Abstract
Understanding the regulation of pancreatic development is key for efforts to develop new regenerative therapeutic approaches for diabetes. Rare mutations in PDX1 and PTF1A can cause pancreatic agenesis, however, most instances of this disorder are of unknown origin. We report de novo heterozygous inactivating mutations in GATA6 in 15/27 (56%) individuals with pancreatic agenesis. These findings define the most common cause of human pancreatic agenesis and establish a key role for the transcription factor GATA6 in human pancreatic development.
The genetic basis for most instances of pancreatic agenesis is unknown; mutations in PDX1 (MIM#260 370) and PTF1A (MIM#609 069) have been reported in only five families1-3. We studied a cohort of 27 individuals with pancreatic agenesis, defined as neonatal diabetes requiring insulin treatment and exocrine pancreatic insufficiency requiring enzyme replacement therapy, born to non diabetic parents. In all subjects for whom pancreatic imaging was performed (n=21), there was a complete absence (n=16) or marked hypoplasia of the pancreas. We found one affected subject to have a homozygous PTF1A splice site mutation, but we identified no mutations in PDX1 in this cohort. A common recessive etiology was unlikely in the remaining 26 affected subjects, as only 5 were known to have consanguineous parents, and none had affected siblings. We therefore hypothesized that pancreatic agenesis resulted, at least in some individuals, from de novo heterozygous mutations.
We initially sought to find de novo mutations by sequencing the exomes of two affected individuals and their unaffected parents. We performed exome capture by in-solution hybridization followed by massively parallel sequencing (Supplementary Methods) and generated between 4.2 and 7.0 billion bases covering 87-90% of the targeted Consensus Coding Sequence bases with at least ten reads (Supplementary Table 1). We called the variants using the Genome Analysis Toolkit and filtered them by removing synonymous variants, variants present in the dbSNP or 1000 Genomes Project databases and variants identified in either parent. This filtering reduced the number of potentially pathogenic de novo mutations to a single heterozygous GATA6 mutation in each subject (Supplementary Table 2). The two variants seen in GATA6 were a missense substitution (p.Thr452Ala) and an 8-bp frameshift deletion (c.1448_1455del). Sanger sequencing confirmed that the mutations were present in the two affected subjects but not in their unaffected parents.
We identified a further 12 heterozygous mutations in 13 affected individuals by sequencing the coding exons and intron-exon boundaries (exons 2-7; the primer sequences used are listed in Supplementary Table 3) of GATA6 in the remaining 24 individuals with pancreatic agenesis of unknown genetic cause. We found these to be missense mutations, frameshift insertions and/or deletions, or splicing mutations (Fig. 1a and Supplementary Table 4). Therefore, we found GATA6 mutations to be present in the majority (15/27 (56%)) of subjects with pancreatic agenesis.
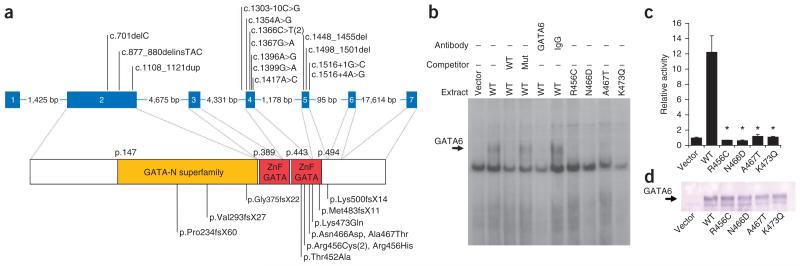
Figure 1. GATA6 mutations causing pancreatic agenesis.
(a) Genomic and protein positions of the 14 GATA6 mutations. (b) Electrophoretic mobility shift assay showing that mutations abolish the binding to a predicted GATA6 binding sequence in the pancreatic HNF4A proximal promoter. We used nuclear extracts from cells transfected with a control vector or vectors expressing GATA6 wild-type (WT) or mutant (Mut) proteins (described at the level of the protein changes shown in fig. 1a). Only wild-type GATA6 formed a retardation complex (arrow) that disappeared after preincubation with unlabeled wild-type but not mutated double-stranded oligonucleotide probes (competitor) and with GATA6 antiserum. Identical results were observed with in vitro translated wild-type and mutant GATA6 proteins using the TFF2 GATA6 binding site (data not shown). (c) Mutated GATA6 does not activate the GATA6-responsive WNT2 promoter-luciferase gene in HeLa cells. *Statistically significant difference in activity as compared to wild type (P < 0.0001). (d) Protein blot showing comparable expression of wild-type and mutant GATA6 proteins.
