Abstract
Despite the predominance of sleep in early infancy, developmental science has yet to play a major role in shaping concepts and theories about sleep and its associated ultradian and circadian rhythms. Here we argue that developmental analyses help us to elucidate the relative contributions of the brainstem and forebrain to sleep-wake control and to dissect the neural components of sleep-wake rhythms. Developmental analysis also makes it clear that sleep-wake processes in infants are the foundation for those of adults. For example, the infant brainstem alone contains a fundamental sleep-wake circuit that is sufficient to produce transitions among wakefulness, quiet sleep, and active sleep. Also, consistent with the requirements of a “flip-flop” model of sleep-wake processes, this brainstem circuit supports rapid transitions between states. Later in development, strengthening bidirectional interactions between the brainstem and forebrain contribute to the consolidation of sleep and wake bouts, the elaboration of sleep homeostatic processes, and the emergence of diurnal or nocturnal circadian rhythms. The developmental perspective promoted here critically constrains theories of sleep-wake control and provides a needed framework for the creation of fully realized computational models. Finally, with a better understanding of how this system is constructed developmentally, we will gain insight into the processes that govern its disintegration due to aging and disease.
Keywords: circadian rhythm, ultradian rhythm, nocturnal, diurnal, sleep homeostasis, fragmentation, consolidation, suprachiasmatic nucleus, brainstem, hypothalamus, development, flip-flop, sleep switch, sleep disorders, Norway rat, Nile grass rat
The mammalian sleep-wake cycle is an ultradian rhythm modulated by a circadian rhythm. These rhythms are expressed ubiquitously among mammals, contributing to and constraining the timing of daily routines such as eating, drinking, locomoting, foraging, reproducing, and parenting (Moore-Ede, Sulzman, & Fuller, 1982; J. S. Takahashi, Turek, & Moore, 2001; Turek & van Reeth, 1996). Among adults, the circadian timing (i.e., diurnal, nocturnal, crepuscular) and daily quantities of sleep and wake vary widely across species (Siegel, 2005; Smale, Lee, & Nunez, 2003). But much less is known about the developmental paths to these adult characteristics. Here we review the development of sleep-wake rhythms to highlight how a focus on developmental change informs our understanding of the neural mechanisms that ultimately subserve these rhythms in adults. We provide evidence that the fundamental brainstem circuit governing sleep-wake ultradian rhythmicity is already functional early in the postnatal period. Moreover, we show that, across early development, this brainstem circuit is increasingly incorporated into homeostatic and circadian systems that depend more heavily on forebrain neural circuits.
Human adults sleep approximately eight hours each day, of which two hours comprise REM sleep. In contrast, human infants sleep approximately 16 hours each day, of which eight hours comprise REM sleep. Nearly 50 years ago, the ontogenetic hypothesis was proposed to account for this predominance of sleep—especially REM sleep—in early infancy (Roffwarg, Muzio, & Dement, 1966). Descriptions of sleep in the infants of other mammalian species, including rats, rabbits, cats, and rhesus monkeys, further attested to the significance of early development for understanding the mechanisms and functions of this basic biological process (Gramsbergen, Schwartze, & Prechtl, 1970; Jouvet-Mounier, Astic, & Lacote, 1970; Meier & Berger, 1965; Shimizu & Himwich, 1968). Nonetheless, developmental concepts have played a surprisingly small role in shaping the theoretical and empirical foundations of the field. Today, otherwise comprehensive reviews of sleep and circadian rhythms typically ignore their early-life expression and related developmental issues. Moreover, when novel hypotheses are introduced to explain the functions of sleep, there is generally little (if any) substantive consideration of development.
There are several advantages to studying sleep-wake rhythms in young animals. First, there is a sense in which infant rats—like flies or nematodes—represent a “simple system” for the study of sleep-wake processes (Hendricks, Sehgal, & Pack, 2000). This “simplicity” affords unique opportunities. For example, because some sleep components (e.g., cortical delta activity) emerge at different developmental ages, we can use this staggered emergence to investigate the necessary and sufficient neural mechanisms that produce each component. As components emerge, we can track and probe interactions among components and the associated changes in neural circuitry to provide a richer and more convincing account of the causal mechanisms involved.
Second, armed with precise developmental accounts of the neural foundations of sleep, our theories and computational models can only benefit. Indeed, we will argue that a truly comprehensive model of sleep-wake cyclicity must accommodate all developmental data: it must account for what develops as well as when and how it develops.
Finally, by helping to build more robust theories and more precise computational models, developmental analyses can inform our understanding of sleep and circadian disturbances across the lifespan. For example, narcolepsy is a sleep disorder characterized, in part, by the fragmentation of sleep-wake states (Mahowald & Schenck, 2005; Taheri, Zeitzer, & Mignot, 2002). Given that fragmentation is also a defining feature of sleep-wake organization in young animals, improved understanding of the development of the sleep-wake system may lead to a better understanding of the rules that govern its disintegration due to aging or disease (Blumberg, Coleman, Johnson, & Shaw, 2007).
Issues pertaining to developmental investigations of sleep and wakefulness
The seeming disorganization of sleep-wake patterns in infant rats, and the absence of differentiated cortical electroencephalographic (EEG) activity until postnatal day (P) 11, led some to argue that sleep is qualitatively distinct in infant and adult rats (for review, see Blumberg & Seelke, 2010). This view, which stands in stark contrast to the notion that neonatal sleep is built upon its early embryological roots (Corner, 1977), is difficult to sustain in light of recent behavioral, electromyographic (EMG), and neurophysiological studies of sleep across the early postnatal period, primarily using Norway rats (e.g., Blumberg, Seelke, Lowen, & Karlsson, 2005; Karlsson, Gall, Mohns, Seelke, & Blumberg, 2005; Mohns & Blumberg, 2010; Seelke & Blumberg, 2008; Tiriac, Uitermarkt, Fanning, Sokoloff, & Blumberg, 2012). Moreover, in an era when sleep is being investigated effectively in flies (Shaw, Cirelli, Greenspan, & Tononi, 2000), nematodes (Raizen et al., 2008), and zebrafish (Yokogawa et al., 2007)—animals that lack a cerebral cortex—it is paradoxical to deny its existence in infant mammals because they lack a particular pattern of cortical EEG activity.
Underlying these differing views of infant sleep is the fundamental question of what sleep is and how it should be measured. For example, some continue to adhere to the notion that the cortical EEG is a special electrographic measure with causal implications for sleep or wakefulness. For those who insist that our definitions of sleep and wakefulness critically depend upon cortical activity, EEG measures are mandatory.
In contrast, others view sleep as primarily a behavioral state that can and should be studied using behavioral criteria (Prechtl, 1974). Proponents of this view do not doubt the value and significance of electrographic criteria, including the cortical EEG, but they do not necessarily equate sleep states with cortical activity. Importantly, Siegel (1999) emphasizes that “the EEG derives its value because of its correlation with behavioral measures of sleep” (p. 89). He further asserts that no matter what the EEG tells us, “If animals are responsive and locomoting, we say they are awake” (p. 89). Consistent with this view, in a longitudinal study of sleep in two strains of rats, strain differences in infant sleep, measured using only behavioral and EMG measures, were similarly expressed by these animals when they were reexamined as adults, measured using both EMG and EEG measures (Dugovic & Turek, 2001). This finding supports the argument for developmental correspondence between sleep-wake mechanisms in infants and adults, independent of the mechanisms responsible for producing differentiated cortical EEG activity (Blumberg & Seelke, 2010).
On the location of the sleep-wake “flip-flop”
In several seminal empirical reports and reviews, Saper and colleagues introduced and developed the concept of the sleep switch (Lu, Sherman, Devor, & Saper, 2006; Saper, Chou, & Scammell, 2001; Saper, Scammell, & Lu, 2005; Saper, Fuller, Pedersen, & Lu, 2010). The metaphorical notion of a switch—and in particular a “flip-flop” switch—was borrowed from electronics to emphasize two ideas: first, that the mechanisms that produce sleep and wake states are mutually inhibitory, and second, that transitions between sleep and wake occur rapidly such that intermediate states are rare. Relying in part on von Economo’s (1930) neurological insights—derived from his postmortem assessments of the brains of patients with encephalitis lethargica—as well as more recent work, Saper and colleagues identified two flip-flops. The first flip-flop governs transitions between sleep and wake states. A second, subsidiary flip-flop governs transitions between the two primary sleep states: REM (or active) sleep and Non-REM (or quiet) sleep (Lu et al., 2006).
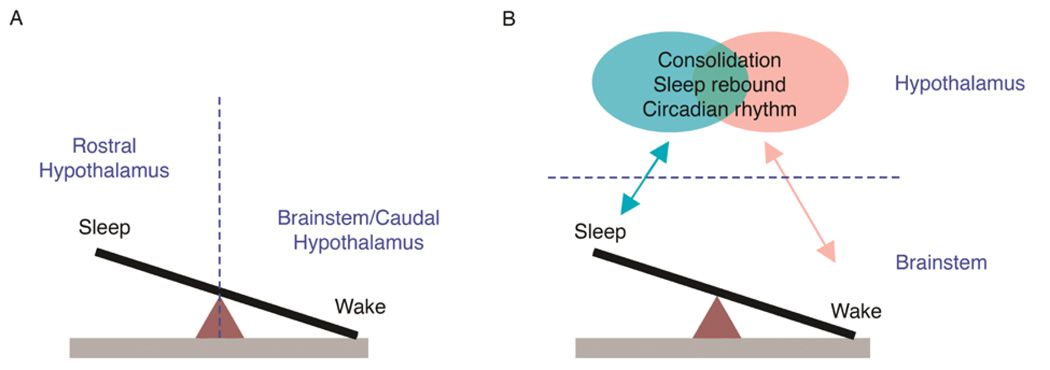
As reported by von Economo (1930), damage to the posterior hypothalamus is associated with excessive sleepiness, thus suggesting that the posterior hypothalamus contains a mechanism that promotes arousal. In contrast, insomnia—a chronic loss of sleep—is associated with damage to the anterior hypothalamus and basal forebrain, thus suggesting that sleep-promoting mechanisms are contained within these areas. Both of these suggestions have subsequently garnered substantial support, and it was Saper’s own group that identified a discrete region within the anterior hypothalamus— the ventrolateral preoptic area (VLPO)—that plays an important role in promoting sleep (Lu, Greco, Shiromani, & Saper, 2000). Figure 1A depicts the fundamental flip-flop governing transitions between sleep and wake. Whereas the wake side of the flip-flop is situated within the brainstem and caudal hypothalamus—including such nuclei as the locus coeruleus (LC), dorsal raphe (DR), and tuberomammilary nucleus (TMN)—the sleep side is depicted as contained exclusively within the rostral hypothalamus, especially the VLPO.
