Abstract
Biologic reactivity to orthopedic implant debris mediates long-term clinical performance of total joint arthroplasty implants. However, why some facets of implant debris are more pro-inflammatory remains controversial such as particle size, shape, base material etc. This precludes accurate prediction and optimal design of modern total joint replacements. We hypothesized that debris particle size can influence adsorbed protein film composition and affect subsequent bioreactivity. We measured size-dependent protein film-adsorption, and adsorbed protein film-dependent cytokine release using equal surface areas of different sized cobalt-chromium-alloy (CoCr-alloy) particle and in vitro challenge of human macrophages (THP-1 and human primary). Smaller 5μm vs 70μm sized particles preferentially adsorbed more serum protein in general (p<0.03), where higher molecular weight serum proteins consistent with IgG were identified. Additionally, 5μm CoCr-alloy particles pre-coated with different protein biofilms (IgG vs albumin) resulted in differential cytokine expression where albumin-coated particles induced more TNF-α and IgG-coated particles induced more IL-1β release from human monocyte/macrophages. In these preliminary in vitro studies we demonstrated the capability of equal surface areas of different particle sizes to influence adsorbed protein composition and that adsorbed protein differences on identical particles can translate into complex differences in bioreactivity. Together this suggests adsorbed protein differences on different sized particles of the same material may be a contributing mechanism by which different sized particles induce differences in reactivity.
Keywords: Cobalt Alloy, Debris, Particle, Adsorbed protein film, Macrophage, IgG, TNF, IL-1b
INTRODUCTION
Biological reactivity (inflammation, fibrous tissue formation, bone loss etc.) to implant debris has been well established to limit long-term implant performance. While metal-on-metal alternatives to traditional metal-on-polymer articulations in total joint replacements reduce the total amount of gravimetric wear, the degree of inflammation generated from debris of these newer designs may differ depending on the type and size of debris [1-4]. If these alternative bearing surfaces produce less gravimetric (volumetric or mass) wear but greater numbers (doses) of smaller particles, the benefits of reduced gravimetric wear may be nullified.
Previous studies have shown that biomaterial type, size and shape influence subsequent tissue bioreactivity [4-10]. While all implant debris induce some degree of biologic reactivity, there is consensus that the more relevant debris to inflammation is less than 10 μm in size, because it is readily phagocytosed leading to inflammatory reactions [11,12]. In addition, metallic debris may be more pro-inflammatory than polymeric debris [13], where macrophage activation and cytokine production have been used as proxies of in vivo inflammatory reactivity. Macrophage cytokines TNF-α, IL-6 and IL-1β are the hallmarks of in vivo inflammation implicated in implant loosening [13]. Recently particle size related reactivity, which has traditionally been reported as inversely proportional to particle size[4-10], has been revisited. New studies indicate that on a particle to particle basis smaller nanometer particles do not induce as much of a response as larger particles in the micron ranges [14]. Furthermore, studies on large vs small and smooth vs rough particles have shown that larger and rougher particles disrupt internal cell lysosomal compartments and thus cause more danger signaling (inflammation, IL-1β) than smaller or smoother particles [15]. However the relationship between particle size and biologic reactivity is complex and likely many faceted, where the interactions of cell-to-particle recognition, adhesion and phagocytosis play a role, as well.
Immediately upon contact, biomaterial surfaces are coated with serum proteins forming a proteinaceous film, referred to in this study as the adsorbed protein film. The composition of adsorbed proteins and subsequent cell-material interactions have been shown to be determined by the physicochemical properties of the biomaterial [16-20]. It is also important to point out that the process of protein adsorption onto the surface of biomaterials is a dynamic one. The biologic reactivity associated with metallic implant degradation can be affected by protein adsorbed protein films (surface and particle) as well as the underlying type of implant material [21,22].
There remains an incomplete understanding of how differences in particle size are translated into differences in reactivity and inflammation in vivo. Does particle size, alone, influence protein binding? We hypothesized that different sized particles of the same biomaterial will adsorb different serum proteins, and these different protein films will mediate subsequent inflammatory responses. We tested our hypothesis by analyzing differential serum protein adsorption on smaller (5μm) and larger (70μm) spherical CoCr-alloy particles and evaluated the differential in vitro reactivity of serum protein adsorption on phagocytosable CoCr alloy particles.
