Abstract
Lipoproteins in plasma transport lipids between tissues, however, only high density lipoproteins (HDL) appear to traverse the blood brain barrier; thus, lipoproteins found in the brain must be produced within the central nervous system. Apolipoproteins E (ApoE) and ApoJ are the most abundant apolipoproteins in the brain, are mostly synthesized by astrocytes and are found on HDL. In the hippocampus and other brain regions lipoproteins help regulate neurobehavioral functions by processes that are lipoprotein receptor-mediated. Moreover, lipoproteins and their receptors also have roles in the regulation of body weight and energy balance, i.e. through lipoprotein lipase (LPL) and the LDL receptor-related protein (LRP). Thus, understanding lipoproteins and their metabolism in the brain provides a new opportunity with potential therapeutic relevance.
Keywords: lipoprotein, metabolism, brain, lipoprotein receptor, neuron, astrocyte
Lipoprotein Metabolism in the Systemic Circulation
Plasma lipoproteins contain lipids and proteins, and transport lipids in a polar environment. Four major classes of lipids are found in lipoproteins: triacylglycerols (TGs), unesterified cholesterol, cholesteryl esters (CE), and phospholipids. Being the most polar, phospholipids are associated with apolipoproteins and surround the less polar lipids. Apolipoproteins serve as enzyme cofactors and receptor ligands. Lipid delivery by lipoproteins and their processing are regulated by lipoprotein receptors located on cell surfaces such as low density lipoprotein receptor and LDL receptor-related protein (LRP), as well as other proteins associating with lipoproteins, e.g. lipoprotein-associated phospholipase A2 (LpPLA2) and serum amyloid A (SAA). Recent evidence also suggests that circulating extracellular microRNAs (miRNAs) are associated with lipoproteins for their transfer between cells 1.
Lipoproteins are traditionally classified by density. The larger lipoprotein particles – chylomicrons and very low density lipoproteins (VLDL) are the TG-rich lipoproteins produced by the intestine and liver. Low density lipoproteins (LDL) are predominantly formed by the hydrolysis of TG-rich lipoproteins by lipoprotein lipase (LPL). High density lipoproteins (HDL) are also secreted by the intestine and liver as nascent ApoA-I/phospholipid discs which incorporate cholesterol through the ATP binding cassette transporter proteins. HDL increases in lipid content and size when TG-rich lipoproteins are processed by LPL, and facilitates reverse cholesterol transport for biliary excretion. Lipoprotein metabolism has been well characterized in plasma, but much less is known about lipoproteins in the brain.
Major Differences between Lipoproteins in Plasma vs. in the Brain
Early studies of lipoproteins in brain focused on particles in the cerebrospinal fluid (CSF) 2–4. Using gel electrophoresis and electron microscopy, CSF lipoproteins were shown to be mostly spherical, resembling the size and density of plasma HDL 4. Unlike plasma, the most abundant apolipoprotein in CSF lipoproteins is ApoE, usually localized to the largest particles3. ApoA-I and ApoA-II are present on smaller particles, and ApoJ distributed across the particle size range. Other apolipoproteins such as ApoA-IV, ApoD, and ApoH are also found in the CSF. In addition, some CSF lipoproteins are found to be associated with amyloid beta (Aβ), suggesting a role for lipoproteins in Aβ polymerization, transport and clearance 2. Another source of lipoproteins in the CSF is the choroid plexus. For example, apo B-containing lipoproteins, which can be identified in CSF, are found at a concentration consistent with porous diffusion enhanced by CSF secretion 5,6.
Despite the existence of the blood-brain barrier (BBB), some of the smaller circulating HDL lipoproteins can enter the brain 2,4. However, when mice are injected with adenovirus containing human apoE isoform 3 (apoE3), ApoE3 protein is found in plasma lipoproteins at high levels, yet remains undetectable in CSF 7. In brain, ApoE is expressed predominantly by astrocytes and microglia, and in reduced quantity in neurons, while ApoJ is expressed in astrocytes, neurons, and the ependymal cells lining the ventricle 8. The majority of the ApoE- and ApoJ-containing lipoproteins found in CSF are thought to originate from surrounding astrocytes. Cholesterol is the most studied lipid in the brain. In the brain, the BBB necessitates that cholesterol homeostasis be maintained by local synthesis, and this process has been studied in detail in mice 9. Cholesterol transporter Niemann Pick type C protein 1 and cholesterol-24S-hydroxylase are essential for cholesterol metabolism in the brain; while changes in the plasma cholesterol concentration or loss of function of ATP-binding cassette AI transporter (ABCA1), scavenger receptor class B, type I (SR-BI), LDL receptor (LDLr), ApoE or ApoA-I have no effect on sterol turnover in the brain 9. Overall, data strongly suggest that during early development, cholesterol originates entirely from local synthesis, but in the adult there is a constant excretion of sterol from the brain into the plasma 9.
