within each lineage. Here, we discuss the evidence for lineage-specific CD4 T-cell memory and summarize the known factors contributing to memory-cell generation, plasticity, and long-term maintenance.
Keywords: CD4 T cell, memory, model, Th1, Th2, Tfh, Treg
I. INTRODUCTION
The immune system is continually confronted by threats from divergent sources. Viruses, bacteria, protozoan and metazoan parasites, fungi, and altered cells from the host's own body each present unique challenges for the host's immune defenses. In response, branches of the adaptive immune system with specialized effector functions have emerged to counter certain categories of threats. This effector specialization is perhaps best illustrated by antibody-secreting plasma cells that broadly target extracellular pathogens and by cytotoxic CD8 T cells that are particularly effective at cell-by-cell elimination of intracellular pathogens. The CD4 T-cell compartment, by contrast, contains at least seven functionally distinct subsets with diverse effector functions.1 These CD4 subsets play integral roles in all branches of adaptive immunity and can directly influence innate responses.1–3 Importantly, CD4 subsets are differentially recruited according to the type of immunologic threat, and multiple subsets with overlapping or disparate functions may be co-recruited. The breadth of their involvement allows CD4 T cells to help coordinate and balance the contribution of each branch of adaptive immunity, helping ensure that each type of immunologic threat is met with an appropriate immune response. If the threat is resolved by a balanced, threat-specific response, then the majority of the adaptive immune effector cells is lost to apoptosis during the contraction phase. Fortunately, a small proportion of the responding cells survives contraction and differentiates into long-lived memory cells. These memory cells persist in greater numbers than their naïve counterparts, and competent memory cells endow the host with enhanced secondary immune responses that are significantly more rapid and efficient than the primary response. Fittingly, these protective memory cells have been referred to as “... the most important biological consequence of the development of adaptive immunity...”4 Conversely, aberrant or dysregulated CD4 T-cell memory is suspected to contribute to multiple chronic or recurring inflammatory and immune-mediated disorders and to the progression of neoplastic disease.5 These remarkably powerful memory cells also provide the critical foundation for vaccine-induced immunity.
Not surprisingly, immune memory cells have been the focus of intense investigation for decades, and significant progress has been made in elucidating the mechanisms underlying their generation and maintenance. Historically, the greatest advances have occurred in the fields of CD8 T-cell and B-cell memory. These fields have benefitted from their limited range of effector phenotypes and robust proliferative capacity, a relatively restricted immunological purview, the early identification and characterization of lineage-specific memory cells, and an inherent phenotypic fidelity between primary and secondary effector cells.6 This contrasts with the broad phenotypic range of CD4 effector cells, a substantially more constrained proliferative capacity, and the limited evidence for memory cells and secondary effector responses for some phenotypes.7 Additionally, in vitro and in vivo phenotypic plasticity has been widely documented in both primary and secondary CD4 effector cells of some lineages.8–17 This makes the broad application of findings from CD8 T-lineage-restricted models to CD4 cells problematic. To overcome some of these concerns, many recent studies have taken compartmentalized approaches to CD4 memory by limiting investigation to similar or overlapping phenotypes.18 Notably, the more nuanced model of CD8 T-cell memory generally remains the guiding framework for CD4 investigations.
Despite the aforementioned challenges, the investigation of CD4 T-cell memory has been successfully guided by several key paradigms, including the classical T-cell response and systems for classifying memory cells according to their effector phenotype, patterns of tissue migration, and capacity for secondary responses. The T-cell response is perhaps the primary paradigm that provides context for the generation of memory. In the section II of this review, we examine the T-cell response and mechanisms operating at key transition points leading to the generation and maintenance of CD4 T-cell memory. In section III, we review current models of CD4 T-cell memory generation and propose the development of an integrated model of CD4 T-cell memory differentiation. In section IV, we cover the functional and migratory divisions of T-cell memory, including the classical central memory (Tcm) and effector memory (Tem) pools, and the more recently characterized tissue-resident memory (Trm) and recirculating memory (Trcm) pools. Finally, key features of secondary memory are summarized in section VI.
It is evident that the characterization of CD4 T-cell memory may be approached using multiple non-exclusive and often complementary methods. Our goal is to review the current literature regarding the generation and maintenance of CD4 T-cell memory in the context of the dominant paradigms guiding this exciting field.
II. EARLY CD4 T-CELL MEMORY DEVELOPMENT
The classical “T-cell response” paradigm provides the framework for understanding the development of CD4 T-cell memory.6,19 The T-cell response is comprised of three phases, which begin when mature naïve CD4 T cells are activated by recognition of antigen in the context of appropriate costimula-tory signals. Activation is followed by rapid clonal proliferation and differentiation into functional effector CD4 T cells in the expansion phase. The primary activation of naïve T cells is often referred to as priming to differentiate it from the more rapid secondary activation of memory cells. Optimal priming requires a complex cascade of signaling events initiated by antigen recognition and perpetuated by cell-to-cell, co-receptor, and cytokine signaling. In CD4 T cells, priming occurs over 1 to 2 days or more and culminates with the installation of a new transcriptional program that endows the T cells with effector functions and a robust proliferative capacity.20 This activated effector program also alters the expression of cell-surface molecules. In mice, for example, this includes permanently inducing the expression of the activation marker CD44, down-regulating the expression of other adhesion molecules such as CD62L and CCR7, and up-regulating molecules such as CD62E and CXCR5 to facilitate trafficking to peripheral sites or lymphofollicular zones, which were previously restricted.21,22 Elimination of the immunologic threat leads to the death of the majority of the expanded effector cells in the contraction phase. A small number of expanded cells survive contraction and persist as a quiescent population in the memory phase. Memory CD4 T cells are maintained in greater numbers than naïve cells and may persist for extended periods of time. These phases are repeated upon antigenic rechallenge, inducing memory cells to undergo a second expansion phase that is remarkably more rapid than the primary expansion and that yields secondary effector cells with enhanced functionality. If the secondary expansion quickly controls the threat, it is again followed by a contraction phase, further enhancing the of size of the secondary memory pool and its capacity for subsequent responses.6,12,19,23–26
Secondary effector cells have been described for most T-cell lineages, with classical memory and secondary responses in the Th1 and Th2 subsets being the most well characterized. The early investigation of Th1 and Th2 T-cell memory responses benefited from experimental models that maintained a high degree of lineage fidelity between primary and secondary effectors, similar to that exhibited by CD8 T cells. In contrast, the early investigation of memory in the Th17, Tfh, and Treg lineages was challenging, owing to a lack of consistent lineage fidelity. It is now widely considered that primary and secondary effectors from these groups can exhibit varying degrees of phenotypic plasticity. While this review focuses on the better-described Th1 and Th2 memory sets, discussions of Th17, Tfh, and Treg memory are included, along with implications and consequences of their apparent plasticity. We note that Treg cells are often considered apart from the other lineages, and their inclusion in this review is consequent to some notable advances in the characterization of Treg memory. Two recent reports employing in vivo studies in mice confirmed that antigen-specific Foxp3+ Treg cells exhibit classical expansion and contraction phases in response to acute viral infections with influenza and vaccinia virus.27,28 These studies demonstrated that a long-lived Treg memory population persists beyond the contraction phase and is capable of mounting enhanced antigen-specific recall responses in these models. In contrast, the nature of memory in the recently characterized Th9 and Th22 lineages is unclear. Although secondary effectors with Th9 and Th22 phenotypes have been reported, further research is necessary to fully characterize their origin and disposition, and a full discussion is best saved for future reviews.
A. Precursor Frequency of Naïve CD4 T Cells Regulates Memory
Naïve CD4 T cells are mature, quiescent, postthymic T lymphocytes. They typically bear T-cell antigen receptors (TCRs) with single-antigen specificity, and CD4 receptors that restrict the antigen recognition capacity of the TCR to only peptides presented on MHC class II molecules, collectively referred to as the peptide-MHCII complex (p:MHCII). Naïve cells are guided by a transcriptional program that maintains their capacity for homeostatic trafficking and antigen surveillance but does not endow the naïve cells with significant effector capacity. Classically, these cells bear the characteristic surface receptors CD62L and CCR7. These receptors are generally believed to promote localization of naïve CD4 T cells to central and regional antigen surveil-lance via continual trafficking through the secondary lymphoid organs (SLOs) via the blood, though a recent study has questioned the functional necessity for CCR7.29–31 This trafficking pattern targets T cells to the paracortical regions of lymph nodes and the parafollicular regions of the spleen, where they scan antigen-presenting cells (APCs), particularly dendritic cells, for cognate antigens.32 Importantly, naïve T cells in mice lack expression of the murine activation marker CD44, while human naïve T cells bear the naïveté-associated surface marker CD45RA but not the activation markers CD45RO or CD69.
The size of the naïve CD4 precursor pool directly affects the magnitude of the primary response and ultimately the size of the memory pool.33 Physiologic numbers of naïve antigen-specific CD4 T cells can vary dramatically between pathogens.34 In mice and humans, the number of naïve CD4 T cells specific for a given epitope is generally in the range of 1–100 per million naïve CD4 T cells; however, the reported number varies significantly by epitope and MHCII allele and may range from 100 to 3000 cells per mouse.33–39 A convincing report from Whitmire et al. suggested that earlier studies may have overestimated precursor frequencies and that a mouse spleen more likely contains approximately 100 naïve CD4 T cells specific for a given epitope.40 Subsequent studies using advanced investigative tools, such as T-cell libraries, have reported similar findings.34 The individual naïve cells within this peptide-specific pool each have a unique TCR, resulting in the possibility of differential strength and duration of TCR signaling during a primary response.35 TCR signaling, as will be discussed later, plays an early critical role in establishing cell lineage and the potential for a cell to persist into memory. This intrinsic TCR diversity within the naïve epitope-specific pool plays a signifi-cant role in generating heterogeneous effector and memory populations. Thus, TCR diversity and precursor frequency may synergistically alter the resulting effector phenotype, the magnitude of the response, and the subsequent predisposition for memory.39
All naïve CD8 T cells are recruited to the primary response regardless of antigen dose or type of infection; however, this has not been conclusively demonstrated for CD4 T cells.41 It is likely that most naïve antigen-specific CD4 cells are activated during the primary response; however, multiple studies have demonstrated that a broad range of fitness is exhibited within the naïve pool, and not all of the naïve antigen-specific cells are represented in the memory pool.1 There is strong evidence that the precursor frequency of antigen-specific cells and the antigen load may have significant effects on the overall activation and differentiation of the precursor pool.36,37,42 An interesting phenomenon observed only in CD4 T cells suggests that, under some conditions, high precursor frequency may actually adversely impact activation and recruitment into memory. In an elegant study by Celli et al.,43 real-time manipulation and two-photon imaging of the interactions between antigen-specific CD4 T cells and antigen-bearing DCs revealed that access to antigen-bearing DCs was a limiting factor in the recruitment and activation of precursor CD4 T cells. In this study, induction of clonal expansion was dependent on a minimum of 6 hours of TCR-p:MHCII interaction, and the frequency and duration of interaction between DCs and CD4 T cells decreased as precursor frequency increased. This finding suggests that increasing precursor frequency also increases interclonal competition for TCR-p:MHCII signaling, which results in shorter T cell-DC interactions and, as discussed in the next section, may ultimately skew selection of CD4 T cells for primary and memory responses. Regardless of T-cell precursor frequency, CD4 T cells do not match the proliferative capacity of their CD8 T-cell counterparts.44 In a model of acute lymphocytic choriomeningitis virus (LCMV) infection in mice, the clonal expansion of CD4 cells specific for the dominant MHC class II epitope may yield a population of up to 10 million cells in the spleen alone.45 It is important to consider, however, that this CD4 expansion was at least 20 times smaller than the concurrent clonal expansion of CD8 T cells, and the disparity persisted into memory. Nonetheless, it is likely that the size of the CD4 precursor pool and the extent of their recruitment into the response determine the magnitude of CD4 T-cell memory.