Figure 1.
Alternative views regarding the location of the fundamental “flip-flop” governing sleep-wake transitions. (A) Currently popular models depict sleep-promoting mechanisms located in the rostral hypothalamus (i.e., VLPO) and wake-promoting mechanisms located in the brainstem and caudal hypothalamus (e.g., LC, TMN). According to this model, a transection placed between the two sides of the flip-flop (e.g., a precollicular transection, denoted by the vertical dashed line), would disable the flip-flop. (B) Developmental considerations lead to a model that places the fundamental sleep-wake flip-flop entirely within the brainstem. Putative brainstem wake-promoting nuclei are largely located within the dorsolateral pontine tegmentum and include LDT, LC, and PB; sleep-promoting nuclei include PO and subLC. According to this model, precollicular transection (now denoted by the horizontal dashed line) does not disable the flip-flop, thereby allowing for cyclic alternations between sleep and wake. This brainstem flip-flop exhibits its greatest autonomy early in development; with age, the flip-flop interacts increasingly and bidirectionally with hypothalamic (and other forebrain) mechanisms to consolidate sleep and wake bouts, allow for the expression of sleep rebound after deprivation, and express circadian rhythmicity. Some of these forebrain circuits can influence sleep and wake states independently of one another, whereas others are likely to be at least partially overlapping.
There is, however, a problem with placing the sleep-side of the flip-flop entirely within the anterior hypothalamus: Early transection studies in cats had indicated the presence of a basic sleep-wake circuit located entirely in the brainstem. Villablanca (1965) transected adult cats in the rostral midbrain and reported sleep and wake states that were qualitatively indistinguishable from such periods in intact cats (although the duration of sleep and wake episodes were altered). To assess sleep and wake states in these cats, he measured muscle tone, muscle twitches, and rapid eye movements. Based on this and subsequent work, Villablanca and colleagues (Villablanca, De Andrés, & Olmstead, 2001) concluded that in transected animals “all polygraphic markers were present which allowed us to identify… behavioral episodes… as true REM sleep” (pp. 721–722). Thus, the brainstem—even when surgically disconnected from the forebrain—is sufficient to support basic components of both sleep and wake.
Evidence for the existence of a fundamental and self-contained sleep-wake circuit in the brainstem is also evident from investigations of sleep-wake processes and their neural substrates in infant rats after complete precollicular transection (Karlsson, Kreider, & Blumberg, 2004; Kreider & Blumberg, 2000; Mohns, Karlsson, & Blumberg, 2006). But what are the key brainstem nuclei that are sufficient to support sleep-wake cyclicity? Findings in infant rats point to a sleep-producing region in the medial medulla—which includes nucleus gigantocellularis and nucleus paramedianus—that interacts with several wake- and sleep-promoting areas in the mesopontine region (Karlsson et al., 2005; Karlsson & Blumberg, 2005). This “medullary inhibitory area” in infants corresponds with that identified in adults (Hajnik, Lai, & Siegel, 2000; Lai & Siegel, 1988).
The wake-promoting mesopontine area in infant rats appears localized to the dorsolateral pontine tegmentum (DLPT), which includes the laterodorsal tegmental nucleus (LDT), LC, and parabrachial nucleus (PB) (Karlsson et al., 2005). A particularly high concentration of wake-promoting neurons was found in LDT. Several neurons associated with twitching were also found in LDT and PB, and DLPT lesions, in addition to reducing the amount of wakefulness, significantly reduced the amount of twitching. Interestingly, recent work in adult rats identified arousal-promoting neurons in the parabrachial nucleus (Fuller, Sherman, Pedersen, Saper, & Lu, 2011); this nucleus deserves closer examination in infants to determine its role in sleep-wake regulation early in development.
Sleep-promoting nuclei in the mesopontine region of adult rats includes the nucleus subcoeruleus (subLC; or sublaterodorsal nucleus, SLD) and nucleus pontis oralis (PO) (Boissard, Fort, Gervasoni, Barbagli, & Luppi, 2003; Luppi et al., 2011). These nuclei have been similarly implicated in sleep regulation in infant rats based on recording, lesioning, or sleep-deprivation methods (Karlsson et al., 2005; Todd, Gibson, Shaw, & Blumberg, 2010). Interestingly, lesions of either nucleus not only decrease durations of wakefulness, but twitching is spared such that it is produced against a background of high muscle tone; this condition resembles “REM without atonia” as described in juvenile and adult rats (Mirmiran, 1982; Morrison, 1988). Finally, a sleep-promoting region in adult rats and mice was identified in the “parafacial zone” that projects to the wake-promoting medial parabrachial nucleus (Anaclet et al., 2012). This newly identified region has not yet been investigated in infants.
To summarize, we now know of several candidate brainstem structures, spanning the medulla and mesopontine region, that likely form a fundamental and self-contained sleep-wake circuit. As we continue to dissect these neural circuits and how they change across development, we should also aim to reveal the real-time interactions among them. Ultimately, our goal should be to understand how these interactions yield the oscillatory “flip-flop” processes that generate sleep-wake cyclicity.
One central theme of this paper, described below and illustrated in Figure 1B, is that the brainstem sleep-wake circuit increasingly interacts bidirectionally with forebrain circuits across early development such that critical aspects of sleep-wake functioning in adults—bout consolidation, sleep rebound, and circadian rhythmicity—are expressed. This process can be visualized by tracking changes in sleep-wake processes across development and the neural mechanisms that subserve these changes. But before we can review these findings, we must first describe the sleep-wake cycle of newborn rats.
Behavioral, electromyographic, and neurophysiological features of the infant rat’s sleep-wake cycle
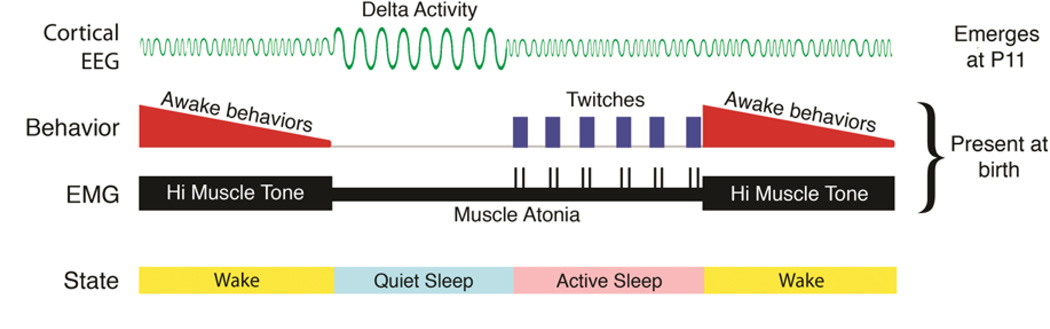
Figure 2 provides a schematic view of sleep and wakefulness across the early postnatal period in rats. First, based on EMG measurements of skeletal muscle tone, we see a simple oscillation between periods of high muscle tone and periods of low muscle tone, or atonia (thick and thin black bars in Figure 2, respectively). Early in postnatal life, all skeletal muscles monitored thus far increase and decrease their tone synchronously, thereby suggesting coherent states of muscle activation throughout the body (reviewed in Blumberg & Seelke, 2010). In fact, even the extraocular muscles, which control eye movements, exhibit fluctuations in tone (Seelke, Karlsson, Gall, & Blumberg, 2005).
Figure 2.
Schematic depiction of state transitions from wake to quiet sleep to active sleep and back to wake in the early postnatal period in Norway rats. Bottom row: Behavioral state categories. Middle rows: Electromyographic (EMG) and behavioral components of the cycle. Skeletal muscle tone fluctuates between high muscle tone during wakefulness and atonia during sleep. At the onset of high muscle tone, wake behaviors are most prevalent, after which they wane and then disappear at the onset of quiet sleep. Twitch movements of the limbs, tail, head, and eyes, which occur in bouts, are observed after a period of behavioral quiescence and mark the onset of active sleep; twitches are also observed as phasic spikes in the EMG record. These two components are observed as early as P2 in rats and require only the brainstem for their full expression. Top row: Beginning at P11, the cortical EEG begins to exhibit delta activity. Even at P11, delta is expressed primarily during the period defined at earlier ages—based on EMG and behavior alone—as quiet sleep.
As also shown in Figure 2, oscillations in muscle tone of newborn rats are tightly coupled with behavior (reviewed in Blumberg and Seelke, 2010). At the onset of high muscle tone, infants typically display high-amplitude, coordinated waking behaviors, including stretching and kicking of the limbs, locomotion, and yawning. Muscle tone typically remains elevated after these overt behaviors have ceased, followed shortly thereafter by rapid decreases toward atonia. Importantly, before P11, atonia with behavioral quiescence provides the only evidence that pups are in quiet sleep.
After a brief period of behavioral quiescence, twitches of the limbs, tail, and head commence. These movements—which are triggered by neurons within the brainstem (Karlsson et al., 2005) and are not mere by-products of a “dreaming” cortex (Blumberg, 2010)—appear quite suddenly and, at early ages, without any further decrease in muscle tone. The twitch movements exhibit complex spatiotemporal structure at individual limb joints, even as early as P2 (Blumberg, Coleman, Gerth, & McMurray, 2013). They are easily detected visually but can also be detected as sharp spikes in the EMG record. When the extraocular muscles were monitored in newborn rats, they also exhibited spike activity corresponding with bouts of twitching elsewhere in the body (Seelke et al., 2005). These latter findings supported the hypothesis that rapid eye movements (REMs) result from twitches of the extraocular muscles, as originally hypothesized many years earlier (Chase & Morales, 1983).
A period of infant sleep is typically terminated by a bout of twitching followed by a sudden burst of high-amplitude movements accompanied by increased muscle tone. This transition marks the end of a single sleep-wake cycle.
