Abstract
Background aims
Cord blood (CB) and amniotic fluid (AF) could represent new and attractive mesenchymal stromal cell (MSC) sources, but their potential therapeutic applications are still limited by lack of standardized protocols for isolation and differentiation. In particular, chondrogenic differentiation has never been deeply investigated.
Methods
MSCs were obtained from CB and AF samples collected during cesarean sections at term and compared for their biological and differentiation properties, with particular interest in cartilage differentiation, in which quantitative real-time polymerase chain reaction and immunohistochemical analyses were performed to evaluate the expression of type 2 collagen, type 10 collagen, SRY-box9 and aggrecan.
Results
We were able to isolate MSCs from 12 of 30 (40%) and 5 of 20 (25%) CB and AF units, respectively. Fluorescence in situ hybridization analysis indicated the fetal origin of isolated MSC strains. Both populations expressed mesenchymal but not endothelial and hematopoietic markers, even though we observed a lower expression of human leukocyte antigen (HLA) I in CB-MSCs. No differences in proliferation rate and cell cycle analysis could be detected. After osteogenic induction, both populations showed matrix mineralization and typical marker expression. Under chondrogenic conditions, pellets derived from CB-MSCs, in contrast with AF-MSCs pellets, were significantly larger, showed cartilage-like morphology and resulted positive for chondrocyte-associated markers, such as type 2 collagen, type 10 collagen, SRY-box9 and aggrecan.
Conclusions
Our results show that CB-MSCs and AF-MSCs collected at term differ from each other in their biological and differentiation properties. In particular, only CB-MSCs showed a clear chondrogenic potential and thus could represent an ideal candidate for cartilage-tissue engineering.
Key Words: amniotic fluid, chondrogenic differentiation, cord blood, mesenchymal stromal cells
Introduction
Mesenchymal stromal cells (MSCs) are multipotent non-hematopoietic progenitors characterized by plastic adhesion, expression of a specific panel of surface antigens and capacity to differentiate into mesenchymal lineages, specifically bone, cartilage and adipose tissue 1, 2, 3. For these reasons, MSCs are considered a promising therapeutic tool for tissue repair and regenerative medicine 4, 5. MSCs were initially isolated from bone marrow (BM) by Friedenstein et al. (6). Although it has been considered for years the main source of MSCs for both experimental and clinical applications, the use of BM-MSCs has disadvantages. First, the procedure for collecting BM from patients and donors is highly invasive and painful. Furthermore, frequency, proliferation and differentiation potential of BM-MSCs decrease with increasing donor age (4). As a consequence, alternative sources from adult and, more importantly, perinatal tissues, have been considered. Among these sources, cord blood (CB) and amniotic fluid (AF), usually regarded as medical waste, could represent new and attractive MSC sources because of their advantages over BM. A large amount of samples can be easily obtained at delivery, with no risk to the donor. Moreover, perinatal tissue–derived MSCs have many advantageous features, such as high proliferation capacity and differentiation potential as the result of their ontogenically younger origin, and no donor age-dependent variations (7). These cells could also be useful for development of in utero stem cell transplantation strategies. Several studies have demonstrated that fetal MSCs harvested in the prenatal period can be processed during the remainder of gestation to obtain autologous tissues for the repair of congenital anomalies soon after birth or in utero 8, 9, 10. Despite this evidence, clinical application of AF-MSCs and CB-MSCs has not yet been achieved, mainly because of the low success rate of isolation. Moreover, the application of these cells for transplant purposes requires extensive characterization, standardization of reproducible differentiation protocols and functional characterization of the differentiated cells. Interestingly, recent studies have shown that MSCs isolated from several prenatal sources represent heterogeneous populations, whose principal differences depend on the source and the donor 11, 12, 13, 14, 15.
MSCs are a promising cell source for cartilage tissue engineering, given their chondrogenic potential. Recently, an increasing number of studies have described that specific properties of MSCs, including chondrogenic capability, are dependent on their origin. In fact, it has been previously demonstrated that MSCs isolated from different tissues do not represent identical cell populations but vary in the expression profile of some growth factors relevant for chondrogenesis 12, 16, 17, 18, 19, 20, 21, 22. Considering these aspects, we hypothesized that the frequency of fetal MSCs correlates with clinical parameters and that term CB-MSCs and AF-MSCs may display differences in their differentiation potential. Therefore, the objectives of this study were to analyze a possible correlation between clinical parameters and the success rate of fetal MSCs isolation, to compare the biological properties of fetal MSCs isolated from term CB and AF samples and, more specifically, to evaluate their capability into cartilage differentiation. Chondrogenic differentiation was induced by means of a three-dimensional pellet culture system submitted to specific stimuli and investigated through histology, immunohistochemistry and gene expression studies of cartilage-associated markers.
Methods
Specimen collection
Both CB and AF samples were collected from volunteer donors during elective cesarean sections at term (37–42 weeks of gestation) at the San Gerardo Hospital (Monza, Italy). Exclusion criteria for collection were presence of clinical chorioamnionitis, prenatally diagnosed chromosome abnormalities and severe oligohydramnios. AF was collected by means of amniotic puncture after transversal incision of the myometrium layer of the lower uterine segment and stored in sterile tubes. CB was collected immediately after delivery of the infant and cord clamping, before the delivery of placenta, and stored in bags (MacoPharma, Rho, Italy) with anticoagulant (citrate-phosphate-dextrose buffer). Collections were performed after maternal consent was given and in accordance with the ethical standards of the hospital ethics committee. Clinical information from each donor was prospectively collected and included date of delivery, gestational age, maternal characteristics, sex and birth weight of the infant and pregnancy details.
Isolation and culture of human MSCs from CB and AF
Mononuclear cells (MNCs) were isolated from whole CB by means of density gradient centrifugation with the use of Ficoll-Hypaque-Plus solution (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The colony-forming unit fibroblast (CFU-F) assay was performed as follows. MNCs were counted with the use of an automated cell counter (Coulter AcT Diff, Beckman Coulter, Brea, CA, USA) and placed in culture at 37°C in a humidified atmosphere containing 5% CO2 at a density of 2 × 106 cells/cm2 into culture dishes (100-mm diameter; Nunc, Rochester, MN, USA) in Dulbecco's modified Eagle's medium (DMEM)–low glucose (Invitrogen, Carlsbad, CA, USA) supplemented with 20% fetal bovine serum (FBS) (Biosera, Ringmer, UK), 1% penicillin-streptomycin (Invitrogen) and 1% L-glutamine (Invitrogen). Dexamethasone (DEX; 10−7 mol/L) (Sigma-Aldrich, St Louis, MO, USA) was added in the primary culture medium for 1 week. Non-adherent cells were removed 48 h after initial plating. After 17 days of culture in medium, CFU-F colonies were scored at ×20 magnification. The cultures, consisting of adherent cells, were maintained in basal medium until they reached 70% confluence and were harvested with the use of 0.05% trypsin (Invitrogen). For subsequent expansion, cells were counted on a hemocytometer and re-plated at a density of 2 × 103cells/cm2 and maintained in culture for several passages.
