Abstract
It is now recognized that extensive expression heterogeneities among cells precede the emergence of lineages in the early mammalian embryo. To establish a map of pluripotent epiblast (EPI) versus primitive endoderm (PrE) lineage segregation within the inner cell mass (ICM) of the mouse blastocyst, we characterised the gene expression profiles of individual ICM cells. Clustering analysis of the transcriptomes of 66 cells demonstrated that initially they are non-distinguishable. Early in the segregation, lineage-specific marker expression exhibited no apparent correlation, and a hierarchical relationship was established only in the late blastocyst. Fgf4 exhibited a bimodal expression at the earliest stage analysed, and in its absence, the differentiation of PrE and EPI was halted, indicating that Fgf4 drives, and is required for, ICM lineage segregation. These data lead us to propose a model where stochastic cell-to-cell expression heterogeneity followed by signal reinforcement underlies ICM lineage segregation by antagonistically separating equivalent cells.
Keywords: mouse, blastocyst, single-cell gene expression profile, inner cell mass, epiblast, primitive endoderm
Mammalian preimplantation development gives rise to three lineages in the blastocyst1; the embryonic epiblast (EPI) and two extraembryonic tissues, the primitive endoderm (PrE) and trophectoderm (TE). Lineage segregation between EPI and PrE occurs within the inner cell mass (ICM) of the blastocyst and involves two successive phases. First, at the morula stage (E2.5; 8-16 cells), the EPI-specific transcription factor Nanog and PrE-specific Gata62, 3 become evident and are expressed by all ICM cells. This overlapping expression persists until E3.5 (64 cells) when two distinct cell populations emerge as PrE precursors activate a sequence of transcription factors (Gata6, Sox17, Gata4 and Sox7)4, and EPI precursors co-express pluripotency-associated factors (e.g. Nanog and Sox2). As EPI and PrE markers establish mutually exclusive expression, they become arranged in a salt-and-pepper distribution2, 3. Even though biased to a specific lineage, ICM cells exhibit a plasticity preceding their sorting to respective positions when the PrE begins to epithelialize at E4.5 (>150 cells)5.
In the mouse this segregation of EPI and PrE lineages is regulated by FGF/MAPK signaling2, 6. Modulation of FGF/MAPK signaling shifts the balance of EPI and PrE cells: excess of Fgf4 converts all ICM cells to adopt a PrE identity7, whereas when FGF signaling is blocked2, 7-13, all ICM cells become Nanog-positive. How the heterogeneity in FGF signaling is established remains an open question. Two, apparently disparate, models have been proposed; a random or cleavage-history-dependent mechanism. Two-to-three ‘waves’ of asymmetric cell divisions (8-to-16-cell, 16-to-32-cell and 32-to-64-cell) generate the ICM cells. Consequently, it has been proposed14 that cells internalized during the first wave exhibit a greater bias towards EPI, whereas cells internalized later are biased to PrE15. This notion was challenged by another study that showed an apparently random generation of EPI and PrE precursors, irrespectively of internalization timing7. Importantly, an absolute correlation between lineage and cleavage pattern has not been evident from any study.
Since the emergence of lineage precursors within the ICM is preceded by stochastic gene expression variability3, we reasoned that single-cell gene expression profiling would be requisite for understanding the mechanisms driving lineage segregation. Recent technical advances enable quantitative gene expression profiling at the single-cell level using qPCR16, microarrays17, 18, or RNAseq19, 20. It is now widely recognized that cell-to-cell expression variation and multi-lineage gene activation exist early on during lineage commitment21-23. Recent single-cell expression studies demonstrated that the expression of key factors is independently regulated in the transition from self-renewal to lineage-committed states in hematopoiesis24, 25, and that early stochastic gene expression is followed by the establishment of a hierarchy during cellular reprogramming26. Although the changes in expression during blastocyst lineage specification began to be characterized at the single-cell level using defined cohorts of genes16, a comprehensive and unbiased view is still missing. Prompted by the availability of characterized lineage-specific markers, and recent studies proposing underlying mechanisms2, 3, 7, 13, 15, 27, we focused our single-cell transcriptomic analysis on the ICM cells of E3.25 (32-50 cells) to E4.5 blastocysts.
Results
Single cell analysis establishes a lineage map
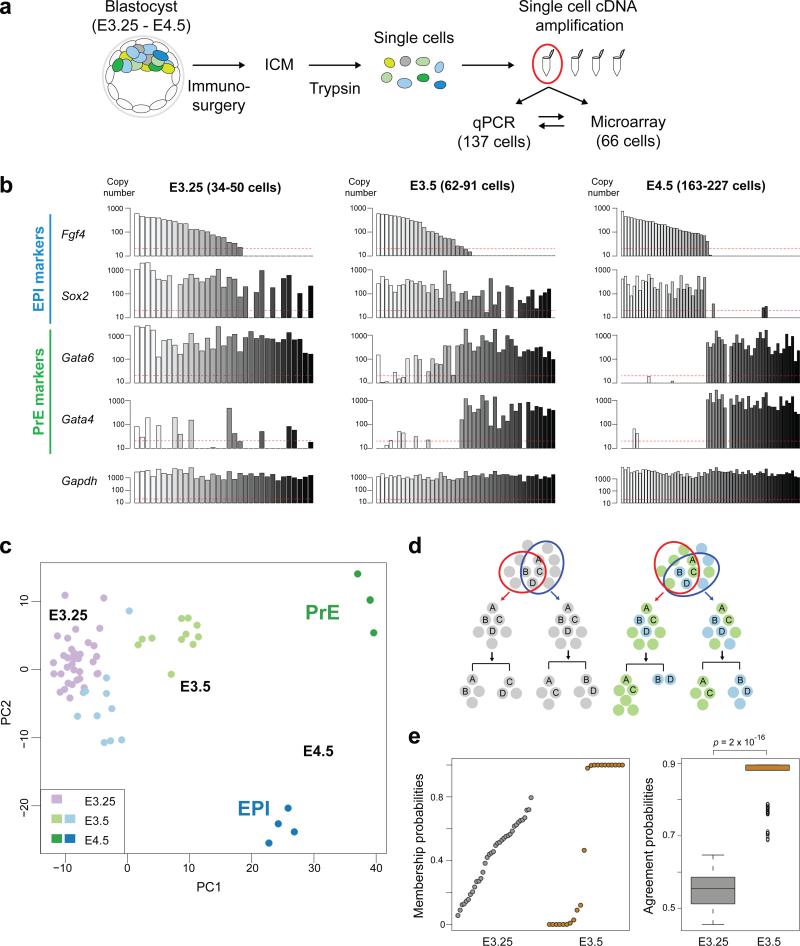
To assess the inherent heterogeneities and population dynamics associated with the emergence of EPI versus PrE cells at the single-cell level, we sought to build on previous methods17, 18 and extend our studies by expression profiling individual cells within the ICM of developing blastocyst-stage mouse embryos. Having formulated a method for collecting live single cells from ICMs recovered by immunosurgery28(Fig. 1a), we established a robust protocol for the amplification of mRNAs from them. Embryos were staged according to the average total number of cells in littermates. Reflecting the quality of the sample preparations, the mRNA isolation protocol produced a representative amplification output for the detection of a control ‘spike’ RNA (Supplementary Fig. S1), a uniform level of expression for housekeeping genes (for example, Gapdh) and bimodal distribution of EPI and PrE lineage-specific gene expression at E4.5 (Fig. 1b). For the ensuing microarray analyses, we selected 66 single-cell samples that provided a linear output for the detection of ‘spike-in’ RNAs with as little as 20 copies (Supplementary Fig. S1; Methods), implying that mRNAs expressed with more than 20 copies could be analysed quantitatively.
Figure 1.