As already discussed, the flip-flop model of Saper and colleagues highlights rapid transitions between behavioral states in adults. In infants we see similarly rapid transitions. For example, in rat pups, periods of behavioral quiescence during quiet sleep are typically terminated by the sudden, coordinated onset of twitching throughout the body; and at the end of a series of twitch bouts, we typically see a rapid transition to wake-related movements and high muscle tone. Thus, even without a measure of cortical activity, the concept of a sleep switch applies with equal force to infants.
One particularly salient cortical EEG measure of sleep is delta activity, a 1–4 Hz oscillating wave that is a defining feature of quiet sleep (also known as Non-REM, delta, and slow-wave sleep). In rats, delta activity is not expressed until P11 (Frank & Heller, 1997; Gramsbergen, 1976; Mirmiran & Corner, 1982; Seelke & Blumberg, 2008), necessitating a reliance on behavioral and EMG measures alone at earlier ages. Therefore, the emergence of delta activity at P11 affords a wonderful opportunity to see whether and how sleep-wake states—measured using behavior and EMG—reorganize as EEG correlates of sleep-wake states emerge.
We monitored sleep-wake activity in infant rats using behavioral, EMG, and EEG measures at P9, P11, and P13, that is, immediately before, during, and immediately after the emergence of delta activity, respectively (Seelke & Blumberg, 2008). As illustrated in Figure 2, the delta component of sleep at P11 is added seamlessly into a ‘slot’ that, at earlier ages, is defined as quiet sleep based only on behavioral and EMG criteria. Again, this elemental sleep-wake circuit—present before P11 in rats and supportive of muscle tone oscillations and bouts of twitching—is contained entirely within the brainstem.
Developmental consolidation of ultradian sleep-wake bouts
One defining feature of early postnatal sleep-wake cyclicity is that infants cycle rapidly between brief, or fragmented, bouts of sleep and wake. Fragmentation, as a distinctive feature of infant sleep, was first documented 60 years ago in human infants (Kleitman & Engelmann, 1953). These investigators found that sleep and wake bouts consolidate significantly over the first several postnatal months. Since then, postnatal consolidation of sleep and wake bouts has been documented in a variety of other mammalian species, including precocial rhesus monkeys, altricial rabbits, Norway rats, and mice, and semi-atricial Nile grass rats (Blumberg et al., 2005; Gramsbergen et al., 1970; Meier & Berger, 1965; Shimizu & Himwich, 1968; Todd, Gall, Weiner, & Blumberg, 2012). In sheep, a precocial species, substantial bout consolidation occurs prenatally (Karlsson, Arnardóttir, Robinson, & Blumberg, 2010).
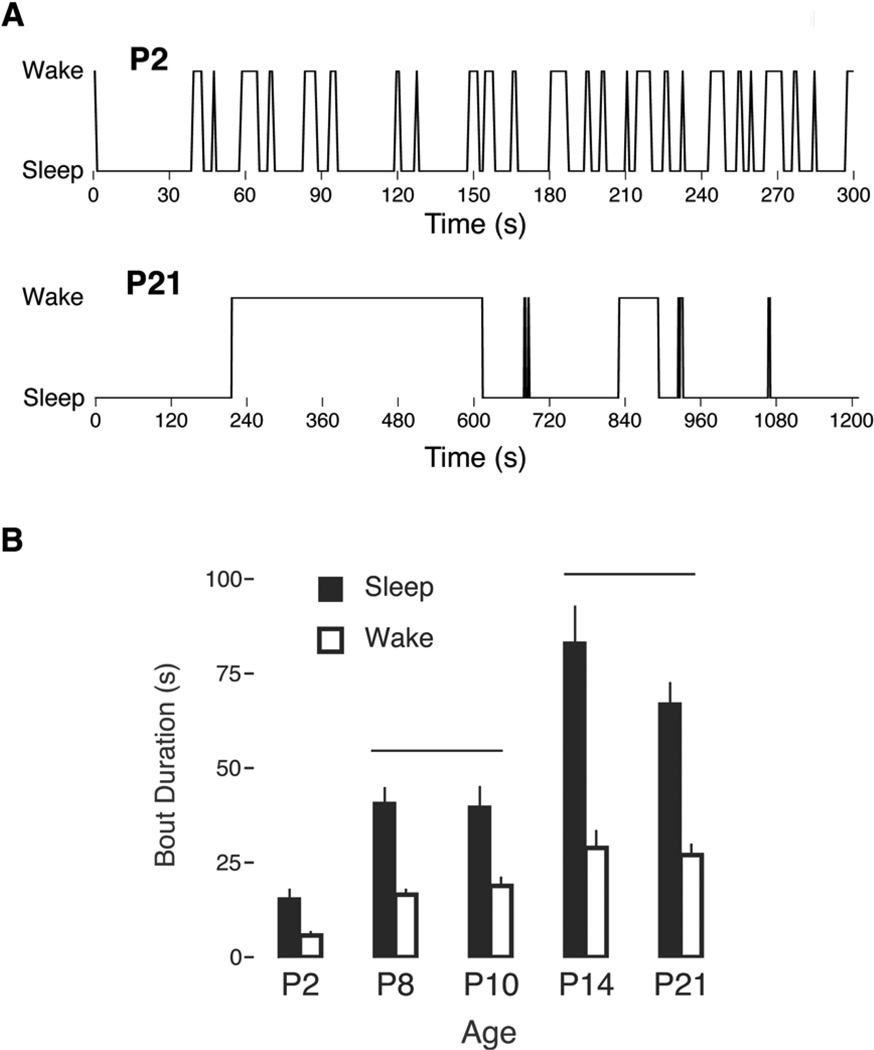
Fragmentation of sleep and wake bouts in infant rats has been documented using behavioral (Gramsbergen et al., 1970) and EMG (Blumberg et al., 2005) measures (Figure 3A). The primary benefit of electromyography is that it allows for precise measurement of low and high muscle tone as a proxy for sleep and wake bout durations, respectively. Accordingly, as shown in Figure 3B, mean sleep and wake bout durations in rats increase more than four-fold over the first two postnatal weeks (Blumberg et al., 2005). This rapid and substantial consolidation requires forebrain participation, as precollicular transections performed at P8 result in fragmented sleep and wake bouts that resemble those expressed at P2 (Karlsson et al., 2004). More precise lesions within the hypothalamus and basal forebrain at P8 also produce fragmented sleep and wake bouts (Mohns et al., 2006).
Figure 3.
(A) Fragmented sleep and wake bouts in a P2 Norway rat (upper) in relation to the relatively consolidated bouts at P21 (lower). Note the different time scales in the two traces. (B) Mean sleep (filled bars) and wake (open bars) bout durations in rats at five postnatal ages. The horizontal lines indicate patterns of significant age differences in sleep and wake durations. Means are presented with standard errors. Adapted from Blumberg, M. S., Seelke, A. M. H., Lowen, S. B., & Karlsson, K. Æ. (2005). Dynamics of sleep-wake cyclicity in developing rats. Proceedings of the National Academy of Sciences of the United States of America 102, 14860–14864.
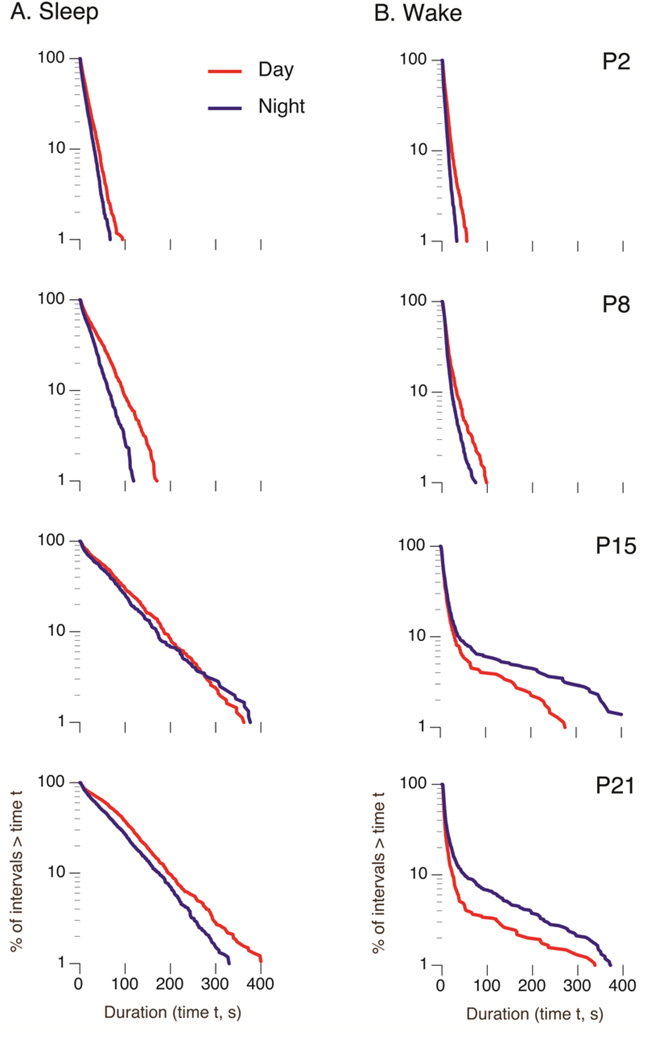
In early development, sleep and wake bouts distribute exponentially, as one would expect of a Markov process in which state transitions occur with a constant probability (Blumberg et al., 2005). Although sleep distributions in several mammalian species, including rats, maintain this exponential distribution into adulthood (Lo et al., 2004), wake distributions uniquely transition from an exponential to a power-law distribution (Blumberg et al., 2005); the developmental changes in the sleep-wake distributions of rats are shown in Figure 4. One characteristic of a power-law distribution is the expression of a small percentage of extremely long bouts. Thus, in effect, the conversion from an exponential to a power-law distribution constitutes a qualitatively distinct form of wake bout consolidation.
Figure 4.
Log-survivor distributions of (A) sleep and (B) wake bout durations in Norway rats across the first three postnatal weeks. Plots also depict values for the day (red) and night (blue). Each plot is constructed from pooled data (620–2213 points per plot). Straight lines on semi-log plots indicate that the data follow an exponential distribution; deviations from a straight line in the wake bouts at P15 and P21 are indicative of power-law distributions. Also, by P15, increased wakefulness at night becomes evident in this nocturnal species. Adapted from Gall, A. J., Todd, W. D., Ray, B., Coleman, C., & Blumberg, M. S. (2008). The development of day-night differences in sleep and wakefulness in Norway rats and the effect of bilateral enucleation. Journal of Biological Rhythms 23, 232–241.