AF specimens were centrifuged and cells were re-suspended in DMEM–low glucose supplemented with 20% FBS, 1% penicillin-streptomycin and 1% L-glutamine, seeded at a concentration of 1 × 106cells/cm2 and incubated at 37°C in 5% CO2 air atmosphere. After 17 days of culture in medium, CFU-F colonies were scored at ×20 magnification. Subsequently, adherent cells were cultured in the same conditions as CB-MSCs.
Proliferation kinetics of MSCs
The cumulative population doubling (PD) levels at each sub-cultivation were calculated from the cell count by use of the equation: PDn = PDn–1+[log(C1/C0)]/log2, wherein C0 = cells number initially seeded and C1 = cells number harvested. The PDs of cells from P2 to P9 were determined. Three sets of cultures were repeated for each sample. The living cell count was performed in a hemocytometer by means of trypan blue dye exclusion (Sigma-Aldrich).
Fluorescence in situ hybridization analysis
CB-MSCs and AF-MSCs isolated from male infants were used to assess contamination of maternal cells. Fluorescence in situ hybridization analysis (FISH) was performed with the use of CEP X (DXZ1) SpectrumGreen/CEP Y (DYZ3) SpectrumOrange probes (Abbott Molecular, Des Plaines, IL, USA).
Karyotype analysis
MSCs were cultured in medium supplemented with 10 μL/mL of colcemid (10 g/mL, Sigma-Aldrich) for up to 6 h. Conventional cytogenetics was performed by the standard procedures and QFQ banding (Q-bands by fluorescence with the use of quinacrine).
Flow cytometric analysis
CB-MSCs and AF-MSCs at passage 3 were labeled with phycoerythrin-conjugated or fluorescein isothiocyanate–conjugated antibodies against CD14 (clone 61D3; eBioscience, San Diego, CA, USA), CD34 (clone 581; BD Biosciences, San José, CA, USA), CD45 (clone HI30; BD Biosciences), CD90 (clone 5E10; eBioscience), CD73 (clone AD2; BD Biosciences), CD105 (clone SN6; eBioscience), CD146 (clone P1H12; BD Biosciences), human leukocyte antigen (HLA)-ABC (HLA I, clone G46-2.6; BD Biosciences), HLA-DR (HLA II, clone G46-6; BD Biosciences), CD31 (clone L133.1; BD Biosciences), CD144 (clone No. 123413, R and D, Minneapolis, MN, USA), vascular endothelial growth factor–receptor 1 (VEGF-R1) (clone No. 49560; R & D) and VEGF-receptor 2 (VEGF-R2) (clone No. 89106, R and D). Isotype antibodies were used as control. Fluorescence-activated cell sorting (FACS) analysis was performed on three CB-MSCs and three AF-MSCs lines, which were selected randomly. Flow cytometric analysis was performed on 20,000 events with the use of a FACScan cytometer, and data were analyzed with the use of CellQuest software (BD Biosciences).
Cell cycle analysis
CB-MSCs and AF-MSCs at passage 4 were harvested 48, 96 and 144 h after seeding and were washed twice with ice-cold phosphate-buffered saline (Gibco, Grand Island, NY, USA). Cells were re-suspended in GM saline solution (phosphate-buffered saline ×1 without CaMg, 1.1 g/L glucose, 0.5 mmol/L ethylenediaminetetra-acetic acid) and permeabilized with ice-cold 70% ethanol, followed by incubation with 2 mL of a solution containing 10 μg/mL propidium iodide plus 25 μL 1 mg/mL RNAse 10,000 units overnight (23).
Flow cytometric analysis was performed on 20,000 events by use of a FACS Calibur (BD Biosciences), and the cell cycle phase distribution was calculated as percentages by a gaussian-modified method (24).
Osteogenic differentiation
CB-MSCs and AF-MSCs at passage 3 were seeded at a density of 6 × 103 cells/cm2 in basal medium, as previously described (25). After 48 h, medium was switched to osteogenic induction medium consisting of DMEM–low glucose (Invitrogen), supplemented with 10% FBS (Biosera), 100 nmol/L DEX (Invitrogen), 10 mmol/L B-glycerol-phosphate (Invitrogen) and 0.05 mmol/L 2-phosphate-ascorbic acid (Invitrogen). The osteogenic differentiation was assessed through the use of alizarin red S (Sigma-Aldrich) staining on day 21 of differentiation. On days 0, 7, 14 and 21, transcript levels for the following genes were analyzed by means of quantitative real-time polymerase chain reaction (Q-RT-PCR): alkaline phosphatase (ALPL), type I collagen (COL1A2), osteonectin (OTN), runt-related transcription factor 2 (RUNX2), osteopontin (OPN) and osteocalcin (OTC).
Adipogenic differentiation
For the adipogenic differentiation, we referred to Pittenger et al. (1). Briefly, CB-MSCs and AF-MSCs at passage 3 were seeded at a density of 2 × 104 cells/cm2 in basal medium. After 24 h, medium was switched to adipogenic induction medium consisting of DMEM–high glucose (Invitrogen) supplemented with 10% FBS (Biosera), 1 μmol/L DEX (Sigma-Aldrich), 1 μmol/L indomethacin (Invitrogen), 500 μmol/L 3-isobutyl-1-methylxantine (Sigma-Aldrich) and 10 μg/mL human recombinant insulin (Sigma-Aldrich). For the detection of adipogenic differentiation, intracellular lipid droplets were stained with the use of oil red O solution (Sigma-Aldrich) on day 21 of differentiation.
Chondrogenic differentiation
CB-MSCs and AF-MSCs at passage 3 were seeded in a 15-mL conical tube at a density of 3 × 105/tube and re-suspended in chondrogenic differentiation medium consisting of DMEM–high glucose (Invitrogen) supplemented with ITS + premix (Collaborative Biomedical Products, Bedford, MA, USA), 1 mmol/L pyruvate (Sigma-Aldrich), 50 μg/mL 2-phosphate–ascorbic acid (Fluka, Sigma-Aldrich), 100 nmol/L DEX (Sigma-Aldrich) and 10 ng/mL transforming growth factor (TGF)-β1 (R and D), as previously described by Gatto et al. (25). Cells were grown as pellets for 3 weeks at 37°C, 5% CO2.
For immunostaining experiments, cartilage pellets were fixed in 4% formaldehyde in phosphate buffer, processed for paraffin embedding and sectioned serially. Five-micron-thick sections were stained with hematoxylin and eosin (Sigma-Aldrich). Primary antibodies used for immuno-localization studies as per established protocols are listed in Supplementary Table S1. Immuno-localization was performed with the use of standard peroxidase/diaminobenzidine (DAB) reaction and counterstained with hematoxylin. Bright-field light microscopy images were obtained with the use of a Zeiss Axiophot epifluorescence microscope (Carl Zeiss, Oberkochen, Germany).