B. TCR Signaling: TCR Avidity, Duration, and Intensity Regulate CD4 T-Cell Memory
Multiple studies have demonstrated that TCR-p:MHCII signaling is one of the most important factors influencing the generation of robust CD4 memory.22,46–48 TCR signaling not only exerts the greatest influence during T-cell priming and polarization but also impacts secondary responses. In the primary response, the unique character of the TCR signaling in each cell differentially affects its selection from the naïve CD4 T-cell pool and helps determine which selected clones may preferentially mount primary and/or memory responses. It also helps drive the progressive increase in functional avidity exhibited by memory CD4 T cells throughout the course of primary and subsequent challenges.35
Most studies of TCR-p:MHCII interactions have used models generating the CD4 T-cell subsets Th1 and Th2.49–55 Primary and memory Th1-cell responses are well characterized and are easily reproduced in many acute viral and intracellular bacterial infections. Full CD4 T-cell priming and maximal proliferation during the expansion phase are dependent on prolonged TCR-p:MHCII signaling. The optimum TCR-p:MHCII interaction occurs over several days and possibly continues throughout the expansion phase when antigens and APCs are not limiting factors.56 Because multiple clones with unique TCRs are recruited during activation, the character of TCR signaling is different for each clone. These differences are important because studies in acute viral infections indicate that only a subset of the antigen-specific TCR clones recruited for the primary response is maintained in the memory phase, and it is vital to understand how and why the clones are selected.49 Some studies have argued that survival to memory is primarily influenced by the functional avidity with which the TCR binds p:MHCII, such that higher avidity clones gain a survival advantage.57,58 From this background it has also been demonstrated that rechallenge progressively yields higher avidity CD4 T-cell clones in the memory pool. In one notable study of LCMV infection by Williams et al.,49 an initial pool of naïve cells with a broad range of avidity became progressively more constrained through the primary response and memory phase, with the surviving clones exhibiting increased avidity.49 Thus, in this model, activated CD4 cells bearing TCRs with lower functional avidity were less likely to persist into long-term memory.
Notably, mounting evidence indicates that the complex TCR-p:MHCII interactions leading to the generation of CD4 T-cell memory encompass more than avidity. Indeed, more recent studies have demonstrated that binding avidity alone does not necessarily promote entry into memory.48,59 Building on their earlier findings,49 Williams et al. recently reported that the LCMV-specific CD4 T-cell memory pool actually contains clones bearing a variety of TCR complexes ranging from low to very high MHCII binding avidity.48 Their investigation revealed that TCR clones, which exhibited a slow rate of TCR-p:MHCII dissociation, or “off-rate,” were preferentially recruited to memory over competing clones, with little regard for binding avidity. Thus, the memory pool is likely initially enriched for TCR clones that are capable of prolonged TCR contact, and perhaps from this pool the higher avidity clones may gain competitive advantage. It is possible that the resulting heterogeneity in the initial memory pool may allow some degree of clonal selection.
Importantly, in some in vivo models, not all CD4 T-cell clones surviving in the memory pool played a prominent effector role in the primary response. Weber et al. reported that Th1 cells with genetically engineered TCRs specific for two closely related Listeria monocytogenes peptides from the Listeria LLO protein were compared: one preferentially mounted a robust primary effector response at the expense of a meager secondary response, while the other mounted a modest primary response but a more robust secondary response.60 This finding was interpreted to suggest that some subsets of responding Th1 cells preferentially function as memory, without mounting a maximal primary response, and that this constitutes evidence of Th1 subset specialization. According to this study, elevated levels of CD5, a negative regulator of TCR signaling, resulted in enhanced memory response at the expense of the primary response. Whether CD5 regulation can yield CD4 effector subsets functionally analogous to the SLEC/MPEC division of CD8 effectors, as discussed in section III.B, remains to be determined. Clearly, multiple mechanisms involving TCR-p:MHCII signaling contribute to the generation of memory CD4 T cells, and further studies are warranted.
C. Costimulatory Signaling and Cytokine Milieu During Priming and Expansion Phase Regulate CD4 T-Cell Memory
TCR-p:MHCII signaling alone is necessary but not sufficient to confer full priming of CD4 T cells. TCR signaling must be reinforced by costimulatory signaling via additional surface molecule interactions, with the APC as well as cytokines in the local milieu. The most well-characterized cell-to-cell signaling pathways involve interactions between the paired CD40-CD40L and B7-CD28 cell surface receptors. CD40 on APCs binds to CD40L (CD154) on T cells, triggering a variety of signaling pathways in both cell types.32,61 This interaction is critical for CD4 T-cell responses, and in the absence of CD40L signaling, the clonal expansion of antigen-specific CD4 T cells in vivo is severely impaired.62 CD4 T cells significantly up-regulate CD40L expression during TCR engagement, and in turn, APCs up-regulate CD40 expression in response to CD40-CD40L interaction. This suggests that a key function of CD40L signaling is to help ensure that non-specific CD4 T-cell activation in minimized. While naïve CD4 T cells form CD40L de novo during priming, new evidence indicates that non-regulatory CD4 effector and memory cells store preformed CD40L that can be rapidly mobilized.63 This likely contributes to the increased efficiency with which CD4 memory T cells mount a secondary response. Conversely, blockade of CD40-CD40L signaling can reduce the magnitude of the primary CD4 responses by up to 90% in mouse models of acute LCMV infection.45 The CD28 signaling pathway has not been fully elucidated; however, its activation is required for maximal clonal expansion of activated CD4 cells and subsequent memory formation.64 Activated antigen-bearing APCs up-regulate B7 expression, and B7 binding to CD28 lowers the threshold sensitivity to TCR-p:MHCII signaling. Thus, the CD4 cells are more sensitive to p:MHCII complexes when the APC is activated.32,51 In addition to lowering the threshold for activation, CD28 signaling strongly up-regulates transcription of IL-2 and inhibitors of apoptosis, thereby increasing the capacity for proliferation and the probability of surviving the contraction phase.65,66
Full CD4 T-cell priming also requires cytokine signaling. During priming, the nature and combination of the cytokine signaling in the local milieu influence the proliferative and effector capacities of the activating cells. The prevailing balance of cytokine signals helps drive the CD4 lineage commitment and, depending on the subset, may influence their capacity for survival into memory.67,68 An array of CD4 lineage-inducing and lineage-defining cytokines and their respective transcription factors have been described for Th1, Th2, Th17, Tfh, and Treg subsets, and these are summarized in numerous reports.1,67,69,70 While the capacities of the Th1 cytokine IL-12, and IFN-γ, as well as the Th2 cytokine IL-4 to drive lineage commitment are well documented, their roles in the generation of CD4 T-cell memory are not well understood. Further, it is challenging to extricate these roles in generating competent effector cells from their subsequent roles in the generation of memory cells. The role of these cytokines in the lineage fidelity and plasticity of secondary responses are further discussed later in this section and again briefly in sections III and IV.
Recently, Liao et al. comprehensively reviewed the role of IL-2 signaling in CD4 T-cell activation and differentiation.71 IL-2 is the most well-characterized T-cell costimulatory cytokine, and it is produced in high quantities by some CD4 subsets upon activation. Its primary functions in CD4 T cells are to act as a growth factor to induce entry into the cell cycle, reinforce TCR signaling, differentially influence the lineage selection of CD4 T cells during activation, and help control the population dynamics of effector cells surviving contraction.72–77 While early studies suggested that IL-2 signaling was unrelated to antigen-induced proliferation,32 more recent studies have demonstrated a role for IL-2 in initial activation as well as expansion and lineage commitment.71,75,76 Compared to CD8 T cells, both naïve and memory CD4 T cells are relatively refractory to IL-2–induced proliferation unless there is concurrent TCR engagement.74 In CD4 T cells, TCR engagement functions to down-regulate cyclin-dependent kinase inhibitors (CDKIs) and up-regulate the expression of key IL-2r subunits, facilitating entry in to the cell cycle and increasing responsiveness to IL-2.71,74 This is important for memory generation, as the strength of IL-2 signaling correlates with the magnitude of the proliferative response exhibited by some CD4 lineages. This is most prominent in Th1 and Th2 cells, in which this response appears to ensure that an optimally expanded effector population is available for entry into memory.68,71 IL-2 signaling during priming also leads to late up-regulation of IL-7rα on the expanded effectors, thereby further enhancing the size of the effector pool that survives to memory.77
Concurrently, the magnitude of IL-2 signaling during activation differentially affects the responsiveness of activated CD4 T cells to lineage-promoting cytokines.68,71 IL-2 signaling increases responsiveness to IL-12 and IL-4 but not IL-17, thereby favoring Th1 and Th2 differentiation over Th17. Increased IL-2 signaling is also required for Treg cell differentiation; thus, it appears that stronger IL-2 signaling preferentially selects for certain lineages to be maintained into memory at the expense of others.71,78 The ratio of IL-2 signaling strength to that of other cytokines also appears to be important in some cases. Studies by Choi et al. indicated that differential signaling by IL-2 and ICOS during priming can regulate Tfh differentiation and memory.78 They reported that lower expression of IL-2rα and increased ICOS signaling during priming and expansion can lead to the preferential development of Tfh cells.
Further supporting the role of IL-2 signaling in the generation of lineage-specific memory, IL-2rα (CD25)–deficient mice experimentally infected with Listeria monocytogenes (LM) exhibit markedly impaired Th1 memory.68,79 Notably, in the same experiment, CD25-deficiency did not inhibit the generation of an interesting set of putative central memory precursors, defined as CD4+ CD44+ CCR7+ CXCR5+ PD-1− cells.68,79 In WT mice, these Tcm-like cells were cogenerated in vivo with Th1 and Tfh (CD4+ CD44+ CCR7+ CXCR5+ PD-1+) cells during the primary response to experimental LM infection. When adoptively transferred to naïve hosts and rechallenged to LM infection, the Tcm-like cells were capable of generating heterogeneous secondary responses of Tcm-like, Th1, and Tfh lineages. The provenance of these Tcm-like cells has been previously debated, with some sources suggesting that they are resting Tfh cells;68 however, this evidence suggests that they may represent an uncommitted, but lineage-restricted, Tcm-like population.68,79 If this assertion stands, then, contrary to some models of CD4 T-cell memory generation, the IL-2–independent Tcm-like pool lacks a defined effector-phenotype intermediate. The adoptive transfer studies conducted by Pepper et al. suggest that CXCR5+/PD-1+ coexpression is tied to a Tfh phenotype, and lack of PD-1 expression yields a Tcm-like population capable of secondary expansion to form Th1, Tfh, and additional Tcm-like cells.68 Given the documented anti-proliferative effect of PD-1 expression, it is reasonable to question whether Tfh memory cells would benefit from maintenance of PD-1 expression, or whether loss of PD-1 expression at the termination of the germinal center reaction would allow these cells to regain the proliferative capacity important for memory responses. Clearly, IL-2 signaling plays important and complex roles in the generation of memory cells of several CD4 lineages. The implications of the intriguing Tcm-like CD4+ CD44+ CCR7+ CXCR5+ PD-1− cells are revisited in section III.