What changes in neural circuitry account for the consolidation of wake bouts across development? To answer this question we can look first to adults, in which an arousal-producing circuit linking the forebrain and brainstem has been identified (Aston-Jones, Chen, Zhu, & Oshinsky, 2001). This circuit includes two hypothalamic nuclei— the suprachiasmatic nucleus (SCN)1 and the dorsomedial hypothalamus (DMH)—as well as the LC in the brainstem. To determine the contribution of this circuit to the development of wake bout consolidation, bilateral lesions of the SCN or DMH were performed in P8 rats with subsequent testing at P21. Lesions of either the SCN (see Figure 5) or DMH produced highly fragmented wake bouts and also prevented the normal development of power-law wake behavior (Gall et al., 2012). Because we had previously demonstrated that destruction of LC terminals eliminates power-law wake behavior without affecting the consolidation of wake bouts (Gall et al., 2009), we concluded that the SCN and DMH consolidate wake bouts independently of the LC.
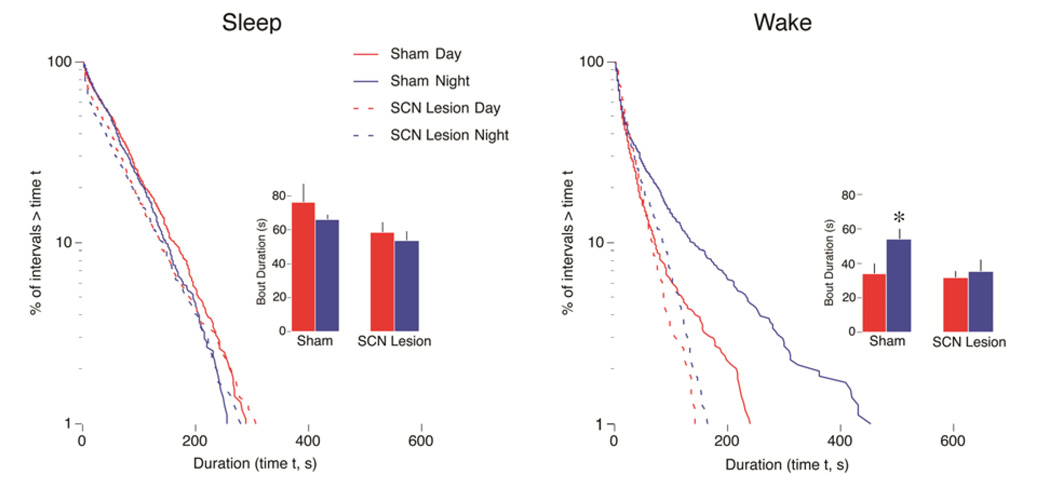
Figure 5.
Effects of SCN lesions on sleep and wake bout distributions in Norway rats. Lesions (or sham surgeries) were performed at P8 with testing at P21. Log-survivor plots are from pooled data (658–937 points per plot). Sham (solid line) and lesioned (dashed line) pups were recorded during the day (red) and at night (blue). Insets present mean sleep bout durations for sham and lesioned pups during the day and at night. * Significant difference from the corresponding daytime value. n = 6 subjects per group. Means are presented with standard errors. Adapted from Gall, A. J., Todd, W. D., & Blumberg, M. S. (2012). Development of SCN connectivity and the circadian control of arousal: A diminishing role for humoral factors? PLoS ONE 7, e45338.
In contrast with wake bout consolidation, disrupting the SCN-DMH-LC circuit had little or no effect on sleep bout consolidation, which is consistent with findings in adult squirrel monkeys with SCN lesions (Edgar et al., 1993). Because precollicular transections at P8 produce highly fragmented wake and sleep bouts (Karlsson et al., 2004), there must be a separate forebrain circuit responsible for the consolidation of sleep bouts. This sleep-promoting circuit, which almost certainly includes the VLPO (Mohns et al., 2006), remains to be fully delineated. Clearly, more work is needed to reveal the points of interaction between the sleep- and wake-promoting circuits.
Fragmented sleep and wake bouts are also a defining feature of narcolepsy, a human neurodegenerative disorder (Mahowald & Schenck, 2005). The co-discovery in the late 1990s of orexin (or hypocretin), led rapidly to the realization that this neurotransmitter is intimately connected with narcolepsy in dogs and humans (for review, see Taheri et al., 2002). Orexin knockout mice were developed quickly and found to exhibit many of the features of human narcolepsy (Chemelli et al., 1999; Willie et al., 2003).
Noting that sleep and wake bout fragmentation characterizes both narcoleptics and infants, we hypothesized that orexin knockout mice would exhibit different developmental patterns of sleep and wake bout consolidation than wild-type controls (Blumberg et al., 2007). This hypothesis proved correct, but only for pups older than 12 days of age. Specifically, between P2 and P12, knockouts and wild-types exhibited substantial and identical consolidation of sleep and wake bouts. However, between P12 and P21, further consolidation of sleep and wake bouts in the knockouts lagged behind that of the wild-types, even as power-law wake behavior emerged in both strains. Therefore, it appears that both orexin-independent and orexin-dependent forebrain mechanisms contribute to consolidation of sleep and wake bouts across early development.
Homeostatic regulation of ultradian sleep-wake processes
When deprived of sleep for long periods of time, humans and other animals exhibit two compensatory responses that are collectively referred to as “sleep homeostasis” (Borbely & Achermann, 1999; Rechtschaffen, Bergmann, Gilliland, & Bauer, 1999). Sleep pressure is an increase in the drive to sleep during the period of deprivation itself, and sleep rebound is an increase in the amount or intensity of sleep after the deprivation procedure has terminated.
Under experimental conditions of high sleep pressure, subjects repeatedly attempt to sleep and experimenters counter each attempt with arousing stimulation. In adult rats, these procedures have revealed areas within the anterior hypothalamus whose neuronal activity profiles are closely associated with the magnitude of sleep pressure (Gvilia, Turner, Mcginty, & Szymusiak, 2006a; Gvilia, Xu, Mcginty, & Szymusiak, 2006b). Such findings in adults give the impression that sleep pressure depends exclusively upon hypothalamic mechanisms, but this is not the case. Specifically, again using transections to separate the brainstem from the hypothalamus, adult cats exhibit increased sleep pressure during deprivation (de Andres, Garzón, & Villablanca, 2003). Thus, the adult brainstem alone is sufficient to support at least some aspects of sleep pressure.
Sleep rebound is variably operationalized as increased sleep time or intensity of delta wave activity (i.e., delta power). Of course, increased delta power can only be used as a marker of recovery sleep at ages when delta activity is expressed, that is, at least P11 in rats. Accordingly, in their examination of sleep homeostasis in developing rats, Frank and colleagues (Frank, Morrissette, & Heller, 1998) began their observations at P12. They found that although delta power did not increase until P24, time in quiet sleep rebounded at P12. But can we assess sleep rebound at even earlier ages when delta waves are not yet expressed?
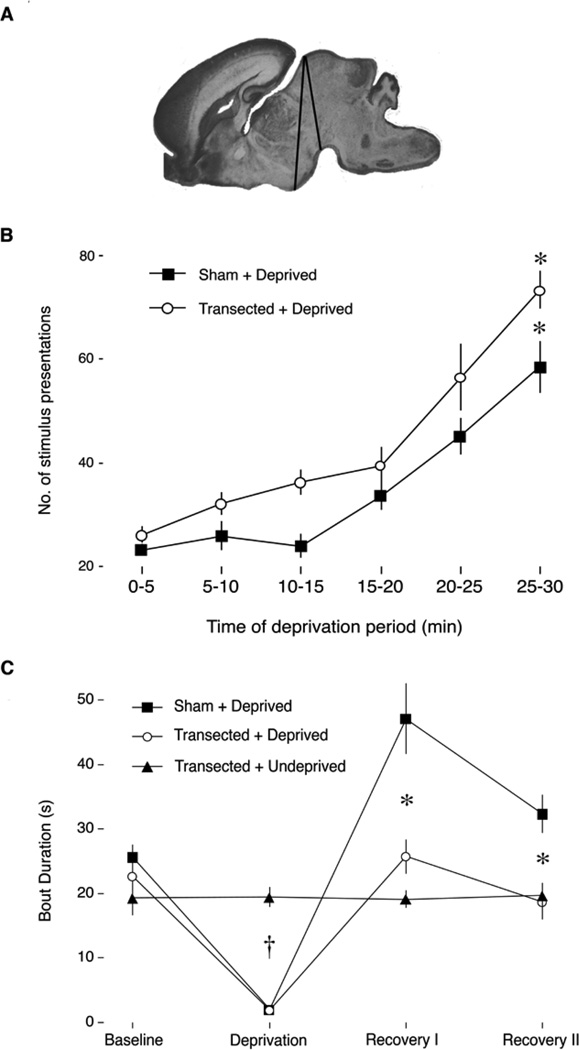
Todd and colleagues (2010) deprived P2 rats of sleep using cold stimulation applied to the snout (see Figure 6). At this age, 30 minutes of sleep deprivation was sufficient to produce significant increases in sleep pressure. Then, when the pups were allowed to sleep they exhibited sleep rebound, expressed as more consolidated sleep bouts. Moreover, and in contrast with sleep pressure, this sleep rebound was eradicated by precollicular transection, thus indicating that the forebrain is required for this form of sleep rebound even this early in development. In fact, we identified anterior hypothalamic nuclei, including the VLPO, that exhibited robust neural activation during sleep rebound.
Figure 6.
Sleep pressure and rebound in P2 Norway rats with and without precollicular transections. (A) Sagittal section of a P2 rat brain to show the anterior-to-posterior range of the transections, denoted by black lines. (B) Mean number of presentations of an arousing stimulus for each 5-min interval during the deprivation period for sham (filled squares) and transected (open circles) groups. In both groups, the number of presentations required to maintain arousal increased significantly over the 30-min deprivation period, indicative of sleep pressure. * Significant difference from the first 5-minute interval. (C) Mean sleep bout durations for three experimental groups during the baseline, sleep deprivation, and recovery periods. Only the Sham+Deprived group exhibited a significant increase in bout duration during the recovery periods, suggesting that neural tissue anterior to the transection is necessary for expressing sleep rebound. † Significant difference from Transected+Undeprived. * Significant difference from Sham+Deprived. Means are presented with standard errors. From Todd, W. D., Gibson, J., Shaw, C., & Blumberg, M. S. (2010). Brainstem and hypothalamic regulation of sleep pressure and rebound in newborn rats. Behavioral Neuroscience, 124, 69–78.