For the assessment of chondrogenic gene expression profile, on days 0, 7, 14 and 21 after induction, transcript levels for type II collagen (COL II), type X collagen (COL X), SRY-box9 (SOX 9) and aggrecan (ACAN) were analyzed by means of Q-RT-PCR.
RNA isolation and Q-RT-PCR
Total RNA was extracted with the use of TRIZOL reagent (Invitrogen), following the manufacturer's protocol; 1 μg of RNA was then reverse-transcribed with the use of a SuperScript II Reverse Transcriptase kit (Invitrogen) in the presence of random hexamers. Quantitative real-time polymerase chain reaction assays were performed in triplicate on an ABI 7900 Real-Time PCR system thermal cycler with the qPCR Mastermix (Applied Biosystems-Invitrogen). All TaqMan gene expression assays were provided by Applied Biosystems (Supplementary Table S2).
After having verified the stable expression of GAPDH, this gene was included as endogenous control. The level of each target gene was normalized to GAPDH levels and expressed relative to the control culture levels (ΔΔCt method).
Statistical analysis
The data were analyzed with the use of SPSS (version 17; SPSS Inc, Chicago, IL, USA). Statistical analysis included χ2 or Fisher exact test for dichotomous variables, t test or one-way analysis of variance for continuous variables. A two-tailed P value of <0.05 or an odds ratio with 95% confidence intervals not inclusive of the unity was considered significant.
Results
Isolation of CB-MSCs and AF-MSCs
We collected CB and AF units from a total of 35 donors during cesarean sections at term. Obstetric and clinical characteristics of the donor population are shown in Table I. Twenty-four donors (69%) had an uncomplicated pregnancy; seven pregnancies were affected by a maternal complication (two cases of gestational diabetes, two cases of pre-gestational hypothyroidism, two cases of mild preeclampsia and one case of epilepsy treated during pregnancy); three cases had a fetal pathology (one fetus with osteogenesis imperfecta, one with hydrocephalous and one with multiple malformations); one pregnancy was affected by severe preeclampsia and severe intrauterine growth restriction.
Table I.
Major obstetric and demographic characteristics of donors.
| Maternal age (years) | 34.7 ± 6.1 |
| Gestational age (weeks) | 38.2 ± 1.9 |
| Neonatal weight (g) | 3080 ± 668 |
| Multiparity (%) | 24 (69%) |
| Male newborn (%) | 19 (54%) |
| Maternal pathologies (%) | 8 (23%) |
| Fetal pathologies (%) | 4 (11%) |
| Elective cesarean section (%) | 27 (77%) |
In a pilot study (data not shown), we demonstrated a consistent decrease in CB-MSCs and AF-MSCs isolation rate when sample volume was <60 mL for CB and 20 mL for AF and when the interval time between sampling and processing was >2 h. According to such preliminary data and evidence from the literature (26), CB units having a volume <60 mL (not adequate, five of 35 units, 14%) and AF samples having a volume <20 mL or visible contamination with blood (not adequate, 15 of 35 units, 43%) were excluded from processing (Table II). The remaining 30 CB and 20 AF units were considered adequate and were processed within 2 h after collection. Both volume and cellularity were significantly lower in AF samples than in CB samples (33.8 ± 22.9 mL versus 88.7 ± 33.5 mL, P < 0.001; 0.5 ± 0.8 × 106 versus 2.5 ± 1.6 × 106 MNCs per milliliter, P < 0.001, respectively). Following our protocol, we isolated MSCs from 12 of 30 processed CB units (40%) and from five of 20 processed AF units (25%); the difference in the overall success of isolation between CB and AF approached significance (34% versus 14%; P = 0.05) (Table II). In two cases, we were able to isolate both CB-MSCs and AF-MSCs from the same donor. In the first set of experiments, we observed a potential difference in the number of CFU-F derived from CB and AF specimens. Therefore, in the next set of experiments, we quantified the colony-forming efficiency (CFE), which is the number of CFU-F formed after seeding MNCs, for 11 CB and 3 AF samples. The CFE was significantly decreased in CB-MNCs (P = 0.01) in comparison with AF-MNCs (Table II).
Table II.
Comparison between the major parameters of CB and AF samples isolation.
| CB (n = 35) | AF (n = 35) | P value | |
|---|---|---|---|
| Adequate (%) | 30/35 (86%) | 20/35 (57%) | 0.01 |
| Successful (%) | 12/30 (40%) | 5/20 (25%) | 0.4 |
| Averall successful (%) | 12/35 (34%) | 5/35 (14%) | 0.05 |
| Volume (mL) | 88.7 ± 33.5 | 33.8 ± 22.9 | <0.001 |
| Cellularity (×10−6/mL) | 2.5 ± 1.6 | 0.5 ± 0.8 | <0.001 |
| CFE (%) | 3.17% | 5.39% | 0.01 |
No differences in the clinical and obstetric features of the donors were observed between successful and unsuccessful samples, either for CB or for AF. No correlations were found between sample volume or neonatal birth weight and the success rate of isolating MSCs. Interestingly, we were able to isolate MSCs from both CB and AF of the fetus affected by osteogenesis imperfecta and from CB of the fetus affected by multiple malformations.