D. Contraction
In models of acute infection, pathogen clearance curtails the primary effector CD4 T-cell response and initiates the transition from the peak of expansion phase to the contraction phase. The length of the CD4 T-cell contraction phase has been variably reported to occur over 1–2 weeks79 or up to 4 weeks,80 during which the cessation of effector proliferation is augmented by the loss of up to 90–95% of the expanded cells.6,79,81 The overall magnitude of CD4 T-cell expansion and contraction are significantly lower than in CD8 T cells, and in some reports the loss of CD4 T cells continued in the memory phase.24,80,82 As recently reviewed by McKinstry et al.,26 the mechanisms underlying CD4 contraction have not been completely elucidated, but they likely include induction of a combination of convergent apoptotic and non-apoptotic death pathways in the lost cells and multiple survival and anti-apoptotic mechanisms in the effector cells surviving to memory. McKinstry et al. also suggested that cell fate is influenced by signaling during priming as well as during the effector stage and resolution of the immunogenic threat. A prior study by Whitmire et al.,80 using a mouse model of acute LMCV infection, demonstrated that a lack of B cells during the contraction phase doubled the rate of Th1 cell contraction and reduced the generation of CD4 T-cell memory. They further demonstrated that B-cell signaling was independent of antigen and antigen–antibody complexes; however, the underlying mechanisms have not been determined. Recent studies using SMARTA transgenic T cells indicate that increased expression of the proapoptotic factor Bim relative to the antiapoptotic factor Bcl-2 contributes to the rapid attrition of lower-affinity Th1 cells following acute Lm-gp61infection in mice, affirming a role for apoptosis in preventing some low-affinity cells from reaching memory.83 Another recent in vivo study in mice suggested that differential expression of CD47 might play a substantial role in non-apoptotic loss of CD4 T cells during contraction.84 In that study, newly activated CD4 cells transiently down-regulated CD47, rendering them susceptible to death by phagocytic cells, unless CD47 expression is rescued by IL-2 signaling. Subsequently, cells lost during the contraction phase tended to be CD47 low, while the resulting memory pool was CD47 high, suggesting that mechanisms that restore CD47 expression also facilitate survival to memory. It is also notable that, while IL-7 signaling is important for CD4 T-cell survival into memory, in vivo IL-7 blockade did not enhance the contraction of antigen-specific cells in vaccinia-infected mice.85
III. DIFFERENTIATION OF MEMORY CD4 T CELLS
The CD4 T cells that survive the contraction phase must undergo another critical transition to become competent memory cells. This transition requires a phenotypic shift from a functionally active, highly proliferative effector state to a quiescent, functionally alert memory state. Unlike naïve cells, memory cells are transcriptionally poised to rapidly regain their proliferative and functional capacity upon re-encounter with antigen. This is due in part to epigenetic alterations occurring during differentiation and to the installation of a transcriptional program distinct from naïve and effector cells that facilitates survival via multiple mechanisms.12,16,27,86–88
Memory CD4 T cells are classically defined as the set of T cells produced during a primary immunogenic challenge that persist and are capable of generating a recall response to secondary challenge.6,89 Using this definition, many early investigators focused on linear models of differentiation from naïve to effector to memory cells, particularly within in the Th1 and Th2 cell paradigm to understand memory. They were able to demonstrate that neither persistence of antigen nor TCR-p:MHCII signaling are required for these CD4 T cells to become long-lived memory cells, and that the removal of antigenic stimulation fostered the transition to memory.90 Subsequently, further characterization of the memory pool has identified multiple additional CD4 effector lineages with multiple tissue-trafficking patterns, a range of effector and proliferative capacities, and plasticity or the interconversion between some CD4 lineages prior to entering and emerging from the memory pool. Thus, over time, ample evidence has accumulated to suggest that the activated CD4 T cells surviving beyond the primary response are plastic and remarkably heterogeneous.91,92
Before reviewing evidence from studies attempting to identify and characterize memory CD4 T cells, it is important to note that it is not clear whether there is a single differentiation pathway by which memory cells are produced. In fact, the exact route by which memory T cells are generated has long been debated, and the demonstration of multiple sets of memory cells and lineage plasticity in primary and secondary effectors complicate the picture. Multiple theoretical models of CD4 memory development have been advanced in an attempt to account for this diversity of long-lived antigen-experienced cells.6,93 We will briefly examine the most widely cited theoretical models of CD4 T-cell memory generation, followed by the associated identifying characteristics of cells destined for and within the memory pool. In later sections, we examine the categorical divisions that have been developed to characterize the resulting phenotypically heterogeneous CD4 memory pool.
A. Models of Memory CD4 T Cell Differentiation
The path or paths by which memory T cells are generated has been debated since their earliest description. Numerous models of memory differentiation have been suggested in attempts to account for various phenomena reported for memory differentiation; however, the exact relation between naïve CD4 T cells, heterogeneous effector cells generated during a primary response, and heterogeneous memory cells generating secondary responses remains unclear.6,79,94 The traditional memory differentiation paradigms reviewed by Kaech et al. have not changed substantially in more than a decade. Multiple studies have since offered refinements to these models, and others have added to the burgeoning complexity of memory CD4 T-cell generation; however, a single unifying theory accounting for the diversity of CD4 T-cell memory has not yet emerged.94 Rather, the evidence suggests that multiple mechanisms of CD4 T-cell memory generation may be differentially employed depending on the effector lineage and inflammatory environment. We focus on variations of the linear differentiation model and the divergent or disparate fate model. These models can be roughly divided into two groups based on pathogen clearance. The former are dependent upon pathogen clearance for memory generation, while the latter contend that memory generation begins prior to, and is independent of, antigen clearance.94
1. Linear Differentiation Model
The traditional linear differentiation model of T-cell development posits that, upon antigen challenge, naïve CD4 T cells become activated and proliferate into effector cells, which then survive the contraction phase and persist as quiescent memory cells.6,94 This model borrows from the CD8 T-cell differentiation model, with the actual transition from effector to memory occurring during and after the contraction phase following pathogen elimination. This model concept also holds that there is lineage fidelity between the primary and secondary effectors. Indeed, the existence of long-lived memory Th1 and Th2 cells capable of mounting competent secondary responses is well described, and several groups have demonstrated that these Th1 memory cells emerge directly from the initial pool of Th1 effector cells generated during the primary response.25,46,79, 95, 96 In one key set of studies using IFN-γ reporter mice, it was demonstrated that naïve endogenous CD4 cells specific for LCMV and LM-OVA yielded Th1 effector cells which then persisted into memory and retained their phenotype upon rechallenge.42,95 These studies were supported by contemporary reports from Lohning et al.,93 who also demonstrated that memory cells can and do emerge from the primary effector population when purified functionally active antigen-specificTh1 or Th2 cells are adoptively transferred to naïve hosts. When primed in vitro or during viral infection in vivo, these antigen-specific cells form memory in the adoptive host and are capable of producing secondary responses when rechallenged. Two caveats should be noted, however. In the latter studies, antigen-specific memory was also formed by activated non-functional Th1 and Th2 cells generated and transferred under the same conditions, and in vivo rechallenge with LCMV caused some memory cells derived from functionally active Th2 cells to adopt a Th1-like phenotype with a shift to IFN-γ production, or co-production with IL-4. This would suggest that at least two memory pools might exist, one generated by effector cells actively producing lineage-defining cytokines, and one by activated cells not producing lineage-defining cytokines. Further, in the data presented by Lohning et al.,93 the secondary effectors generated by the transferred non-cytokine secretors from both Th1 and Th2 groups were equally capable of IFN-γ production comparable to the memory cells derived from the cytokine-producing Th1 group. Notably, the Th2-derived cells co-produced significant amounts of IL-4 regardless of the cytokine-producing capacity of the adoptively transferred progenitor cells. While it is likely that the linear differentiation model accurately characterizes the differentiation of certain CD4 T-cell memory subsets, there is significant evidence that some subsets are generated through alternative differentiation pathways.
2. The Decreasing Potential/Progressive Differentiation Model
The decreasing potential model suggests that, within the framework of linear differentiation, primary effector cells become progressively differentiated with increasing strength and duration of TCR and costimulatory signaling.58,94 In this model, the ability of effector cells to generate memory is progressively restricted in proportion to signaling, with failure of memory generation coincident with maximal signaling and the terminal differentiation of the majority of the responding cells. A recent study in CD4 T cells focused on the progressive differentiation model variant,58 which may also account for the differential production of the central memory (Tcm) and effector memory (Tem) pools.79 The results suggested that only cells of intermediate differentiation are able to respond to memory-supporting survival signals and that terminally differentiated effector cells are not maintained into memory.58 Thus, progressively greater levels of activation-associated signaling drive CD4 T-cell terminal differentiation at the expense of responsiveness to the survival signals present at the termination of the primary response. The capacity for effector cells to survive into memory is then dependent upon the point at which antigen is cleared. Another variation on this model, proposed by Moulton et al., suggested that shorter duration of antigen exposure generated less-differentiated precursors which preferentially yielded CD62LHi Tcm cells, while an increasing duration of antigen exposure progressively favored CD62LLo Tem cells.92 Although this model was referred to as a divergent model, it more closely follows the paradigms of linear differentiation than the divergent differentiation or disparate fate models discussed next.
Notably, an additional level of complexity may also arise from these models. Because naïve T cells sharing antigen specificity bear different receptors, reside in different locations and signaling milieu, and may have different interactions with antigen, the naïve cells are inherently subject to different levels of signaling. Differential signaling means that not all naïve cells are equally recruited or activated and may give rise to a variety of effectors phenotypes, each with its own response with regard to terminal differentiation and memory formation.58,79 As some cells in this model would not be terminally differentiated, differential signaling could also provide a mechanism to account for plasticity in recall responses should the balance of extracellular lineage-determining signals change. Differential signaling could also address some of the inconsistent findings of Lohning et al.,93 described above, with regard to the generation of memory by non-cytokine–producing cells and cytokine shifts. In LCMV infection, Th2 cells constitute a minor component of the CD4 T-cell response that is dominated by Th1-associated signaling. Thus, the adoptively transferred Th2 cells with the capacity for memory would be less differentiated and capable of responding to the Th1-cyotkines dominating the inflammatory milieu of secondary responses to LCMV.
3. Divergent Differentiation/Disparate Fate Model
According to the divergent differentiation model, the initial proliferation of activated cells during the expansion phase yields heterogeneous progeny with different capacities for differentiation to either effector or memory cells. In this model, the dividing, newly activated cell differentially distributes factors between the daughter cells, with one of the cells receiving factors which preferentially equip it for survival into memory. In this way, a subset of responding antigen-specific cells with a competitive advantage for generating memory are created during the initial response. This, then, occurs without regard for duration of antigen exposure and is independent of antigen clearance. A growing body of evidence supports this model, and perhaps the most convincing evidence is derived from studies examining asymmetric cell division in which the memory- and lineage-associated factors in the parent cell are unequally distributed to the daughter cells. Chang et al. investigated whether newly activated CD4 T cells equally distributed their receptors among daughter cells during the initial cell following activation.93,94 Using CD4 T cells with transgenic Leishmania-specific TCRs, they demonstrated that the distribution of specific activation-associated signaling receptors was asymmetric in vivo and that the disparity in receptor distribution was evident during initial divisions of the expansion phase. These insights revealed a potential mechanism by which multiple phenotypes could arise from the activation of a single antigen-specific CD4 T cell.
Asymmetric cell division was subsequently suggested to contribute to the diversity of CD4 effector and memory lineages, with the range of effector phenotypes and the propensity for memory influenced by the number of sequential asymmetric divisions.78,97 While Choi et al. did not examine the effects on CD4 T-cell fate, they did suggest that multiple repetitions of this phenomenon during expansion may help explain the concurrent emergence of Bcl6+ Tfh and Blimp1+ Th1 CD4 effector lineages within two cell divisions of activation.78 This would support arguments that asymmetric cell division may underlie the generation of some types of memory cells.18 Others have demonstrated asymmetric division at work in several T-cell models; however, they are largely restricted to CD8 T-cell investigations and target the development of the well-defined memory precursor effector cells (MPECs) and short-lived effector cells (SLECs) which have been characterized only in the CD8 lineage.98,99 Even so, some findings with regard to linkage between the nature of TCR signaling and asymmetric distribution of fate-determining factors fit well within the previously discussed framework of TCR signaling and differential clonal selection for memory in CD4 T cells.99 The implications of asymmetric cell division on the interpretation of CFSE-based cell proliferation studies was recently addressed, with a review of several mathematical models devised to account for this phenomenon.100 Further investigation into asymmetric cell division in CD4 T cells is necessary and will continue to yield instructive insights into memory CD4 T-cell formation.