All together, these results make clear that the two components of sleep homeostasis—pressure and rebound—are dissociable very early in development. It is also clear that the two forms of sleep rebound—increased sleep duration and delta power—exhibit very different developmental profiles. More work is needed to understand the degree of overlap in the neural mechanisms controlling these two forms of sleep rebound across early development, both before and after the emergence of cortical delta activity.
Finally, the neuromodulator adenosine has been heavily implicated in sleep pressure as well as in the increases in sleep duration and delta power that characterize sleep rebound (Basheer, Strecker, Thakkar, & McCarley, 2004). Adenosinergic agonists have sleep-promoting effects in numerous sites throughout the brain (Marks & Birabil, 2000; Scammell et al., 2001; Strecker et al., 2000). However, during periods of sleep deprivation, increases in adenosine levels appear to be specific to the basal forebrain and cortex, with sharp decreases in those levels occurring during sleep rebound (Porkka-Heiskanen, Strecker, & McCarley, 2000). Unfortunately, little is currently known about the functions of adenosine in early infancy and how they relate to the changing expression and neural mechanisms of sleep pressure and rebound across early development.
Circadian sleep-wake rhythms: Development and evolution
In addition to its aforementioned role in ultradian sleep-wake rhythms, the SCN is most familiar as a circadian pacemaker (Stephan & Zucker, 1972). The SCN modulates a variety of physiological and behavioral processes on a 24-hour cycle. The cyclicity is endogenous as, even in vitro, SCN neural tissue expresses a ~24-hour rhythm of activity that first develops around embryonic day (E) 22 in rats, with circadian rhythms of glucose metabolism being detectable as early as E19 (Reppert & Schwartz, 1984; Shibata & Moore, 1987). These fetal rhythms are synchronized to the light-dark cycle via the mother’s circadian system (Christ, Korf, & Gall, 2012; Reppert, 1985; Reppert & Schwartz, 1983; 1986b). It is not yet clear how the mother synchronizes the fetal circadian system. Signals from several maternal endocrine organs (i.e., pituitary, adrenals, thyroid-parathyroids, ovaries, and pineal) appear to have been ruled out (Reppert & Schwartz, 1986a), although timed injections of melatonin or dopamine into SCN-lesioned mothers are capable of entraining her fetuses (Davis & Mannion, 1988; Viswanathan, Weaver, Reppert, & Davis, 1994). More recently, it has been suggested that maternal feeding may play an important role in entrainment of the fetal SCN (Ohta et al., 2008).
Postnatally, but before P8, rat dams continue to entrain pups’ circadian rhythms, whereas after P8 light overrides maternal influences to become the predominant entraining stimulus (Duncan, Banister, & Reppert, 1986; Ohta, Honma, Abe, & Honma, 2002; K. K. Takahashi & Deguchi, 1983). Also during the first postnatal week, the retinohypothalamic tract (RHT) develops rapidly, and the strength of connectivity between the RHT and SCN increases as well (Hannibal & Fahrenkrug, 2004; Speh & Moore, 1993).
The fact that the rat’s SCN exhibits rhythmicity at birth suggested that sleep-wake circadian rhythmicity would also be detectible by then (Reppert, 1985; Reppert, Weaver, & Rivkees, 1988). Indeed, we found that day-night differences in sleep and wakefulness could be detected as early as P2 (Gall, Todd, Ray, Coleman, & Blumberg, 2008). However, these day-night differences were expressed in an unexpected way. Specifically, both sleep and wake bouts were shorter at night than during the day, resulting in substantially faster ultradian cycling at night. It was not until pups were two weeks of age, after light becomes the predominant entraining stimulus, that they began exhibiting the species-typical nocturnal pattern comprising longer wake bouts and shorter sleep bouts at night (Figure 4) (Gall et al., 2008).
Having documented the developmental emergence of nocturnal sleep-wake rhythms in Norway rats, we wondered how these rhythms would emerge developmentally in a closely related diurnal species. Our guiding idea was that a developmental comparative approach could potentially help to reveal the neural mechanisms underlying species differences in circadian preference. The central mystery here is that the SCN of all species studied thus far, whether nocturnal or diurnal, is more active during the day than during the night. Thus, species differences in circadian preference must lie downstream of the SCN (Smale et al., 2003).
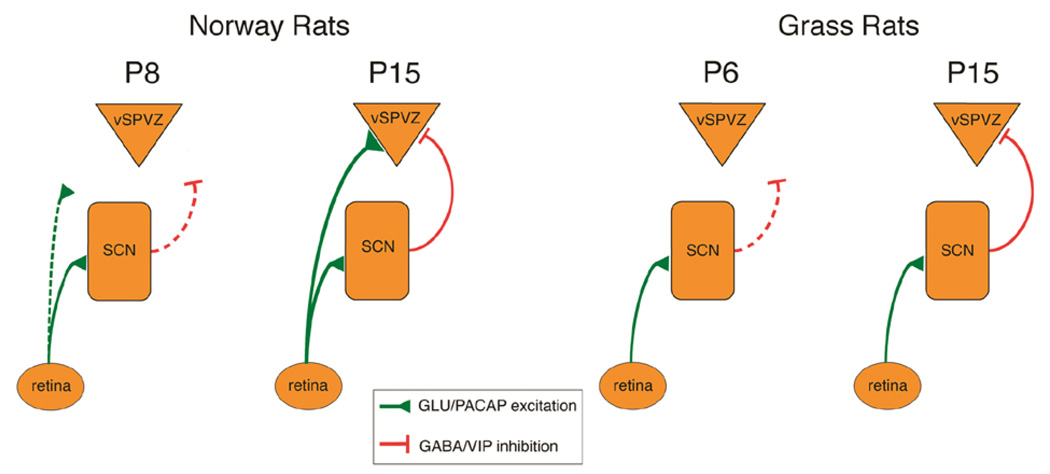
For our comparison, we chose the diurnal Nile grass rat (Arvicanthis niloticus), a Murid rodent that is closely related to Norway rats and about whose circadian biology much has been learned in recent years (Smale et al., 2003). Similar to Norway rats, the day-night sleep-wake pattern of grass rats emerged over the first two postnatal weeks: the initially slightly longer daytime wake bouts at P2 became substantially longer between P8 and P15 (Todd et al., 2012). Next, using Fos immunohistochemistry, we compared day-night differences in neural activity at P8 and P15 within the SCN and an interconnected adjacent structure, the ventral subparaventricular zone (vSPVZ). Previous work in adult grass rats had shown that the SCN and vSPVZ exhibit an anti-phase activity pattern across the day and night (i.e., when one is active, the other is not), whereas these structures exhibit an in-phase activity pattern across the day and night in adult Norway rats (Schwartz, Nunez, & Smale, 2004). We found that the developmental emergence of these activity patterns mirrored the behavioral findings, emerging between P8 and P15 in both species.
In Norway rats, the RHT sends excitatory connections directly from the retina to the SCN (Hannibal, Moller, & Ottersen, 2000) (see Figure 7). In turn, the SCN sends a predominately inhibitory connection to the vSPVZ (Hermes, Kolaj, Doroshenko, Coderre, & Renaud, 2009). Noting that parallel excitatory connections from the retina to the SCN and vSPVZ could explain the in-phase SCN-vSPVZ activity pattern, we predicted that the RHT connection to the vSPVZ would develop by the end of the second postnatal week in concert with the emergence of the in-phase activity pattern. Using retinal tracing and immunohistochemical staining for presynaptic terminals expressing PACAP, a neurotransmitter released by the RHT that is colocalized with glutamate (Hannibal et al., 2000), we found strong support for this prediction (Todd et al., 2012). But what might account for the anti-phase activity pattern in grass rats? We predicted that grass rats would develop little or no RHT projections to the vSPVZ nor PACAP terminals at the vSPVZ, allowing the inhibitory projection from the SCN to the vSPVZ to produce the anti-phase pattern. Again, our data supported this prediction.
Figure 7.
Proposed model of developmental and species differences in neural connections among retina, SCN, and ventral subparaventricular zone (vSPVZ) in Norway rats and Nile grass rats. Green lines: presumed excitatory connections releasing glutamate (GLU) and pituitary adenylate cyclase (PACAP). Red lines: presumed inhibitory connections releasing GABA and vasoactive intestinal peptide (VIP). Dashed lines denote developing or relatively weak connections. From Todd, W. D., Gall, A. J., Weiner, J. A., & Blumberg, M. S. (2012). Distinct retinohypothalamic innervation patterns predict the developmental emergence of species-typical circadian phase preference in nocturnal Norway rats and diurnal Nile grass rats. The Journal of Comparative Neurology, 520, 3277–3292.
All together, these findings suggested that species differences in the developmental wiring of the RHT system contribute to species differences in circadian preference. To further test our hypothesis and broaden our perspective, we performed a retrospective analysis of the existing literature on retinal tracing in a variety of nocturnal and diurnal species. We discovered a general pattern such that nocturnal species, such as Norway rats, possess a strong direct connection between the RHT and the vSPVZ, and diurnal species, such as grass rats, lack a strong direct connection. This analysis also suggested that the maturity of young at birth (i.e., altriciality vs. precociality) and the timing of RHT development (i.e., prenatal vs. postnatal) modulate the functioning of this connection and the development of species-typical circadian preference (Todd et al., 2012). Additional work in a diversity of species is needed to rigorously test our hypothesis and assess the downstream consequences of species differences in RHT connectivity for other neural structures.