MATERIALS AND METHODS
Particle preparation for adsorbed protein film adsorption analysis
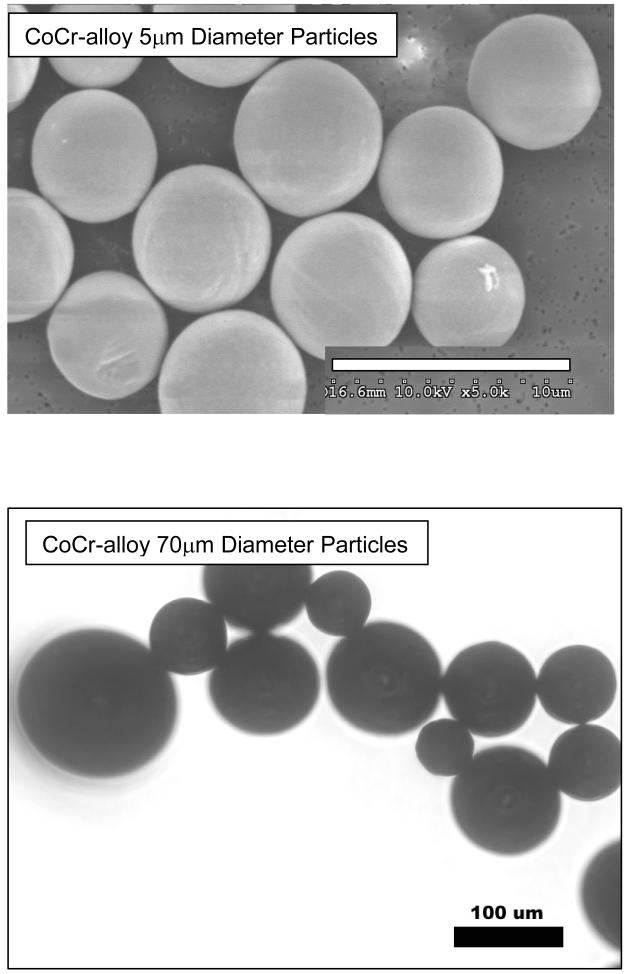
For adsorbed protein film analysis of adsorption kinetics on large and small particles, smooth spherical Cobalt-Chromium-Molybdenum-alloy (ASTM F-75) (CoCr-alloy) of an average mean size of 5μm and 70μm diameter particles (Starmet Corp.; Concord, MA) were used. >99% of 5μm particles were <10μm and >99% of 70μm particles were <100μm. Particle sizes were confirmed by scanning electron microscopy SEM, (Hitachi SN-5000), see Figure 1. For differential serum adsorption experimentation, differences in particle size were selected to maximize the chance of preliminary hypothesis testing on widely different sized particles with verifiably similar surface morphologies (smooth and spherical) while at the same time using large enough particles to accurately account for equal exposure areas to serum using weight calculations of particle number (i.e. the larger the smallest size particle the better). For protein adsorption, stock amounts of 5 μm and 70 μm particles were aliquoted to approximate equal surface areas of 600 cm2. For cell reactivity testing only phagocytosable CoCr-alloy 5μm diameter spherical particles were used to test the bioreactivity relevance of different adsorbed proteins. Particles were cleaned using a 3 step process: 1) metal-safe detergent (Alconox), 2) sonication in 70% ethanol for 1 hr followed by a 24 hour 70% ethanol soak at room temperature (25°C), and 3) a 24 hour soak in PyrocleanTM (a detergent for endotoxin removal). Each step was followed by triple washing with deionized water. Vacuum dried particles were autoclaved at the end of the cleansing process and tested to verify that they were Lipopolysaccharide (LPS) free using a quantitative limulus assay analysis, Kinetic QCL: <0.05Eu (Pyrogent 5000, Lonza).
Figure 1.
Scanning electron micrographs (SEM) of (a) 5μm Co-alloy spherical particles and (b) Transmission light microscopy images of 70μm diameter Co-alloy spherical particles, where light microscopy was used in real time to view particle migration and motion and confirm that particles were not stuck together.
Serum adsorbed protein film analysis
Human serum samples were obtained from peripheral blood obtained intravenously from n=6 healthy subject volunteers using Rush IRB approved informed consent (4 males and 2 females, ages 23–54). CoCr-alloy particles of 5μm and 70μm diameter were incubated in serum at 37°C for 7 days with continuous gentle side-to-side (see-saw) rocking, at 1Hz (Labquake). After 168 hours of incubation time to allow for steady state protein adsorption-desorption kinetics, i.e. Vroman effects [23,24], the serum protein-coated particles were transferred to a 50mL centrifuge tube and washed 3 times with sterile, cold PBS to remove loose non-adherent proteins. Greater than 95% of adsorbed protein film proteins were eluted from the alloy particles for analysis with 500=L 8M urea and 40mM Tris-HCl, as verified by secondary 2%SDS elution at 100°C and SDS-Page. The eluted proteins were then desalted with 1500μL cold acetone. The eluent was centrifuged at 13,500g and air-dried in a vacuum to ultimately form a protein pellet. An electrophoretic analysis of the adsorbed protein layer was then performed. 50μL of a 2% SDS sample buffer was added to each sample pellet. Pellets were analyzed using 10-20% polyacrylamide gels (SDS-PAGE; BioRad, Hercules, CA). 40μL of sample from each material surface and 7.2ng (BioRad) of calibration marker was loaded into 1-D polyacrylamide gels. PAGE gels were subsequently fixed and stained with GelCode (Pierce, Rockford, IL). Gel densitometry was conducted with a transparency adapted scanner (420oe, Periferal Dynamics Inc., Plymouth Meeting, PA) and Scion Image (PC-NIH Image; NIH, Bethesda MD) image processing software was used for determination of total and peak protein amounts.
To determine IgG levels, pellets were rehydrated with PBS and a 40mM chaps solution. 100 μL of sample was placed in a maxisorp plate in triplicate. Total IgG was determined using manufacturer’s instructions. Briefly, sample adsorption to the plate was allowed to take place over 72 hours on an orbital shaker at room temperature. Horse-radish peroxidase bound anti-IgG (R&D Systems) was then placed in each well and incubated for 2 hours after blocking with bovine albumin. Amounts of IgG were then measured with an Elisa plate reader at 450nm 20 minutes following TMB-substrate addition. Standards of IgG (R&D Systems) were also performed to determine approximate IgG concentrations of original serum samples. Original serum samples were also measured for amounts of IgG (ELISA) after being diluted 1:32 with PBS. IgG and total protein amounts were determined using a protein bioassay kit following manufacturer’s protocols (Pierce* BCA Protein Assay).
THP-1 macrophage cell line
A THP-1 (ATCC) human monocyte cell line was used for dose testing. Approximately 200,000 cells/well were plated in 48-well plates in 350μL DMEM medium/well, 10% AB serum. Cells were treated with a phorbol ester (12-O-tetradecanoylphorbol-13-acetate, TPA) (200nM final concentration) for 48h to stimulate differentiation into adherent macrophages. Cell supernatants were collected at 24 hours and assayed for TNF-α and IL-1β production as an indicator of bioreactivity (R&D Systems, Minneapolis, MN) following manufacturer’s protocols.
Primary Human Monocytes/Macrophages
Blood samples were obtained from healthy volunteers with Institutional Review Board approved, informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient and CD14+ monocytes were isolated from PBMCs by negative selection using magnetic bead antibody cocktail for CD3, CD7, CD16, CD19, CD56, CD123 and Glycophorin A (Miltenyi Biotec). Isolated human primary monocytes were assessed for >90% purity using FACS.