Morphology and immunophenotypic characterization of CB-MSCs and AF-MSCs
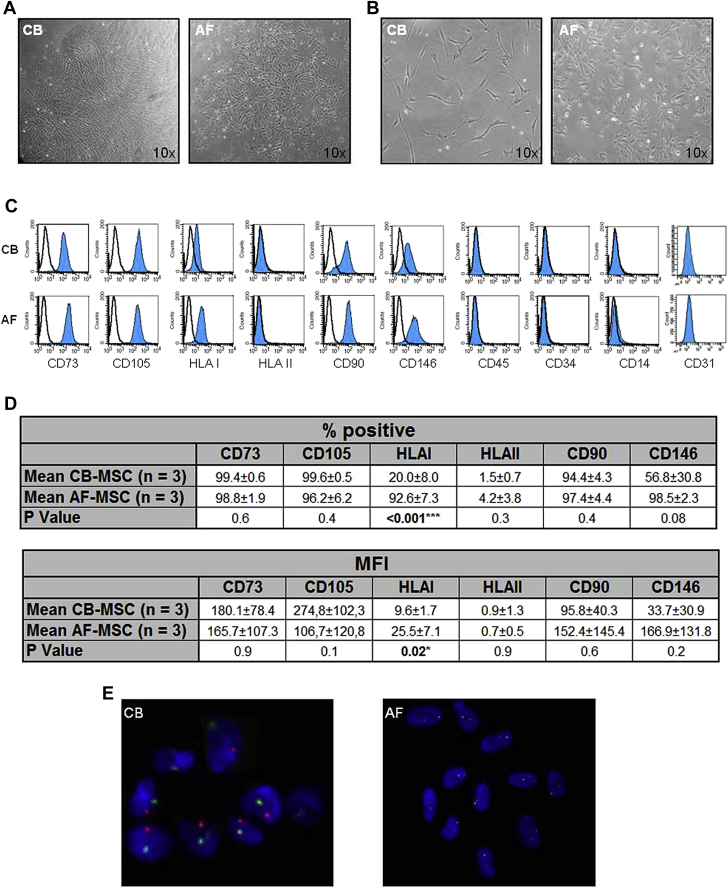
To compare the characteristics of CB-MSCs and AF-MSCs, we considered only the cell strains derived from healthy donors. The MSC strains used in the majority of the assays of our study were selected randomly from the isolated cell lines, with 3 of 12 used for CB-MSCs and 3 of 5 used for AF-MSCs. CB-MSC and AF-MSC colonies were detected, respectively, at 16.2 ± 2.6 days (range, 14–21 days, n = 12) and 17.0 ± 2.7 days (range, 15–21 days, n = 5) after initial MNCs plating. The primary cultured cells from AF were round, spindle or polygonal in shape and were characterized by a more irregular morphology. Conversely, the primary cultured CB-MSCs were a more homogeneous population (Figure 1A). After passaging, CB-MSCs were uniformly fibroblast-like, whereas the described differences of AF-MSCs concerning the morphology were observed throughout the culture (Figure 1B). For further characterization, surface protein expression of MSCs from the three CB and the three AF samples at passage 3 was examined by flow cytometry. Selected antigens representing a CB-MSC line and an AF-MSC line are depicted in Figure 1C. Greater than 90% of CB-MSCs and AF-MSCs expressed the typical MSCs marker proteins CD73, CD105 and CD90. MSCs derived from both sources did not display expression of hematopoietic antigens (CD45, CD34, CD14 and HLA II). MSCs were also negative for typical endothelial markers such as CD31, CD144 (vascular endothelial cadherin), VEGF-R1 and VEGF-R2 (Figure 1C and data not shown). HLA I was expressed by a significantly lower percentage of CB-MSCs compared with AF-MSCs (20.0% ± 8.0% versus 92.6% ± 7.3%; P < 0.001). This was observed for the mean fluorescence intensity of HLA I expression, which was significantly lower for CB-MSCs compared with AF-MSCs (9.6 ± 1.7 versus 25.5 ± 7.1, P = 0.02) (Figure 1D). Regarding the expression of CD146, a typical surface antigen appearing on perivascular cells and pericytes, a wide range of differences was observed in the CB-MSC and AF-MSC samples tested. To exclude maternal contamination in the culture of CB-MSCs and AF-MSCs, FISH analysis was performed to identify XY chromosomes in MSCs isolated from a male neonate. This test showed an XY incidence of 100%, which indicates the fetal origin of both types of perinatal MSCs (Figure 1E).
Figure 1.
Morphology, immunophenotype and cytogenetic analysis of CB-MSCs and AF-MSCs. (A) Morphology of CFU-F generated from CB-MSCs and AF-MSCs isolated after initial plating. (B) Morphology of isolated CB-MSCs and AF-MSCs (passage 1). (C) Representative surface immunophenotype of CB-MSCs and AF-MSCs obtained from the same donor at passage 3. Open histograms show isotype controls and colored histograms show tested samples. (D) Comparison of the surface protein expression in MSCs derived from three CB and three AF samples; percentage (%) of positive cells and mean fluorescence intensity (MFI) are shown. ∗P < 0.05; ∗∗∗P < 0.001. (E) Representative XY FISH on isolated nuclei from CB-MSCs and AF-MSCs derived from a male fetus shows the presence of both X and Y chromosomes.
Proliferation of CB-MSCs and AF-MSCs
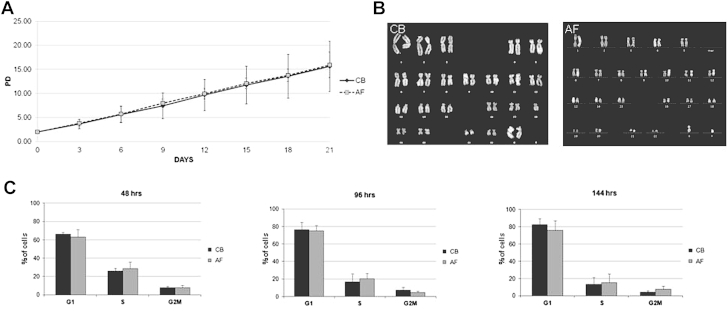
Adherent cells from eight CB-MSC and three AF-MSC lines were passaged up to eight times, and PDs were calculated. CB-MSCs and AF-MSCs divided for an extensive period of time in vitro. No differences in the proliferation rate were observed between CB-MSCs and AF-MSCs (Figure 2A). The karyotype of two of three CB-MSC and two of three AF-MSC strains that were selected randomly was analyzed at different culture passages (between PD 20 and PD 25) to evaluate the occurrence of spontaneous chromosomal alterations during expansion. All analyzed samples remained genetically stable (Figure 2B). To confirm that CB-MSCs expansion capability was similar to that of AF-MSCs, we performed a cell cycle analysis at different time points for three AF-MSC and three CB-MSC lines. The cell cycle distribution pattern was similar for CB-MSCs and AF-MSCs (Figure 2C). For both AF-MSCs and CB-MSCs, the majority of cells were in the G0/G1 phase of the cell cycle (63% ± 8% and 66% ± 2%, respectively) at 48 h after seeding, and the remaining cells were similarly distributed in SG2M phase. Likewise, no differences in the cell cycle analysis could be detected between CB-MSCs and AF-MSCs at 96 and 144 h after seeding.
Figure 2.
Proliferation, senescence and cell cycle phase distribution of CB-MSCs and AF-MSCs. (A) Expansion curve of CB-MSCs and AF-MSCs. Graph represents mean ± standard deviation of eight CB donors (continuous line) and three AF donors (dotted line). (B) Representative Q-banded karyotype analysis of CB-MSCs and AF-MSCs in culture at standard density shows genetic stability for both populations. (C) Cell cycle analysis. Percentage of cells in the different phases of the cell cycle was assessed by means of propidium iodide (PI) flow cytometric assay. Analysis was performed on at least 20,000 cells for each sample at 48, 96 and 144 h after replating. Graph represents mean ± standard deviation of three CB donors and three AF donors.