Circadian sleep-wake rhythms: Humoral and non-humoral SCN functions
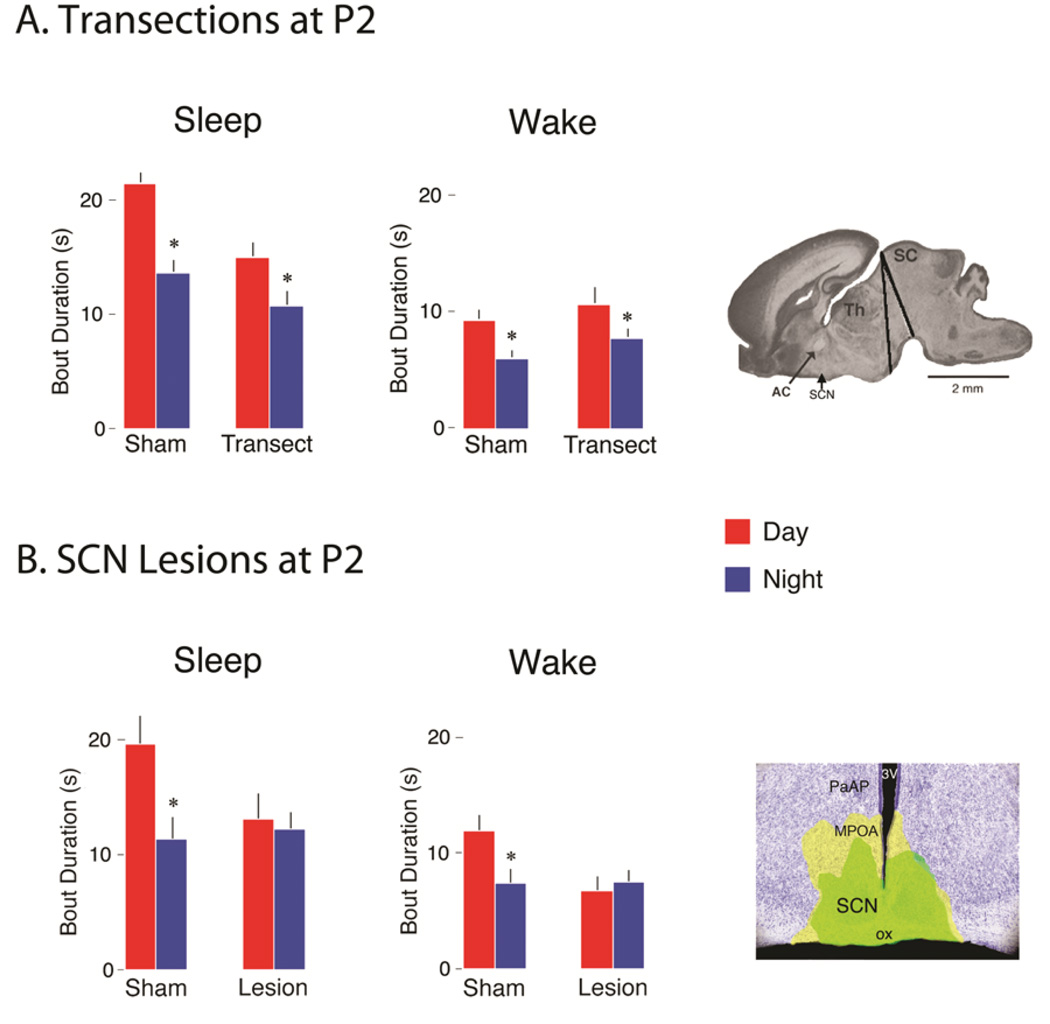
Although the SCN is considered a “master clock” that exerts downstream control of the circadian rhythms of the brain and peripheral organs, its activity is nonetheless modulated by behavioral state (Deboer, Vansteensel, Détári, & Meijer, 2003). We explored this issue in rat pups using a paradigm comprising strong arousing stimulation applied to the pups’ snout. At P2, such stimulation evoked increased neural activity in the LC and DMH, but not the SCN (Todd et al., 2010). In contrast, by P8, the same stimulation evoked activity in all three structures; moreover, activation of the SCN at this age was blocked by prior inactivation of the LC (Gall et al., 2012). These results suggested that interconnectivity among the three structures increases over the first postnatal week, which we confirmed with anatomical tracing.
The functional implications of this emerging LC-DMH-SCN connectivity for infant ultradian and circadian sleep-wake rhythms was probed further using precollicular transections and focal lesions (Gall et al., 2012). First, recall that P2 rats exhibit shorter sleep and wake bouts at night than during the day, resulting in faster sleep-wake cycling at night. Whereas transections caudal to the SCN failed to disrupt this rapid nighttime cycling, SCN lesions did (see Figure 8); this result suggested that the SCN was communicating with brainstem neural circuits via humoral factors (Silver, LeSauter, Tresco, & Lehman, 1996). But because we observed that transections caudal to the SCN at P8 now eliminated any day-night differences in sleep-wake behavior, we were led to hypothesize that the humoral influence of the SCN on sleep-wake behavior had waned.
Figure 8.
Effects of precollicular transections and SCN lesions on day-night differences in sleep and wake bout durations in P2 Norway rats. (A) Mean sleep and wake bout durations for sham and transected pups during the day (red bars) and at night (blue bars). * Significant difference from corresponding daytime value. n = 6 subjects per group. Far right: The range of transections in the sagittal plane. Abbreviations: AC: anterior commissure; SCN: suprachiasmatic nucleus; Th: thalamus; SC: superior colliculus. (B) Mean sleep and wake bout durations for sham and SCN-lesioned pups during the day (red bars) and at night (blue bars). Surgeries were performed at P1. * Significant difference from corresponding daytime value. n = 5 subjects per group. Far right: Photograph of a coronal section showing the extent of the bilateral electrolytic SCN lesions in this experiment; the smallest (green-filled area) and largest (yellow-filled area) lesions are shown. Abbreviations: SCN: suprachiasmatic nucleus; 3V: third ventricle; MPOA: medial preoptic area; ox: optic chiasm; PaAP: Anterior part of parvicellular nucleus. Means are presented with standard errors. Adapted from Gall, A. J., Todd, W. D., & Blumberg, M. S. (2012). Development of SCN connectivity and the circadian control of arousal: A diminishing role for humoral factors? PLoS ONE, 7, e45338.
One possible cause of the apparent loss of SCN humoral influence involves inductive alterations in SCN tissue resulting from the development of direct neural connectivity with other structures (e.g., the DMH). (It is also possible that humoral effects persist beyond the first postnatal week but are masked by the overriding influence of direct neural connections.) If the SCN does indeed lose its capacity to release humoral factors over development, such a loss could explain the preference among investigators for using fetal SCN grafts and the reduced efficacy of SCN grafts for restoring rhythmicity over the first postnatal week in hamsters (Romero, Lehman, & Silver, 1993). Regardless, at the present time we envision the development of circadian rhythmicity as a process whereby the fundamental sleep-wake oscillator in the brainstem comes increasingly under the direct neural influence of the SCN and associated forebrain structures.
Conclusions
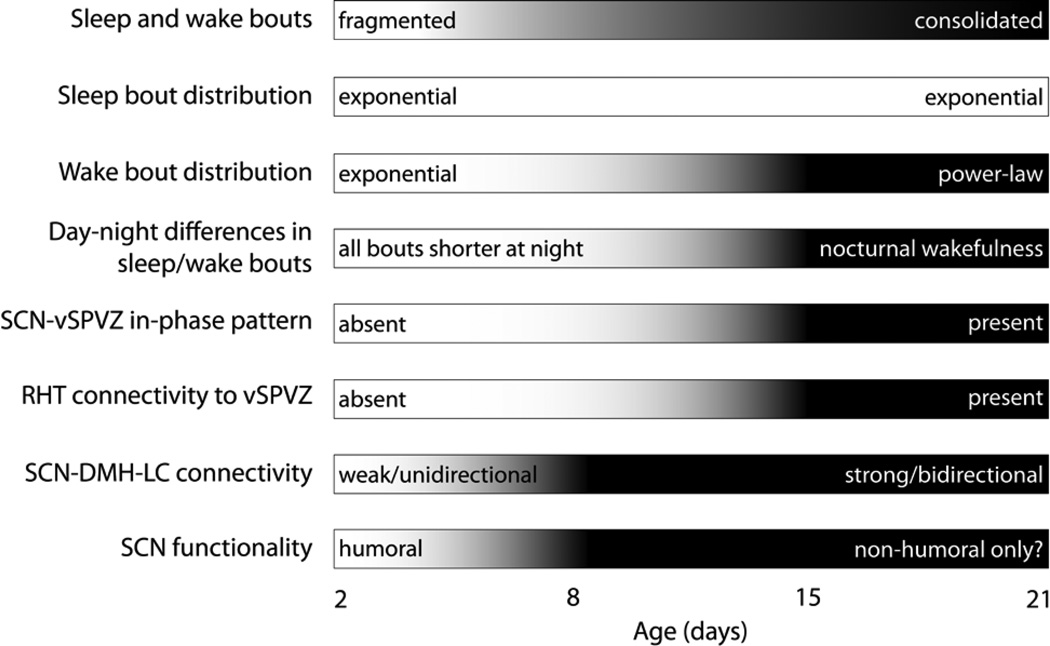
As summarized in Figure 9, the development of ultradian and circadian sleep-wake rhythms in Norway rats comprises complex interactions among multiple processes. One overarching theme that we have emphasized in this review concerns the relative contributions of brainstem and forebrain mechanisms to sleep-wake processes across developmental time. In general, the consolidation of sleep and wake bouts, the emergence of circadian preference, and the transition from an exponential to a power-law wake bout distribution all depend on the elaboration of forebrain circuits and the strengthening of forebrain connections with the brainstem. In addition, at P2, the two dimensions of sleep homeostasis—pressure and rebound—are dissociable, with the brainstem sufficient to express sleep pressure and the forebrain necessary to express sleep rebound.
Figure 9.
Summary of the relative developmental changes in ultradian and circadian sleep-wake rhythmicity and associated neural substrates in Norway rats.
The analysis presented here reinforces the view that sleep-wake processes are, first and foremost, sensorimotor processes. Fluctuations in muscle tone and phasic motor activity (i.e., twitches of the limbs, rapid eye movements) are the pillars of sleep-wake cyclicity, both in terms of their early developmental expression and the brainstem mechanisms that produce them. As we have seen, the sufficiency of the brainstem to produce the basic sleep-wake oscillations of early development is demonstrated most dramatically using transections that sever all connections with the forebrain. Although transections are perhaps crude by comparison with other available techniques for investigating neural function, its power derives from the remarkably complex and integrated behavioral repertoires expressed by transected infants and adults (Bignall, 1974; Bignall & Schramm, 1974; Hicks & D’Amato, 1970).