Isolated human monocytes from n=3 subjects were collected and isolated and allowed to differentiate into attached monocyte/macrophages (CD14+) for 48 hours (with human M-CSF at a working concentration of 50ng/mL). Cells were challenged with 5μm sized particles at doses of 1 particle/cell and 5 particles/cell. Monocytes/Macrophages were also challenged with protein coated particles, where CoCr-alloy 5μm spherical particles were incubated with different adsorbed proteins: human AB serum (Gembio Bioproducts), human albumin (Lee Biosolutions), IgG (Sigma), and fibronectin (American Diagnostica). These three proteins were selected as proxies of major components in human serum; 1) albumin represents a bulk less bioactive protein of serum protein, 2) immunoglobulins are key components of immuno-reactive serum protein and 3) fibronectin represents components of adhesion serum proteins [25]. Particles 5μm in size were incubated with 200ug of each protein in a 50 μL PBS solution for 48 hours at 37° C. Subsequently, particles were gently washed with sterile PBS to remove non-adherent protein then both differentiated human THP-1 macrophages and isolated primary human monocytes/macrophages were challenged with 5μm-sized particles at a concentration of 5 particles/cell . Supernatants were collected at 1 hour and 24 hours and Luminex multiplex assays were performed to determine secreted TNF-α, IL-6 and IL-1β (Millipore) production using manufacturer instructions.
All reactivity testing was conducted in triplicate. Statistical analysis between groups was performed for parametric data using one way ANOVA with Newman-Keuls Multiple comparison test of pairs at a significance level of p=0.05. And non-parametric data Kruskal-Wallis testing with post-test Dunn’s comparison of groups was performed at a p<0.05 confidence level (Graphpad Prism).
RESULTS
Adsorbed protein film analysis
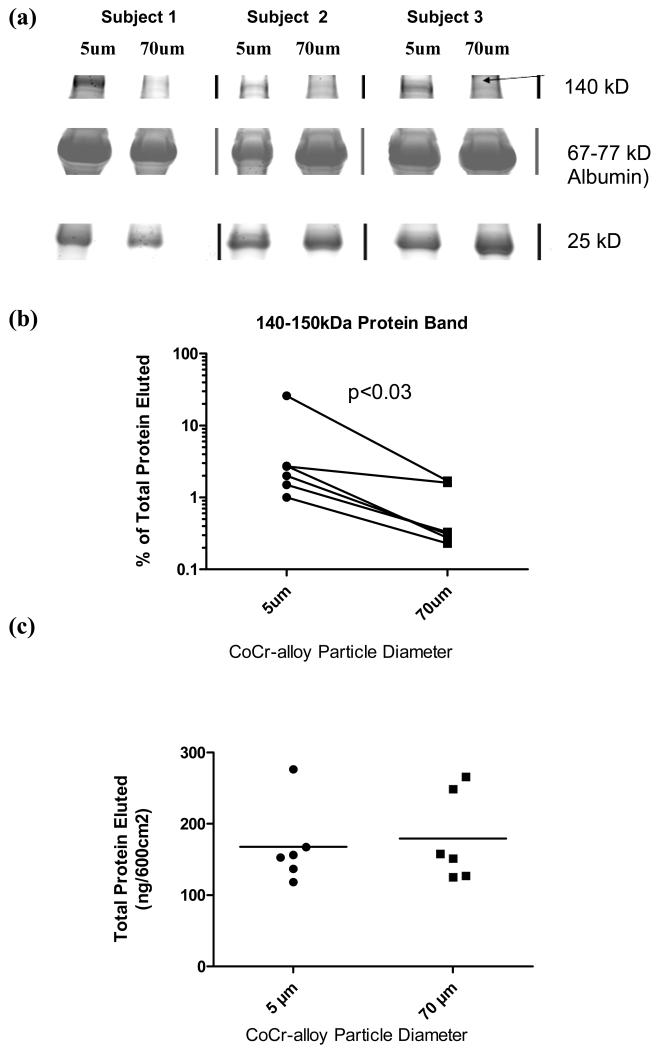
Adsorbed serum protein films demonstrated subject-dependent compositional differences on 5μm and 70μm diameter Co-Cr alloy particles. Electrophoresis results of protein elution from different size particles show that there were approximately 20 general ranges of proteins (prominent protein bands) detected on the surfaces of the metal alloys tested. Densitometric analysis of the 20 adsorbed protein bands revealed that among the 6 subjects tested, the only statistically significant difference in the intensity of protein(s) binding was within the range of approximately 150 kDa molecular weight (p < 0.05) when normalized to the total amount of protein adsorbed for each subject, Figure 2a and 2b. Data was normalized to total protein to account for differences in total protein in each individuals serum.
Figure 2.
Analysis of eluted serum proteins from n= 6 subjects adsorbed onto 5um and 70um Co-alloy particles demonstrated (a) an example of eluted serum proteins from 5um and 70um particles from 3 of 6 subjects, SDS-PAGE, showing a higher weight (140-150kD) serum protein preferentially bound by smaller-sized (5 μm) CoCr-alloy particles compared to other protein that did not vary. (b) There was a statistically decreased amount of 140-150 kD sized proteins on 70um sized CoCr-alloy particles compared to 5um (p<0.03), Wilcoxon matched pairs test for non-parametric data. (c) However, there was no significant difference in the total amount of adsorbed protein.
Total protein adsorption concentrations for 5μm and 70μm diameter CoCr-alloy particles were determined by electrophoresis using known protein standards for extrapolation. Equal surface areas of different particle sizes (5μm vs 70μm diameter) did not result in any statistically significant total adsorbed protein differences (Figure 2c).
Adsorbed Particle IgG analysis
Total serum IgG for each subject as determined by ELISA ranged from 378 μg/ml to 552 μg/ml with an average of 456±63 μg/mL. However, the total serum protein measurements did not vary significantly between subjects.