B. Terminal Effector and Memory Precursor CD4 T Cells
In the CD8 T-cell memory field, the identification of two distinct effector CD8 T-cell populations that emerge during the expansion phase was a critical development.101 The first population, the short-lived effector cells (SLECs), are lost during contraction, while the second population, the memory precursor effector cells (MPECs), survive contraction and subsequently differentiate into competent memory cells. CD8 SLECs and MPECs are distinguished by differential expression patterns of cell surface receptors. SLECs are characterized by high expression of the senescence-associated molecule KLRG-1 and low expression of CD127, the alpha subunit of the IL-7 receptor. In contrast, MPECs are characterized by low expression levels of KLRG-1 and high expression of CD127. A remarkable amount of productive investigation has focused on the mechanisms underlying the differential production of SLECs and MPECs. While the discovery of SLECs and MPECs was a key step toward elucidating the generation of CD8 T-cell memory, an analogous paradigm broadly applicable to the field of CD4 T-cell memory has been elusive.
Recently, a similar division within the primary CD4 Th1 effector pool was reported by Kaech et al.46 Using an LCMV model of acute viral infection, they identified a pool of primary effectors with memory precursor-like phenotype, which persisted beyond the contraction phase and exhibited a superior capacity for secondary effector and proliferative responses. These memory precursors were identified based on the expression levels of the cell surface molecules Ly6c and PSGL-1 and the transcription factor T-bet. The memory precursors were PSGL-1Hi Ly6cLo and T-betInt, while the remainder of terminally differentiated Th1 effector cells were PSGL-1Hi/ Ly6cHi/T-betHi. Ly6c expression is regulated by T-bet expression, suggesting that greater T-bet signaling during priming yields a robust, terminally differentiated effector phenotype, which exhibits a greater proliferative history during the primary response. In contrast, moderately attenuated T-bet signaling yields smaller initial populations that are longer-lived and retain significant proliferative capacity for secondary responses. When Kaech et al. compared the level of Tbx21 gene (encodes T-bet) expression levels between the groups at D8 and D60 post infection, they found that Tbx21 levels were nearly identical in the Ly6cLo/T-betInt groups at both times, and they offered this as evidence that the transcriptional program allowing entry into Th1 the memory pool was established early in the T-cell response. Sharing further similarity to the CD8 T-cell paradigm, the Ly6cLo T-betInt population eventually gives rise to a Th1 population with a central memory-like phenotype (CD62LHi CCR7Hi), with differential localization to the splenic T-cell zones, while the Ly6cHi/T-betHi cells localize to the red pulp and can potentially give rise to effector memory cells.
However, Kaech et al. noted two important distinctions between CD8 SLECs and MPECs, and Th1 Ly6cHi/T-betHi effector and Ly6cLo/T-betInt memory precursors. The memory pool of Th1 cells always contains a fraction of Ly6cHi/T-betHi cells whose frequency rapidly declines to a low but steady state. Although the Th1 pool initially exhibits heterogeneity of CD127 expression, all cells became CD127Hi regardless of phenotype when adoptively transferred. When cells from the Ly6cHi/ T-betHi or Ly6cLo/T-betInt were separately adoptively transferred to naïve mice, they found that only the Ly6CLo/T-betInt cells exhibited prolonged survival and subsequently generated a subpopulation of Ly6cHi/T-betHi cells. The Ly6cHi/T-betHi eventually accounted for approximately 40% of the memory cells in the absence of antigen. Thus, they contend that Th1 memory cells may continually repopulate an effector pool in addition to generating a central-memory-like pool. A possible mechanism was suggested by the ability of Type I interferons to non-specifically induce Ly6C expression. They did not report the level of CD62L or CCR7 expression on the new Ly6cHi/ T-betHi effector pool generated from the transferred Ly6cLo/T-betInt cells. It is possible that the memory-generated pool of Ly6cHi/T-betHi effectors was CD127Hi and was responsible for the apparent conversion of Ly6cHi/T-betHi primary cells to CD127Hi. Thus, it is possible that elevated CD127 expression may still be a viable marker for memory precursors in these cells. It is likely that the identification of CD4 memory effector precursors will dramatically expand the CD4 memory field as it did the CD8 field, and further research is necessary. To this end, it would be interesting to investigate the role of TCR-p:MHCII avidity, dissociation rates, and CD5 expression, as discussed in section II.B, in the generation of these recently characterized Th1 Ly6cHi/T-betHi effectors and Ly6cLo/T-betInt CD4 memory precursors.
Many groups have investigated whether true lineage-committed Tfh memory cells exist, and different experimental models have generated conflicting results (briefly reviewed by Hale et al.18). While examining the role of Ly6C expression in CD4 memory precursor subsets memory, Marshall et al. reported that Tfh cells generated in their experiments exhibited functional attenuation, and it was not clear whether the Tfh cells persisting into memory maintained lineage fidelity or regained effector functions upon rechallenge.46 Contemporary studies using Listeria monocytogenes models or influenza infection in Il21-reporter mice have suggested the CXCR5+ memory Tfh population could repopulate both Th1 (CXCR5−) and Tfh populations.102 Hale et al. expanded on Marshall's findings by distinguishing Th1 effector and memory from Tfh cells based on differential expression of Ly6C, granzyme B, T-bet, CXCR5, and Bcl-6 expression.18, 46 They confirmed the lineage specificity of CXCR5−/Ly6cHi/granzyme B+/T-betHi/Bcl-6Lo cells as Th1 precursors and CXCR5+/Ly6cLo/ granzyme B−/CXCR5+/Bcl-6Hi cells as Tfh memory precursors. These Th1 and Tfh memory cells were generated from a clonal population of SMARTA transgenic cells and generally maintained lineage fidelity, and secondary effector functions mirrored primary effector functions. Interestingly, both Th1 and Tfh cells exhibited demethylation of IFN-γ and IL-21 gene loci at all time points following activation. In concert with the findings of Luthje et al.,102 Hale et al. also reported that Th1 memory cells predominantly formed a Th1 recall response, whereas a considerable amount of variability was reported in the phenotype of the Tfh recall responses, with a significant number acquiring a Th1 phenotype.18
C. An Integrated Model for CD4 T-Cell Memory Differentiation
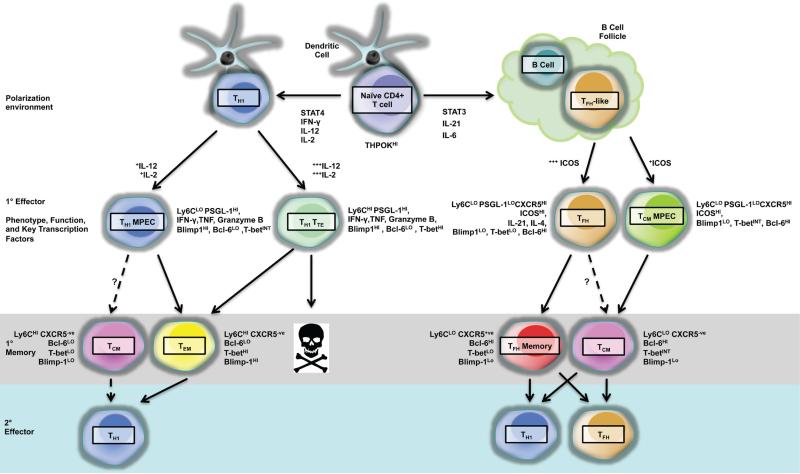
Based on the discussions in sections II.C and III.B, it is clear that, when considered separately, the current models of T-cell memory generation cannot account for the range of phenomena reported in recent CD4 T-cell memory literature. It is likely that multiple pathways of memory generation contribute to the range of phenotypes and capacity for plasticity reported in the memory pool, and that complementary and divergent developmental pathways are necessary to provide flexibility to this critical central component of the immune system. Although data for memory in some CD4 T-cell lineages are scant, and the relationship between certain lineages remains unclear, it is reasonable to attempt to reconcile the empirical evidence with our guiding conceptual model of memory CD4 T-cell generation. To this end, we propose the development of an integrated model of CD4 T-cell memory differentiation (Fig. 1). The initial framework for this model, as depicted in Figure 1, would incorporate recent evidence that the differentiation of memory Th1 and Tfh cells is dictated by several factors: (1) the duration and intensity of signaling triggered by exposure to IL-2, IL-12, and IL-21, and (2) the interaction of CD4 T cells with B cells.18,46,67,68 In this model, the exposure of activated CD4 T cells to IL-12 and varying concentrations of IL-2 induces intermediate to high levels of T-bet and Blimp1, which in turn inhibit the expression of Bcl-6. The increased T-bet:Bcl-6 ratio drives the generation of terminally differentiated T-betHi Th1 SLECs or the T-betInt MPECs, which further differentiate into Tem or potential Tcm memory cells, depending upon the interaction with B cells. Alternatively, under IL-2-limiting conditions, exposure to IL-21 and interactions with B cells, CD4 T cells differentiate into a plastic population of Tfh-like cells that give rise to Tfh memory or Tcm cells. Upon subsequent exposure to antigens, cells from the Tfh, Tcm, and Tem pools may then differentiate into Th1 effectors and Tfh effectors. It is our intention that this integrated model of memory differentiation will account for phenotypic plasticity and will be adapted as new data become available to address the complex relationships between additional lineages.
FIG. 1. An integrated model of CD4 T-cell memory differentiation.
TH1: Exposure of CD4 T cells to IL-12 and IL-2 leads to T-bet up-regulation and subsequent TH1 cell differentiation; while sustained interaction of CD4 T cells with DCs and varying levels of IL-2 and IL-12 signaling lead to the differentiation of TH1 terminal effectors or TH1 memory precursors. TH1 memory precursors differentiate into TEM cells. TFH: Sustained interaction of CD4 T cells with B cells up-regulates Bcl-6 and represses T-bet expression, which favors TFH-cell differentiation or development of TCM cells. After pathogen clearance, the cells undergo contraction, and the fraction of TH1 and TFH cells that survive become long-lived memory cells of TCM, TEM, and TFH memory phenotypes. Upon secondary antigen encounter, TH1 memory CD4 T cells secondary expansion are thought to give rise to more TH1 like 2° effector cells, while TFH memory cells are more plastic and can give rise to TH1 and TFH 2° effector cells.
D. Epigenetic Basis of Memory CD4 T-Cell Features
One of the hallmarks of resting memory T cells is that their functional and metabolic quiescence is linked to the maintenance of a poised transcriptional state from which effector and proliferative functions can be rapidly regained. As concisely reviewed by Youngblood et al.,86,103 investigations into the mechanisms underlying this poised state have focused on the epigenetic alterations occurring during activation and lineage commitment. In CD4 T cells, depending on the lineage, it is likely that many key epigenetic alterations also function to ensure lineage fidelity, to prevent detrimental gene expression, or to facilitate the expression of previously suppressed genes.86,103–107 It is also likely that commonly studied epigenetic modifications, including DNA methylation states, histone modifications, and microRNA expression, each play an important role in T-cell memory.108,109
Extensive investigations by Wei et al. examined paired suppressive and facilitative histone methylation states across a range of genes in CD4 T cells, including activation-associated genes and lineage-associated genes encoding IFN-γ, IL-4, IL-17, RORγt, and Foxp3.104 They found that cells expressing lineagespecific genes tended to exhibit consistent facilitative epigenetic modification of those genes, while the same cells did not always exhibit compensatory suppressive modification of the signature genes typically expressed by other lineages. They reported that suppressive methylation of the interferon gamma locus prevented IFN-γ expression in non-IFN-γ-secreting lineages; however, IL-4 was only epigenetically suppressed in Th1 and Th17 cells, but not in Treg cells. These findings suggested that epigenetically driven lineage specificity was limited to certain subsets of lineage-associated genes and only occurred in some lineages. Thus, some CD4 lineages were not epigenetically prevented from expressing transcription factors or cytokines traditionally associated with other lineages, suggesting a mechanism for phenotypic plasticity in secondary effectors.