Lipoprotein Synthesis, Assembly and Metabolism in Astrocytes and Other Cell Types in the Brain
Astrocytes are specialized star-shaped glial cells that surround and support neurons to provide essential nutrients for neuronal growth and function. Astrocytes are also considered to be the major sites for synthesis of lipoprotein constituents and lipoprotein assembly in the brain. However, recent evidence suggests that at least for cholesterol, astrocytes and neurons cooperate in the regulation of its synthesis and redistribution in the brain 10. Specifically, selected enzymatic steps and precursors in the biosynthesis of cholesterol differ in cultured astrocytes vs. neurons. In addition, different mechanisms appear to regulate cholesterol efflux from neurons and astrocytes, reflecting the different roles these cell types play in brain cholesterol homeostasis. For example, astrocytes produce and release ApoE, while neurons metabolize cholesterol to 24(S)-hyroxycholesterol. Cholesterol efflux from astrocytes is facilitated by apolipoproteins alone or lipoprotein particles, while cholesterol removal from neurons is triggered only by lipoprotein particles. ABCA1- and ABCG1-regulated cholesterol efflux occurs only in astrocytes while ABCG4-mediated cholesterol efflux happens only in neurons 11. Furthermore, the newly synthesized cholesterol is rarely converted to CE, and is quickly re-distributed among various cell types within the brain (reviewed in 10). Moreover, the half-life of brain-derived cholesterol is much longer (up to 5 years) compared to that of days in the periphery, with extensive re-distribution and transportation via ATP-binding cassette transporters and HDL-like lipoproteins respectively, as the main mechanism to maintain homeostasis.
Apolipoproteins are expressed at higher levels in astrocytes than the rest of the brain. The mRNA and protein levels of ApoE and ApoJ are quite age-dependent - with ApoJ increasing ~5–10 fold and ApoE levels dropping with aging 12. ApoJ is a ubiquitous multifunctional glycoprotein and its expression in the brain is up-regulated in response to neuronal damage, brain injury and other stress; and ApoJ has been proposed to play a role in Aβ clearing 13. ApoJ-containing lipoprotein particles usually contain the least amount of lipid, while ApoE-containing lipoproteins carry the most lipid, and are the largest in size 14.
The role of ApoE-containing lipoproteins in the brain has been extensively studied and reviewed. In brief, three major functions have been suggested for astrocyte-derived ApoE-containing lipoproteins: 1) the transfer of phospholipids and cholesterol via ATP-binding cassette (ABC) transporters such as ABCA1 and ABCG1 15; 2) interaction with the LDL receptor superfamily of proteins located on the surface of neurons to facilitate axonal growth and neuronal survival 16; and 3) interaction with the LDL receptor-related protein 1 (LRP1)-dependent cellular uptake pathway, in the deposition of amyloid plaques 17,18. Although there is minimal direct interaction between ApoE and soluble Aβ in CSF 19, apoE isoforms in ApoE-containing lipoprotein complexes can regulate the metabolism of soluble Aβ bycompeting for the binding of LRP1 with Aβ in astrocytes 19,20.
ApoE knockout mice placed on a diet enriched in homocysteine to induce oxidative stress, show impaired learning and memory 21. Out of the three major isoforms of apoE – apoE2, apoE3, and apoE4, apoE4 confers the major risk for Alzheimer’s disease (AD). The expression of apoE4 allele usually results in increased expression of apoC1 22 Mice overexpressing human apoC1 also display impaired learning and memory 23. Interestingly apoC1−/− mice also show impaired hippocampal-dependent memory with no gross changes in brain morphology or brain cholesterol levels, but increased expression of the proinflammatory marker tumor necrosis factor-α 24.
With all the evidence discussed above several major questions remain: 1) What is the role of neurons in the synthesis and regulation of lipoprotein metabolism? 2) Can different lipoprotein particles enter neurons or be recognized by surface markers on neurons? 3) What are the major functions of lipoproteins in the brain – is it simply lipid delivery and/or are they carriers for various biological molecules? Some of the answers to these questions may relate to the existence and properties of certain lipid structures in the brain, and the cell type and region-specific expression of lipoprotein receptors in the brain.
Lipid Rafts and Neuronal Porosomes
Lipid rafts are cholesterol enriched domains in bio-membranes that serve as the preferential clustering site of membrane signaling proteins. Lipid rafts exist in both neurons and astrocytes 25. In addition to cholesterol and sphingolipids, saturated fatty acids are enriched in lipid rafts. In systemic circulation, lipid rafts serve as a signaling platform linking lipoprotein metabolism to atherosclerosis 26. In neurodegenerative diseases, lipid raft disarrangement may be an early marker for diagnosis 27. Size-exclusion chromatography and electron microscopy have been used to study the lipid composition of nascent HDL formed by ABCA1. The proportions of free cholesterol, glycerophosphocholine, and sphingomyelin are similar between nascent HDL and lipid rafts 28, suggesting a possible role of lipid rafts in lipoprotein assembly in addition to their role in facilitating lipoprotein-mediated lipid exchange.