The genetic evidence to support the pathogenicity of the GATA6 mutations is very strong. First, in 12/15 affected subjects, both parental DNA samples were available, and a combination of Sanger sequencing and a microsatellite analysis (Supplementary Methods) established that in all of these subjects, the mutations had arisen de novo. Second, five mutations are insertions or deletions resulting in a premature termination codon and three are predicted to cause aberrant splicing (Supplementary Methods). Third, none of the mutations has been reported in 1,094 population controls (from the 1000 Genomes Project database).
GATA6 is a transcription factor that includes two tandem GATA zinc fingers that together function as a DNA-binding domain. All six missense mutations that we found affect residues on the DNA binding surface (Supplementary Fig. 1) that are conserved across vertebrate orthologs of GATA6 and in all human GATA proteins (Supplementary Fig. 2). Consistent with this observation, GATA6 proteins carrying four different missense mutations did not bind to GATA6 recognition sites and were unable to activate a GATA6-responsive promoter (Fig. 1b-d). Thus, genetic and molecular studies indicate that pancreatic agenesis is caused by inactivating GATA6 mutations.
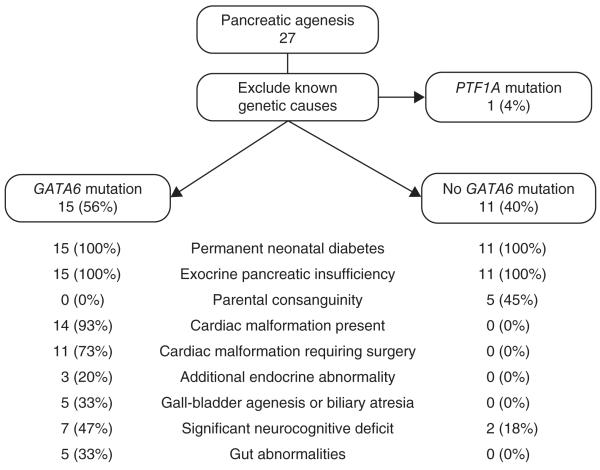
In addition to pancreatic agenesis (Supplementary Fig. 3), we frequently observed other phenotypes in individuals with GATA6 mutations (Fig. 2). The most common phenotypes (seen in 14/15 affected subjects) were congenital cardiac defects, particularly outflow tract malformations such as atrial septal defects, ventral septal defects or tetralogy of Fallot. A clinical syndrome of pancreatic agenesis and congenital heart malformations has been previously described in six individuals4, but the only prior reports of GATA6 mutations were in nine individuals with congenital heart malformations who accounted for 1.5% of all individuals tested in those studies5-7. There is no previous report of endocrine or exocrine pancreatic failure in any individual with a GATA6 mutation. Other features present in our cohort included congenital biliary tract anomalies, gut developmental disorders including hernia, neurocognitive abnormalities and additional endocrine abnormalities (Supplementary Table 4). These findings, therefore, implicate GATA6 in the development of multiple organ systems, including the biliary tract, gut, pituitary and thyroid, as well as the pancreas.
Figure 2. Clinical characteristics of the pancreatic agenesis cohort.
In addition to pancreatic agenesis, GATA6 mutations cause several other phenotypes. The precise malformations seen in each subject are listed in Supplementary Table 4.
It was notable that the 11 affected subjects without GATA6 mutations rarely (2/11) had extra-pancreatic features and were more likely to be born to consanguineous parents than individuals with these mutations (5/11 subjects without GATA6 mutations compared to 0/15 subjects with GATA6 mutations), suggesting there is at least one as yet unidentified recessive subtype of isolated pancreatic agenesis.