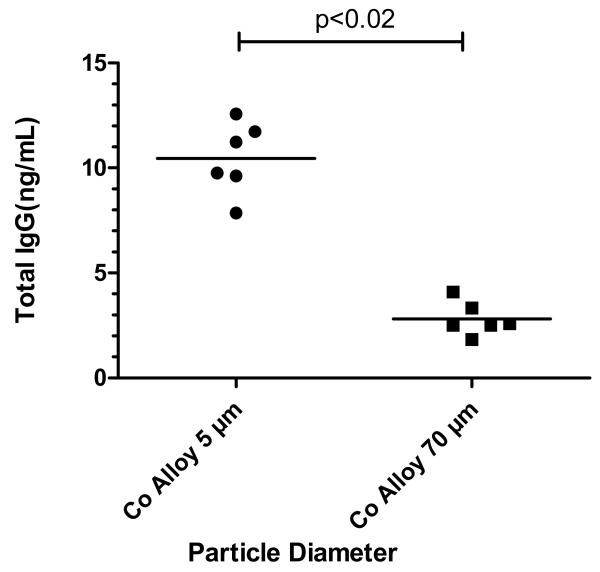
Differential adsorption of IgG onto 5μm and 70μm diameter spherical CoCr-alloy particles was measured after incubation in serum for 168 hours at 37°C as previously described. Our results show statistically significant amounts of IgG (p<0.02) adsorbed by the smaller size (5μm) CoCr-alloy particles compared to the large size (70μm) CoCr-alloy particles, Figure 3. Adsorption of IgG was approximately 4 times greater for the 5μm smaller sized particles compared to their 70μm large-sized particles. Our findings were qualitatively similar to the results obtained using densitometry of elecrophoresis gel results, which showed a higher amount of protein bound to smaller sized particles with respect to higher molecular weight ranges associated with IgG, p<0.05, Figure 2b vs Figure 3.
Figure 3.
Quantitative determination of total eluted IgG (ng/cm2) adsorbed onto CoCr-alloy beads of 70μm and 5μm diameter in size were measured with ELISA. These results were comparable to relative amounts of 150kD proteins shown in Fig 5, where 5μm beads preferentially adsorbed IgG, p<0.02 t-test, unpaired data.
THP-1 macrophage responses
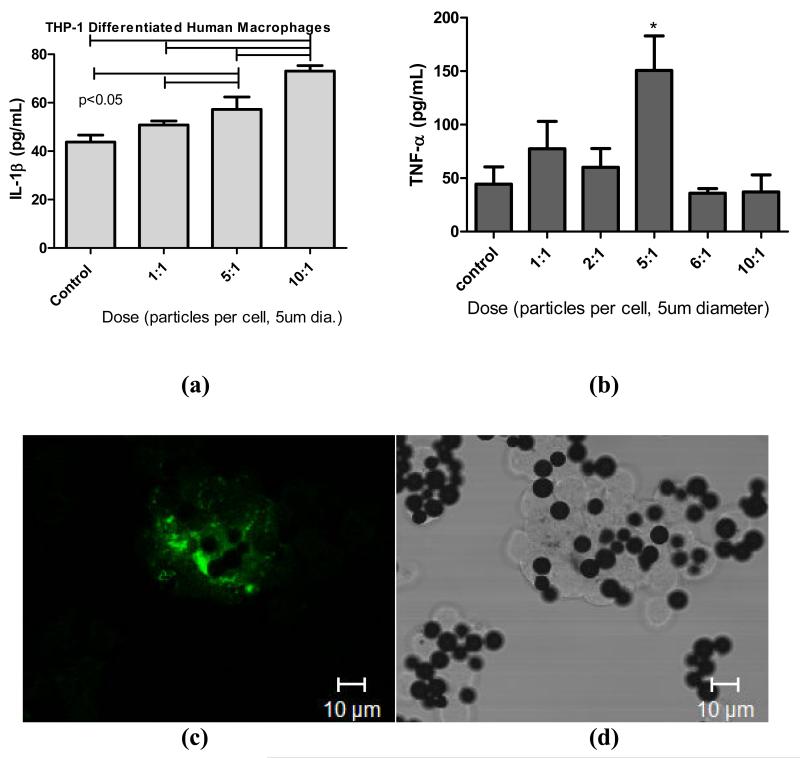
To determine the optimal particle dose for subsequent particle bioreactivity of adsorbed protein films we challenged human THP-1 macrophages with increasing concentration of particles for 24 hours using IL-1β and TNF-α as measures of inflammatory reactivity (Figure 4). There was a maximal IL-1β dose response to 5μm particles at 10 particles per cell (24 hours) and 5 particles per cell for TNF-α (1 hour). Confocal microscopy coupled with transmission light microscopy was used to determine that THP-1 macrophages were able to phagocytose particles as early as 1-2 hours post challenge. 5μm spheres demonstrated that both active phagocytosis and lysosome activity (fluorescently labeled OVA) were associated with CoCr-alloy phagocytosis at 1 hour time post challenge. These responses are limited to particle dose comparison and positive controls with LPS alone and LPS biofilm only compared using primary human cells.
Figure 4.
(a). IL-1b responses of differentiated THP-1 human macrophages over 24 hours when challenged with increasing particle dose of 5μm particles, where maximal stimulation is shown to occur at 10 particles/cell. Note p<0.001 where indicated by bars, one way ANOVA with Newman-Keuls Multiple comparison test of pairs. (b) TNF- α responses of differentiated THP-1 human macrophages at 1 hour post challenge with 5μm diameter particles, where maximal stimulation is shown to occur at 5 particles/cell. Note p<0.05 where indicated by bars, one way ANOVA with Newman-Keuls Multiple comparison test of pairs. (c) Fluorescent confocal microscopy and (d) transmission light microscopy of the same field of THP-1 macrophages after 2 hours of challenge with 5um sized spheres showing active phagocytosis and lysosome activation (fluorescently labeled OVA) associated with particle phagocytosis.