Porosomes are universal secretory portals at the plasma membrane that facilitate the transient docking and fusion of membrane-bound secretory vesicles to exchange intravesicular contents to the outside 29. In neurons, 12 to 17-nm cup-shaped porosome structures are present at the presynaptic membrane where 40–50 nm synaptic vesicles transiently dock and fuse to release neurotransmitters 30. The neuronal porosome complex has been isolated, its composition determined, and it has been structurally and functionally reconstituted in artificial lipid membranes 31,32. Cholesterol was found to be an integral component of the neuronal porosome complex, and critical to the stability of the porosome/fusion pore 33,34. Porosomes are also found in astrocytes with similar structure but smaller in size (10–15 nm) 35,36. Proteomic analysis of neuronal porosomes revealed the absence of any typical lipoprotein-associated proteins, yet this does not exclude the possibility that porosomes can serve as a platform for lipoprotein-mediated lipid exchange in the brain.
Lipoprotein Receptors in the CNS
The LDL receptor superfamily of proteins are a class of single membrane spanning receptors that bind Apo B100 or ApoE and also endocytose a variety of distinct extracellular proteins. In peripheral tissues, the tissue or cell type-specific presence of different lipoprotein receptors dictates where lipoprotein particles will be docked and lipoprotein-mediated lipid exchange occur. For example, HDL binds to a different set of cell surface receptors, some for cholesterol efflux, i.e. ABCA1 and ABCG1; and others for uptake and degradation, i.e. scavenger receptor class B type 1 (SR-B1). Some of these receptors are also expressed in the brain.
Scavenger receptor class B type 1 (SR-BI)
In the periphery, SR-BI mediates the selective uptake of HDL-associated CE independent from HDL internalization. In the brain, SR-BI is expressed in endothelial cells and contributes to the selective uptake of HDL-associated CE and vitamin E (α-tocopherol), once HDL traffics through the BBB 37–39. The importance of SR-BI in regulating systemic and brain vitamin E metabolism is supported by the observation that SR-BI deficient mice display higher plasma levels of α-tocopherol accompanied by decreases in α-tocopherol in several tissues and the brain 38. Together with ABCA1 and ApoA-1, SR-BI can modulate the polarized sterol mobilization at the BBB 40. SR-BI in the brain also appears to mediate phosphatidylcholine uptake from LDL or HDL resulting in increases in fatty acid content 41. This process provides a route for generating a pool of polyunsaturated fatty acids (PUFA)-containing lipids for transport into deeper brain regions.
Low density lipoprotein receptor (LDLr)
LDLr knock out (LDLr−/−) mice show no major deficits in sensory or motor function, but exhibit increased locomotor activity 42. LDLR−/− mice also show a decrease in learning and memory regardless of diet 43. Cholesterol-enriched diets increase plasma cholesterol levels, which relate to an increase in oxidative stress, decrease in the mitochondrial complex I and II activities in the cerebral cortex, and cognitive impairment 43. In aged LDLr−/− mice that are exposed to cholesterol-enriched diets earlier in life, antioxidant imbalance and oxidative damage are evident by a marked increase in lipid peroxidation and an increase in acetylcholinesterase activity in the prefrontal cortex. Both working and spatial preference as well as procedural memory is impaired without alterations in motor function 44.
Very low-density lipoprotein receptor (VLDLr) and apolipoprotein E receptor 2 (ApoEr2)
VLDLr binds TG-rich lipoproteins but not LDL, and in the periphery, also serves as a remnant lipoprotein receptor. The VLDLr in various tissues usually functions in concert with LPL. In the brain, various mutations in VLDLr have been associated with disequilibrium syndrome (DES) 45,46, autosomal recessive cerebellar hypoplasia leading to mental retardation 47, and quadrupedal locomotion in humans 48. In developing brain, the VLDLr plays a role in neuronal migration along with ApoEr2, a cell surface receptor and part of the low density lipoprotein receptor family with a 50% sequence homology to VLDLr. VLDLr/ApoEr2 double knockout mice have a disruption of cortical layering and cerebellar dysmorphology 49. VLDLr knockout mice alone display contextual fear conditioning deficits and a moderate defect in long term potentiation (LTP) 50. ApoEr2 knockout mice display similar deficits in contextual fear conditioning and a more pronounced defect in LTP 50, and decreased dendritic spine density in cortical layers II/III 51 yet cholesterol and PUFA concentrations throughout the brain are not different 52. An earlier study using radiolabelled fatty acids incorporated into lipoproteins suggest indirectly that circulating fatty acids within VLDL's do not enter the brain in the awake rodent 53. These data suggest that deficits in VLDLr−/− and ApoEr2−/− mice are more related to the reelin-dependent pathway that regulates processes of neuronal migration and positioning, and synaptic plasticity through modulation of N-Methyl-D-aspartic acid (NMDA) receptor activity 50,54 in complex with the post-synaptic protein PSD-95 54 in the brain.
LDL receptor-related protein 1 (LRP1)
LRP1 is an endocytic receptor that mediates the cellular uptake of various ligands including chylomicron remnants. In brain, LRP1 is expressed at high levels in both glial and neuronal cells to mediate endocytosis of ligands, regulate calcium influx into neurons after stimulation with NMDA, interact with amyloid precursor protein to regulate Aβ clearing, and interact with PSD-95 to regulate synaptic transmission 55–59. LRP1 forebrain knockout mice (using CaMKII-driven Cre) display impaired brain lipid metabolism with decreased cholesterol and TG in cortex, an age-dependent decrease in spine density in pyramidal neurons in the cortex and CA1 region in the hippocampus, and progressive neurobehavioral abnormalities characterized by impaired memory, motor function, and reduced LTP 60.