This work uncovers an essential function of GATA6 in human pancreas development. In contrast to the observations reported here, Gata6 heterozygous-null mice show no obvious phenotype8, whereas Gata6 homozygous-null mice die during gastrulation, thus precluding the investigation of the role of this transcription factor in mouse pancreatic organogenesis8. Earlier studies have nevertheless provided indirect evidence that Gata6 has a role in mouse pancreas development. Gata6 is expressed in embryonic pancreatic multipotent progenitors9, and one study using tetraploid complementation revealed fewer cells expressing Pdx1 in the ventral foregut of Gata6−/− embryos compared to heterozygous embryos9. Another study showed that transgenic overexpression of a chimeric protein formed by Gata6 and the engrailed repressor inhibits pancreas development10. A conclusive demonstration that Gata6 is essential for the development of mouse pancreas requires further work.
The functional studies of missense DNA-binding-domain mutations and the identification of frameshift and splicing mutations suggest that heterozygous GATA6 mutations result in loss of function and cause pancreatic agenesis through haploinsufficiency. This is in contrast to PDX1 and PTF1A mutations, where pancreatic agenesis results from a complete absence of functional protein as a result of homozygous inactivating mutations. Homozygous-null mouse models closely resemble the human phenotypes caused by biallelic mutations in PDX1, PTF1A, NEUROD1 and NEUROG3 (refs. 1,2,11,12). In contrast, as is seen with GATA6, discrepant phenotypes between mouse and man are seen in haploinsufficient forms of monogenic diabetes resulting from mutations in the transcription factor genes HNF1A, HNF4A or HNF1B (reviewed in ref. 13).
Current efforts to develop replacement therapies for diabetes focus on inducing functional endocrine cells from adult somatic cells through the expression of key transcription factors14 or through recapitulation of human pancreatic development from pluripotent cells15. Both strategies have largely exploited the knowledge of transcriptional regulators of pancreatic differentiation in mice. The key role of GATA6 in the development of the human pancreas provides new knowledge to develop tools for regenerative medicine in diabetes.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the families who participated in this study. We are grateful to A. Damhuis, A. Moorhouse and K. Paszkiewicz for their expert technical assistance. We thank H. Yamagishi (Keio University, Japan) for providing the GATA6 plasmid. S.E.F. was the Sir Graham Wilkins, Peninsula Medical School Research Fellow. S.E. and A.T.H. are employed as core members of staff within the National Institute for Health Research-funded Peninsula Clinical Research Facility. The research leading to these results received funding from Diabetes UK, the Wellcome Research Leave Award for Clinical Academics (ref 067463/Z/2/Z) and the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number 223211 (Collaborative European Effort to Develop Diabetes Diagnostics, CEED3) and grant agreement number FP7-PEOPLE-ITN-2008 (Marie Curie Initial Training Networks, Biology of Liver and Pancreatic Development and Disease).
Footnotes
COMPETING FINANCIAL INTERESTS: The authors declare no competing financial interests.
References
- 1.Sellick GS, et al. Nat. Genet. 2004;36:1301–1305. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- 2.Stoffers DA, et al. Nat. Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 3.Thomas IH, et al. Pediatr. Diabetes. 2009;10:492–496. doi: 10.1111/j.1399-5448.2009.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian M, et al. Am. J. Med. Genet. A. 2010;152a:340–346. doi: 10.1002/ajmg.a.33194. [DOI] [PubMed] [Google Scholar]
- 5.Kodo K, et al. Proc. Natl. Acad. Sci. USA. 2009;106:13933–13938. doi: 10.1073/pnas.0904744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin X, et al. J. Hum. Genet. 2010;55:662–667. doi: 10.1038/jhg.2010.84. [DOI] [PubMed] [Google Scholar]
- 7.Maitra M, et al. Pediatr. Res. 2010;68:281–285. doi: 10.1203/PDR.0b013e3181ed17e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrisey EE, et al. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt AJ, et al. BMC Dev. Biol. 2007;7:37. doi: 10.1186/1471-213X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decker K, et al. Dev. Biol. 2006;298:415–429. doi: 10.1016/j.ydbio.2006.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubio-Cabezas O, et al. Diabetes. 2011;60:1349–1353. doi: 10.2337/db10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubio-Cabezas O, et al. Diabetes. 2010;59:2326–2331. doi: 10.2337/db10-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Servitja JM, Ferrer J. Diabetologia. 2004;47:597–613. doi: 10.1007/s00125-004-1368-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, et al. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, et al. Nat. Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.