Single-cell expression analysis of the lineage segregation within the inner cell mass of the mouse blastocyst. (a) Schematic of the experimental method of single-cell isolation and gene expression profiling. cDNA was processed, stored and used for qPCR and microarray analyses. (b) Gene expression profiles of 137 cells isolated from the ICM at E3.25 (33 cells from 4 embryos), E3.5 (43 cells from 3 embryos) and E4.5 (61 cells from 3 embryos) analysed by qPCR. Each bar represents the expression of indicated genes in individual cells, with the same horizontal positions representing the same cells. Red line indicates the minimal level of gene expression detectable quantitatively (20 copies). (c) Principal component analysis (PCA) plot of the microarray expression profiles characterizing the relative position of individual cells from blastocysts (66 cells including 36 cells from 6 embryos at E3.25, 22 cells from 3 embryos at E3.5, and 8 cells from one embryo at E4.5) in a map of lineage segregation. Note that the PCA was performed in an unsupervised manner, i.e., without information on cell stage or lineage. (d) Schematic of the cluster stability analysis to identify subpopulations among cells. If distinguishable subgroups exist (marked in green and blue in the right scheme), repeated bootstrap-sampled unsupervised clustering segregates them reproducibly (right panel). If repeated clustering produces incongruent results, no stably identifiable subgroups exist (left, grey). (e) Results of the cluster stability analysis (using a version of k-means clustering, partitioning around medoids, with k = 2) for E3.25 and E3.5 cells. (Left) Membership probabilities of each cell in the consensus clustering. Each dot represents the relative frequency at which a cell was assigned to one of the two consensus clusters in 250 random samplings. For E3.5, these frequencies had a bimodal distribution at 0 and 1, while for E3.25, they were diffuse. (Right) Boxplot of cluster agreement score of 250 random samplings with the consensus. Consistently high agreement was seen for E3.25, whereas the score was close to random expectation for E3.25. The agreement score distributions between E3.25 and E3.5 were significantly different (p = 2 × 10−16, Wilcoxon test).
The data obtained from qPCR analysis of a total of 137 single cells ranging from E3.25 to E4.5 revealed distinct behaviors in gene expression dynamics as the two ICM lineages arise (Fig. 1b). At least two distinct mechanisms can give rise to bimodal lineage-specific gene expression. In the first, bimodal gene expression is achieved from an initial state whereby all ICM cells express certain genes, followed by resolution into mutually-exclusive lineage-specific patterns, presumably through lineage-specific gene repression. This was the case for Sox2 and Gata6, in agreement with the findings of Guo et al. (2010)16. Alternatively, we noted cases where initially negligible level of gene expression evolves into lineage-specific gene activation and mutually-exclusive expression. This was the case for Gata4. Notably, the expression of Fgf4 gene was detected only in some cells at E3.25, therefore presaging the segregation of EPI or PrE progenitors at E3.5.
Among the 154 single-cell samples (see Methods for details), cRNAs derived from the highest quality 66 individual ICM cells (as assessed by expression of spike RNA) were hybridized to the GeneChip Mouse Genome 430 2.0 arrays. Overall, 10,958 distinct mRNAs were detected above background in these samples. The single-cell data established a transcriptome map of lineage segregation between EPI and PrE in the mouse blastocyst. To visualise the main features of this map, we used principal component (PC) projections of individual cells based on the expression of the 100 most variable genes in all cells (Fig. 1c). In this map, PC1 approximately corresponded to the stage of development (time), whereas PC2 aligned with the lineage difference (EPI or PrE). These data reveal that the EPI and PrE lineages become progressively segregated within a cohort of initially equivalent ICM cells during E3.25-E4.5 blastocyst stages.
Unsupervised clustering of the data obtained from single ICM cells at E3.5 and E4.5 (22 and 8 cells, respectively) using the expression of the 100 most variable genes identified two stable clusters, which we conclude corresponded to EPI and PrE lineages based on the expression of markers for each lineage. Thus, these data collectively provide the most comprehensive unbiased list of markers for EPI or PrE lineage at E3.5 and E4.5 (Supplementary Table S1). An unsupervised clustering stability analysis (Fig. 1d) demonstrated that ICM cells in E3.5 embryos showed strong evidence for falling into two clusters, while those at E3.25 did not reproducibly segregate into clusters (Fig. 1e). These data therefore reveal that at E3.25 ICM cells are not readily distinguishable in terms of their gene expression profile. Consequently, the transcriptome data do not favour what would be predicted from a model of predetermination15, in which distinct ‘waves’ of cell divisions generate distinctly identifiable types of inner cells; however, the data also do not exclude the possibility that more subtle differences – e.g. in single messages, or in other molecules - between ICM cells could underlie their eventual cell fate specification (see Discussion).
Progressive establishment of correlation
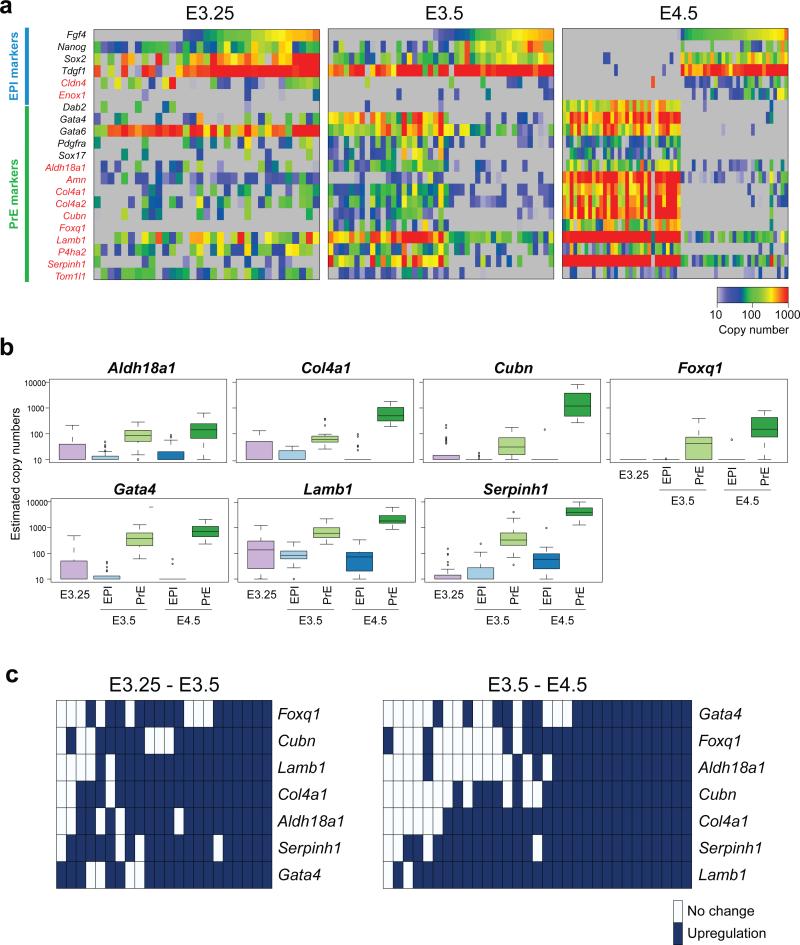
To begin to unravel the general principles of lineage emergence and segregation within the early mouse embryo, we validated several lineage markers newly identified in the microarray analysis of 66 cells (Supplementary Table S1) using qPCR for a total of 137 single cells (Fig. 2a). Genes analysed included: Cldn4 and Enox1 for EPI, and Aldh18a1, Amn, Col4a1, Col4a2, Cubn, Foxq1, Lamb1, P4ha2, Serpinh1 and Tom1l1 for PrE. Among them, the PrE-specific expression of Amn, Cubn, Col4 is in agreement with immunofluorescence staining in Gerbe et al. (2008)29, and that of Lamb1 with Artus et al. (2011)30. Immunostaining of Serpinh1 and P4ha2 also confirmed their specific expression in PrE at E4.5 (Supplementary Fig. S2). Differentially expressed lineage-specific markers exhibited stochastic expression that appeared uncorrelated between genes, early in the lineage segregation process (Fig. 2a).
Figure 2.