Novel Roles of Lipoproteins in the CNS: Lipoprotein Metabolism in the Neurons
Many of the lipoprotein receptors discussed above are expressed in both astrocytes and neurons. Even though astrocytes are considered to be the major cells of larger lipoprotein assembly, increasing evidence suggests that lipoprotein components synthesized by neurons might be important in regulating lipoprotein metabolism in the brain. Data from neuron-specific modification of LRP1 discussed above are in line with such hypothesis.
With the recent development of the apoE knockout and knockin mouse models, it is feasible to relate the ApoE protein made in different cell types in the brain to its potential unique biological activities 61. ApoE knock-in mice have been generated on a background of apoE−/− mice to express human apoE isoforms either in astrocytes or neurons. Expression of the human ApoE3 isoform protects the neuronal synapses and dendrites from the excitotoxicity seen in apoE deficient mice. Astrocyte-derived ApoE4 displays protective effects similar to ApoE3, whereas neuronal expression of ApoE4 results in loss of cortical neurons after an excitotoxic challenge 62. Neuronal apoE4 knockin mice also display more severe and widespread deficits in dendritic arborization as well as spine density and morphology, than astrocyte apoE4 knockin mice 63. The cellular source of ApoE also impacts its role in the clearance of Aβ. During the Aβ clearing process, astrocyte-derived ApoE gets drained into the perivascular space (PVS) while neuronal ApoE4 does not, and when these knockin mice are crossed with amyloid precursor protein (APP) transgenic mice, both neuronal and astrocyte-derived ApoE4 are found to be co-localized in the PVS, and astrocytes take up the neuronal ApoE4 bound to Aβ, but not neuronal ApoE4 alone 64.
Recent studies in neuron-specific LRP1−/− mice suggest another role for lipoprotein metabolism in the CNS. LRP1 forebrain knockout mice display increased food intake, decreased energy consumption and decreased leptin signaling, resulting in obesity 65. Direct injection of cre lentivirus into the arcuate nucleus of the hypothalamus of LRP1 Flox-P mice that leads to region-specific neuronal LRP1 deletion resulted in increased food intake, markedly increased NPY and AgRP gene expression, and greater body weight gain. Neuronal inactivation of LRP1 via the synapsin-driven cre also leads to increases in food intake, but these mice die prematurely between 6~9 mo due to hyper-catabolism66.
The potential role of lipoprotein-derived lipid in the CNS regulation of energy balance is most strongly supported by data from a neuron-specific LPL deficient mouse model 67. Deficiency of LPL, the rate limiting enzyme in the hydrolysis of TG-rich lipoproteins in the periphery 68 in neurons increases food intake, reduces energy expenditure, up-regulates neuropeptide AgRP and NPY gene expression, reduces PUFA concentrations in TG and FFA pools in the hypothalamus, reduces TG-rich lipoprotein-derived lipid uptake in the hypothalamus, and results in obesity even on a chow diet 67. LPL mRNA expression is detected in many regions of the brain, and in both neurons and astrocytes, with the hippocampal area having the highest levels of expression 69. Very interestingly, general LPL knockout mice rescued from neonatal lethality by somatic gene transfer (no exogenous or endogenous LPL expression in the brain) display impaired learning and memory function compared to other neuron-specific or general knockout mouse models of many lipoprotein receptors described earlier 69. These neuron-specific LPL deficient mice also show reductions of the presynaptic marker synaptophysin (instead of the postsynaptic marker PSD95) in the hippocampus, a decreased number of presynaptic vesicles, and reductions of vitamin E content in the brain 70.
Phenotypes related to the specific functions of lipid-related genes in the brain are summarized in Table 1. Based on the data accrued from these genetically-modified mice and the data from ex vivo experimentation discussed above, a number of common themes emerge: 1) Most of the lipoprotein receptors that recognize larger particles (LDLr, VLDLr, ApoER2 and LRP1) relate to certain types of neurobehavioral functions 2) Only the traditional HDL receptor SR-BI and the LRP1 seem to modify brain cholesterol, TG, and vitamin E content. 3) Although the lipoprotein receptors for larger lipoproteins are not essential for maintaining brain lipid content, various knockout mice do display phenotypes in response to dietary perturbations, implying a potential link between lipoprotein metabolism in the CNS and dietary lipids.
Table 1.