Building on the findings of Wei et al.,104 subsequent studies by Hale et al. recently demonstrated that differential epigenetic alteration of the Gzmb locus in Th1 and Tfh cells during the primary response to LCMV was maintained in memory.18 This modification, Th1 Gzmb demethylation, helped maintain lineage fidelity during the secondary response and facilitated the rapid re-expression of granzyme B in Th1 cells while suppressing granzyme B expression in Tfh cells.18 Youngblood et al. recently studied competing epigenetic changes occurring during differentiation of Th1 versus Th2 cells.100 They found that the interferon gamma locus in Th1 effector cells was modified by demethylation and transcription-facilitating open histone modifications, while the locus in Th2 cells maintained transcriptionally suppressive methylation and closed histone modifications. Concurrently, the activity of DNA methyltransferase 1 in Th1-polarized cells results in methylation of IL-4 and FoxP3 loci, thereby suppressing Th2 and Treg differentiation and enforcing a Th1 phenotype.86 These findings provide further evidence that epigenetic modifications occurring during the primary response affect genes that are key to determining the range of secondary effector responses available to memory cells.
Clearly, epigenetic modifications do not globally enforce all aspects of lineage-specific gene expression in CD4 T cells, and a lack of suppressive epigenetic modification appears to confer considerable latitude for some cell types, particularly Th17 and Tregs, to express genes associated with other CD4 lineages. Furthermore, interesting data suggest that epigenetic changes that occur during memory development may actually sensitize some memory cells to epigenetic modifications to which their naïve counterparts are refractory. Investigation of Treg secondary responses in humans have indicated that naïve (CD45RA+) Tregs exhibited stable suppressive methylation of the RORC locus when stimulated under Th17-polarizing conditions, whereas the locus was demethylated in memory (CD45RA−) Tregs under similar circumstances.110 Thus, naïve human Tregs were refractory to in vitro Th17 repolarization, whereas previously activated Tregs were surprisingly susceptible. As previously mentioned, the problem of lineage infidelity and plasticity has complicated the investigation of memory in several lineages, but it has perhaps been most problematic with regard to the burgeoning field of Treg cells, and it is likely that epigenetic modifications underlie a significant proportion of this plasticity.104,111–113 While investigations of epigenetic modification have greatly expanded our understanding of CD4 T-cell memory and hold great promise for expanding our understanding of the well-described lineages as well as elucidating the origins of poorly defined memory cells lacking clear evidence of lineage commitment.68,79,86 Youngblood et al. expressed concern that many epigenetic investigations utilized in vitro polarized CD4 lineages and that these may not be representative of in vivo gene regulation.86 We echo these concerns and suggest that future investigations continue to explore the dynamic epigenetic alterations occurring during primary and secondary CD4 T-cell responses in vivo, as well as those influencing transitions between functionally defined sets of memory cells such as Tem, Tcm, Trm, and Trcm discussed in section IV.
E. Maintenance of CD4 T-Cell Memory
The maintenance of CD4 memory cells is not completely understood, and it is likely that multiple mechanisms differentially contribute to the proliferative renewal and homeostatic turnover of various memory populations. Maintenance of memory CD4 T cells is dependent upon homeostatic TCR signaling and multiple cytokines including IL-7 and IL-15.42,114–118 Studies using mice transgenically altered to allow inducible TCR signaling blockade have demonstrated that homeostatic (non-specific) TCR signaling is not required for primary effector CD4 cells to transition to memory; however, it is required for CD4 memory-cell homeostatic turnover and longevity. The mechanisms have not been fully elucidated, but it has been reported that TCR signaling blockade during memory decreases responsiveness to IL-7 signaling.119,120 IL-7 is required during the transition from CD4 T-cell effector to memory in the SLOs and target tissues, and again for long-term maintenance of CD4 memory.93,114,118,121,122 Similar to memory CD8 T cells, memory CD4 T cells require IL-15 for long-term maintenance, and the relative strengths of IL-7 and IL-15 signaling may significantly alter the rate of homeostatic turnover of memory CD4 T cells and influence the proliferation of secondary responses.16,117,123 The sources of IL-7 signaling are incompletely characterized, but have been investigated by multiple groups using several techniques.124–127 The cumulative evidence indicates that stromal tissues in multiple SLOs including lymph nodes, spleen, and bone marrow produce IL-7. Clearly, memory T cells reside in numerous additional tissues, and there is interesting evidence that at least a fraction of the memory CD4 T-cell pool actively traffics to the SLOs for targeted interaction with IL-7–producting stromal cells.124 IL-15 is produced by a variety of cells, including antigen-presenting cells, bone marrow stromal cells, and epithelial cells in the skin and respiratory and gastrointestinal tracts, and IL-15 has been demonstrated to elicit chemotaxis in T cells.123
Combined TCR signaling and IL-7 and IL-2 cytokine signaling have also been shown to suppress pro-apoptotic pathways during the transition to memory in human CD4 T cells. Riou et al. demonstrated that the CD3/CD28 and IL-2/IL-7 signaling pathways induce inhibitory phosphorylation of the transcription factor FoxO3a,53 thus preventing FoxO3a from activating transcription of Bim and FasL while concurrently inducing the inhibitory phosphorylation of target STATs downstream of FoxO3a.128 Riou et al. also demonstrated that the degree of inhibitory FoxO3a phosphorylation was significantly greater in the Tcm compared to Tem, and they suggested a differential need for antiapoptotic signaling to promote the survival of each group. FoxO3a phosphorylation is expected to reduce the induction of one of its target gene, the CDKI p27Kip1. Interestingly, loss of p27Kip1 prevented slow attrition in the number of memory CD4 T cells by reducing apoptosis; however, the underlying mechanisms are unclear.129
As discussed in the next section, the majority of memory CD4 T cells are maintained in the SLOs. The spleen appears to be the primary reservoir for circulating memory cells of all types, with progressively fewer cells maintained in the lymph nodes, mucosa-associated lymphoid tissues, and peripheral target tissues, respectively. A substantial fraction of memory CD4 T-cell traffic to bone marrow, where they are maintained indefinitely and have been suggested to constitute a secondary lymphoid organ.130 Memory CD4 T cells constitute up to 2% of the bone marrow and have recently been characterized as CD4+ CD44Hi CD62LLo CD69Hi Ly6CHi CD49bHi.125,131,132 This finding suggests that memory CD4 T cells are similar to the Th1 Tem memory cells, with an enhanced capacity for bone marrow homing; however, further studies to characterize the prevailing transcriptional programs with respect to lineage-determining transcription factors should prove instructive. While CD69 and CD49b expression on these memory cells is reported to facilitate homing to niches containing IL-7-expressing BM stromal cells,132 memory maintenance is also facilitated by CD11c+ dendritic cells, which may also provide antigen presentation for secondary responses.133
A mechanism for recruitment of memory cells from the bone marrow for homeostatic surveillance and secondary responses has not been elucidated but would most likely follow venous drainage, as the bone marrow is not drained by lymphatic vessels.130 Early studies of T-80 antibody-labeled helper T cells in the tibial bone marrow of domestic sheep demonstrated a relatively high rate of egress from the marrow with wide dissemination to SLOs under homeostatic conditions.134 Notably, a significant population of marrow-origin T cells labeled by transfusion in one tibia was recovered from remote and contralateral bone marrow samples. It is likely that this homeo-static trafficking is enhanced under inflammatory conditions, as demonstrated for conventional SLOs. A study in a mouse influenza model demonstrated that memory CD4 T cells recruited from SLOs to lung tissues for secondary responses include both antigen-specific and nonantigen-specific cells.135 This indicates that inflammatory signaling may induce memory cells in SLOs to traffic to target tissues, thereby enhancing peripheral immunosurveillance independent of antigen specificity.
IV. DIVISIONS OF CD4 MEMORY
Competent memory CD4 T cells may variously be defined by the phenotype of their effector lineage, homeostatic trafficking pattern, tissue distribution, or capacity for secondary responses. The classical distinction between Tcm and Tem has been augmented with tissue-resident memory (Trm) and recirculating memory (Trcm).87,136–140
A. Central versus Effector Memory
The first T-cell memory classification system devised by Sallusto et al. divided the memory pool into Tcm and Tem.137 Briefly, Tcm is characterized by trafficking between SLOs via the blood. Markers of Tcm include high expression of the lymphoid homing cell-surface receptors CD62L and CCR7. These cells have great proliferative potential and upon rechallenge their effector cytokine production favors IL-2 rather than lineage-specific cytokines. In contrast, Tem is characterized by trafficking between the spleen and nonlymphoid tissues, arriving from the lymphoid tissues via the blood and returning via lymphatics. CD62L and CCR7 are absent or poorly expressed by Tem while alternative adhesion molecules that facilitated entry into nonlymphoid target tissues are up-regulated.141–143 Tem express lineage-specific transcriptional programs and produce appropriate cytokines upon rechallenge, but tend to lack the full proliferative potential of Tcm.
The cytokines and transcription factors linked to effector lineage commitment concurrently influence entrance into the Tcm and Tem memory pools. There is evidence that IL-21signaling and Bcl-6 expression favor a Tcm phenotype, whereas Tem differentiation can be driven by the IL-2/Stat5 pathway.144–146 Even though the surface marker CCR7 has long been characterized as a critical component underpinning the characteristic SLO homing ability of naïve T cells and Tcm cells, it is worth noting that the actual role of CCR7 in guiding memory T cells to the lymph nodes has been called into question.29,30,137
Interestingly, in the previously discussed studies by Lohning et al.93 and Harrington et al.,95 memory cells generated by cytokine-producing progenitors were predisposed to maintaining a CD62LLo phenotype. This finding suggests that cytokine-producing effector cells tend to populate the Tem pool rather than Tcm. In the latter study, non-IFN-γ-producing Th1 progenitors exhibited a lower propensity for maintaining a CD62LLo status, suggesting that memory formed by this group may enter the Tcm pool. While functional effector cells appear to be favored for entry into the Tem, it also appears that, under some circumstances, they may shift from the Tem pool to the Tcm pool, which appears to differentially favor the more functional effectors. A dynamic differentiation model proposed by Schwendemann et al. suggested that that human peripheral blood CD4 cells could differentiate from Tcm from cells within the circulating Tem pool.147
B. CD4 T Resident Memory and Recirculating Memory
Trm is a recently characterized population that lacks CD62L and CCR7 and appears to traffic to target tissues, particularly the skin and mucosae, but does not recirculate.148–151 Originally reported in CD8 T cells, Trm cells were characterized by their expression of the adhesion molecule CD103, a receptor reported to facilitate their retention within epithelia.87,136 It has been challenging to apply the CD103 marker to memory CD4 T cells, as they may express it at undetectable or low levels, particularly in the skin and mucosae.138,139,152,153 CD4 Trm cells appear to share tissue distribution with CD8 Trm; however, they exhibit different localization within the tissues.23,87,139,140,154 CD4 primary effector and effector memory cells typically localize to the dermis and submucosa, while their CD8 analogues exhibit intraepithelial localization. Thus, CD4 cells, though present in sites complementary to CD8 Trm, they lack the characteristic CD8 Trm marker CD103, and they have ready access back to circulation via lymphatics and blood vessels.136
Curiously, despite this relatively unrestricted access to circulation in the dermis and submucosa, and the existence of a clearly demonstrated circulating memory pool, it is apparent that a fraction of the CD4 memory cells in these target tissue locations do not traffic back into circulation in parabiosis experiments, and this fraction appears to represent Trm.139,155 Some authors have subsequently suggested that the large pool of memory Treg cells in non-lymphoid organs represent a subset of the recently described CD4+ Trm, as they do not appear to recirculate.156
The two tissues in which conventional CD4 Trm are most well characterized are the lung and the skin, while relatively little is known about gut-specific Trm.