Correlation and hierarchy of gene expression is progressively established during lineage segregation within the ICM of the mouse blastocyst. (a) Expression of lineage-specific markers analysed by single-cell qPCR (137 cells in total including 33 cells from 4 embryos at E3.25, 43 cells from 3 embryos at E3.5, and 61 cells from 3 embryos at E4.5). Genes marked in red represent newly identified lineage markers. Each column represents the expression profile of an individual cell, with the color-code at the right bottom representing the estimated copy number for each gene. (b) Progressive upregulation of newly identified PrE differentiation marker genes. Box plots showing the expression level for each gene, collected for each stage from single-cell qPCR analysis (137 cells in total including 33 cells from 4 embryos for E3.25, 21 and 22 cells from 3 embryos for E3.5 EPI and PrE, and 30 and 31 cells from 3 embryos for E4.5 EPI and PrE, respectively). (c) Hierarchical relationships of the activation of PrE differentiation marker genes. Each column represents one cell, dark blue indicates upregulation of genes during the transition from E3.25 to E3.5 (left) or from E3.5 to E4.5 (right). Upregulation during a transition was operationally defined as a gene expression value more than the midpoint of the average expression levels for E3.25 and E3.5 cells, or for E3.5 and E4.5 cells, respectively (see Methods and Supplementary Fig. S3 and S4 for detailed method). Hierarchy in gene activation was significantly stronger at the E3.5 to E4.5 transition than at the E3.25 to E3.5 transition (p = 2 × 10−16, t-test).
We identified several lineage markers that allow characterisation of the stage of PrE differentiation, because these genes were progressively activated during lineage specification (Fig. 2b). These marker genes were defined in two steps (see Methods for details); after screening the microarray data for lineage-specific genes that were progressively upregulated from E3.25 to E3.5, and to E4.5, the identified candidate genes were verified by qPCR of additional single-cell cDNA samples. This allowed identification of 7 PrE differentiation stage markers (Fig. 2b) whose gene expression is progressively upregulated during the PrE lineage differentiation. It should be noted that the comparable EPI markers were more difficult to identify, because E3.25 ICM cells more closely resemble the E3.5 EPI than the PrE cohort, and upregulation of the expression of EPI markers is generally limited during differentiation (Fig. 1c).
Using these 7 PrE differentiation stage markers, we examined potentially hierarchical relationships of the activation in the lineage markers by asking whether the genes could be ordered so that within each individual cell, expression of a gene is only seen if the preceding gene is activated (Fig. 2c; see Methods and Supplementary Fig. S3 and S4 for detailed methods). Remarkably, an approximate hierarchy in gene activation was observed at the E3.5 to E4.5 transition, while evidence for hierarchy was much weaker at E3.25 to E3.5 (p = 2 × 10-16, t-test), suggesting that the activation of lineage-specific marker gene expression establishes a hierarchical relationship only at the late blastocyst stage.
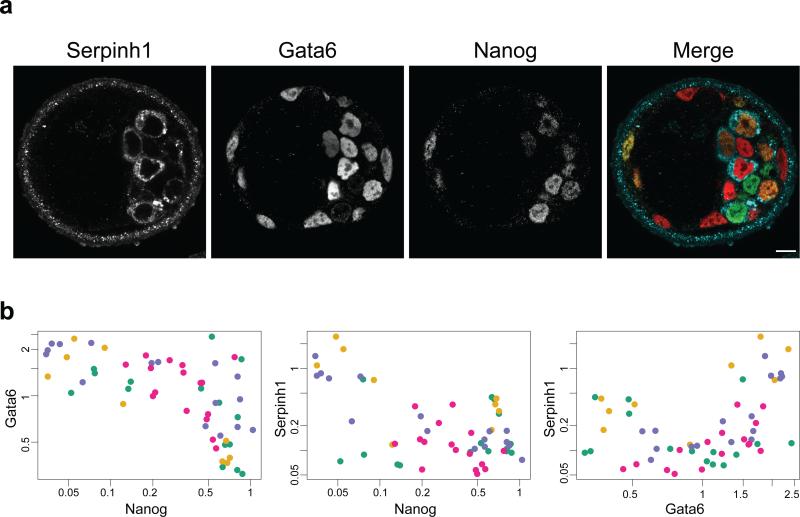
We also wished to evaluate variability in the expression of the lineage markers at the protein level. To do so, we performed a quantitative analysis of protein expression of a newly identified PrE marker, Serpinh1 (also known as heat shock protein 47, Hsp47)31, in relation to the lineage markers, Gata6 and Nanog (Fig. 3a). Serpinh1 is localized exclusively in the cytoplasm of PrE cells in E4.5 blastocysts (Supplementary Fig. S2a), in agreement with its reported function as a chaperone for collagen synthesis. To evaluate any potential variability in protein expression during EPI versus PrE segregation, E3.5 blastocysts (having a total of 70-90 cells) were immunostained simultaneously for Serpinh1, Gata6 and Nanog, as well as DNA and cell membrane for z-axis normalisation and cell/nucleus segmentation. This allowed us to perform quantitative measurements of the levels of protein expression for 56 individual ICM cells derived from 4 embryos (Fig. 3b, and Supplementary Video S1; see Methods for details). While positive or negative correlation of protein expression levels is evident between Nanog and Gata6, Nanog and Serpinh1, and Gata6 and Serpinh1, high variability in their expression levels at E3.5 does not allow separation of the two cell populations, in contrast to E4.5 ICM cells (see Supplementary Fig. S2). This is consistent with our findings made at the RNA level (Fig. 2a), and favours a model in which EPI and PrE lineages stochastically emerge within a cohort of initially equivalent ICM cells, rather than being predetermined by two distinct division histories15.
Figure 3.
Heterogeneity in protein expression level of the EPI and PrE markers. (a) A single-section immunofluorescence image of the E3.5 blastocyst simultaneously stained for Serpinh1 (a newly identified PrE marker), Gata6 and Nanog. In the merged image, Serpinh1, Gata6 and Nanog are labelled in blue, red and green, respectively. Scale bar; 10 μm. (b) Quantitative plots showing the normalized mean fluorescent intensity of Gata 6 relative to Nanog, Serpinh1 relative to Nanog, and Serpinh1 relative to Gata6. Each dot represents one blastomere with different colours representing different embryos (56 cells from 4 embryos at E3.5). The expression intensity value of the respective gene is normalized against the level of DAPI signal. The average background fluorescence level is 0.032, 0.001 and 0.027 for Gata6, Nanog and Serpinh1, respectively. Correlation of protein expression levels is evident between Nanog and Gata6, Nanog and Serpinh1, and Gata6 and Serpinh1 (r = -0.62 and p = 3 × 10−7, r = - 0.46 and p = 3 × 10−4, and r = 0.46 and p = 3 × 10−4, respectively; Pearson's correlation coefficient).
Cell position influences gene expression
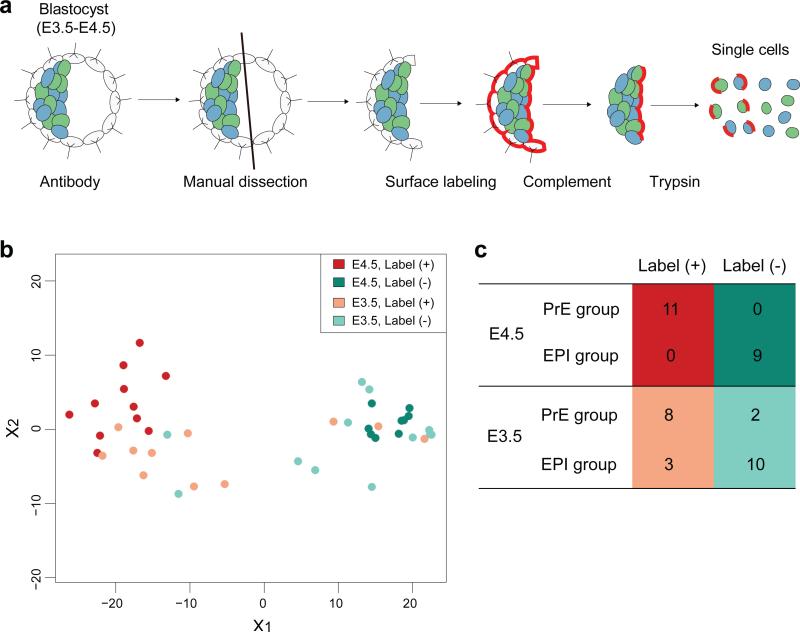
Positional information has been proposed to play a prominent role in the patterning of early embryos2, 3, 32. However, there are currently limited data3, 29 to suggest that a cell's position within the ICM influences its overall gene expression. To address this question and determine whether gene expression differences within the ICM reflect the position of individual cells, we established a method to identify, selectively isolate and expression profile cells located on the surface of the ICM adjacent to the blastocyst cavity versus those located deeper within the ICM (Fig. 4a). Expression profiling and comparison of these two populations revealed that cells facing the blastocyst cavity more closely resembled the PrE lineage from E3.5 onwards (Fig. 4b,c). These data therefore suggest that positional information may play an instructive role influencing the differential gene expression observed within the ICM at E3.5.