It is possible that sleep-wake “centers” migrate to more rostral structures as development proceeds (Bignall, 1974). However, the available evidence points to a more complex, interactive scheme in which bidirectional interactions between brainstem and forebrain systems increase progressively with age, resulting in a brainstem system that is modified by its interactions with the forebrain yet still retains its capacity to function with some autonomy after transection. Thus, from both a developmental and evolutionary perspective, the brainstem may comprise an elemental system upon which the forebrain system is built. Importantly, this perspective is consistent with a flip-flop model of sleep switching, but it leads to a different view regarding the precise brainstem and forebrain circuitry subserving sleep-wake transitions (see Figure 1).
To say that the brainstem circuit is elemental is not to say that it is hardwired or innate. This circuit, too, must develop its intrinsic and extrinsic connections to produce the tonic and phasic motor activity depicted in Figure 2. It seems clear that these connections are established largely during the prenatal period in rats and elaborated across early ontogeny, although few developmental details are currently known. Regardless, the brainstem circuit is elemental only in the sense that it is a necessary component of the sleep-wake system upon which all additional components are built.
The period during which brainstem circuits are integrated with forebrain circuits may be one of instability and potential vulnerability. For example, sudden infant death syndrome (SIDS) has a peak incidence of 2 to 4 months (Kinney & Thach, 2009), coinciding with the period in human infants when sleep and wake bouts consolidate and circadian rhythmicity first develops (Kleitman & Engelmann, 1953). One currently popular model of SIDS—the Triple-Risk Model—posits how converging stressors may result “in the asphyxia of a vulnerable infant who has defective cardiorespiratory or arousal defense systems during a critical developmental period when immature defense mechanisms are not fully integrated” (Kinney & Thach, 2009, p. 57). Perhaps the transition to consolidation and circadian control of arousal, dependent as it is on emerging forebrain modulation of brainstem circuits (Gall et al., 2012), represents just such a critical developmental period that, under certain circumstances, can compromise the ability of an infant to arouse from sleep.
We now know a great deal about the brainstem and forebrain circuits controlling sleep and wake states as well as the pathophysiological conditions that arise when that circuitry is compromised. What is still missing, however, is a sense of how the various neural components interact dynamically, in real time, to produce transitions among sleep and wake states. Also missing is an understanding of how ultradian, homeostatic, and circadian systems influence one another. Since these systems develop at different rates, a developmental approach to understanding these systems is especially useful to elucidate their neural mechanisms and mutual dependencies. Critically, for a variety of reasons, it is very difficult to disentangle these systems experimentally in human infants (Jenni, Borbély, & Achermann, 2004). Therefore, infant rats and mice, which can be experimentally manipulated and are born in a relatively immature state, will continue to provide important information concerning the development and neural control of ultradian and circadian sleep-wake rhythms.
One major theme of this review is that our efforts to identify the neural sleep-wake circuit and model its functioning will prove more successful if we focus greater attention on the initial capacity of the brainstem circuit alone to generate these states. Armed with a developmentally informed computational model, we will be better able to achieve a full understanding of how the brainstem and forebrain interact to produce and regulate sleep-wake rhythms. This effort will lead not only to a greater understanding of sleep and wakefulness across the lifespan in health and disease, but will also help us achieve the additional goals of explaining their evolutionary diversity and functional significance across the animal kingdom (Blumberg, 2012; Capellini, Barton, Mcnamara, Preston, & Nunn, 2008; Lesku, Roth, Amlaner, & Lima, 2006; Siegel, 2005).
Acknowledgments
Preparation of this article was made possible by a research grant (HD63071) and an Independent Scientist Award (MH66424) from the National Institutes of Health. We thank Greta Sokoloff, Alex Tiriac, Janet Best, Jim Shaffery, and Deena Schmidt for helpful comments and suggestions.
Footnotes
Although the SCN is most familiar to many as the “master” circadian clock, it also modulates ultradian rhythms (Edgar, Dement, & Fuller, 1993; Gall, Todd, & Blumberg, 2012).
Contributor Information
Mark S. Blumberg, Departments of Psychology and Biology and Delta Center, The University of Iowa, Iowa City, Iowa, 52242 USA
Andrew J. Gall, Department of Psychology, Michigan State University, East Lansing, Michigan 48824
William D. Todd, Department of Neurology and Division of Sleep Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts 02215
References
- Anaclet C, Lin JS, Vetrivelan R, Krenzer M, Vong L, Fuller PM, Lu J. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. Journal of Neuroscience. 2012;32:17970–17976. doi: 10.1523/JNEUROSCI.0620-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky M. A neural circuit for circadian regulation of arousal. Nature Neuroscience. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Progress in Neurobiology. 2004;73(6):379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bignall K. Ontogeny of levels of neural organization: The righting reflex as a model. Experimental Neurology. 1974;42(3):566–573. doi: 10.1016/0014-4886(74)90079-x. [DOI] [PubMed] [Google Scholar]
- Bignall KE, Schramm L. Behavior of chronically decerebrated kittens. Experimental Neurology. 1974;42:519–531. doi: 10.1016/0014-4886(74)90075-2. [DOI] [PubMed] [Google Scholar]
- Blumberg MS. Beyond dreams: Do sleep-related movements contribute to brain development? Frontiers in Neurology. 2010;1:140. doi: 10.3389/fneur.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS. Homology, correspondence, and continuity across development: The case of sleep. Dev Psychobiol. 2012:92–100. doi: 10.1002/dev.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AMH. The form and function of infant sleep: From muscle to neocortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. The Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 391–423. [Google Scholar]
- Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Current Biology. 2013;23:2100–2109. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Coleman C, Johnson E, Shaw C. Developmental divergence of sleep-wake patterns in orexin knockout and wild-type mice. European Journal of Neuroscience. 2007;25:512–518. doi: 10.1111/j.1460-9568.2006.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AMH, Lowen SB, Karlsson KÆ. Dynamics of sleep-wake cyclicity in developing rats. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. European Journal of Neuroscience. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- Borbely A, Achermann P. Sleep homeostasis and models of sleep regulation. Journal of Biological Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- Capellini I, Barton RA, Mcnamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. 2008;62:1764–1776. doi: 10.1111/j.1558-5646.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M, Morales F. Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep. Science. 1983;221:1195–1198. doi: 10.1126/science.6310749. [DOI] [PubMed] [Google Scholar]
- Chemelli R, Willie J, Sinton C, Elmquist J, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Christ E, Korf H-W, Gall von C. When does it start ticking? Ontogenetic development of the mammalian circadian system. Prog Brain Res. 2012;199:105–118. doi: 10.1016/B978-0-444-59427-3.00006-X. [DOI] [PubMed] [Google Scholar]
- Corner M. Sleep and the beginnings of behavior in the animal kingdom -- Studies of ultradian motility cycles in early life. Progress in Neurobiology. 1977;8:279–295. doi: 10.1016/0301-0082(77)90008-9. [DOI] [PubMed] [Google Scholar]
- Davis FC, Mannion J. Entrainment of hamster pup circadian rhythms by prenatal melatonin injections to the mother. American Journal of Physiology. 1988;255:R439–R448. doi: 10.1152/ajpregu.1988.255.3.R439. [DOI] [PubMed] [Google Scholar]
- de Andres I, Garzón M, Villablanca J. The disconnected brain stem does not support rapid eye movement sleep rebound following selective deprivation. Sleep. 2003;26:419–425. doi: 10.1093/sleep/26.4.419. [DOI] [PubMed] [Google Scholar]
- Deboer T, Vansteensel MJ, Détári L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nature Neuroscience. 2003;6:1086–1090. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Turek FW. Similar genetic mechanisms may underlie sleep-wake states in neonatal and adult rats. Neuroreport. 2001;12:3085. doi: 10.1097/00001756-200110080-00021. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Banister MJ, Reppert SM. Developmental appearance of light-dark entrainment in the rat. Brain Research. 1986;369:326–330. doi: 10.1016/0006-8993(86)90544-5. [DOI] [PubMed] [Google Scholar]
- Economo von C. Sleep as a problem of localization. Journal of Nervous and Mental Disease. 1930;71:249–259. [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. Journal of Neuroscience. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Heller H. Development of diurnal organization of EEG slow-wave activity and slow-wave sleep in the rat. American Journal of Physiology. 1997;273:R472–R478. doi: 10.1152/ajpregu.1997.273.2.R472. [DOI] [PubMed] [Google Scholar]
- Frank M, Morrissette R, Heller H. Effects of sleep deprivation in neonatal rats. American Journal of Physiology. 1998;44:R148–R157. doi: 10.1152/ajpregu.1998.275.1.R148. [DOI] [PubMed] [Google Scholar]
- Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. The Journal of Comparative Neurology. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Joshi B, Best J, Florang V, Doorn J, Blumberg MS. Developmental emergence of power-law wake behavior depends upon the functional integrity of the locus coeruleus. Sleep. 2009;39:920–926. doi: 10.1093/sleep/32.7.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Todd WD, Blumberg MS. Development of SCN connectivity and the circadian control of arousal: A diminishing role for humoral factors? PLoS ONE. 2012;7:e45338. doi: 10.1371/journal.pone.0045338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Todd WD, Ray B, Coleman C, Blumberg MS. The development of day-night differences in sleep and wakefulness in Norway rats and the effect of bilateral enucleation. Journal of Biological Rhythms. 2008;23:232–241. doi: 10.1177/0748730408316954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen A. The development of the EEG in the rat. Dev Psychobiol. 1976;9:501–515. doi: 10.1002/dev.420090604. [DOI] [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Dev Psychobiol. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Gvilia I, Turner A, Mcginty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. Journal of Neuroscience. 2006a;26:3037–3044. doi: 10.1523/JNEUROSCI.4827-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gvilia I, Xu F, Mcginty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. Journal of Neuroscience. 2006b;26:9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnik T, Lai Y, Siegel JM. Atonia-related regions in the rodent pons and medulla. Journal of neurophysiology. 2000;84:1942–1948. doi: 10.1152/jn.2000.84.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Melanopsin containing retinal ganglion cells are light responsive from birth. Neuroreport. 2004;15:2317–2320. doi: 10.1097/00001756-200410250-00003. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Moller M, Ottersen OP. PACAP and glutamate are co- stored in the retinohypothalamic tract. The Journal of Comparative Neurology. 2000;418:147–155. [PubMed] [Google Scholar]