Osteogenic and adipogenic differentiation capacity of CB-MSCs and AF-MSCs
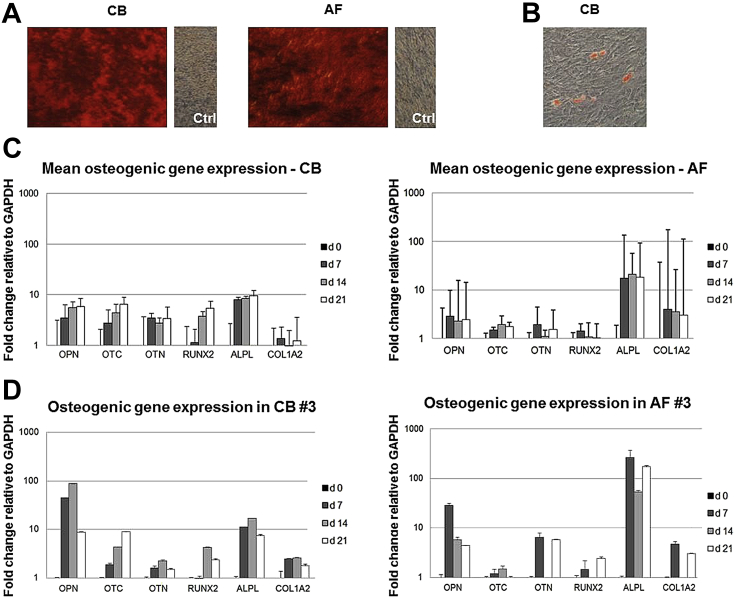
To investigate the in vitro differentiation potential, CB-MSCs and AF-MSCs from different samples were grown for 3 weeks in osteoblastic and adipocytic differentiation medium. After osteogenic differentiation, both CB-MSC and AF-MSC cultures showed extracellular matrix mineralization that could be detected by alizarin red staining (Figure 3A).
Figure 3.
Osteogenic and adipogenic differentiation of CB-MSCs and AF-MSCs. (A) Osteogenic differentiation of CB-MSCs and AF-MSCs demonstrated by deposition of mineralized matrix detected by means of the alizarin red S method. (B) Adipogenic differentiation of CB-MSCs is demonstrated by the accumulation of few lipid vacuoles stained by oil red O. Only one of eight CB-MSC lines was positive for oil red O staining. (C) Expression of osteogenesis-related genes detected by RT-PCR after 7, 14 and 21 days of culture (CB-MSCs, n = 6; AF-MSCs, n = 5). (D) Expression of osteogenesis-related genes in CB-MSCs and AF-MSCs isolated from the same donor.
To further analyze osteogenic differentiation at the molecular level, transcript levels of selected genes involved in bone ontogeny were determined for OPN, OTC, OTN, RUNX2, ALPL and COL IA at 7, 14 and 21 days by means of Q-RT-PCR (Figure 3C). This analysis showed the expected expression profiles of the osteoblast phenotype for CB-MSCs (n = 6). In this cell population, ALPL, OPN, OTC, OTN and RUNX2 showed a nearly constant upregulated expression level. This was also the case for osteogenic differentiation of AF-MSCs (n = 5), in which the expression levels of ALPL, OPN, OTC, OTN and RUNX2 were upregulated over time. The transcript levels of the different genes analyzed showed an extremely high variability between the different CB-MSC and AF-MSC lines. In a single case, we were able to evaluate osteogenic transcript levels in differentiated CB-MSCs and AF-MSCs isolated from the same donor, showing a similar pattern (Figure 3D). Cells cultured in adipogenic medium were stained with oil red O to detect intracytoplasmatic lipid droplets. After adipogenic induction, only 13% of CB-MSC lines (one of eight samples analyzed) showed an adipogenic phenotype. In this sample, however, only small oil droplets were formed within the cells, and the frequency of positive cells was low (Figure 3B). None of the AF-MSCs displayed an adipogenic phenotype under differentiation conditions (n = 5).
Chondrogenic differentiation capability of CB-MSCs and AF-MSCs
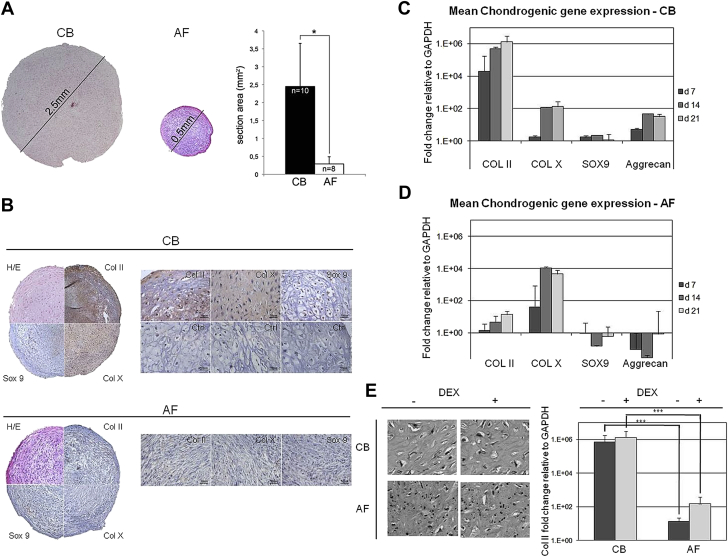
To investigate the chondrogenic differentiation capability, MSCs from both sources were cultured in the presence of TGF-β1 to generate a solid spherical three-dimensional tissue structure that can be harvested and processed for histology, immunohistochemistry and molecular analysis. Both CB-MSCs and AF-MSCs generated typical pellets when cultured as micromasses under chondrogenic conditions. After 21 days of culture, a significant increase in pellet size was found in CB-MSCs–derived pellets. Conversely, the pellets obtained from AF-MSCs were smaller and did not grow significantly during the culture (section areas: 2.45 ± 1.2 mm2 versus 0.29 ± 0.2 mm2, respectively, CB-MSC versus AF-MSC pellets, P < 0.05; Figure 4A). Cartilage-like cells within lacunae were observed in sections of pellets generated from CB-MSCs but not in pellets from AF-MSCs (Figure 4B). As expected, pellets derived from CB-MSCs but not from AF-MSCs stained diffusely positive for COL II, COL X and SOX-9 (Figure 4B). In addition, expression analysis of COL II, COL X, SOX-9 and ACAN at different time points (day 7, day 14 and day 21) confirmed that chondrogenic differentiation was induced only in CB-MSCs (Figure 4C,D). In particular, the strong upregulation of COL II expression observed after chondrogenic induction in CB-MSCs was absent in the case of AF-MSCs at each time point (day 7, CB-MSCs versus AF-MSCs, P = 0.00007; day 14, CB-MSCs versus AF-MSCs, P = 0.00008; day 21, CB-MSCs versus AF-MSCs, P = 0.000000003; Figure 4C,D). Also ACAN and SOX-9 were exclusively expressed by CB-MSCs pellets. SOX-9 expression was detected at day 7 and day 14 after induction, in the earlier stages of chondrogenesis. These data were also confirmed by the comparison performed on cartilage pellets derived from CB-MSCs and AF-MSCs generated from the same donor (data not shown). Because isolation of CB-MSCs and AF-MSCs was performed in the presence and in the absence of DEX, respectively, additional experiments were also performed to evaluate whether DEX could affect cellular commitment. These experiments demonstrated that CB-MSCs isolated in the absence of DEX maintained their chondrogenic differentiation capability, as evidenced by histology of the cartilage pellet and expression of COL II (Figure 4E). On the other side, the addition of DEX to AF-MSC cultures did not improve the quality of the pellet, as evidenced by histological and molecular data (Figure 4E).