Neurological Phenotype of Mice with Genetic Modification of Lipoprotein- Related Genes
| Gene Modified |
Loss or Gain of Function |
Cell or Tissue specificity |
Brain Phenotype | Behavioral Phenotype |
Notes | Refs |
|---|---|---|---|---|---|---|
| apoE | Loss | whole body | ↓ neuronal synapses & dendrites from excitotoxicity | ↓ learning & memory | On a homocysteine enriched diet | 21 |
| apoE3 in apoE−/− | Replacement | Whole body | Protects against ↓ neuronal synapses & dendrites from excitotoxicity | 62 | ||
| apoE4 in apoE−/− | Replacement | Astrocyte | Protects against ↓ neuronal synapses & dendrites from excitotoxicity | 63 | ||
| apoE4 in apoE−/− | Replacement | Neuron | ↓ cortical neurons after an excitotoxic challenge, more severe ↓ in dendritic arborization & spine density & morphology | 63 | ||
| ApoC1 | Gain | Whole body | ↓ learning & memory | 23 | ||
| ApoC1 | Loss | Whole body | ↓ learning & memory | 24 | ||
| SR-BI | Loss | Whole body | ↑ brain α-tocopherol | 38 | ||
| LDLr | Loss | Whole body | ↑ in lipid peroxidation, ↑ in oxidative stress | ↓ learning & memory, ↑ locomotor activity | Cholesterol enriched diet for metabolic phenotypes | 42,43, 44 |
| VLDLr | Loss | Whole body | ↓ contextual fear conditioning & a moderate ↓ in long term potentiation (LTP) | 50 | ||
| ApoEr2 | Loss | Whole body | ↓ dendritic spine density in cortical layers II/III | contextual fear conditioning ↓ & moderate ↓ in long term potentiation (LTP) | 50, 51, 52 | |
| VLDLr /ApoEr2 | Loss | Whole body | disruption of cortical layering & cerebellar dysmorphology | 49 | ||
| LRP1 | Loss | CaMKII-driven Cre, forebrain | obesity, ↑ food intake | ↓ memory, motor function, & ↓ LTP | ↓ cholesterol & TG in cortex, | 60, 65 |
| LRP1 | Loss | synapsin-driven cre | ↑ food intake | Died earlier due to hyper-catabolism | 66 | |
| LRP1 | Loss | hypothalamus | ↑ food intake, obesity | ↑ AgRP & NPY levels | 65 | |
| LPL | Loss | Whole body rescued | ↓ learning & memory function | 69 | ||
| LPL | Loss | Neuron | ↑ food intake, obesity | ↓ PUFA, increased AgRP, NPY | 67 |
Concluding remarks and future perspectives
Several key questions arise from the evidence discussed above with regards to the exact role of neurons in the synthesis and regulation of lipoprotein metabolism, the entry way of different lipoprotein particles in neurons or the major functions of lipoproteins in the brain (see outstanding questions box). We suspect that some of the answers to these questions may relate to the existence and properties of certain lipid structures in the brain, and the cell type and region-specific expression of lipoprotein receptors in the brain, and are a fruitful area for future investigation.
In conclusion, lipoprotein metabolism is important, tightly regulated, and involved in many functional processes in the brain. The coordinated synthesis, assembly, remodeling, and transport of lipoprotein particles in the brain are evident, but detailed mechanisms remain to be elucidated (Figure 1). Considering the essential role of LPL in peripheral lipoprotein metabolism, the functional roles of brain LPL protein in both energy balance (a proposed hypothalamic mechanism) and cognitive function (a proposed hippocampal mechanism), and the data from other lipoprotein receptors, it is tempting to suggest that lipoprotein-derived molecules provide signals to mediate essential pathways in different brain regions that contributes to neuronal plasticity underlying many important brain functions.
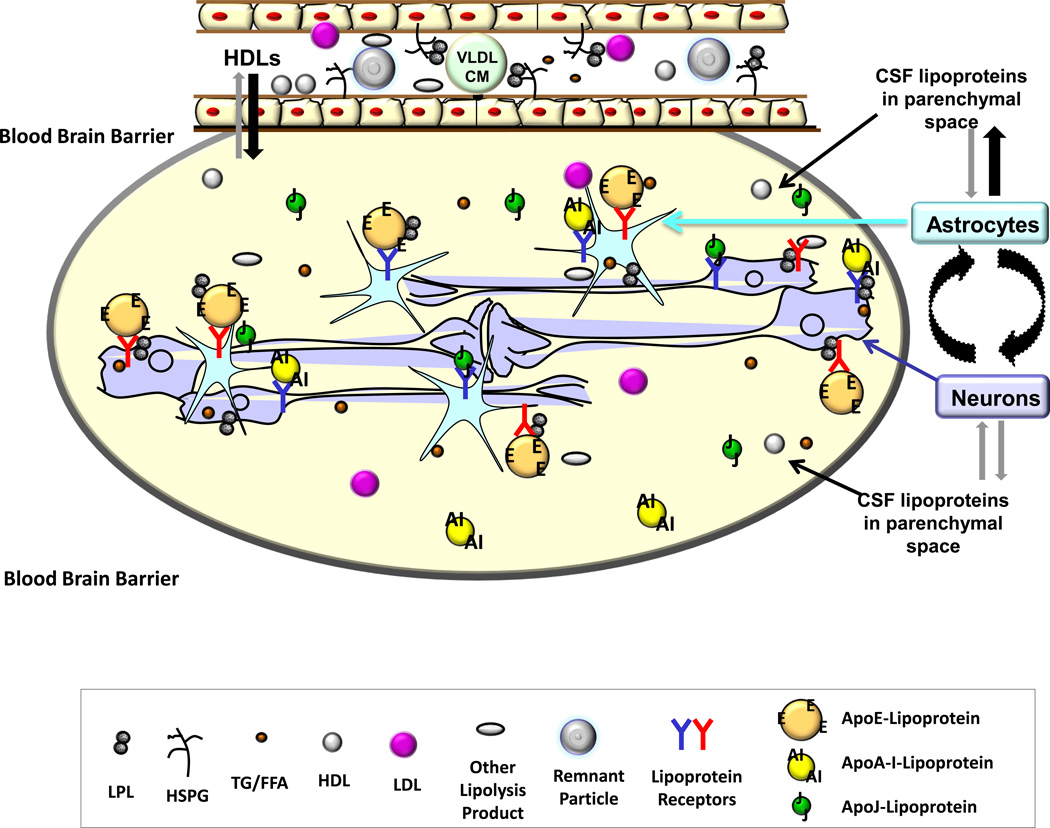
Figure 1. Lipoprotein Metabolism in the CNS.