Figure 4.
Cell position influences gene expression. (a) Schematic of the method to label the cells on the surface of the ICM facing the blastocyst cavity. Immunosurgery was combined with manual bisection and isolation of the embryonic half of the blastocyst, followed by fluorescent labelling of the exposed surface cell layer (see Methods for details). (b) Multi-dimensional scaling plot of the labelled and non-labelled E3.5 and E4.5 inner cells, based on the expression of 10 highly variable genes, as identified from the E3.5 and 4.5 microarray data (Cotl1, Cth, Cubn, Fgf4, Lama1, Morc1, Pdgfra, Sepinh1, Sox17, Srgn), and quantified by additional single-cell qPCR measurements (43 cells in total including 23 cells from 6 embryos at E3.5, and 20 cells from 2 embryos at E4.5). (c) Number of label positive and negative cells in PrE and EPI groups, in which the lineage identity is assigned by marker gene expressions. Clear segregation of the PrE and EPI cells at E4.5 indicates that this labelling method can clearly distinguish the PrE cells from the EPI cells in the E4.5 blastocyst. In E3.5, label positive cells are strongly enriched in the PrE group (odds ratio 12, p = 0.01, Fisher's exact test).
Fgf4 is required for EPI versus PrE segregation
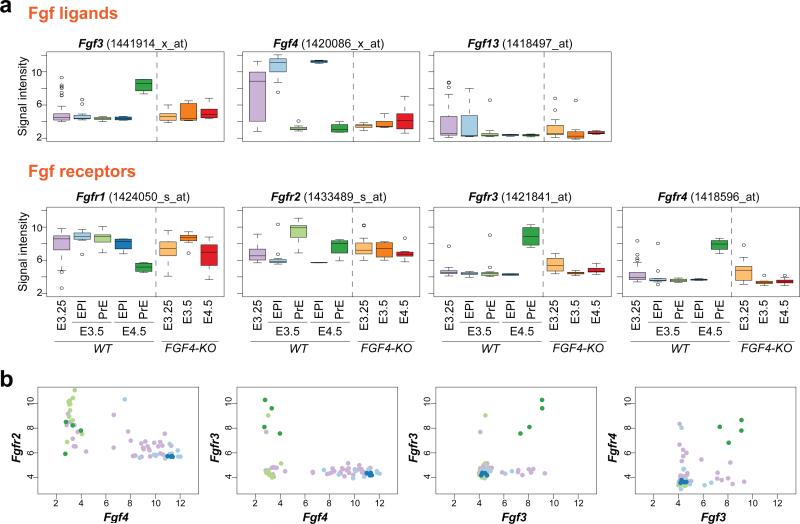
Next, we wished to identify the symmetry breaking signals driving lineage segregation within ICM cells. To do this we sought to characterize the genes that segregate into two distinct ICM populations at the earliest stage, corresponding to E3.5 in our analysis. Fgf4 was identified as one of such genes exhibiting the greatest differential expression between EPI and PrE cells (Fig. 1b and 2a; Supplementary Table S1). To comprehensively characterise the involvement of Fgf signalling in the EPI versus PrE lineage segregation, the expression of all Fgf ligands, receptors and downstream cytoplasmic signalling components in the developing blastocyst were analysed using the 66 single-cell ICM transcriptome data (Fig. 5a and Supplementary Fig. S5). Several Fgf ligands (Fgf3, 4 and 13) and all Fgf receptors (Fgfr1 – 4) were found to be differentially expressed within the ICM, thus possibly contributing to the EPI versus PrE lineage segregation. By contrast, cytoplasmic signalling components exhibited no differential expression, suggesting that any differential regulation would predominantly be at the post-transcriptional level. The overlapping expression of ligands and receptors suggests the presence of redundant functions within Fgf signalling pathway components. A statistically significant correlation (positive or negative) in gene expression levels is discernible at the single cell level for Fgf4 against Fgfr2 (in agreement with Guo et al. (2010)16), Fgf4 against Fgfr3, Fgf3 with Fgfr3, and Fgf3 with Fgfr4 at E3.5 and E4.5 ICMs (Fig. 5b). Among those genes expressed in the blastocyst, Fgf4 and Fgfr2 exhibit differential expression the earliest (E3.25), followed by Fgfr1. The higher variability, and bimodality (Fig. 5b), in the expression of Fgf4 than of Fgfr2 at E3.25 suggests that Fgf4 may be the driver for the observed differential gene expression and EPI versus PrE lineage segregation.
Figure 5.
Comprehensive characterisation of expression of Fgf signalling components in the early mouse embryo. (a) Box plots showing the mRNA expression levels of Fgf ligands and receptors detectable in the early mouse embryo, collected for each stage from single-cell microarray analysis (66 WT cells including 36 cells from 6 embryos for E3.25, 11 and 11 cells from 3 embryos for E3.5 EPI and PrE, and 4 and 4 cells from one embryo for E4.5 EPI and PrE cells, respectively; and 35 Fgf4−/− cells including 17 cells from 3 embryos for E3.25, 8 cells from one embryo for E3.5 and 10 cells from one embryo for E4.5). Those Fgf ligands whose expression level is negligible are shown in Supplementary Fig. S5. (b) Scatter plots with each dot representing the mRNA expression levels of specific Fgf ligand and receptor pairs in one blastomere. The colour code of the dot is the same throughout this study, shown in inset of Fig.1c, with pink representing E3.25 cells, light blue and green E3.5 EPI and PrE cells, and blue and green E4.5 EPI and PrE cells, respectively. Those with statistically significant correlation (positive or negative) are shown (r = -0.77, p = 7 × 10−7 (Fgf4 vs. Fgfr2); r = -0.42, p = 2 × 10−2 (Fgf4 vs. Fgfr3); r = 0.82, p = 4 × 10−8 (Fgf3 vs. Fgfr3); r = 0.76, p = 1 × 10−6 (Fgf3 vs. Fgfr4); Pearson's correlation coefficient).