Figure 4.
Chondrogenic differentiation capability of CB-MSCs and AF-MSCs. (A) On the left, pellets generated from CB-MSCs and AF-MSCs are shown. Hematoxylin and eosin staining of pellets induced by culture with TGF-β1 for 3 weeks. On the right, mean section area of pellets derived from CB-MSCs (black bar, n = 10, from n = 7 different donors) and AF-MSCs (white bar, n = 8, from n = 3 different donors) are shown. ∗P < 0.05. (B) Immunostaining of CB-MSC (upper) and AF-MSC (lower) pellets with anti–COL II, anti–COL X and anti–SOX 9. (C) Expression of chondrogenesis-related genes detected by RT-PCR at 7, 14 and 21 days of culture in CB-MSC pellets (pellets n = 3 for each time point, derived from three CB-MSC strains). (D) Expression of chondrogenesis-related genes detected by RT-PCR at 7, 14 and 21 days of culture in AF-MSC pellets (pellets n = 4 for each time point, derived from three AF-MSC strains). (E) Histology and expression of chondrogenesis-related genes in CB-MSCs and AF-MSCs treated or not treated with DEX (CB-MSCs without DEX, pellets n = 2 derived from two CB-MSC strains; CB-MSCs with DEX, pellets n = 4 derived from three CB-MSC strains; AF-MSCs without DEX, pellets n = 3 derived from three AF-MSC strains; AF-MSCs with DEX, pellets n = 2 derived from one AF-MSC strain). ∗∗∗P < 0.001.
Discussion
In the present study, we evaluated whether CB and AF collected at term of pregnancy could represent a useful and accessible source of fetal MSCs. Furthermore, the biological properties and differentiation potential of MSCs derived from these two fetal sources were compared. When we applied strict criteria for the selection of the samples to be processed, we were able to isolate MSC colonies from 40% and 25% of the CB and AF units, respectively. Likewise, Zhang et al. (26) achieved a 90% rate of success in isolation of CB-MSCs through the use of similar selection criteria (CB volume ≥90 mL and storage time before processing ≤2 h). Other studies have reported isolation yields ranging from <10% to as high as 90%, demonstrating a lack of consensus in the isolation and culture protocols 26, 27, 28, 29, 30, 31. The lower efficiency in AF-MSCs isolation compared with CB-MSCs can be attributed to the heterogeneous population of cells from fetal origin contained in the AF 32, 33. Furthermore, the total volume and the cellularity were significantly lower in AF samples, which could have affected the isolation rates. Several studies demonstrated higher efficiency in the isolation of MSCs from preterm amniotic fluid, usually collected during second-trimester amniocenteses; this difference is probably explained by the decreased proportion of viable cells in AF with advancing gestational age 33, 34. It is noteworthy, however, that although term AF is a waste product, which can be easily collected during delivery with no risk for the donor, AF collected from amniocentesis could not be considered for donation because of the risks related to the procedure. To further improve the success of MSCs isolation from both sources, we evaluated whether isolation was influenced by any features of the donor or the sample and found no correlation with analyzed obstetric characteristics.
Considering that MSCs are already entering the clinical arena, an extensive characterization and standardization of reproducible in vitro and in vivo differentiation protocols is mandatory for potential application of these cells. We demonstrated that although the two MSC populations can be considered similar in regard to growth kinetics, osteogenic differentiation capacity and absence of adipogenic differentiation, they clearly diverge in their isolation rate, colony-forming efficiency, morphology, immunophenotype (HLA-class I) and, more interestingly, chondrogenic differentiation capacity.
First, the CFE was significantly higher in AF than in CB samples, which suggests a higher frequency of MSCs in the nucleated cells of AF than in those of CB, even though the isolation rate in AF samples was lower. Cells isolated from the two sources showed, otherwise, a comparable proliferation rate and cell cycle status. Both AF-MSCs and CB-MSCs expressed the typical mesenchymal surface proteins and lacked expression of the hematopoietic and endothelial markers. Notably, however, significant differences were observed in the expression of HLA-class I, with lower expression in CB-MSCs compared with that in AF-MSCs. However, it has been previously demonstrated that fetal MSCs lack or exhibit very low expression of HLA-class I 35, 36. Furthermore, AF-MSCs and CB-MSCs successfully differentiated into osteoblasts, as confirmed by positive alizarin red S staining and upregulated expression of the principal osteogenic genes. Conversely, none of the AF-MSC and only one CB-MSC line tested showed adipogenic differentiation capability. Our data are, overall, in agreement with the literature 11, 26, 27, 37 and might be explained by the ontogenic age of these cells (38). Only few studies have shown successful adipogenesis in CB-MSCs, with reduced formation of lipid droplets 6, 39, 40. On the other hand, sporadic work reported successful adipogenic differentiation of AF-MSCs isolated from second-trimester AF samples 32, 41, whereas adipogenesis of full-term AF-MSCs has never been investigated.