1. Lung
In mice, a subset of CD69Hi CD11aHi (LFA-1) CD4 memory T cells bearing transgenic TCRs specific for influenza H1 hemaglutinin were recently demonstrated to remain in the lung following resolution of H1N1 PR8 influenza infection.155 These cells preferentially trafficked to the lung when adoptively transferred to naïve mice and did not migrate during parabiosis experiments up to 3 weeks in duration. These cells were classified as lung-tissue memory cells and exhibited enhanced proliferative and functional antiviral responses compared to circulating spleen-derived CD4 memory cells. A recent study comparing Trm cells in patient-derived human skin and lung-tissue biopsies demonstrated a robust Trm population in both tissues. Both shared surface expression patterns of Tem cells; however, VLA-1 was preferentially expressed in lung Trm but not skin cells.150 The lung cells did not express the T-cell skin-homing marker CLA or intestinal-homing marker α4β7, suggesting that VLA-1 may be a marker for human lung Trm.148,149,157,158 When stimulated ex vivo by microbeads coated with α-CD2, α-CD3, and α-CD28, the lung Trm cells were capable of triple cytokine production of IFN-γ, IL-2, and TNF-α. Smaller fractions of the lung Trm were capable of producing IL-17, IL-4, or IL-17.
2. Skin
The CD4 Trcm in the initial study by Kaede et al. were defined as CD4+/CD44Hi/CCR7Int/CD62LInt/ CD69−/ CD103(+/−)/E-selectin ligand+. Bromely et al.,demonstrated two distinct CD4 memory cell populations within the skin, while exit of Trcm from the skin was CCR7-dependent, a Trm (CD4+/CD69+/ CD103+/CCR7−) population did not exit the skin.138 They noted that some CD4+ cells entering the draining lymph node were CD103+; however, these cells were also CD69−, suggesting that the combination of CCR7−/CD103+/CD69+ were necessary for indefinite retention within the skin compartment. Interestingly, the Trcm population maintained homing markers for both lymphoid tissue (CD62L/CCR7) and skin homing markers (CCR4 and E-selectin ligands). Upon ex vivo stimulation with α-CD3 and α-CD28, the Trcm produced IL-2 and up-regulated CD40L in vitro, but did not express cytokines IFN-γ or IL-10. The proliferative capacity was not reported, and additional studies using antigen-specific cells would improve the characterization of this newly reported memory population.
In contrast to Trm, a recently characterized subset of memory CD4 T cells within the skin-resident pool is also capable of actively exiting the skin and trafficking to lymph nodes or inflamed skin.139 It has been suggested that this subset be non-exclusively classified as recirculating memory T cells (Trcm).138,139 It is possible that, as the Trm pool characterization progresses, the Trcm classification may serve to distinguish this subset from a CD8-like CD4 Trm population (CD62L−/CCR7−/ CD103Hi), which enters but does not exit the skin, and from the CD4 Tem population (CD62L−/CCR7−/CD103−), which enters but is not retained in the skin.138,139 The categorization of CD4 T-cell memory by functionally and migrationally defined subsets greatly facilitates investigation and discussion. These memory divisions are not rigid, and pathways likely exist for transition between divisions.147 We expect that the divisions will continue to be modified as new memory populations, such as the previously described bone-marrow-homing memory CD4 T cells, are more fully characterized.125,132
V. SECONDARY CD4 T-CELL RESPONSES
Competent memory CD4 T cells produce protective secondary CD4 T-cell responses, which are more rapid and efficient but no less influential than primary responses. Secondary CD4 T cells can exhibit rapid cytokine production to help recruit and orchestrate threat-specific responses. Some memory CD4 T-cell subsets accelerate and enhance CD8 T-cell responses, while others facilitate rapid B-cell responses, and under some conditions, the secondary CD4 T cells may even function via direct cytolytic pathways.159 In contrast to the prolonged interaction required for naïve CD4 T-cell activation, memory-cell TCR-p:MHCII interactions are rapid and efficient during a secondary encounter. A growing body of evidence suggests that the strength and type of secondary challenge affects the generation and maintenance of CD4 T cells in a similar capacity to the primary challenge. A strong primary response followed by a strong secondary response appears to yield the greatest net increase in memory cell numbers, functional avidity, and longevity.116 It has also been demonstrated that the frequency and nature of subsequent secondary challenges may further shift the memory-cell phenotype from central memory to effector memory. Mueller et al. reviewed several studies summarizing how the quick removal of antigen during infection, or administration of single-dose peptide vaccination, tends to elicit a Tcm phenotype, while multiple repeated infections and prime-boost protocols for peptide vaccination elicit a progressive phenotypic shift to Tem with each subsequent exposure.87
Ravkov and Williams reported that the magnitude of the secondary response is strongly influenced by the strength and length of secondary challenge, with shorter duration of antigen exposure and lower levels of inflammation resulting in attenuated CD4 T-cell responses.160 They found that rapid pathogen clearance by cytotoxic CD8 T cells impaired the ability of the CD4 cells to mount a secondary response.160 Their data suggested that some memory CD4 T cells may need a duration of antigen exposure in excess of 48 hours to mount an optimal Th1 secondary response. Kim et al., subsequently reported that recovered mice previously infected with a strain of LCMV Armstrong did not mount a significant secondary response when rechallenged with LCMV Armstrong, but it did mount a robust secondary response to Listeria monocytogenes genetically modified to express the LCMV peptide GP61-80 (Lm-gp61).116 They found a similar but less robust augmentation of secondary expansion following primary infection with LM-gp61 and rechallenge with LCMV Armstrong. They also found that the robust secondary response to heterologous challenge subsequently generated memory cells with higher TCR affinity and greater turnover rates (assessed by BRDU incorporation at 75 and 200 days) compared to primary responses and homologous rechallenge. There were no differences between groups with regard to expression levels of CD122 or CD127, or Bcl-2. They also reported that heterologous challenge resulted in significantly greater secondary effector and secondary memory cell generation and survival in the target organs, particularly the liver. However, despite the strong secondary response, the size of the memory Th1 cell population generated by heterologous rechallenge did continue to decline, though less so than the homologous challenge. It is important to point out that the mice exposed to the homologous challenge were adequately protected, and a costly (energetic) secondary challenge was not needed; however, the quantity and quality of secondary memory cells did decline over time. Further, in both primary and secondary responses, the Th1 memory cells progressively became more sensitive to antigen stimulation. The implications for these findings are not clear. As vaccinologists commonly employ strategies using separate immunizing agents in prime and boost vaccines, further work with heterologous challenge models is necessary to fully elucidate the mechanisms underlying these divergent responses and provide a rational basis for future vaccine design.
VI. CONCLUSIONS AND FUTURE DIRECTIONS
It is well established that CD4 T cells orchestrate several key aspects of the humoral and cell-mediated immune response in a multitude of ways. CD4 T cells coordinate the interplay of innate immune cells, humoral immunity, and cytotoxicity such that the contribution of each component is tailored to a specific threat. This orchestration is critical as the potential threats are diverse; the most effective type of initial response is tailored to the threat and the type of memory that will be protective is likely to also be threat dependent. The importance of CD4 T cells in immune defense is graphically illustrated by the profound immune deficiencies seen in human patients with HIV/AIDS as well as SIV and related viral infections in nonhuman primates, and the need to understand CD4 T cell responses cannot be overstated. Immunologists have made significant strides in unraveling the complexities of the CD4 T-cell response. More recently, this has been particularly true with the advancement of our understanding of the differentiation of distinct CD4 T-cell subsets in response to a spectrum of infectious insults. Still, the molecular and cellular basis of CD4 T-cell memory is poorly understood, and its study is complicated by the presence of numerous functionally distinct subsets of effectors that might differ in their phenotype, genetic stability, half-life, maintenance requirements, and trafficking patterns, as well as their intrinsic abilities to differentiate into memory cells. It remains unclear whether all subsets of effector CD4 T cells differentiate into memory, and whether those that do become memory retain their lineage-defining differentiation signatures after their transition to memory. We expect that developments such as the recent identification and characterization of CD4 memory precursor cells in Th1 and Tfh lineages will facilitate further investigation in this regard. It also remains to be determined to what degree various memory CD4 T cells maintain lineage fidelity between primary and secondary responses. Other important but less understood aspects of CD4 T-cell memory are the differentiation pathways of central, effector, resident, and recirculating memory cells, and the importance of CD4 T-cell memory in the maintenance of memory B cells. The development of murine models using multiple concurrent gene-expression reporter constructs could provide insights into these relationships.
Due to the sheer breadth of the CD4 T-cell memory field, the focus of this review was, by necessity, largely limited to infectious agents generating Th1-like and complementary Tfh and Treg responses. Numerous investigations reviewed elsewhere have also examined the role of CD4 T cells, particularly Treg cells, in tumor immunology and immune-mediated diseases.5 More broadly, the role of memory CD4 T cells in diverse conditions such as immunity to helminth infection,161 the progression of asthma,162 and immune-mediated diseases such as psoriasis have been investigated;163 however, the role of cross- and auto-reactive memory CD4 T cells in immune defense and immunopathology generally remains understudied. The field of CD4 T-cell memory is an exciting area of research, and it is expected that emerging technologies will lead to a better understanding of the physiology of CD4 T-cell memory. In turn, new research is expected to foster the development of novel vaccine approaches to elicit and maintain durable and effective CD4 T-cell memory, as well as identify targets for interventional immuotherapeutics.
ACKNOWLEDGMENTS
The authors would like to acknowledge Eui Ho Kim and Erin Plisch in the Suresh Molecular Immunology Laboratory for their helpful discussions of CD4 T-cell memory. Work was supported by PHS grants AI048785 and AI101976 to MS. DJG was supported by the NIH Institutional Training Grant T32OD010423, and MMT was supported by the NIH Virology Training Grant T32AI078985.