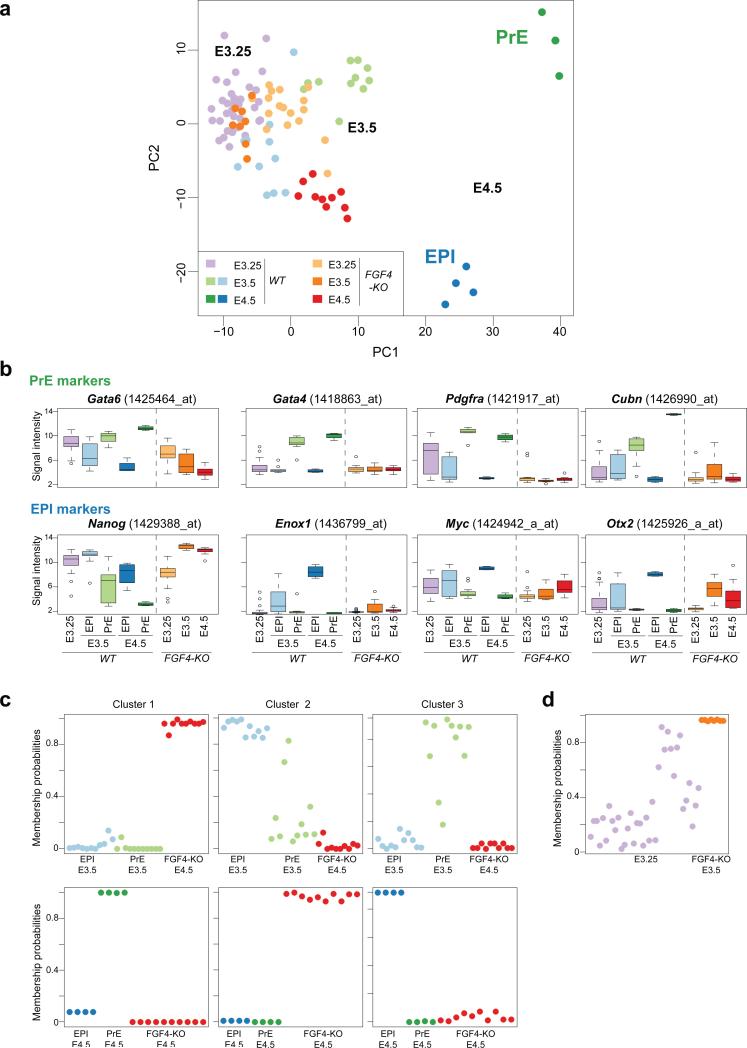
We recently demonstrated that Fgf4 is required for the establishment of a salt-and-pepper distribution of EPI/PrE lineage precursors at E3.5, as well as the specification of PrE within the ICM13. To comprehensively characterize the impact of loss of Fgf4 on EPI versus PrE lineage segregation we performed single-cell gene expression analyses on the ICMs of Fgf4−/− mutant embryos. The expression profiles of individual ICM cells derived from Fgf4−/− blastocysts at E3.25-E4.5 were overlaid on the lineage map established using the wild-type single-cell expression profiles (shown in Fig. 1c). The samples’ coordinates allowed us to characterize the differentiation status of Fgf4−/− ICM cells. Surprisingly we noted that the differentiation not only of PrE but also of EPI cell lineage was arrested in Fgf4−/− mutants (Fig. 6a), indicating that Fgf4 is required for segregating these two lineages. Moreover, in Fgf4−/− mutants, expression of PrE lineage-specific markers was significantly suppressed and maintained at the level of WT E3.25, whereas loss of Fgf4 had a more variable effect on EPI markers (Fig. 6b). It should be noted that although E4.5 Fgf4−/− ICM cells are positioned relatively close to EPI cells in the 2-dimensional PCA projection (Fig. 6a), their expression profiles are significantly distinct from E3.5 and E4.5 WT EPI cells (Fig. 6c), indicating that Fgf4−/− ICM cells are not simply differentiating into the EPI lineage. Similarly, although E3.5 Fgf4−/− cells appear to overlap with E3.25 WT cells, a more detailed analysis of their expression profiles indicates that they represent a distinct population (Fig. 6d). Moreover, E3.25 Fgf4−/− ICM cells appear to be distributed differently from WT cells, suggesting that there might be a distinct role for Fgf signalling at an early stage. Additional qPCR analysis of Fgfr2, Nanog and Gata6 expression in E3.25 and E3.5 ICM cells (Supplementary Fig. S6) revealed that while in WT cells their gene expression levels show positive or negative correlation at the single-cell level, Fgf4−/− cells tend to lose such correlations. These data suggest the requirement of Fgf signalling in establishing the gene regulatory network for EPI versus PrE lineage segregation. Loss of Fgf4 alone does not induce compensatory expression of other Fgf ligands, and the expression pattern of other Fgf signalling components are generally unaltered at the E3.25/3.5 stage (Fig. 5a and Supplementary Fig. S5). Genes downregulated in E3.5 Fgf4−/− cells (Supplementary Table S2) would include putative targets of Fgf signalling in the early mouse embryo. Collectively, our data suggest that heterogeneity in the expression, and thus availability, of Fgf4 is critical for lineage segregation and couples it to the salt-and-pepper distribution of EPI/PE cells within the E3.5 ICM13.
Figure 6.
Fgf4 is required for driving lineage segregation between EPI and PrE in the early mouse embryo. (a) PCA plot of the microarray expression profiles of Fgf4−/− cells (35 Fgf4−/− cells including 17 cells from 3 embryos for E3.25, 8 cells from one embryo for E3.5 and 10 cells from one embryo for E4.5) overlaid on the EPI versus PrE lineage map established using WT cell profile (66 WT cells including 36 cells from 6 embryos for E3.25, 11 and 11 cells from 3 embryos for E3.5 EPI and PrE, and 4 and 4 cells from one embryo for E4.5 EPI and PrE cells, respectively). Note that the position of WT cells is identical to that shown in Fig.1c and is used here as a reference map. (b) Impact of the loss of Fgf4 on the expression of lineage markers analysed by microarray. Box plots show the expression of PrE and EPI markers (including differentiation markers), collected for each stage from single-cell microarray analysis (similarly to Fig. 5a). (c) Cluster stability analysis (250 random samplings) for Fgf4−/− E4.5 cells together with WT E3.5 EPI and PrE cells (upper row), or with E4.5 EPI and PrE cells (lower row). Shown are the membership probabilities of the consensus clustering, analogous to the analysis in Fig. 1e. Unsupervised clustering faithfully recovers the grouping into WT E3.5 EPI cells, WT E3.5 PrE cells, WT E4.5 EPI cells, WT E4.5 PrE cells and Fgf4−/− E4.5 cells. (d) Cluster stability analysis (250 random samplings) for Fgf4−/− E3.5 cells together with WT E3.25 cells. Shown are the membership probabilities of the consensus clustering. The analysis demonstrates that Fgf4−/− E3.5 cells form a single, tight cluster.
Discussion
In this study we have developed a framework for the isolation of single cells from the ICMs of developing mouse blastocysts, expression profiling and data analysis. The data represent the first comprehensive and unbiased single-cell resolution lineage map of the ICM of mammalian blastocyst. The finding that inner cells at E3.25 show no apparent distinction favours a model of stochastic and progressive segregation of EPI and PrE lineages7. However, these data do not exclude the possibility that some difference may exist among cells within the ICM at E3.25, as was postulated by Guo et al. (2010)16 based on the inverse correlation between Fgf4 and Fgfr2, which we also noted in our samples (see Fig. 5b). The statistical cell sub-population analysis used in this study provides evidence against a consistent, widespread gene expression pattern reflecting predetermination or lineage commitment at E3.25, although our analysis would not detect a difference that is restricted to a small number of genes or non-mRNA molecules. E3.25 ICM cells, however, do not exhibit a “uniform” gene expression status, perhaps reminiscent of the ground state of ES cells33, but instead are a mixture of cells with stochastic gene expression variability. Stochastic fluctuations of gene expression may offer a greater repertoire of combinatorial gene expression23, which may underlie the developmental plasticity and highly regulative capacity of the preimplantation mouse embryo before E4.534.
Our single-cell data allowed us to comprehensively identify EPI and PrE lineage markers. Newly identified genes that are specifically expressed early in the PrE differentiation include extra-cellular matrix components and factors involved in their synthesis. Presumptive PrE cells may need to produce a large amount of structural proteins that need to be incorporated into the basement membrane at the interface between the newly forming PrE epithelial layer and adjacent inner EPI cells.
We also determined the impact of loss of a key signalling molecule, Fgf4, through the analysis of ICM cells in a mutant13. Embryos lacking Fgf4, or the effector Grb22, exhibit a profound defect within the ICM characterized by an absence of PrE cells, a phenotype which can be recapitulated using Fgf signalling inhibitors9. If Fgf4 and Grb2 are critical non-redundant points in the pathway, an open question pertains the identity of the receptors and downstream intracellular effectors required for transducing the Fgf4/Grb2 signal, the cells in which the pathway is normally active, and the mechanism by which Fgf4 positive and negative cells are generated in E3.25 ICMs.
It is now well established that molecular heterogeneities presage marker restriction and lineage segregation3, 35. Live-imaging of a fluorescent reporter for the PrE-lineage reporter embryos demonstrated that cell sorting and position-dependent regulation of gene expression may help resolve the molecular heterogeneities into the pattern3. A combination of live imaging embryos expressing lineage-specific fluorescent reporters and single-cell gene expression profiling should eventually allow dissection of the underlying mechanisms. A recent study investigating neural tube patterning in zebrafish revealed that cell sorting rearranges an initial mixture of different neural progenitors formed by heterogeneous signalling activity into sharply bordered domains36. Thus, the generation and resolution of molecular heterogeneities might represent a conserved mechanism for driving pattern formation in various contexts during embryonic development across species37.