Due to the blood brain barrier (BBB), the exchange of lipoprotein particles between the systemic circulation and the CNS is minimal, with some smaller HDL-like particles able to traverse. Most of the lipoproteins inside the CNS originate from astrocytes, although many of the lipoprotein constituents can be synthesized and processed differently in neurons. Lipoprotein particles are constantly synthesized, assembled, exchanged, and modified between astrocytes and neurons. Astrocytes can secrete lipoprotein particles into the cerebrospinal fluid (CSF) or reabsorb the smaller particles for remodeling and reloading of lipids. Lipoprotein receptors and lipoprotein lipase (LPL) located on the surface of astrocytes and neurons appear to play a regulatory role in lipoprotein metabolism in the CNS, effects that are brain region-specific.
Box: Outstanding questions.
What is the role of neurons in the synthesis and regulation of lipoprotein metabolism?
Can different lipoprotein particles enter neurons or be recognized by surface markers on neurons?
What are the major functions of lipoproteins in the brain – is it simply lipid delivery and/or are they carriers for various biological molecules?
What is the relative contribution of TG-rich lipoprotein fatty acid delivery to the brain vs. albumin-bound FFA?
Is cholesterol turnover different in gray 330 vs. white matter?
What is the mechanism by which apoE4 contributes to the natural history of Alzheimer’s Disease?
Highlights.
Lipoproteins in the brain are mostly made in the brain.
Lipoprotein composition in the brain is different from that in the circulation.
Neurons and astrocytes coordinate lipoprotein metabolism in the brain.
Lipoproteins help regulate neurobehavioral function and energy balance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr. Opin. Lipidol. 2012;23:91–97. doi: 10.1097/MOL.0b013e328350a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladu MJ, et al. Lipoproteins in the central nervous system. Ann. N.Y. Acad. Sci. 2000;903:167–175. doi: 10.1111/j.1749-6632.2000.tb06365.x. [DOI] [PubMed] [Google Scholar]
- 3.Neely MD, Montine TJ. CSF lipoproteins and Alzheimer's disease. J. Nutr. Health Aging. 2002;6:383–391. [PubMed] [Google Scholar]
- 4.Koch S, et al. Characterization of four lipoprotein classes in human cerebrospinal fluid. J. Lipid Res. 2001;42:1143–1151. [PubMed] [Google Scholar]
- 5.Felgenhauer K, et al. Evaluation of the blood-CSF barrier by protein gradients and the humoral immune response within the central nervous system. J. Neurol. Sci. 1976;30:113–128. doi: 10.1016/0022-510x(76)90259-8. [DOI] [PubMed] [Google Scholar]
- 6.Rapoport SI, Pettigrew KD. A heterogenous, pore-vesicle membrane model for protein transfer from blood to cerebrospinal fluid at the choroid plexus. Microvasc. Res. 1979;18:105–119. doi: 10.1016/0026-2862(79)90020-7. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, et al. Apolipoprotein E does not cross the blood-cerebrospinal fluid barrier, as revealed by an improved technique for sampling CSF from mice. Am. J. Physiol Regul. Integr. Comp Physiol. 2012;303:R903–R908. doi: 10.1152/ajpregu.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vance JE, Hayashi H. Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim. Biophys. Acta. 2010;1801:806–818. doi: 10.1016/j.bbalip.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Quan G, et al. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res. Dev. Brain Res. 2003;146:87–98. doi: 10.1016/j.devbrainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Pfrieger FW, Ungerer N. Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res. 2011;50:357–371. doi: 10.1016/j.plipres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, et al. Cholesterol efflux is differentially regulated in neurons and astrocytes: implications for brain cholesterol homeostasis. Biochim. Biophys. Acta. 2013;1831:263–275. doi: 10.1016/j.bbalip.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott DA, et al. Apolipoproteins in the brain: implications for neurological and psychiatric disorders. Clin. Lipidol. 2010;51:555–573. doi: 10.2217/CLP.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calero M, et al. Apolipoprotein J (clusterin) and Alzheimer's disease. Microsc. Res. Tech. 2000;50:305–315. doi: 10.1002/1097-0029(20000815)50:4<305::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.DeMattos RB, et al. Purification and characterization of astrocyte-secreted apolipoprotein E and J-containing lipoproteins from wild-type and human apoE transgenic mice. Neurochem. Int. 2001;39:415–425. doi: 10.1016/s0197-0186(01)00049-3. [DOI] [PubMed] [Google Scholar]
- 15.Vance JE, Hayashi H. Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim. Biophys. Acta. 2010;1801:806–818. doi: 10.1016/j.bbalip.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi H, et al. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J. Neurosci. 2007;27:1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtzman DM, et al. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martiskainen H, et al. Targeting ApoE4/ApoE receptor LRP1 in Alzheimer's disease. Expert. Opin. Ther. Targets. 2013;17:781–794. doi: 10.1517/14728222.2013.789862. [DOI] [PubMed] [Google Scholar]