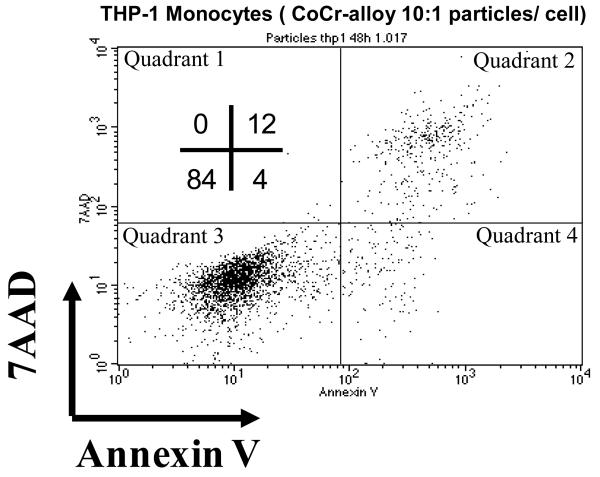
The viability of THP-1 macrophages was assessed using flow cytometry analysis of 7AAD (7-Aminoactinomycin D) and Annexin V. 7-AAD passes through disrupted cell membranes and stains DNA and is a measure of cell viability while Annexin V is used to detect cell surface phosphatidylserine which is expressed during apoptosis and other forms of cell death. A dose of 10 particles per cell decreased the viability of THP-1 macrophages to 84%, Figure 5. Thus a challenge concentration below 10 particles per cell was used for further testing of protein adsorption bioreactivity.
Figure 5.
THP-1 were 84% viable after treatment with CoCr alloy particles at a dose of 10:1 where apoptosis was determined by 7AAD uptake and Annexin V surface expression analyzed by FACS and expressed as percent of cells positive for either marker 48 hours after treatment. Data are representative results of one of three independent experiments.
Primary macrophages response
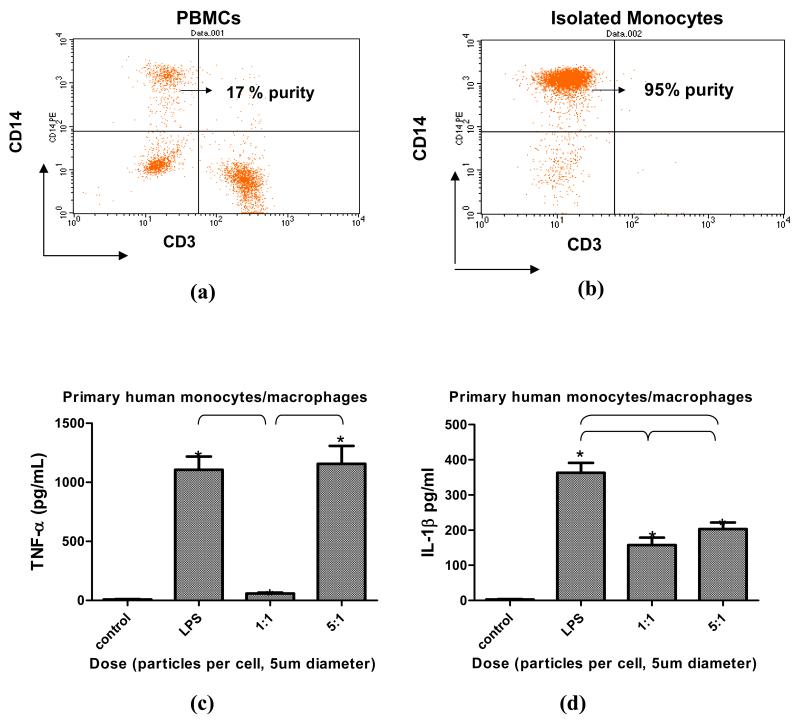
Primary human monocyte/macrophages (CD14+) TNF-α and IL-1β cytokine responses to 5μm CoCr-alloy particles were measured in n=3 subjects, Figure 6. The cytokine response of each subject varied in magnitude. However, the pattern of results was similar in that an increased dose of 5μm sized particles at 5 particles/cell produced a greater TNF-α and IL-1β than a dose of 1 particle per cell, p<0.05, Figure 6. Larger particles of 70um diameter were not tested in culture because the particle size was too large to enable phagocytosis by single cells in vitro.
Figure 6.
(a) Flow cytometry of Ficol separated human PBMCs labeled with CD14 and CD3 demonstrated 17% of primary human PBMCs were CD14+ monocytes. (b) After isolation using magnetic bead separation (AutoMacs, Milteni) the purity of CD14+ monocytes was increased to 95% and used for subsequent testing. (c) TNF-α and (d) IL-1β responses of isolated differentiated primary human monocyte/macrophages challenged with 5 μm sized particles after 1hr (TNF-α) and 24 hrs (IL-1β), (n=3 subjects). Maximal stimulation occurred at a dose of 5 particles/cell. Note p<0.05 where indicated by bars or by * indicating comparison to controls using one way ANOVA with Newman-Keuls Multiple comparison test of pairs.