ABBREVIATIONS
- Ab
antibody
- Ag
antigen
- IFN-γ
interferon gamma
- IL-
interleukin
- KO
knock out
- Tcm
central memory T cell
- Tem
effector memory T cell
- Tfh
T follicular helper cell
- Th
helper T cell
- Trcm
recirculating memory T cell
- Treg
T regulatory cell
- Trm
resident memory T cell
- WT
wild type
REFERENCES
- 1.McKinstry KK, Strutt TM, Swain SL. The potential of CD4 T-cell memory. Immunology. 2010 May;130(1):1–9. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nature Rev Immunol. 2012 Feb;12(2):136–48. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinstry KK, Dutton RW, Swain SL, Strutt TM. Memory CD4 T cell-mediated immunity against influenza A Virus: more than a little helpful. Archivum Immunologiae et Therapiae Experimentalis. 2013 May 25; doi: 10.1007/s00005-013-0236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy K, Travers P, Walport M, Janeway C. Janeway's immunobiology. 8th ed. xix. Garland Science; New York: 2012. p. 868. [Google Scholar]
- 5.Caserta S, Borger JG, Zamoyska R. Central and effector memory CD4 and CD8 T-cell responses to tumor-associated antigens. Critical Rev Immunology. 2012;32(2):97–126. doi: 10.1615/critrevimmunol.v32.i2.10. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996 Apr 5;272(5258):54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 7.Stockinger B, Kassiotis G, Bourgeois C. CD4 T-cell memory. Sem Immunol. 2004 Oct;16(5):295–303. doi: 10.1016/j.smim.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Tan C, Gery I. The unique features of Th9 cells and their products. Crit Rev Immunol. 2012;32(1):1–10. doi: 10.1615/critrevimmunol.v32.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Daniel V, Sadeghi M, Opelz G. Plasticity and overlap of in vitro-induced regulatory T-cell markers in healthy humans. Transplantation Proc. 2013 Jun;45(5):1816–21. doi: 10.1016/j.transproceed.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 10.Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nature Immunol. 2013 Apr;14(4):372–9. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannons JL, Lu KT, Schwartzberg PL. T follicular helper cell diversity and plasticity. Trends Immunol. 2013 May;34(5):200–7. doi: 10.1016/j.it.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamane H, Paul WE. Memory CD4+ T cells: fate determination, positive feedback and plasticity. Cell Molec Life Sci. 2012 May;69(10):1577–83. doi: 10.1007/s00018-012-0966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakayamada S, Takahashi H, Kanno Y, O'Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012 Jun;24(3):297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hori S. Regulatory T cell plasticity: beyond the controversies. Trends Immunol. 2011 Jul;32(7):295–300. doi: 10.1016/j.it.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Putheti P, Awasthi A, Popoola J, Gao W, Strom TB. Human CD4 memory T cells can become CD4+IL-9+ T cells. PloS One. 2010;5(1):e8706. doi: 10.1371/journal.pone.0008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLeod MK, Kappler JW, Marrack P. Memory CD4 T cells: generation, reactivation and reassignment. Immunology. 2010 May;130(1):10–5. doi: 10.1111/j.1365-2567.2010.03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009 Jun;21(3):281–5. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013 Apr 18;38(4):805–17. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia S, DiSanto J, Stockinger B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity. 1999 Aug;11(2):163–71. doi: 10.1016/s1074-7613(00)80091-6. [DOI] [PubMed] [Google Scholar]
- 20.Jelley-Gibbs DM, Lepak NM, Yen M, Swain SL. Two distinct stages in the transition from naive CD4 T cells to effectors, early antigen-dependent and late cytokine-driven expansion and differentiation. J Immunology. 2000 Nov 1;165(9):5017–26. doi: 10.4049/jimmunol.165.9.5017. [DOI] [PubMed] [Google Scholar]
- 21.Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003 Mar 17;197(6):751–62. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nature Immunol. 2009 Apr;10(4):375–84. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013 Sep 25; doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nature Med. 2001 Aug;7(8):913–9. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 25.Topham DJ, Doherty PC. Longitudinal analysis of the acute Sendai virus-specific CD4+ T cell response and memory. J Immunology. 1998 Nov 1;161(9):4530–5. [PubMed] [Google Scholar]
- 26.McKinstry KK, Strutt TM, Swain SL. Regulation of CD4+ T-cell contraction during pathogen challenge. Immunological Rev. 2010 Jul;236:110–24. doi: 10.1111/j.1600-065X.2010.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez AM, Zhu J, Huang X, Yang Y. The development and function of memory regulatory T cells after acute viral infections. J Immunology. 2012 Sep 15;189(6):2805–14. doi: 10.4049/jimmunol.1200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brincks EL, Roberts AD, Cookenham T, Sell S, Kohlmeier JE, Blackman MA, Woodland DL. Antigen-specific memory regulatory CD4+Foxp3+ T cells control memory responses to influenza virus infection. J Immunol. 2013 Apr 1;190(7):3438–46. doi: 10.4049/jimmunol.1203140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999 Oct 1;99(1):23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 30.Vander Lugt B, Tubo NJ, Nizza ST, Boes M, Malissen B, Fuhlbrigge RC, Kupper TS, Campbell JJ. CCR7 plays no appreciable role in trafficking of central memory cd4 T cells to lymph nodes. J Immunology. 2013 Aug 9; doi: 10.4049/jimmunol.1200938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caucheteux SM, Torabi-Parizi P, Paul WE. Analysis of naive lung CD4 T cells provides evidence of functional lung to lymph node migration. Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):1821–6. doi: 10.1073/pnas.1221306110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Ann Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 33.Kwok WW, Tan V, Gillette L, Littell CT, Soltis MA, LaFond RB, Yang J, James EA, DeLong JH. Frequency of epitope-specific naive CD4(+) T cells correlates with immunodominance in the human memory repertoire. J Immunol. 2012 Mar 15;188(6):2537–44. doi: 10.4049/jimmunol.1102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med. 2009 Jul 6;206(7):1525–34. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013 May 9;153(4):785–96. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative selection and peptide chemistry determine the size of naive foreign peptide-MHC class II-specific CD4+ T cell populations. J Immunol. 2010 Oct 15;185(8):4705–13. doi: 10.4049/jimmunol.1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2012 May 1;188(9):4135–40. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins MK, Moon JJ. Response to comment on The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2013 Mar 1;190(5):1896. doi: 10.4049/jimmunol.1290080. [DOI] [PubMed] [Google Scholar]
- 39.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007 Aug;27(2):203–13. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitmire JK, Benning N, Whitton JL. Precursor frequency, nonlinear proliferation, and functional maturation of virus-specific CD4+ T cells. J Immunol. 2006 Mar 1;176(5):3028–36. doi: 10.4049/jimmunol.176.5.3028. [DOI] [PubMed] [Google Scholar]
- 41.van Heijst JW, Gerlach C, Swart E, Sie D, Nunes-Alves C, Kerkhoven RM, Arens R, Correia-Neves M, Schepers K, Schumacher TN. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient. Science. 2009 Sep 4;325(5945):1265–9. doi: 10.1126/science.1175455. [DOI] [PubMed] [Google Scholar]
- 42.van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T Cells. Current Opinion Immunol. 2009 Apr;21(2):167–72. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celli S, Lemaitre F, Bousso P. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 2007 Oct;27(4):625–34. doi: 10.1016/j.immuni.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Powell TJ, Brown DM, Hollenbaugh JA, Charbonneau T, Kemp RA, Swain SL, Dutton RW. CD8+ T cells responding to influenza infection reach and persist at higher numbers than CD4+ T cells independently of precursor frequency. Clinical Immunol. 2004 Oct;113(1):89–100. doi: 10.1016/j.clim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Whitmire JK, Murali-Krishna K, Altman J, Ahmed R. Antiviral CD4 and CD8 T-cell memory: differences in the size of the response and activation requirements. Philosophical Trans Royal Soc London Series B, Biological Sciences. 2000 Mar 29;355(1395):373–9. doi: 10.1098/rstb.2000.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, Kaech SM. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011 Oct 28;35(4):633–46. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Lymphoid reservoirs of antigen-specific memory T helper cells. Nature Immunol. 2007 Jul;8(7):753–61. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- 48.Kim C, Wilson T, Fischer KF, Williams MA. Sustained interactions between T cell receptors and antigens promote the differentiation of CD4(+) memory T cells. Immunity. 2013 Sep 19;39(3):508–20. doi: 10.1016/j.immuni.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008 Apr;28(4):533–45. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naive murine CD4+ T cells. J Immunol. 1999 Aug 15;163(4):1817–26. [PubMed] [Google Scholar]
- 51.Lanzavecchia A, Lezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999 Jan 8;96(1):1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 52.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nature Rev Immunol. 2003 Dec;3(12):939–51. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 53.Riou C, Yassine-Diab B, Van grevenynghe J, Somogyi R, Greller LD, Gagnon D, Gimmig S, Wilkinson P, Shi Y, Cameron MJ, Campos-Gonzalez R, Balderas RS, Kelvin D, Sekaly RP, Haddad EK. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007 Jan 22;204(1):79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Umeshappa CS, Xiang J. Regulators of T-cell memory generation: TCR signals versus CD4+ help? Immunol Cell Biol. 2011 Jul;89(5):578–80. doi: 10.1038/icb.2011.28. [DOI] [PubMed] [Google Scholar]
- 55.Baumgartner CK, Yagita H, Malherbe LP. A TCR affinity threshold regulates memory CD4 T cell differentiation following vaccination. J Immunology. 2012 Sep 1;189(5):2309–17. doi: 10.4049/jimmunol.1200453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005 May 16;201(10):1555–65. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004 Nov;21(5):669–79. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nature Rev Immunology. 2002 Dec;2(12):982–7. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 59.Kim C, Williams MA. Nature and nurture: T-cell receptor-dependent and T-cell receptor-independent differentiation cues in the selection of the memory T-cell pool. Immunology. 2010 Nov;131(3):310–7. doi: 10.1111/j.1365-2567.2010.03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber KS, Li QJ, Persaud SP, Campbell JD, Davis MM, Allen PM. Distinct CD4+ helper T cells involved in primary and secondary responses to infection. Proc Natl Acad Sciences U S A. 2012 Jun 12;109(24):9511–6. doi: 10.1073/pnas.1202408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009 May;229(1):152–72. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995 Dec 7;378(6557):617–20. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 63.Koguchi Y, Buenafe AC, Thauland TJ, Gardell JL, Bivins-Smith ER, Jacoby DB, Slifka MK, Parker DC. Preformed CD40L is stored in Th1, Th2, Th17, and T follicular helper cells as well as CD4+ 8- thymocytes and invariant NKT cells but not in Treg cells. PloS One. 2012;7(2):e31296. doi: 10.1371/journal.pone.0031296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pagan AJ, Pepper M, Chu HH, Green JM, Jenkins MK. CD28 promotes CD4+ T cell clonal expansion during infection independently of its YMNM and PYAP motifs. J Immunol. 2012 Sep 15;189(6):2909–17. doi: 10.4049/jimmunol.1103231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995 Jul;3(1):87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 66.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003 Jul 1;171(1):166–74. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 67.Oestreich KJ, Weinmann AS. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nature Rev Immunol. 2012 Nov;12(11):799–804. doi: 10.1038/nri3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011 Oct 28;35(4):583–95. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sethi A, Kulkarni N, Sonar S, Lal G. Role of miRNAs in CD4 T cell plasticity during inflammation and tolerance. Frontiers Genet. 2013;4:8. doi: 10.3389/fgene.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009 Aug;39(8):2076–82. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 71.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013 Jan 24;38(1):13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998 Aug;9(2):229–37. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 73.Firpo EJ, Koff A, Solomon MJ, Roberts JM. Inactivation of a Cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Molec Cell Biol. 1994 Jul;14(7):4889–901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gesbert F, Moreau JL, Theze J. IL-2 responsiveness of CD4 and CD8 lymphocytes: further investigations with human IL-2Rbeta transgenic mice. Int Immunol. 2005 Aug;17(8):1093–102. doi: 10.1093/intimm/dxh289. [DOI] [PubMed] [Google Scholar]
- 75.Malek TR, Yu A, Scibelli P, Lichtenheld MG, Codias EK. Broad programming by IL-2 receptor signaling for extended growth to multiple cytokines and functional maturation of antigen-activated T cells. J Immunol. 2001 Feb 1;166(3):1675–83. doi: 10.4049/jimmunol.166.3.1675. [DOI] [PubMed] [Google Scholar]
- 76.Dooms H, Kahn E, Knoechel B, Abbas AK. IL-2 induces a competitive survival advantage in T lymphocytes. J Immunol. 2004 May 15;172(10):5973–9. doi: 10.4049/jimmunol.172.10.5973. [DOI] [PubMed] [Google Scholar]
- 77.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007 Mar 19;204(3):547–57. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011 Jun 24;34(6):932–46. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nature Immunol. 2011 Jun;12(6):467–71. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009 Feb 15;182(4):1868–76. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swain SL, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley LM. From naive to memory T cells. Immunol Rev. 1996 Apr;150:143–67. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 82.Kamperschroer C, Quinn DG. Quantification of epitope-specific MHC class-II-restricted T cells following lymphocytic choriomeningitis virus infection. Cell Immunol. 1999 May 1;193(2):134–46. doi: 10.1006/cimm.1999.1458. [DOI] [PubMed] [Google Scholar]