Our single-cell data showed that ICM cells maintain the same level of gene expression variability despite the lack of Fgf4 (standard deviation of log2 expression measurements of the 100 most variable genes in E3.25: 1.7±1.2 (WT) versus 1.5±1.2 (Fgf4−/−)), suggesting that Fgf4 is not required for generating the initial molecular heterogeneity. Consequently, it would be conceivable to separate early blastocysts with cell-to-cell gene expression variability into two phases. In the first phase, expression of individual genes exhibits stochastic variability, possibly independent from one another. While in the second phase, a correlation of gene expressions gradually emerges, likely due to the activation of lineage-specific signalling cascades (e.g. Fgf) (Fig. 7). The second phase may correspond to the blastocyst stage in which a “salt-and-pepper” pattern of expression2 can be defined by the onset of Gata4 expression, and restriction of Gata6 to Gata4-positive PrE lineage “precursors”, or cells with a propensity to contribute to the emergent PrE. However, as demonstrated in this study (see Figs. 2 and 3), when evaluated with a number of genes/proteins simultaneously, ICM cells at this stage still exhibit a high-degree of expression variability, and future studies would require a comprehensive and quantitative description of molecular heterogeneities. Taken together, we propose that an initial phase of stochastic gene expression followed by signal reinforcement may drive lineage segregation by antagonistically separating a cohort of initially equivalent cells (Fig. 7). Thus the inherent molecular heterogeneity, and subsequent salt-and-pepper pattern of lineage precursors, within the ICM may form the foundation for segregating distinct EPI and PrE lineages within an initially equivalent population of cells.
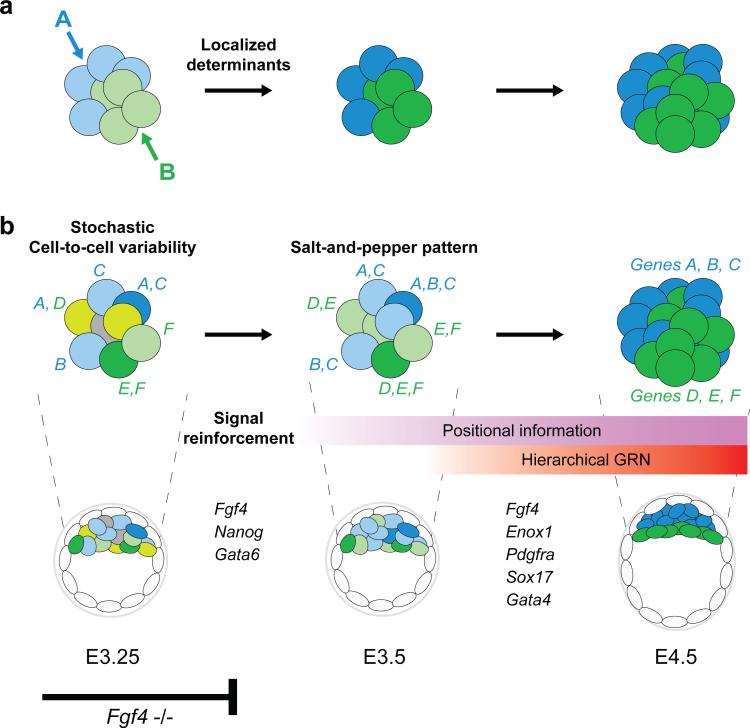
Figure 7.
Schematic model for EPI vs. PrE lineage segregation in the early mouse embryo, contrasting to mechanisms for embryo patterning in non-mammalian species. (a) In many non-mammalian species, localized determinants play a key role in embryonic patterning. (b) In the ICM of the mouse blastocyst, EPI and PrE lineages are progressively segregated within a cohort of initially equivalent cells. Cell-to-cell variability generated by stochastic onset of gene expression (genes A, B, C represent the lineage marker for blue cells, whereas D, E, F for green cells) is progressively enhanced by signalling activities and feedbacks as well as cell-cell interactions, and forms salt and pepper pattern, with emerging two populations. This process eventually leads to establishing two distinct cell lineages (blue or green cells) with specific gene regulatory networks in the context of positional information. In the absence of Fgf4, reinforcement by the signalling cascade may fail and lineage segregation is halted without differentiation into either of the two lineages.
METHODS
Embryo collection and staging
BL/6×C3H F1 mice, or heterozygous mice with ablation of Fgf4 allele13, were bred naturally and embryos were recovered at E3.25, E3.5 or E4.5 by flushing oviducts or uteri. After removal of the zona pellucida with pronase (0.5% w/v Proteinase K (Sigma, P8811), 0.5% PVP-40 (Fluka, 81420) in HEPES-buffered KSOM (FHM; EMD Millipore, Zenith Biothech, MR-024-D)), the ICM was isolated from blastocysts by immunosurgery according to Solter and Knowles (1975)28. Briefly, blastocysts were incubated for 10-30 min at 37 °C in KSOM (EMD Millipore; Zenith Biothech, MR-121-D) containing anti-mouse lymphocyte serum (Cedarline, CL2301, 1:5-10), followed by wash with FHM and 15-30 min incubation at 37 °C in KSOM supplemented with guinea pig complement serum (Cedarline, CL5000F, 1:2-8). After removing the lysed TE cells by repeated pipetting, ICM was further dissociated into single blastomeres by pipetting in HBS (25 mM Hepes, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM Dextrose, 0.9 mM CaCl2, 0.5 mM MgCl2, pH 7.05) supplemented with 1 mM EDTA (H-EDTA) after 5-7 min incubation at 37 °C in H-EDTA supplemented with 1% Trypsin (Sigma-Aldrich, T-4549). It usually took 80-90 min to isolate and lyse ICM cells and recover their RNAs after sacrificing the mouse, and great care is taken to minimize the time. The developmental stage of embryos subjected to the single-cell gene expression analysis was defined as follows. Upon recovery, an average-size embryo was selected for subsequent experiments, and the remaining littermates were fixed in PBS supplemented with 4% paraformaldehyde (Electron Microscopy Sciences, 19208) and stained in PBS with DAPI (Molecular Probes, D3571, 1:1,000) as well as with either Alexa Fluor 633 or 564 Phalloidin (Molecular Probes, A22284 or A22283, respectively, 1:100-200). The total cell number of each embryo was counted and an average cell number of the littermates, but excluding samples with the maximum and minimum cell numbers, was determined and used to represent the developmental stage of the experimental sample for single-cell analysis.
The European Molecular Biology Laboratory animal facility is operating according to international animal welfare rules (Federation for Laboratory Animal Science Associations guidelines and recommendations). Requirements of formal control of the German national authorities and funding organizations are satisfied and controlled by the Institutional Animal Care and Use Committee.
Single-cell cDNA amplification
Single-cell cDNA amplification was performed as previously described17, 18. Briefly, single blastomeres were lysed in individual tubes without purification, and first-strand cDNAs were synthesized using a modified poly(dT)-tailed primer. The unincorporated primer was digested by exonuclease and the second strands were generated with a second poly(dT)-tailed primer after poly(dA) tailing of the first-strand cDNAs. cDNAs were amplified by PCR, first with poly(dT)-tailed primers, and subsequently with primers bearing the T7 promoter sequence. The resulting cDNA products were used for further quantitative real-time PCR (qPCR), or for generating biotin-labeled cRNAs to hybridize to the GeneChip Mouse Genome 430 2.0 Arrays (Affymetrix). Primer sequences for qPCR are provided in Supplementary Table S3. In all cDNA amplification experiments, poly(A)-tailed RNAs artificially designed from Baccilus subtilis genes were added to each sample as spike RNAs to monitor the amplification process, and this allowed us to estimate the copy number of the gene transcripts analysed. A mixture of four distinct spike RNAs, Lys, Thr, Phe and Dap (American Type Culture Collection 87482, 87483, 87484, 87486) were prepared and added to each sample as a mixture of 1000, 100, 20 and 5 copies, respectively. Samples (162 cells) were collected from a total of 12 embryos (52 single cells from 6 embryos at E3.25, 48 cells from 3 embryos at E3.5 and 62 cells from 3 embryos at E4.5) in 12 independent experiments, each time collecting the sample single cells from one embryo. Those samples (8 cells) in which efficiency of the amplification of the spike RNAs or Gapdh was substantially lower were excluded from the analysis, resulting in a total of 154 single-cell samples (50 cells from 6 embryos at E3.25, 43 cells from 3 embryos at E3.5 and 61 cells from 3 embryos at E4.5). Among them, the samples of the highest quality with a linear output for the detection of spike RNAs of as little as 20 copies were selected for microarray (66 cells; 36 cells from 6 embryos at E3.25, 22 cells from 3 embryos at E3.5 and 8 cells from one embryo at E4.5) (Supplementary Fig. S1). A total of 137 single-cell samples were used for qPCR analysis, including 33 cells derived from 4 embryos at E3.25, 43 cells from 3 embryos at E3.5 and 61 cells from 3 embryos at E4.5. Forty-nine single-cell samples were shared in both of the microarray and qPCR analyses. For the single-cell analysis of Fgf4−/− embryos, sample single cells (35 cells) were collected from a total of 5 Fgf4−/− embryos (17 cells from 3 embryos at E3.25, 8 cells from one embryo at E3.5 and 10 cells from one embryo at E4.5) in 5 independent experiments, each time collecting the sample cells from one embryo, and the absence of Fgf4 expression was confirmed by qPCR prior to the microarray analysis. For additional qPCR analysis performed in Fig. S6, only 9 cells, derived from one embryo, out of 17 cells were used for Fgf4−/− E3.25 ICM cells, because cDNAs for the remaining 8 cells were used up. In Fig S6, the gene expression levels are normalized to that of Gapdh (x or y = 0), and those mRNAs whose amplification resulted in a Ct value > 30 were considered to be undetectable.