Primary macrophages and reactivity to specific adsorbed protein films
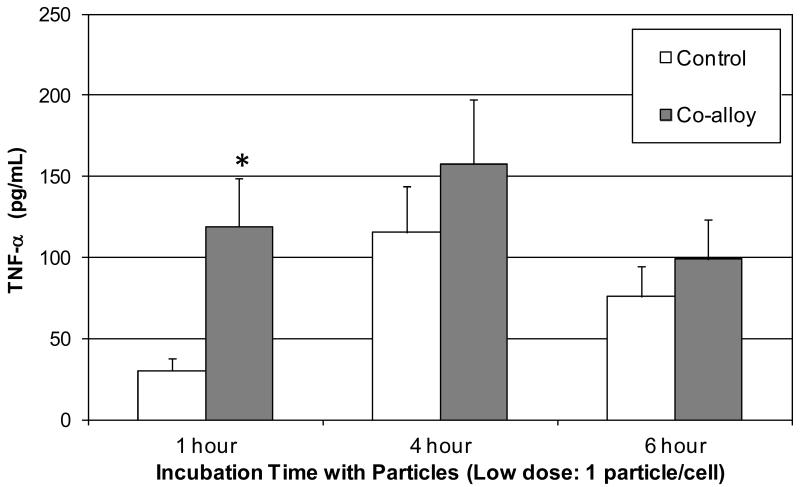
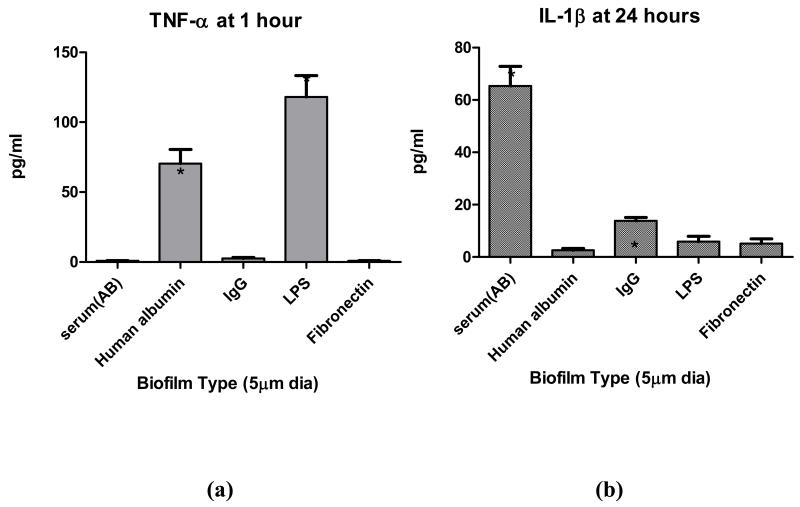
To determine if observed adsorbed protein film and reactivity differences can be produced by using a single size of CoCr-alloy particle (i.e. 5μm diameter particle) coated with different adsorbed protein films, i.e. AB serum, albumin, IgG, fibronectin or LPS (positive control) 5μm sized CoCr-alloy particles were incubated in these respective common human serum proteins for 48 hours and then used to challenge primary human monocytes/macrophages (CD14+, n=3 subjects). A time course was used to establish the most effective time to detect TNF-α released from primary (non-primed) human monocytes/macrophages in vitro, Figure 7. TNF-α and IL-1β release was assessed at 1 hour and 24 hours respectively at a dose of 5 particles/cell (5um dia). The greatest TNF-α response at 1 hour was to albumin and LPS particle films, Figure 8. At 24 hours the greatest IL-1β response was to particles with adsorbed human AB serum (i.e. a complex milieu of human serum proteins). While this implicates IgG in reactivity differences of different size, given that IgG adsorbed protein films induced significantly higher IL-1β than albumin, it also demonstrates the highest IL-1β response to particles was when coated with multiple serum proteins. These results of primary cells show that TNF-α and IL-1β released from primary human monocytes/macrophages can vary significantly with type of adsorbed protein film.
Figure 7.
Time Course: Human primary monocytes (n=3) dosed with a low concentration of particles (1 particle per cell) demonstrated maximal TNF-α response difference with untreated controls at 1 hour (5 μm diameter CoCr-alloy spherical particles). Note * = p<0.05 in comparison to all other groups using one way ANOVA with Newman-Keuls Multiple comparison test of pairs.
Figure 8.
Adsorbed protein film dependent differences in (a) TNF-α and (b) IL-1β responses of differentiated primary human monocyte/macrophages (n=3 subjects) using 5 μm diameter CoCr-alloy spherical particles. These preliminary results of adsorbed protein film differences were produced by incubation in different serum proteins for 48 hours. Serum albumin and LPS produced the greatest amount of TNF-α whereas AB human serum and IgG adsorbed protein film produced the greatest amount of IL-1β. Note * = p<0.05 in comparison to all other groups using one way ANOVA with Newman-Keuls Multiple comparison test of pairs.
DISCUSSION
In support of our original hypothesis, we found that particle size can influence protein binding characteristics and that different adsorbed proteins on the same type of particle can induce variable inflammatory responses (i.e. serum albumin adsorbed protein film increased TNF-α and IgG increased IL-1β). While compositional differences in the adsorbed protein films associated with polymeric and metal implant materials have been previously reported[26], this is the first report of experimentally determined bulk constitutive differences between the adsorbed serum proteins formed on differing sizes of a single material. Overall, total amounts of adsorbed serum protein on particles of different sizes (but same surface areas) did not vary between subjects (Figure 5a). Thus, surface properties affected by metal particle size are likely a significant determinant of adsorbed protein film composition. Factors related to the particle size and material such as surface chemistry, surface charge, and microstructure variation likely contribute to differences in the protein binding properties.
Differential amounts of IgG eluted from the adsorbed serum protein film of 5μm and 70μm CoCr-alloy particles further illustrate the influence of particle size on adsorbed protein film properties. Smaller 5μm sized particles bound larger proteins particularly in the W150kD range with significantly greater affinity than the larger particles. These differences may be due in part to changes in opsonized particle surface charge [27]. Gel electrophoresis and ELISA testing confirmed that particle size influences adsorption kinetics of specific proteins such as IgG and cell culture testing of IgG adsorbed protein film increased the reactivity of CoCr-alloy particles. It is likely that IgG proteins play a role in the in vivo reactivity of CoCr-alloy particles. Past investigations have shown that opsonization of particles with serum proteins (i.e.Immunoglobulins) compared to un-opsonized particles leads to an increase in inflammatory responses [21,28]; however, it remained unknown to what degree, if any, changes in the kinds of proteins in this layer effects subsequent bioreactivity of particulate implant debris.
Low dose particle challenge was used in an attempt to more closely model the subtle innate immune response that has been long established as the primary cause of implant inflammation and failure over the long term[29-31]. It is likely that if macrophages (and other cells) reacted to aseptic low dose particle challenge in vivo, with highly high amounts of inflammatory cytokines (e.g. >1000’s pg/mL TNF-α and IL-1β), then total joint replacement implants would not last very long (<1yr) without causing extreme inflammation. In this investigation we attempted to determine if (in a simplified model) particle size affects biofilm composition and if that composition change can translate into differences in reactivity at lower doses. Our preliminary evidence shows that particle size differences can affect human serum protein adsorption kinetics and that altering this protein “biofilm” has the potential to change the bioreactivity of particulate debris, but further study is required to determine if this is important clinically. However there are several limitations and caveats to these findings. First, the particles used in this study were made of implant grade alloy, but were larger than typical metal implant debris. Metal and ceramic particles have generally been characterized as an order of magnitude smaller than polymer particles (at approximately <0.05um in diameter, i.e. in the nanometer range)[32-35]. But some CoCr-alloy corrosion products (e.g. chromium-phosphate ) range in size from sub-micron to aggregates of particles up to 500 micrometers [36,37]. Additionally the particles used in this investigation were cleaned with acid (endotoxin cleaning) and thus were fully surface-oxidized. It is unclear to what degree particles produced by wear and corrosion in vivo are oxidized prior to protein adsorption, if at all. While SEM-EDS analysis did not indicate any demonstrable chemical differences in alloy make-up between the large and small particles, it is possible that chemical/microstructure differences in the CoCr-alloy particles of different sizes were present and mediate protein adsorption. Thus the clinical applicability of our findings have yet to be established and further work must be conducted to determine if the same phenomenon takes place in vivo and with particles in the submicron and nanometer size ranges. However, at smaller size ranges controlling for surface oxidation, shape and surface roughness is technically challenging and may be experimentally prohibitive.