- Hendricks J, Sehgal A, Pack A. The need for a simple animal model to understand sleep. Progress in Neurobiology. 2000;61:339–351. doi: 10.1016/s0301-0082(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Hermes M, Kolaj M, Doroshenko P, Coderre E, Renaud LP. Effects of VPAC2 receptor activation on membrane excitability and GABAergic transmission in subparaventricular zone neurons targeted by suprachiasmatic nucleus. Journal of neurophysiology. 2009;102:1834–1842. doi: 10.1152/jn.91261.2008. [DOI] [PubMed] [Google Scholar]
- Hicks S, amp; D’Amato C. Motor-sensory and visual behavior after hemispherectomy in newborn and mature rats. Experimental Neurology. 1970;29:416–438. doi: 10.1016/0014-4886(70)90069-5. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Borbély AA, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. American journal of physiology Regulatory, integrative and comparative physiology. 2004;286:R528–38. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience. 2005;130:275–283. doi: 10.1016/j.neuroscience.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Arnardóttir H, Robinson SR, Blumberg MS. Dynamics of sleep-wake cyclicity across the fetal period in sheep (Ovis aries) Dev Psychobiol. 2010;53:89–95. doi: 10.1002/dev.20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biology. 2005;3:e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Kreider J, Blumberg MS. Hypothalamic contribution to sleep-wake cycle development. Neuroscience. 2004;123:575–582. doi: 10.1016/j.neuroscience.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. The New England Journal of Medicine. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitman N, Engelmann T. Sleep characteristics of infants. Journal of Applied Physiology. 1953;6:269–282. doi: 10.1152/jappl.1953.6.5.269. [DOI] [PubMed] [Google Scholar]
- Kreider J, Blumberg MS. Mesopontine contribution to the expression of active “twitch” sleep in decerebrate week-old rats. Brain Research. 2000;872:149–159. doi: 10.1016/s0006-8993(00)02518-x. [DOI] [PubMed] [Google Scholar]
- Lai Y, Siegel JM. Medullary regions mediating atonia. Journal of Neuroscience. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesku JA, Roth TC, Amlaner CJ, Lima SL. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. The American Naturalist. 2006;168:441–453. doi: 10.1086/506973. [DOI] [PubMed] [Google Scholar]
- Lo C-C, Chou T, Penzel T, Scammell TE, Strecker RE, Stanley HE, Ivanov PC. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17545–17548. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Greco M, Shiromani P, Saper C. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. Journal of Neuroscience. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for conrol of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Clément O, Sapin E, Gervasoni D, Peyron C, Léger L, et al. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Medicine Reviews. 2011;15:153–163. doi: 10.1016/j.smrv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Mahowald M, Schenck C. Insights from studying human sleep disorders. Nature. 2005;437:1279–1285. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- Marks GA, Birabil CG. Infusion of adenylyl cyclase inhibitor SQ22,536 into the medial pontine reticular formation of rats enhances rapid eye movement sleep. Neuroscience. 2000;98:311–315. doi: 10.1016/s0306-4522(00)00093-2. [DOI] [PubMed] [Google Scholar]
- Meier G, Berger R. Development of sleep and wakefulness patterns in the rhesus monkey. Experimental Neurology. 1965;12:257–277. doi: 10.1016/0014-4886(65)90071-3. [DOI] [PubMed] [Google Scholar]
- Mirmiran M. In: “Oneiric” behavior during active sleep induced by bilateral lesions of the pontine tegmentum in juvenile rats. Koella W, editor. Basil: Presented at the Sleep: Sixth European Congress of Sleep Research; 1982. pp. 236–239. [Google Scholar]
- Mirmiran M, Corner M. Neuronal discharge patterns in the occipital cortex of developing rats during active and quiet sleep. Brain Research. 1982;255:37–48. doi: 10.1016/0165-3806(82)90074-8. [DOI] [PubMed] [Google Scholar]
- Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in newborn rats. Journal of Neuroscience. 2010;30:3438–3449. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohns EJ, Karlsson KÆ, Blumberg MS. The preoptic hypothalamus and basal forebrain play opposing roles in the descending modulation of sleep and wakefulness in infant rats. European Journal of Neuroscience. 2006;23:1301–1310. doi: 10.1111/j.1460-9568.2006.04652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM, Fuller C. The clocks that time us. Cambridge: Harvard University Press; 1982. [Google Scholar]
- Morrison A. Paradoxical sleep without atonia. Archives Italiennes de Biologie. 1988;126:275–289. [PubMed] [Google Scholar]
- Ohta H, Honma S, Abe H, Honma K. Effects of nursing mothers on rPer1 and rPer2 circadian expressions in the neonatal rat suprachiasmatic nuclei vary with developmental stage. European Journal of Neuroscience. 2002;15:1953–1960. doi: 10.1046/j.1460-9568.2002.02016.x. [DOI] [PubMed] [Google Scholar]
- Ohta H, Xu S, Moriya T, Iigo M, Watanabe T, Nakahata N, et al. Maternal feeding controls fetal biological clock. PLoS ONE. 2008;3:e2601. doi: 10.1371/journal.pone.0002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Prechtl HFR. The behavioural states of the newborn infant. Brain Research. 1974;76:185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
- Raizen D, Zimmerman J, Maycock M, Ta U, You Y, Sundaram M, Pack A. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann B, Gilliland M, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- Reppert SM. Maternal entrainment of the developing circadian system. Annals of the New York Academy of Sciences. 1985;453:162–169. doi: 10.1111/j.1749-6632.1985.tb11808.x. [DOI] [PubMed] [Google Scholar]
- Reppert SMS, Weaver DRD, Rivkees SAS. Maternal communication of circadian phase to the developing mammal. Psychoneuroendocrinology. 1988;13:63–78. doi: 10.1016/0306-4530(88)90007-8. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Schwartz WJ. Maternal coordination of the fetal biological clock in utero. Science. 1983;220:969–971. doi: 10.1126/science.6844923. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Schwartz WJ. The suprachiasmatic nuclei of the fetal rat: characterization of a functional circadian clock using 14C–labeled deoxyglucose. Journal of Neuroscience. 1984;4:1677–1682. doi: 10.1523/JNEUROSCI.04-07-01677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Schwartz WJ. Maternal endocrine extirpations do not abolish maternal coordination of the fetal circadian clock. Endocrinology. 1986a;119:1763–1767. doi: 10.1210/endo-119-4-1763. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Schwartz WJ. Maternal suprachiasmatic nuclei are necessary for maternal coordination of the developing circadian system. Journal of Neuroscience. 1986b;6:2724–2729. doi: 10.1523/JNEUROSCI.06-09-02724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Romero M-T, Lehman M, Silver R. Age of donor influences ability of suprachiasmatic nucleus grafts to restore circadian rhythmicity. Developmental Brain Research. 1993;71:45–52. doi: 10.1016/0165-3806(93)90103-h. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. TRENDS in Neurosciences. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Saper C, Fuller P, Pedersen N, Lu J. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Nunez A, Smale L. Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience. 2004;127:13–23. doi: 10.1016/j.neuroscience.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. European Journal of Neuroscience. 2005;22:911–920. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Cirelli C, Greenspan R, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Shibata S, Moore RY. Development of neuronal activity in the rat suprachiasmatic nucleus. Developmental Brain Research. 1987;431:311–315. doi: 10.1016/0165-3806(87)90220-3. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Himwich H. The ontogeny of sleep in kittens and young rabbits. Electroencephalography and Clinical Neurophysiology. 1968;24:307–318. [PubMed] [Google Scholar]
- Siegel JM. The evolution of REM sleep. In: Lydic R, Baghdoyan H, editors. Handbook of Behavioral State Control. Boca Raton: CRC Press; 1999. pp. 87–100. [Google Scholar]
- Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. Journal of Biological Rhythms. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Speh J, Moore R. Retinohypothalamic tract development in the hamster and rat. Developmental Brain Research. 1993;76:171–181. doi: 10.1016/0165-3806(93)90205-o. [DOI] [PubMed] [Google Scholar]
- Stephan F, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesion. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker RER, Morairty SS, Thakkar MMM, Porkka-Heiskanen TT, Basheer RR, Dauphin LJL, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behavioural Brain Research. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annual Review of Neuroscience. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Turek FW, Moore RY, editors. Handbook of behavioral neurobiology: Circadian clocks. Vol. 12. New York: Kluwer Academic/Plenum; 2001. [Google Scholar]
- Takahashi KK, Deguchi TT. Entrainment of the circadian rhythms of blinded infant rats by nursing mothers. Physiology and Behavior. 1983;31:373–378. doi: 10.1016/0031-9384(83)90204-4. [DOI] [PubMed] [Google Scholar]
- Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Current Biology. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd WD, Gall AJ, Weiner JA, Blumberg MS. Distinct retinohypothalamic innervation patterns predict the developmental emergence of species-typical circadian phase preference in nocturnal Norway rats and diurnal Nile grass rats. The Journal of Comparative Neurology. 2012;520:3277–3292. doi: 10.1002/cne.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd WD, Gibson J, Shaw C, Blumberg MS. Brainstem and hypothalamic regulation of sleep pressure and rebound in newborn rats. Behavioral Neuroscience. 2010;124:69–78. doi: 10.1037/a0018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, van Reeth O. Circadian rhythms. In: Fregly MJ, Blatteis CM, editors. Handbook of physiology: Environmental physiology. Oxford: Oxford University Press; 1996. pp. 1329–1360. [Google Scholar]
- Villablanca J. The electrocorticogram in the chronic cerveau isolé cat. Electroencephalography and Clinical Neurophysiology. 1965;19:576–586. doi: 10.1016/0013-4694(65)90243-9. [DOI] [PubMed] [Google Scholar]
- Villablanca J, De Andrés I, Olmstead C. Sleep-waking states develop independently in the isolated forebrain and brain stem following early postnatal midbrain transection in cats. Neuroscience. 2001;106:717–731. doi: 10.1016/s0306-4522(01)00329-3. [DOI] [PubMed] [Google Scholar]
- Viswanathan N, Weaver DR, Reppert SM, Davis FC. Entrainment of the fetal hamster circadian pacemaker by prenatal injections of the dopamine agonist SKF 38393. Journal of Neuroscience. 1994;14:5393–5398. doi: 10.1523/JNEUROSCI.14-09-05393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie J, Chemelli R, Sinton C, Tokita S, Williams S, Kisanuki Y, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Yokogawa T, Marin W, Faraco J, Pezeron G, Appelbaum L, Zhang J, et al. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biology. 2007;5:2379–2397. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]