Immunofluorescence staining and quantitative protein expression analysis
Embryos were fixed for 10 min at room temperature in PBS supplemented with 4% paraformaldehyde (for Serpinh1, Gata6 and Nanog; Electron Microscopy Sciences, 19208) or for 15-20 min at 4 °C in PBS supplemented with 10% TCA (for P4ha2; WAKO, 206-08082), and washed in PBS with 0.1% Tween 20 (PBS-T). After permeabilisation for 20-30 min at room temperature in PBS supplemented with 0.25% Triton X-100, embryos were blocked for 1 hour at room temperature in PBS with 3% BSA, and then incubated with mouse monoclonal anti-Hsp47 (Serpinh1, Enzo Life Sciences, ADI-SPA-470, 1:200), goat polyclonal anti-Gata6 (R&B Systems, AF1700, 1:100), rabbit polyclonal anti-Nanog (Cosmo Bio, REC-RCAB0002PF, 1:100), and/or rabbit polyclonal anti-P4ha2 (Abcam, ab118711, 1:100-200) dissolved in the blocking solution for overnight at 4 °C. After washing with PBS-T, embryos were further incubated with Alexa Fluor 488 donkey anti-mouse (Molecular Probe, A21202, 1:200), Alexa Fluor 555 donkey anti-goat (Molecular Probe, A21432, 1:200), Alexa Fluor 647 donkey anti-rabbit (Molecular Probe, A31573, 1:200), Alexa Fluor 488 goat anti-rabbit (Molecular Probe, A11008, 1:100), Atto 425 phalloidin (Sigma-Aldrich, 66939, 1:200) and/or DAPI (Molecular Probes, D3571, 1:200-1000) in the blocking solution for 1 hour at 37 °C. The representative images shown in Fig. 3a and Fig. S2 have been replicated by 5 independent experiments.
Fluorescence images were acquired using a confocal microscope (LSM 710 or 780; Carl Zeiss) equipped with a 40× water immersion C-Apochromat 1.2 NA objective. The pinhole was open to the 1-μm thickness of the stack, and when the z stack was acquired (for Fig. 3 and Supplementary Video S1), the interval used between stacks was 0.45 μm. Image analysis was performed using IMARIS (Bitplane) or ImageJ. Quantitative protein expression analysis (Fig. 3b) was performed as follows, with modification to the method used in Dietrich and Hiiragi (2007)35. Cell membrane and nucleus were segmented based on phalloidin and DAPI signals, respectively. The protein expression level in the cytosol or nucleus was measured as the average of the mean florescent intensities within the defined segments in the five slices separated with equal distance along the entire z-axis of the cell or nucleus, and normalized against the average of mean DAPI intensities measured in the same way (56 cells from 4 embryos at E3.5 in 4 independent experiments). The background intensity was defined as the average of mean fluorescence intensities of 15 randomly chosen spots located outside of the embryo, divided by the average of all mean DAPI intensities. QuickTime movie (Supplementary Video S1) showing the entire z-scans of immunofluorescence staining was generated using Photoshop (CS5, Adobe, San Jose, CA).
Labeling of the cells located on the surface of the inner cell mass facing to the blastocyst cavity
After removal of the zona pellucida with a brief treatment of pronase, blastocysts were incubated for 25 min at 37 °C in KSOM with anti-mouse antibody (Cedarline, CL2301, 1:8), and manually bisected using a 27-G needle in KSOM containing HEPES38. The surface of the resultant embryos containing polar TE and ICM was stained by 2 × 1 sec incubation in KSOM supplemented with Cell Mask (Invitrogen, C10045, 1:100), followed by 15-20 min incubation at 37 °C in KSOM supplemented with guinea pig complement (Cedarline, CL5000F, 1:2). After removal of the lysed TE by pipetting, the ICM was dissociated into single blastomeres by 5 min incubation in H-EDTA followed by 7 min incubation at 37 °C in H-EDTA supplemented with 0.05% Trypsin, and further pipetting in FHM. Fluorescently-labeled outer or non-labeled inner cells were identified under confocal microscopy, and gene expression was analysed using single-cell cDNA amplification and qPCR as described above. Samples (43 cells) were collected from a total of 8 embryos (23 cells from 6 embryos at E3.5 and 20 cells from 2 embryos at E4.5) in 8 independent experiments, each time collecting the sample cells from one embryo. Occasionally, outer cells seemed to be not entirely removed, due possibly to the modified immunosurgery protocol, and those single cell samples in which qPCR detected the expression of TE markers (e.g. Cdx2 and Id2) were eliminated from further analysis.
Statistical analyses
All statistical procedures were developed by a statistician (W.H.), carefully checked for robustness both to (i) choice of method and (ii) natural variability in the data, and the analyses were performed using R/Bioconductor software. An R package named Hiiragi2013.pdf including the complete data and software scripts (‘vignettes’) is available as an executable document at www.bioconductor.org. No statistical method was used to predetermine sample size. The experiments were not randomized, and the investigators were not blinded to allocation during experiments and outcome assessment. Statistical tests were chosen to meet the properties of the data. t-tests were performed with the Welch approximation to the degrees of freedom to allow for unequal variances. Extensive data exploration and visualisation provided no indication of heteroskedasticity-induced problems.
Microarray data processing was performed using the RMA algorithm implemented in the Bioconductor package affy39. Data quality was verified using the package arrayQualityMetrics40. Cluster stability analysis was performed by applying the unsupervised clustering method partitioning around medoids (PAM) to B = 250 resampled data sets (each containing a random subset of 67% of cells, sampled without replacement), constructing a consensus clustering, and comparing the B individual clustering results with the consensus. Specifically, for each sample, its cluster assignment probabilities were computed, and for each of the B clusterings, their agreement with the consensus was measured by the Euclidean dissimilarity D of the membership matrices, i.e., the square root of the minimal sum of the squared differences of U and all column permutations of V, where U and V are the cluster membership matrices. The cluster agreement scores shown in Figs. 1e and 6c are 1−D/M, where M is an upper bound for the maximal Euclidean dissimilarity. Computations were performed using the R package clue41.
For the analysis of hierarchical relationship among gene activations, the differentiation stage markers were first identified as follows: (1) expressed in only one of the lineages at E4.5, and (2) expressed an average fold-change of at least 8 from E3.25 to E4.5, as well as average fold-changes of at least 1.4 in the individual transitions from E3.25 to E3.5, and from E3.5 to E4.5. We then used qPCR of additional single-cell cDNA samples for validation, and identified 7 PrE differentiation stage markers (Fig. 2b) whose gene expression is progressively upregulated during the PrE lineage differentiation, without change in the EPI lineage. For each of the 7 genes, the average levels in the conditions E3.25, E3.5 (PrE) and E4.5 (PrE) were computed, and two thresholds were defined corresponding to the midpoint between the averages of E3.25 and E3.5, and the midpoint between the average of E3.5 and E4.5 (see Supplementary Fig. S3). Data were binned into two states on and off as follows. For either the E3.25 to E3.5 transition or the E3.5 to E4.5 transition, a gene was considered on in a cell if its expression value exceeded the threshold associated with the transition. For a particular ordering of the seven identified genes, a hierarchy mismatch score was defined by counting the number of instances when an on gene preceded an off gene in the ordering. The minimum score was determined over all 7! = 5040 possible orderings, and normalized to the range from 0 to 1 by dividing it by the number of gene pair comparisons. All possible orders with the minimum score are depicted in Supplementary Fig. S4. To assess the statistical significance of the observed difference between the hierarchy mismatch score of the E3.25 to E3.5 transition and that of the E3.5 to E4.5 transition, the procedure was bootstrap-resampled.