In this study we demonstrated that adsorbed serum proteins can be dependent on particle size and that differences in adsorbed protein films can produce differences in macrophage inflammatory reactivity. The exact mechanism(s) by which this size-related adsorption phenomena occur are unknown at present, but are likely due to changes in surface charge inherent to different sized particles that have been previously shown to play a role in protein film adsorption kinetics [23,38]. CoCr-alloy particle opsonization with IgG produced more IL-1β and albumin opsonization produced more TNF-α, respectively (p<0.05). This suggests there may be a complex relationship between adsorbed proteins and resultant inflammatory responses that modulate intracellular reactivity where there can be differential pathogen recognition (PAMP) associated TNF reactivity vs more danger signaling (DAMP) IL-1 β associated inflammatory responses. The mechanism(s) associated with how different sized particles and different adsorbed proteins elicit different kinds of inflammatory responses (DAMP/inflammasome IL-1β vs PAMP/NFκβ TNF-α) are currently under further investigation.
ACKNOWLEDGEMENTS
We would like to acknowledge the support of the Crown Family Chair of Orthopedics and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR060782. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reference List
- [1].Greenwald AS, Garino JP. Alternative bearing surfaces: the good, the bad, and the ugly. J Bone Joint Surg Am. 2001;83-A(Suppl 2 Pt 2):68–72. doi: 10.2106/00004623-200100022-00002. [DOI] [PubMed] [Google Scholar]
- [2].Matthews JB, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Comparison of the response of three human monocytic cell lines to challenge with polyethylene particles of known size and dose. J Mater Sci Mater Med. 2001 Mar;12(3):249–58. doi: 10.1023/a:1008967200706. [DOI] [PubMed] [Google Scholar]
- [3].Matthews JB, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Comparison of the response of primary murine peritoneal macrophages and the U937 human histiocytic cell line to challenge with in vitro generated clinically relevant UHMWPE particles. Biomed Mater Eng. 2000;10(3-4):229–40. [PubMed] [Google Scholar]
- [4].Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Macrophage/particle interactions. Effect of size, composition and surface area. J Biomed Mater Res. 1994;28:81–90. doi: 10.1002/jbm.820280111. [DOI] [PubMed] [Google Scholar]
- [5].González O, Smith RL, Goodman SB. Efffect of size, concentration, surface area, and volume of polymethylmethacrylate paticles on human macrophages in vitro. J Biomed Mater Res. 1996;30:463–73. doi: 10.1002/(SICI)1097-4636(199604)30:4<463::AID-JBM4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- [6].Green TR, Fisher J, Matthews JB, Stone MH, Ingham E. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J Biomed Mater Res. 2000 Sep;53(5):490–7. doi: 10.1002/1097-4636(200009)53:5<490::aid-jbm7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- [7].Lee J-M, Salvati EA, Betts F, DiCarlo EF, Doty SB, Bullough PG. Size of metallic and polyethylene debris in failed cemented total hip replacements. J Bone Joint Surg. 1992;74B:380–4. doi: 10.1302/0301-620X.74B3.1587882. [DOI] [PubMed] [Google Scholar]
- [8].Sabokbar A, Pandey R, Athanasou NA. The effect of particle size and electrical charge on macrophage-osteoclast differentiation and bone resorption. J Mater Sci Mater Med. 2003 Sep;14(9):731–8. doi: 10.1023/a:1025088418878. [DOI] [PubMed] [Google Scholar]
- [9].Schmalzried TP, Campbell P, Schmitt AK, Brown IC, Amstutz HC. Shapes and dimensional characteristics of polyethylene wear particles generated in vivo by total knee replacements compared to total hip replacements. J Biomed Mater Res. 1997;38(3):203–10. doi: 10.1002/(sici)1097-4636(199723)38:3<203::aid-jbm4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [10].Tomazic-Jezic VJ, Merritt K, Umbreit TH. Significance of the type and the size of biomaterial particles on phagocytosis and tissue distribution. J Biomed Mater Res. 2001 Jun 15;55(4):523–9. doi: 10.1002/1097-4636(20010615)55:4<523::aid-jbm1045>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [11].Caicedo MS, Pennekamp PH, McAllister K, Jacobs JJ, Hallab NJ. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte costimulatory molecule expression and release of proinflammatory cytokines critical to metal-induced lymphocyte reactivity. J Biomed Mater Res A. Oct 20;2009 doi: 10.1002/jbm.a.32627. [DOI] [PubMed] [Google Scholar]
- [12].Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67(2):182–8. [PubMed] [Google Scholar]
- [13].Kaufman AM, Alabre CI, Rubash HE, Shanbhag AS. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. J Biomed Mater Res A. 2008 Feb;84(2):464–74. doi: 10.1002/jbm.a.31467. [DOI] [PubMed] [Google Scholar]
- [14].Brown C, Lacharme-Lora L, Mukonoweshuro B, Sood A, Newson RB, Fisher J, et al. Consequences of exposure to peri-articular injections of micro- and nano-particulate cobalt-chromium alloy. Biomater. 2013 Nov;34(34):8564–80. doi: 10.1016/j.biomaterials.2013.07.073. [DOI] [PubMed] [Google Scholar]