- 83.Jay DC, Mitchell DM, Williams MA. Bim mediates the elimination of functionally unfit Th1 responders from the memory pool. PloS One. 2013;8(6):e67363. doi: 10.1371/journal.pone.0067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van VQ, Baba N, Rubio M, Wakahara K, Panzini B, Richard C, Soucy G, Franchimont D, Fortin G, Torres AC, Cabon L, Susin S, Sarfati M. CD47(low) status on CD4 effectors is necessary for the contraction/resolution of the immune response in humans and mice. PloS One. 2012;7(8):e41972. doi: 10.1371/journal.pone.0041972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tripathi P, Mitchell TC, Finkelman F, Hildeman DA. Cutting Edge: Limiting amounts of IL-7 do not control contraction of CD4+ T cell responses. J Immunol. 2007 Apr 1;178(7):4027–31. doi: 10.4049/jimmunol.178.7.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Youngblood B, Hale JS, Ahmed R. T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology. 2013 Jul;139(3):277–84. doi: 10.1111/imm.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annual Rev Immunol. 2013;31:137–61. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 88.Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG, Anderson SM, Wei L, Sun H, O'Shea JJ, Schwartzberg PL. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011 Oct 28;35(4):622–32. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Swain SL. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity. 1994 Oct;1(7):543–52. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 90.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999 Nov 12;286(5443):1381–3. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 91.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009 Dec 18;31(6):859–71. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moulton VR, Bushar ND, Leeser DB, Patke DS, Farber DL. Divergent generation of heterogeneous memory CD4 T cells. J Immunol. 2006 Jul 15;177(2):869–76. doi: 10.4049/jimmunol.177.2.869. [DOI] [PubMed] [Google Scholar]
- 93.Lohning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Hofer T, Radbruch A, Zinkernagel RM, Hengartner H. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med. 2008 Jan 21;205(1):53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nature Rev Immunol. 2002 Apr;2(4):251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 95.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008 Mar 20;452(7185):356–60. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 96.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature Immunol. 2010 Jan;11(1):83–9. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang JT. Polarity and lymphocyte fate determination. Curr Opin Cell Biol. 2012 Aug;24(4):526–33. doi: 10.1016/j.ceb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oliaro J, Van Ham V, Sacirbegovic F, Pasam A, Bomzon Z, Pham K, Ludford-Menting MJ, Waterhouse NJ, Bots M, Hawkins ED, Watt SV, Cluse LA, Clarke CJ, Izon DJ, Chang JT, Thompson N, Gu M, Johnstone RW, Smyth MJ, Humbert PO, Reiner SL, Russell SM. Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J Immunol. 2010 Jul 1;185(1):367–75. doi: 10.4049/jimmunol.0903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.King CG, Koehli S, Hausmann B, Schmaler M, Zehn D, Palmer E. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity. 2012 Oct 19;37(4):709–20. doi: 10.1016/j.immuni.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bocharov G, Luzyanina T, Cupovic J, Ludewig B. Asymmetry of cell division in CFSE-based lymphocyte proliferation analysis. Frontiers Immunol. 2013;4:264. doi: 10.3389/fimmu.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 2003 Dec;4(12):1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 102.Luthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, Nutt SL. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nature Immunol. 2012 May;13(5):491–8. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 103.Youngblood B, Hale JS, Akondy R. Using epigenetics to define vaccine-induced memory T cells. Curr Opin Virol. 2013 Jun;3(3):371–6. doi: 10.1016/j.coviro.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O'Shea JJ, Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009 Jan 16;30(1):155–67. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nature Rev Immunol. 2009 Feb;9(2):91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 106.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nature Immunol. 2002 Jul;3(7):643–51. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 107.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012 Nov 16;37(5):785–99. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 108.Liu X, Nurieva RI, Dong C. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunol Rev. 2013 Mar;252(1):139–45. doi: 10.1111/imr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Podshivalova K, Salomon DR. MicroRNA regulation of T-lymphocyte immunity: modulation of molecular networks responsible for T-cell activation, differentiation, and development. Crit Rev Immunol. 2013;33(5):435–76. doi: 10.1615/critrevimmunol.2013006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmidl C, Hansmann L, Andreesen R, Edinger M, Hoffmann P, Rehli M. Epigenetic reprogramming of the RORC locus during in vitro expansion is a distinctive feature of human memory but not naive Treg. Eur J Immunol. 2011 May;41(5):1491–8. doi: 10.1002/eji.201041067. [DOI] [PubMed] [Google Scholar]
- 111.He H, Ni B, Tian Y, Tian Z, Chen Y, Liu Z, Yang X, Lv Y, Zhang Y. Histone methylation mediates plasticity of human FOXP3 Treg cells by modulating signature gene expressions. Immunology. 2013 Oct 24; doi: 10.1111/imm.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009 Oct 29;114(18):3727–35. doi: 10.1182/blood-2009-05-219584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gratz IK, Rosenblum MD, Abbas AK. The life of regulatory T cells. Ann N Y Acad Sci. 2013 Apr;1283:8–12. doi: 10.1111/nyas.12011. [DOI] [PubMed] [Google Scholar]
- 114.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci U S A. 2004 Jun 22;101(25):9357–62. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008 Dec 19;29(6):848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 116.Kim C, Jay DC, Williams MA. Stability and function of secondary Th1 memory cells are dependent on the nature of the secondary stimulus. J Immunol. 2012 Sep 1;189(5):2348–55. doi: 10.4049/jimmunol.1200244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007 Apr 16;204(4):951–61. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003 Dec 15;198(12):1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nature Immunol. 2003 Jul;4(7):680–6. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 120.Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proc Natl Acad Sci U S A. 2010 Jan 12;107(2):827–31. doi: 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK, Rosenblum MD. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol. 2013 May 1;190(9):4483–7. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003 Dec 15;198(12):1807–15. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001 Jan 1;97(1):14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 124.Mazzucchelli RI, Warming S, Lawrence SM, Ishii M, Abshari M, Washington AV, Feigenbaum L, Warner AC, Sims DJ, Li WQ, Hixon JA, Gray DH, Rich BE, Morrow M, Anver MR, Cherry J, Naf D, Sternberg LR, McVicar DW, Farr AG, Germain RN, Rogers K, Jenkins NA, Copeland NG, Durum SK. Visualization and identification of IL-7 producing cells in reporter mice. PloS One. 2009;4(11):e7637. doi: 10.1371/journal.pone.0007637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009 May;30(5):721–30. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 126.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nature Immunol. 2007 Nov;8(11):1255–65. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 127.Withers DR, Gaspal FM, Mackley EC, Marriott CL, Ross EA, Desanti GE, Roberts NA, White AJ, Flores-Langarica A, McConnell FM, Anderson G, Lane PJ. Cutting edge: lymphoid tissue inducer cells maintain memory CD4 T cells within secondary lymphoid tissue. J Immunol. 2012 Sep 1;189(5):2094–8. doi: 10.4049/jimmunol.1201639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caserta S, Zamoyska R. Memories are made of this: synergy of T cell receptor and cytokine signals in CD4(+) central memory cell survival. Trends Immunol. 2007 Jun;28(6):245–8. doi: 10.1016/j.it.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 129.Jatzek A, Tejera MM, Singh A, Sullivan JA, Plisch EH, Suresh M. p27(Kip1) negatively regulates the magnitude and persistence of CD4 T cell memory. J Immunol. 2012 Dec 1;189(11):5119–28. doi: 10.4049/jimmunol.1201482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005 Jul;26(7):360–6. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 131.Shinoda K, Tokoyoda K, Hanazawa A, Hayashizaki K, Zehentmeier S, Hosokawa H, Iwamura C, Koseki H, Tumes DJ, Radbruch A, Nakayama T. Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc Natl Acad Sci U S A. 2012 May 8;109(19):7409–14. doi: 10.1073/pnas.1118539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hanazawa A, Lohning M, Radbruch A, Tokoyoda K. CD49b/CD69-dependent generation of resting t helper cell memory. Frontiers Immunol. 2013;4:183. doi: 10.3389/fimmu.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feuerer M, Beckhove P, Mahnke Y, Hommel M, Kyewski B, Hamann A, Umansky V, Schirrmacher V. Bone marrow microenvironment facilitating dendritic cell: CD4 T cell interactions and maintenance of CD4 memory. Int J Oncol. 2004 Oct;25(4):867–76. [PubMed] [Google Scholar]
- 134.Pabst R, Miyasaka M, Dudler L. Numbers and phenotype of lymphocytes emigrating from sheep bone marrow after in situ labelling with fluorescein isothiocyanate. Immunol. 1986 Oct;59(2):217–22. [PMC free article] [PubMed] [Google Scholar]
- 135.Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology. 2005 Sep 30;340(2):296–306. doi: 10.1016/j.virol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 136.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013 Jan;34(1):27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 137.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999 Oct 14;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 138.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013 Feb 1;190(3):970–6. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carbone FR, Mackay LK, Heath WR, Gebhardt T. Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Curr Opin Immunol. 2013 Jun;25(3):329–33. doi: 10.1016/j.coi.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 140.Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nature Immunol. 2013 Oct;14(10):978–85. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 141.Brinkman CC, Rouhani SJ, Srinivasan N, Engelhard VH. Peripheral tissue homing receptors enable T cell entry into lymph nodes and affect the anatomical distribution of memory cells. J Immunol. 2013 Sep 1;191(5):2412–25. doi: 10.4049/jimmunol.1300651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grindebacke H, Stenstad H, Quiding-Jarbrink M, Waldenstrom J, Adlerberth I, Wold AE, Rudin A. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J Immunol. 2009 Oct 1;183(7):4360–70. doi: 10.4049/jimmunol.0901091. [DOI] [PubMed] [Google Scholar]
- 143.Gehad A, Al-Banna NA, Vaci M, Issekutz AC, Mohan K, Latta M, Issekutz TB. Differing requirements for CCR4, E-selectin, and alpha4beta1 for the migration of memory CD4 and activated T cells to dermal inflammation. J Immunol. 2012 Jul 1;189(1):337–46. doi: 10.4049/jimmunol.1102315. [DOI] [PubMed] [Google Scholar]
- 144.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nature Immunol. 2010 Feb;11(2):114–20. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009 Aug 21;325(5943):1006–10. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012 Feb 13;209(2):243–50. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schwendemann J, Choi C, Schirrmacher V, Beckhove P. Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. J Immunol. 2005 Aug 1;175(3):1433–9. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- 148.Shin H, Iwasaki A. Tissue-resident memory T cells. Immunological Rev. 2013 Sep;255(1):165–81. doi: 10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008 Jan 11;319(5860):198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 150.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PloS One. 2011;6(1):e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2013 Sep 25; doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011 Sep 8;477(7363):216–9. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 153.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005 May 1;174(9):5444–55. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 154.Yang L, Yu Y, Kalwani M, Tseng TW, Baltimore D. Homeostatic cytokines orchestrate the segregation of CD4 and CD8 memory T-cell reservoirs in mice. Blood. 2011 Sep 15;118(11):3039–50. doi: 10.1182/blood-2011-04-349746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011 Dec 1;187(11):5510–4. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nature Immunol. 2013 Oct;14(10):1007–13. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997 Oct 30;389(6654):978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 158.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010 Mar 15;207(3):553–64. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McKinstry KK, Strutt TM, Kuang Y, Brown DM, Sell S, Dutton RW, Swain SL. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J Clin Invest. 2012 Aug 1;122(8):2847–56. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ravkov EV, Williams MA. The magnitude of CD4+ T cell recall responses is controlled by the duration of the secondary stimulus. J Immunol. 2009 Aug 15;183(4):2382–9. doi: 10.4049/jimmunol.0900319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zaph C, Rook KA, Goldschmidt M, Mohrs M, Scott P, Artis D. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol. 2006 Jul 1;177(1):511–8. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li Y, Hua S, He X, Li Y, Sun Y. The variation of CD4+ memory T cells in the development of asthma in mice (P6233). J Immunol. 2013 May;190(62):1. 2013. [Google Scholar]
- 163.Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clinical Rev Allergy Immunol. 2013 Apr;44(2):183–93. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]