To our knowledge, chondrogenic differentiation of human AF-MSCs and CB-MSCs has never been rigorously compared concerning the peculiar properties of the chondroid pellets generated in vitro. On the basis of our protocol, only CB-MSCs efficiently differentiated into cartilage when they were condensed into a single aggregate and exposed to TGF-β1. In fact, pellets derived from CB-MSCs, compared with AF-MSCs, were significantly larger, showed typical cartilage-like morphology and expressed transcripts for proteoglycans and chondrocyte-associated markers, such as COL II, COL X, SOX-9 and ACAN. SOX-9 is a key transcriptional regulator in early chondrogenesis, with an important role in transactivating cartilage-specific genes during chondrocyte differentiation 42, 43. In our study, gene expression profiles of SOX-9, detected at early stages of differentiation, COL II and ACAN correlated with a high in vitro potential of CB-MSCs to differentiate into chondrocytes. On the contrary, pellets derived from AF-MSCs expressed low levels of cartilage-specific genes. It is also interesting to note that in our study, the hypertrophy-associated gene COL X was detected in the early stages of the chondrogenic differentiation process in CB-MSCs and in AF-MSCs. Previous studies have reported similar data showing that human MSCs cultured in pellets under standard chondrogenic conditions expressed COL X transcripts throughout the pellet culture, including in the undifferentiated MSCs 44, 45. Indeed, in AF-MSC–derived pellets, cells were not differentiated and immunohistochemistry analysis did not reveal the presence of COL X protein expression, which was also true for COL II and SOX-9. In contrast, in CB-MSCs pellets, COL X–positive chondrocytes were detected by means of immunolocalization. As previously described for other cell sources, we could also confirm for CB-MSCs that in vitro protocols of chondrogenesis produce derived chondrocytes that undergo hypertrophy 16, 46. These data demonstrate that although MSCs from the two sources analyzed here may be considered similar in the majority of the biological aspects, they clearly diverge in their chondrogenic differentiation capability. Furthermore, in one case, we were able to do a direct comparison of chondrogenic potential of CB-MSCs and AF-MSCs from the same neonatal donor, confirming that the difference shown in this study cannot be attributed to a general difference between donors. The use of DEX in the establishment of the CB-MSCs and the absence of DEX in the AF-MSCs isolation protocol may be another critical factor in determining the quality of the generated cartilage pellets. The choice to include or exclude DEX in the isolation protocols was based on the available literature (26). DEX has been shown to stimulate chondrogenic differentiation of equine-, rat- and human-derived MSCs in vitro (47). Whether short-term exposure to DEX during isolation of MSCs strains, before the subsequent exposure to a chondrogenic induction protocol, increases the chondrogenic potential of MSCs is undetermined. To address this question, we evaluated whether the addition of DEX in the isolation protocol of AF-MSCs or the exclusion of DEX during the isolation of CB-MSCs could change the subsequent chondrogenic potential. Thus, we were able to conclude that the addition of DEX in the first week of cell culture does not appear to be a critical factor in determining differences between the chondrogenic capabilities of both MSCs. The high capacity of human CB-MSCs, among various tissue-derived MSCs, to differentiate into chondrocytes has been described by other groups 12, 20, 21, 26. Interestingly, Berg et al. 48, 49 reported that equine CB-MSCs have superior chondrogenic potential compared with MSCs of other biological origin, confirming similarities between different large animals species. To our knowledge, MSCs derived from human AF isolated at term of pregnancy have not been extensively investigated concerning their potential for cartilage regeneration 34, 50. A few studies have characterized the ability of different multipotent stem cell populations derived from the first or second trimester of pregnancy to undergo chondrogenic differentiation 51, 52, 53. Following a chondrogenic differentiation protocol similar to ours, Kolambkar et al. (54) reported that amniotic fluid–derived stem cells were able to produce a cartilage-like matrix in specific culture conditions, even though less efficiently compared with BM-derived MSCs (54). Similarly, human AF-MSCs have been shown to produce cartilage in TGF-β3 containing fibrin hydrogel, even if with a lower potential compared with other MSC populations (51). Therefore, a possible explanation for our results may be that AF-MSC populations isolated from amniotic fluid at term of the pregnancy have lost their chondrogenic potential or contain only a small proportion of cells still able to maintain this property. Furthermore, in our study, we have adopted the most widely applied culture system for chondrogenesis 16, 17; however, it would be interesting, for future studies, to explore alternative strategies for the enhancement of chondrogenesis in AF-MSCs such as increased TGF-β concentration, addition of bone morphogenetic proteins and a combination of TGF-β and insulin-like growth factor-1 (46). According to our results, even if AF cells appear to contain a higher number of clonogenic precursors compared with CB, AF-MSCs completely fail to differentiate into cartilage, which shows that their multipotent capacity is limited to osteogenic differentiation. Conversely, the less clonogenic term CB-MSCs showed great chondrogenic potential, making them a good candidate stem cell population for therapeutic and biotechnological applications in cartilage repair. In vivo studies on cartilage formation and repair with the use of CB-MSCs are now necessary to support the results obtained in this study through the evaluation of in vivo chondrogenesis, ultimately confirming their therapeutic potential.
Acknowledgments
This work was supported by the Italian Telethon Foundation to MS (TCP 07004). MS is Assistant Telethon Scientist.
Disclosure of interests: The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jcyt.2014.02.008.
Supplementary data
References
- 1.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 3.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Caplan A.I. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sensebe L., Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87(Suppl 9):S49–S53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 7.Gucciardo L., Lories R., Ochsenbein-Kolble N., Done E., Zwijsen A., Deprest J. Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG. 2009;116:166–172. doi: 10.1111/j.1471-0528.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Gao F., Ma L., Jiang J., Miao J., Jiang M. Therapeutic potential of in utero mesenchymal stem cell (MSCs) transplantation in rat foetuses with spina bifida aperta. J Cell Mol Med. 2012;16:1606–1617. doi: 10.1111/j.1582-4934.2011.01470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deprest J., De Coppi P. Antenatal management of isolated congenital diaphragmatic hernia today and tomorrow: ongoing collaborative research and development: Journal of Pediatric Surgery Lecture. J Pediatr Surg. 2012;47:282–290. doi: 10.1016/j.jpedsurg.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Kunisaki S.M. Congenital anomalies: treatment options based on amniotic fluid-derived stem cells. Organogenesis. 2012;8:89–95. doi: 10.4161/org.22238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kern S., Eichler H., Stoeve J., Kluter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 12.Rebelatto C.K., Aguiar A.M., Moretao M.P., Senegaglia A.C., Hansen P., Barchiki F. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 13.Sessarego N., Parodi A., Podesta M., Benvenuto F., Mogni M., Raviolo V. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica. 2008;93:339–346. doi: 10.3324/haematol.11869. [DOI] [PubMed] [Google Scholar]