- [15].Caicedo MS, Samelko L, McAllister K, Jacobs JJ, Hallab NJ. Increasing both CoCrMo-alloy particle size and surface irregularity induces increased macrophage inflammasome activation in vitro potentially through lysosomal destabilization mechanisms. J Orthop Res. 2013 Oct;31(10):1633–42. doi: 10.1002/jor.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001 Mar;83-A(3):428–36. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- [17].Kao WJ. Evaluation of protein-modulated macrophage behavior on biomaterials: designing biomimetic materials for cellular engineering. Biomater. 1999 Dec;20(23-24):2213–21. doi: 10.1016/s0142-9612(99)00152-0. [DOI] [PubMed] [Google Scholar]
- [18].Holgers KM, Ljungh A. Cell surface characteristics of microbiological isolates from human percutaneous titanium implants in the head and neck. Biomater. 1999 Jul;20(14):1319–26. doi: 10.1016/s0142-9612(99)00033-2. [DOI] [PubMed] [Google Scholar]
- [19].Andrade JD, Hlady V. Springer-Verlag; Berlin: 1986. Protein adsorption and materials biocompatibility: a tutorial review and suggested hypothesis. [Google Scholar]
- [20].Ratner BD, Johnson AB, Thomas LJ. Biomaterial surfaces. Journal of Biomedical Materials Research. 1987;21:59–90. [PubMed] [Google Scholar]
- [21].Maloney WJ, Sun DH, Nakashima Y, James R, Smith RL. Effects of serum protein opsonization on cytokine release by titanium-alloy particles. J Biomed Mater Res. 1998 Sep 5;41(3):371–6. doi: 10.1002/(sici)1097-4636(19980905)41:3<371::aid-jbm5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- [22].Rezania A, Healy KE. Integrin subunits responsible for adhesion of human osteoblast-like cells to biomimetic peptide surfaces. J Orthop Res. 1999 Jul;17(4):615–23. doi: 10.1002/jor.1100170423. [DOI] [PubMed] [Google Scholar]
- [23].Hallab N, Bundy K, O’Connor K, Moses RL. Biofilm composition and cellula morphology as related to cellular adhesion to biomaterials. J Long Term Effects Med Impl. 1995;5(1):87–8. [PubMed] [Google Scholar]
- [24].Hallab NJ, Bundy KJ, O’Connor K, Moses RL, Jacobs JJ. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001 Feb;7(1):55–71. doi: 10.1089/107632700300003297. [DOI] [PubMed] [Google Scholar]
- [25].Fabrizius-Homan DJ, Cooper SL. Competitive adsorption of vitronectin with albumin, fibrinogen, and fibronectin on polymeric biomaterials. Journal of Biomedical Materials Research. 1991;25:953–71. doi: 10.1002/jbm.820250804. [DOI] [PubMed] [Google Scholar]
- [26].Sun DH, Nakashima Y, Maloney WJ, Goodman SB, Trindade MCD, Smith RL. Trans 43rd Ann Ortho Res Soc. Fransisco: 1997. Opsonization of particulate debris: protein identification by 2-D gel analysis; p. 324. [Google Scholar]
- [27].Thiele L, Diederichs JE, Reszka R, Merkle HP, Walter E. Competitive adsorption of serum proteins at microparticles affects phagocytosis by dendritic cells. Biomater. 2003 Apr;24(8):1409–18. doi: 10.1016/s0142-9612(02)00525-2. [DOI] [PubMed] [Google Scholar]
- [28].Kobzik L, Huang S, Paulauskis JD, Godleski JJ. Particle opsonization and lung macrophage cytokine response. In vitro and in vivo analysis. J Immunol. 1993 Sep 1;151(5):2753–9. [PubMed] [Google Scholar]
- [29].Willert HG, Semlitsch M. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res. 1977;11:157–64. doi: 10.1002/jbm.820110202. [DOI] [PubMed] [Google Scholar]
- [30].Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007 Jul;460:240–52. doi: 10.1097/BLO.0b013e31804b4147. [DOI] [PubMed] [Google Scholar]
- [31].Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop. 2001 Dec;(393):71–7. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- [32].Catelas I, Medley JB, Campbell PA, Huk OL, Bobyn JD. Comparison of in vitro with in vivo characteristics of wear particles from metal-metal hip implants. J Biomed Mater Res B Appl Biomater. 2004 Aug 15;70(2):167–78. doi: 10.1002/jbm.b.20036. [DOI] [PubMed] [Google Scholar]
- [33].Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg [Am] 2000 Apr;82(4):457–76. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- [34].Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomater. 2007 Dec;28(34):5044–8. doi: 10.1016/j.biomaterials.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Brown C, Fisher J, Ingham E. Biological effects of clinically relevant wear particles from metal-on-metal hip prostheses. Proc Inst Mech Eng [H ] 2006 Feb;220(2):355–69. doi: 10.1243/095441105X63291. [DOI] [PubMed] [Google Scholar]
- [36].Urban RM, Jacobs JJ, Sumner DR, Peters CL, Voss FR, Galante JO. The bone-implant interface of femoral stems with non-circumferential porous coating: a study of specimens retrieved at autopsy. J Bone Joint Surg [Am] 1996;78-A(7):1068–81. doi: 10.2106/00004623-199607000-00012. [DOI] [PubMed] [Google Scholar]
- [37].Urban RM, Jacobs J, Gilbert JL, Rice SB, Jasty M, Bragdon CR, et al. Characterization of solid products of corrosion generated by modular-head femoral stems of different designs and materials. In: Marlowe DE, Parr JE, Mayor MB, editors. STP 1301 Modularity of Orthopedic Implants. Philadelphia; ASTM: 1997. pp. 33–44. [Google Scholar]
- [38].Hallab NJ, Skipor A, Jacobs JJ. Interfacial kinetics of titanium- and cobalt-based implant alloys in human serum: metal release and biofilm formation. J Biomed Mater Res. 2003 Jun 1;65A(3):311–8. doi: 10.1002/jbm.a.10429. [DOI] [PubMed] [Google Scholar]