- 19.Verghese PB, et al. ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc. Natl. Acad. SciUSA. 2013;110:E1807–E1816. doi: 10.1073/pnas.1220484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladu MJ, et al. Preferential interactions between ApoE-containing lipoproteins and Abeta revealed by a detection method that combines size exclusion chromatography with non-reducing gel-shift. Biochim. Biophys. Acta. 2012;1821:295–302. doi: 10.1016/j.bbalip.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evola M, et al. Oxidative stress impairs learning and memory in apoE knockout mice. Pharmacol. Biochem. Behav. 2010;96:181–186. doi: 10.1016/j.pbb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, et al. A common Hpa I RFLP of apolipoprotein C-I increases gene transcription and exhibits an ethnically distinct pattern of linkage disequilibrium with the alleles of apolipoprotein E. J. Lipid Res. 1999;40:50–58. [PubMed] [Google Scholar]
- 23.Abildayeva K, et al. Human apolipoprotein C-I expression in mice impairs learning and memory functions. J. Lipid Res. 2008;49:856–869. doi: 10.1194/jlr.M700518-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Berbee JF, et al. Apolipoprotein CI knock-out mice display impaired memory functions. J. Alzheimers. Dis. 2011;23:737–747. doi: 10.3233/JAD-2010-100576. [DOI] [PubMed] [Google Scholar]
- 25.Sebastiao AM, et al. Lipid rafts, synaptic transmission and plasticity: impact in age-related neurodegenerative diseases. Neuropharmacology. 2013;64:97–107. doi: 10.1016/j.neuropharm.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 26.Lemaire-Ewing S, et al. Lipid rafts: a signalling platform linking lipoprotein metabolism to atherogenesis. Atherosclerosis. 2012;221:303–310. doi: 10.1016/j.atherosclerosis.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Marin R, et al. Lipid raft disarrangement as a result of neuropathological progresses: A novel strategy for early diagnosis? Neuroscience. 2013;245:26–39. doi: 10.1016/j.neuroscience.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Sorci-Thomas MG, et al. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J. Lipid Res. 2012;53:1890–1909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, et al. Neuronal porosome proteome: Molecular dynamics and architecture. J. Proteomics. 2012;75:3952–3962. doi: 10.1016/j.jprot.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho WJ, et al. Conformation states of the neuronal porosome complex. Cell Biol. Int. 2010;34:1129–1132. doi: 10.1042/CBI20100510. [DOI] [PubMed] [Google Scholar]
- 31.Cho WJ, et al. EM 3D contour maps provide protein assembly at the nanoscale within the neuronal porosome complex. J. Microsc. 2008;232:106–111. doi: 10.1111/j.1365-2818.2008.02088.x. [DOI] [PubMed] [Google Scholar]
- 32.Cho WJ, et al. Structure, isolation, composition and reconstitution of the neuronal fusion pore. Cell Biol. Int. 2004;28:699–708. doi: 10.1016/j.cellbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Jeremic A, et al. Cholesterol is critical to the integrity of neuronal porosome/fusion pore. Ultramicroscopy. 2006;106:674–677. doi: 10.1016/j.ultramic.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Cho WJ, et al. Neuronal fusion pore assembly requires membrane cholesterol. Cell Biol. Int. 2007;31:1301–1308. doi: 10.1016/j.cellbi.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JS, et al. Porosome in astrocytes. J. Cell Mol. Med. 2009;13:365–372. doi: 10.1111/j.1582-4934.2008.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho WJ, et al. Nanoscale 3D contour map of protein assembly within the astrocyte porosome complex. Cell Biol. Int. 2009;33:224–229. doi: 10.1016/j.cellbi.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Goti D, et al. Scavenger receptor class B, type I is expressed in porcine brain capillary endothelial cells and contributes to selective uptake of HDL-associated vitamin E. J. Neurochem. 2001;76:498–508. doi: 10.1046/j.1471-4159.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- 38.Mardones P, et al. Alpha-tocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)-deficient mice. J. Nutr. 2002;132:443–449. doi: 10.1093/jn/132.3.443. [DOI] [PubMed] [Google Scholar]
- 39.Balazs Z, et al. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J. Neurochem. 2004;89:939–950. doi: 10.1111/j.1471-4159.2004.02373.x. [DOI] [PubMed] [Google Scholar]
- 40.Panzenboeck U, et al. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J. Biol. Chem. 2002;277:42781–42789. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- 41.Sovic A, et al. Scavenger receptor class B, type I mediates uptake of lipoprotein-associated phosphatidylcholine by primary porcine cerebrovascular endothelial cells. Neurosci. Lett. 2004;368:11–14. doi: 10.1016/j.neulet.2004.04.097. [DOI] [PubMed] [Google Scholar]