- 14.Campagnoli C., Roberts I.A., Kumar S., Bennett P.R., Bellantuono I., Fisk N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 15.in 't Anker P.S., Noort W.A., Scherjon S.A., Kleijburg-van der Keur C., Kruisselbrink A.B., van Bezooijen R.L. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–852. [PubMed] [Google Scholar]
- 16.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 17.Mackay A.M., Beck S.C., Murphy J.M., Barry F.P., Chichester C.O., Pittenger M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 18.Heng B.C., Cao T., Lee E.H. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells. 2004;22:1152–1167. doi: 10.1634/stemcells.2004-0062. [DOI] [PubMed] [Google Scholar]
- 19.Koga H., Muneta T., Nagase T., Nimura A., Ju Y.J., Mochizuki T. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 20.de Mara C.S., Duarte A.S., Sartori-Cintra A.R., Luzo A.C., Saad S.T., Coimbra I.B. Chondrogenesis from umbilical cord blood cells stimulated with BMP-2 and BMP-6. Rheumatol Int. 2013;33:121–128. doi: 10.1007/s00296-011-2328-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang J.F., Wang L.J., Wu Y.F., Xiang Y., Xie C.G., Jia B.B. Mesenchymal stem/progenitor cells in human umbilical cord blood as support for ex vivo expansion of CD34(+) hematopoietic stem cells and for chondrogenic differentiation. Haematologica. 2004;89:837–844. [PubMed] [Google Scholar]
- 22.Fernandes A.M., Herlofsen S.R., Karlsen T.A., Kuchler A.M., Floisand Y., Brinchmann J.E. Similar properties of chondrocytes from osteoarthritis joints and mesenchymal stem cells from healthy donors for tissue engineering of articular cartilage. PLoS One. 2013;8:e62994. doi: 10.1371/journal.pone.0062994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavecchio M., Natoli C., Ubezio P., Erba E., D'Incalci M. Dynamics of cell cycle phase perturbations by trabectedin (ET-743) in nucleotide excision repair (NER)-deficient and NER-proficient cells, unravelled by a novel mathematical simulation approach. Cell Prolif. 2007;40:885–904. doi: 10.1111/j.1365-2184.2007.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ubezio P. Microcomputer experience in analysis of flow cytometric DNA distributions. Comput Programs Biomed. 1985;19:159–166. doi: 10.1016/0010-468x(85)90007-8. [DOI] [PubMed] [Google Scholar]
- 25.Gatto F., Redaelli D., Salvade A., Marzorati S., Sacchetti B., Ferina C. Hurler disease bone marrow stromal cells exhibit altered ability to support osteoclast formation. Stem Cells Dev. 2012;21:1466–1477. doi: 10.1089/scd.2011.0555. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Hirai M., Cantero S., Ciubotariu R., Dobrila L., Hirsh A. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112:1206–1218. doi: 10.1002/jcb.23042. [DOI] [PubMed] [Google Scholar]
- 27.Bieback K., Kern S., Kluter H., Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 28.Erices A., Conget P., Minguell J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 29.Secco M., Zucconi E., Vieira N.M., Fogaca L.L., Cerqueira A., Carvalho M.D. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146–150. doi: 10.1634/stemcells.2007-0381. [DOI] [PubMed] [Google Scholar]
- 30.Morigi M., Rota C., Montemurro T., Montelatici E., Lo Cicero V., Imberti B. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28:513–522. doi: 10.1002/stem.293. [DOI] [PubMed] [Google Scholar]
- 31.Manca M.F., Zwart I., Beo J., Palasingham R., Jen L.S., Navarrete R. Characterization of mesenchymal stromal cells derived from full-term umbilical cord blood. Cytotherapy. 2008;10:54–68. doi: 10.1080/14653240701732763. [DOI] [PubMed] [Google Scholar]
- 32.Kim J., Lee Y., Kim H., Hwang K.J., Kwon H.C., Kim S.K. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gosden C.M. Amniotic fluid cell types and culture. Br Med Bull. 1983;39:348–354. doi: 10.1093/oxfordjournals.bmb.a071847. [DOI] [PubMed] [Google Scholar]
- 34.In't Anker P.S., Scherjon S.A., Kleijburg-van der Keur C., de Groot-Swings G.M., Claas F.H., Fibbe W.E. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 35.Chen P.M., Yen M.L., Liu K.J., Sytwu H.K., Yen B.L. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18:49. doi: 10.1186/1423-0127-18-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z.Y., Teoh S.H., Chong M.S., Schantz J.T., Fisk N.M., Choolani M.A. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27:126–137. doi: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 37.Karagianni M., Brinkmann I., Kinzebach S., Grassl M., Weiss C., Bugert P. A comparative analysis of the adipogenic potential in human mesenchymal stromal cells from cord blood and other sources. Cytotherapy. 2013;15:76–88. doi: 10.1016/j.jcyt.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Moerman E.J., Teng K., Lipschitz D.A., Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee O.K., Kuo T.K., Chen W.M., Lee K.D., Hsieh S.L., Chen T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 40.Goodwin H.S., Bicknese A.R., Chien S.N., Bogucki B.D., Quinn C.O., Wall D.A. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7:581–588. doi: 10.1053/bbmt.2001.v7.pm11760145. [DOI] [PubMed] [Google Scholar]
- 41.De Gemmis P., Lapucci C., Bertelli M., Tognetto A., Fanin E., Vettor R. A real-time PCR approach to evaluate adipogenic potential of amniotic fluid-derived human mesenchymal stem cells. Stem Cells Dev. 2006;15:719–728. doi: 10.1089/scd.2006.15.719. [DOI] [PubMed] [Google Scholar]
- 42.Huang W., Chung U.I., Kronenberg H.M., de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc Natl Acad Sci U S A. 2001;98:160–165. doi: 10.1073/pnas.011393998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng L.J., Wheatley S., Muscat G.E., Conway-Campbell J., Bowles J., Wright E. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 44.Mwale F., Stachura D., Roughley P., Antoniou J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res. 2006;24:1791–1798. doi: 10.1002/jor.20200. [DOI] [PubMed] [Google Scholar]
- 45.Barry F., Boynton R.E., Liu B., Murphy J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 46.Boeuf S., Richter W. Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Res Ther. 2010;1:31. doi: 10.1186/scrt31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derfoul A., Perkins G.L., Hall D.J., Tuan R.S. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24:1487–1495. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- 48.Berg L., Koch T., Heerkens T., Bessonov K., Thomsen P., Betts D. Chondrogenic potential of mesenchymal stromal cells derived from equine bone marrow and umbilical cord blood. Vet Comp Orthop Traumatol. 2009;22:363–370. doi: 10.3415/VCOT-08-10-0107. [DOI] [PubMed] [Google Scholar]
- 49.Buechli M.E., Lamarre J., Koch T.G. MicroRNA-140 expression during chondrogenic differentiation of equine cord blood-derived mesenchymal stromal cells. Stem Cells Dev. 2013;22:1288–1296. doi: 10.1089/scd.2012.0411. [DOI] [PubMed] [Google Scholar]
- 50.You Q., Tong X., Guan Y., Zhang D., Huang M., Zhang Y. The biological characteristics of human third trimester amniotic fluid stem cells. J Int Med Res. 2009;37:105–112. doi: 10.1177/147323000903700112. [DOI] [PubMed] [Google Scholar]
- 51.Park J.S., Shim M.S., Shim S.H., Yang H.N., Jeon S.Y., Woo D.G. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-beta3. Biomaterials. 2011;32:8139–8149. doi: 10.1016/j.biomaterials.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 52.Kunisaki S.M., Fuchs J.R., Steigman S.A., Fauza D.O. A comparative analysis of cartilage engineered from different perinatal mesenchymal progenitor cells. Tissue Eng. 2007;13:2633–2644. doi: 10.1089/ten.2006.0407. [DOI] [PubMed] [Google Scholar]
- 53.Preitschopf A., Zwickl H., Li K., Lubec G., Joo G., Rosner M. Chondrogenic differentiation of amniotic fluid stem cells and their potential for regenerative therapy. Stem Cell Rev. 2012;8:1267–1274. doi: 10.1007/s12015-012-9405-4. [DOI] [PubMed] [Google Scholar]
- 54.Kolambkar Y.M., Peister A., Soker S., Atala A., Guldberg R.E. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol. 2007;38:405–413. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.