Supplementary Material
ACKNOWLEDGEMENTS
We thank A. Courtois for help with image analysis, R. Niwayama for quantitative protein expression analysis, S. Salvenmoser and R. Bloehs for technical assistance, and EMBL Genomics Core Facility for technical support. We also thank the members of the Hiiragi, Hadjantonakis, Huber and Saitou laboratories for helpful and stimulating discussions. Work in the laboratory of T. H. is supported by the Max Planck Society, European Research Council under the European Commission FP7, Stem Cell Network North Rhine Westphalia, German Research Foundation (Deutsche Forschungsgemeinschaft), and World Premier International Research Center Initiative, Ministry of Education, Culture, Sports, Science and Technology, Japan. Work in the laboratory of A.-K.H. is supported by the National Institutes of Health (NIH) NIH RO1-HD052115 and RO1-DK084391 (AKH) and NYSTEM. W.H. acknowledges funding from the European Commission FP7-Health through the RADIANT project. Y.O. is supported by Naito and Uehara Memorial Foundation fellowships, and by Marie Curie FP7 IIF fellowship (no. 273193).
Footnotes
AUTHOR CONTRIBUTIONS
Y. O., A.T. and T.H. designed the study, Y.O. performed most of the experiments, A. T., K. K. and M. S. contributed to establishing the method of single-cell gene expression analysis in the mouse preimplantation embryo, Y.O., A.T., M.K. and P.X. collected the single-cell samples, W.H. and A.K.O. performed statistical analysis, and M.J.A.-B. contributed to initial analyses of the data. Y.O., A.-K.H. and T.H. wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Accession number. The microarray data have been deposited to ArrayExpress database with the accession number E-MTAB-1681.
References
- 1.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- 2.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Developmental cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrode N, et al. Anatomy of a blastocyst: Cell behaviors driving cell fate choice and morphogenesis in the early mouse embryo. Genesis. 2013;51:219–233. doi: 10.1002/dvg.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabarek JB, et al. Differential plasticity of epiblast and primitive endoderm precursors within the ICM of the early mouse embryo. Development. 2012;139:129–139. doi: 10.1242/dev.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development. 2010;137:3351–3360. doi: 10.1242/dev.050146. [DOI] [PubMed] [Google Scholar]
- 7.Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development. 2010;137:715–724. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]
- 8.Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5082–5087. doi: 10.1073/pnas.95.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng AM, et al. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 11.Feldman B, Poueymirou W, Papaioannou VE, DeChiara TM, Goldfarb M. Requirement of FGF-4 for postimplantation mouse development. Science. 1995;267:246–249. doi: 10.1126/science.7809630. [DOI] [PubMed] [Google Scholar]
- 12.Wilder PJ, et al. Inactivation of the FGF-4 gene in embryonic stem cells alters the growth and/or the survival of their early differentiated progeny. Developmental biology. 1997;192:614–629. doi: 10.1006/dbio.1997.8777. [DOI] [PubMed] [Google Scholar]
- 13.Kang M, Piliszek A, Artus J, Hadjantonakis AK. FGF4 is required for lineage restriction and salt-and-pepper distribution of primitive endoderm factors but not their initial expression in the mouse. Development. 2013;140:267–279. doi: 10.1242/dev.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chisholm JC, Houliston E. Cytokeratin filament assembly in the preimplantation mouse embryo. Development. 1987;101:565–582. doi: 10.1242/dev.101.3.565. [DOI] [PubMed] [Google Scholar]
- 15.Morris SA, et al. Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6364–6369. doi: 10.1073/pnas.0915063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo G, et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Developmental cell. 2010;18:675–685. doi: 10.1016/j.devcel.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Kurimoto K, et al. An improved single-cell cDNA amplification method for efficient high-density oligonucleotide microarray analysis. Nucleic acids research. 2006;34:e42. doi: 10.1093/nar/gkl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurimoto K, Yabuta Y, Ohinata Y, Saitou M. Global single-cell cDNA amplification to provide a template for representative high-density oligonucleotide microarray analysis. Nature protocols. 2007;2:739–752. doi: 10.1038/nprot.2007.79. [DOI] [PubMed] [Google Scholar]
- 19.Tang F, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nature methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 20.Tang F, et al. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nature protocols. 2010;5:516–535. doi: 10.1038/nprot.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelkmans L. Cell Biology. Using cell-to-cell variability--a new era in molecular biology. Science. 2012;336:425–426. doi: 10.1126/science.1222161. [DOI] [PubMed] [Google Scholar]
- 22.Eldar A, Elowitz MB. Functional roles for noise in genetic circuits. Nature. 2010;467:167–173. doi: 10.1038/nature09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu M, et al. Multilineage gene expression precedes commitment in the hemopoietic system. Genes & development. 1997;11:774–785. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- 24.Pina C, et al. Inferring rules of lineage commitment in haematopoiesis. Nature cell biology. 2012;14:287–294. doi: 10.1038/ncb2442. [DOI] [PubMed] [Google Scholar]
- 25.Moignard V, et al. Characterization of transcriptional networks in blood stem and progenitor cells using high-throughput single-cell gene expression analysis. Nature cell biology. 2013;15:544. doi: 10.1038/ncb2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buganim Y, et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012;150:1209–1222. doi: 10.1016/j.cell.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frankenberg S, et al. Primitive endoderm differentiates via a three-step mechanism involving Nanog and RTK signaling. Developmental cell. 2011;21:1005–1013. doi: 10.1016/j.devcel.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:5099–5102. doi: 10.1073/pnas.72.12.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerbe F, Cox B, Rossant J, Chazaud C. Dynamic expression of Lrp2 pathway members reveals progressive epithelial differentiation of primitive endoderm in mouse blastocyst. Developmental biology. 2008;313:594–602. doi: 10.1016/j.ydbio.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 30.Artus J, Piliszek A, Hadjantonakis AK. The primitive endoderm lineage of the mouse blastocyst: sequential transcription factor activation and regulation of differentiation by Sox17. Developmental biology. 2011;350:393–404. doi: 10.1016/j.ydbio.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widmer C, et al. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13243–13247. doi: 10.1073/pnas.1208072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarkowski AK, Wroblewska J. Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. Journal of embryology and experimental morphology. 1967;18:155–180. [PubMed] [Google Scholar]
- 33.Silva J, Smith A. Capturing pluripotency. Cell. 2008;132:532–536. doi: 10.1016/j.cell.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wennekamp S, Mesecke S, Nedelec F, Hiiragi T. A self-organization framework for symmetry breaking in the mammalian embryo. Nature reviews. Molecular cell biology. 2013;14:454–461. doi: 10.1038/nrm3602. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich JE, Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- 36.Xiong F, et al. Specified neural progenitors sort to form sharp domains after noisy shh signaling. Cell. 2013;153:550–561. doi: 10.1016/j.cell.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kay RR, Thompson CR. Forming patterns in development without morphogen gradients: scattered differentiation and sorting out. Cold Spring Harbor perspectives in biology. 2009;1:a001503. doi: 10.1101/cshperspect.a001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohnishi Y, et al. Small RNA class transition from siRNA/piRNA to miRNA during pre-implantation mouse development. Nucleic acids research. 2010;38:5141–5151. doi: 10.1093/nar/gkq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 40.Kauffmann A, Gentleman R, Huber W. arrayQualityMetrics--a bioconductor package for quality assessment of microarray data. Bioinformatics. 2009;25:415–416. doi: 10.1093/bioinformatics/btn647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornik K. A CLUE for CLUster Ensembles. Journal of Statistical Software. 2005;14 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.