- 42.Elder GA, et al. Increased locomotor activity in mice lacking the low-density lipoprotein receptor. Behav. Brain Res. 2008;191:256–265. doi: 10.1016/j.bbr.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de OJ, et al. Positive correlation between elevated plasma cholesterol levels and cognitive impairments in LDL receptor knockout mice: relevance of cortico-cerebral mitochondrial dysfunction and oxidative stress. Neuroscience. 2011;197:99–106. doi: 10.1016/j.neuroscience.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Moreira EL, et al. Age-related cognitive decline in hypercholesterolemic LDL receptor knockout mice (LDLr-/-): evidence of antioxidant imbalance and increased acetylcholinesterase activity in the prefrontal cortex. J. Alzheimers. Dis. 2012;32:495–511. doi: 10.3233/JAD-2012-120541. [DOI] [PubMed] [Google Scholar]
- 45.Ali BR, et al. A missense founder mutation in VLDLR is associated with Dysequilibrium Syndrome without quadrupedal locomotion. BMC. Med. Genet. 2012;13:80. doi: 10.1186/1471-2350-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moheb LA, et al. Identification of a nonsense mutation in the very low-density lipoprotein receptor gene (VLDLR) in an Iranian family with dysequilibrium syndrome. Eur. J. Hum. Genet. 2008;16:270–273. doi: 10.1038/sj.ejhg.5201967. [DOI] [PubMed] [Google Scholar]
- 47.Boycott KM, et al. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am. J. Hum. Genet. 2005;77:477–483. doi: 10.1086/444400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozcelik T, et al. Mutations in the very low-density lipoprotein receptor VLDLR cause cerebellar hypoplasia and quadrupedal locomotion in humans. Proc. Natl. Acad. SciUSA. 2008;105:4232–4236. doi: 10.1073/pnas.0710010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trommsdorff M, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 50.Weeber EJ, et al. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 2002;277:39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- 51.Dumanis SB, et al. ApoE receptor 2 regulates synapse and dendritic spine formation. PLoS. One. 2011;6:e17203. doi: 10.1371/journal.pone.0017203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahman T, et al. The very low density lipoprotein receptor is not necessary for maintaining brain polyunsaturated fatty acid concentrations. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:141–145. doi: 10.1016/j.plefa.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Purdon D, et al. No evidence for direct incorporation of esterified palmitic acid from plasma into brain lipids of awake adult rat. J. Lipid Res. 1997;38:526–530. [PubMed] [Google Scholar]
- 54.Beffert U, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Castaneda A, et al. Identification of the low density lipoprotein (LDL) receptor-related protein-1 interactome in central nervous system myelin suggests a role in the clearance of necrotic cell debris. J. Biol. Chem. 2013;288:4538–4548. doi: 10.1074/jbc.M112.384693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuo M, et al. Involvement of low-density lipoprotein receptor-related protein and ABCG1 in stimulation of axonal extension by apoE-containing lipoproteins. Biochim. Biophys. Acta. 2011;1811:31–38. doi: 10.1016/j.bbalip.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Lillis AP, et al. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Q, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi H, et al. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J. Neurosci. 2007;27:1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Q, et al. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci. 2010;30:17068–17078. doi: 10.1523/JNEUROSCI.4067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang Y. Roles of apolipoprotein E4 (ApoE4) in the pathogenesis of Alzheimer's disease: lessons from ApoE mouse models. Biochem. Soc. Trans. 2011;39:924–932. doi: 10.1042/BST0390924. [DOI] [PubMed] [Google Scholar]
- 62.Buttini M, et al. Cellular source of apolipoprotein E4 determines neuronal susceptibility to excitotoxic injury in transgenic mice. AmJPathol. 2010;177:563–569. doi: 10.2353/ajpath.2010.090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain S, et al. Cellular source-specific effects of apolipoprotein (apo) E4 on dendrite arborization and dendritic spine development. PLoS. One. 2013;8:e59478. doi: 10.1371/journal.pone.0059478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rolyan H, et al. Amyloid-beta protein modulates the perivascular clearance of neuronal apolipoprotein E in mouse models of Alzheimer's disease. J. Neural Transm. 2011;118:699–712. doi: 10.1007/s00702-010-0572-7. [DOI] [PubMed] [Google Scholar]
- 65.Liu Q, et al. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS. Biol. 2011;9:e1000575. doi: 10.1371/journal.pbio.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.May P, et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol. Cell Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, et al. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metab. 2011;13:105–113. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am. J. Physiol Endocrinol. Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Eckel RH. Lipoprotein Lipase in the Brain and Nervous System. Annu. Rev. Nutr. 2012 doi: 10.1146/annurev-nutr-071811-150703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xian X, et al. Presynaptic defects underlying impaired learning and memory function in lipoprotein lipase-deficient mice. J. Neurosci. 2009;29:4681–4685. doi: 10.1523/JNEUROSCI.0297-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]